Abstract
To develop novel antibiotic peptides useful as therapeutic drugs, the enantiomeric analogue of pleurocidin (Ple), which is a well known 25-mer antimicrobial peptide, was designed for proteolytic resistance by D-amino acids substitution. The proteolytic resistance was confirmed by using HPLC after the digestion with various proteases. To investigate the antibiotic effect of L- and D-Ple, the antibacterial activity and hemolytic effect were tested against human erythrocytes. The D-Ple showed a decreased antibacterial activity and a dramatically decreased hemolytic activity compared with L-Ple. The hemolytic effect of analogue was further confirmed by using calcein leakage measurement with liposome. To elucidate these results, the secondary structure of the peptides was investigated by using circular dichroism spectroscopy. The results revealed that D-Ple, as well as L-Ple, had typical α-helical structures which were mirror images, with a different helicity. These results suggested that the discrepancy of the structure between the two peptides made their antibacterial activity distinct.
Keywords: anti-bacterial agents, hemolysis, molecular structure, pleurocidin, structure-activity relationship
Introduction
Surface-active peptides, i.e. peptides that bind to and affect amphiphilic surfaces, such as membranes, receptors, etc., have been extensively studied in recent years (DeGrado et al., 1981; Kaiser et al., 1988). A common feature of many of these compounds is their characteristic helical amphiphilic secondary structure, which is often induced by the respective target (Bessalle et al., 1990). Also, some representatives of the compounds are cationic wide-range cytolytic peptides isolated from mammalian phagocytes, e.g. defensins (Ganz et al., 1990), insects, e.g. melittins (Habermann et al., 1972), cecropins (Steiner et al., 1981), sarcotoxins (Okada et al., 1985), and amphibians, e.g. magainins (Zasloff et al., 1987). The target of these surface-active peptides appears to be the cellular lipid bilayer membrane.
Pleurocidin (Ple) (GWGSFFKKAAHVGKHVGKAALTHYL-NH2) is one of the most well-known not only as a α-helical cationic antibacterial, but also as surface-active peptides derived from the skin mucous of the winter flounder (Pleuronectes americanus) (Cole et al., 1997). This peptide has been reported to exhibit a broad range of antibacterial activity, to display a primary sequence homology, with dermaseptins, from the skin of the arboreal frog Phyllomedusa bicolor (Mor et al., 1991) and ceratatoxins (Marchini et al., 1993), and also to possess an amphipathic α-helical structure (Yoshida et al., 2001). Due to the importance of the structural features of Ple, this peptide has been studied regarding the correlation between its antibacterial activity and its membrane-disrupting activity (Saint et al., 2002).
On the other hand, although natural antimicrobial peptides including Ple had strong antimicrobial activities, these peptides were very sensitive to enzymatic degradation by proteases in serum, and furthermore, these problems created difficulty in developing peptidic therapeutic agents. To overcome these problems, D-amino acids were substituted for the L-amino acids of antimicrobial peptides. It has been reported that this substitution can improve the antimicrobial activity and proteolytic stability (Hong et al., 1999). Thus, the substitution of D-amino acid could be a strategy for developing proteases-resistant antimicrobial peptides for clinical therapeutic use (Jung et al., 2007b).
The aim of this study is to compare the antibacterial activity between D-Ple and L-Ple.
Materials and Methods
Peptide synthesis
Peptides were synthesized by the solid-phase method using 9-fluorenyl-methoxycarbonyl (Fmoc)-chemistry (Merrifield et al., 1986). Rink amide 4-methyl benzhydrylamine (MBHA) resin (0.55 mmol/g) was used as the support to obtain a C-terminal amidate peptide. The coupling of Fmoc-amino acids was performed with N-hydroxybenzotriazole (HOBt) and dicyclohexylcarbodiimide (DCC). Amino-acid side chains were protected as follows: tert-butyl (Asp), trityl (Gln), and tert-butyloxycarbonyl (Lys). Deprotection and cleavage from the resin were carried out using a mixture of trifluoroacetic acid, phenol, water, thioanisole, 1,2-ethanedithiol, and triisopropylsilane (88:2.5:2.5:2.5:2.5:2.0, v/v) for 2 h at room temperature. The crude peptide was repeatedly washed with a diethylether, dried in a vacuum, and purified using a reversed-phase preparative HPLC on a Waters 15-µm Deltapak C18 column (19 × 30 cm). The purity of the peptide was checked by analytical reversed-phase HPLC on an Ultrasphere C18 column (Beckman, 4.6 × 25 cm). The purified peptides were hydrolyzed with 6 M-HCl for 22 h at 110℃, and then dried in a vacuum. The residues were dissolved in 0.02 M HCl and subjected to an amino acid analyzer (Hitachi, 8500 A, Japan). Peptide concentration was determined by amino acid analysis. The molecular weights of the synthetic peptides were determined using a matrix-assisted laser desorption ionization (MALDI)-mass spectrometer (Jungblut et al., 1997). Confirmation that all-D-Ple was synthesized completely was examined with various proteases in HPLC. These proteases were alpha-chymotrypsin (EC 3.4.21.1, Sigma), endoproteinase Glu-C from Staphylococcus aureus V8 (EC 3.4.21.19, Sigma) and trypsin (EC 3.4.21.4, Sigma).
Bacterial strains and antibacterial activity assay
Staphylococcus epidermidis (KCTC 1917) was obtained from the Korean Collection for Type Cultures (KCTC) of the Korea Research Institute of Bioscience and Biotechnology (KRIBB), Daejeon, Korea. Escherichia coli (ATCC 25922), Escherichia coli O-157 (ATCC 43895), Staphylococcus aureus (ATCC 25923), Enterococcus faecium (ATCC 29212), and Pseudomonas aeruginosa (ATCC 27853) were obtained from the American Type Culture Collection (ATCC) (Manassas, VA). Enterococcus faecium, methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa were clinically isolated from patients of the Kyungpook National University Hospital, Daegu, Korea. Bacterial cells (2 × 107 cells/ml) were inoculated into a Mueller-Hinton broth and 0.1 ml/well was dispensed onto 96-well microtiter plates. The bacteria numbers were calculated by measurement of turbidity with a spectrophotometer (DU530, Beckman, Fullerton, CA) (Park et al., 1986). MICs (minimum inhibitory concentrations) were determined by a serial two-fold dilution of test compounds, following the recommendations of the Clinical and Laboratory Standards Institute (CLSI) (CLSI, 2005). After 24 h of incubation at 37℃, the minimal concentration of compound required to prevent the growth of a given test organism was determined and was defined as MIC. The growth was assayed with a microtiter ELISA Reader (Molecular Devices Emax, CA) by monitoring absorption at 620 nm (Sung et al., 2006).
Circular dichroism (CD) analysis
CD spectra of the peptides were recorded using a spectropolarimeter (Jasco J720, Japan). All samples were maintained at 25℃ during the analysis. Four scans per sample were performed over a wavelength range of 190-250 nm at 0.1 nm intervals. The spectra were measured in 0% and 50% (v/v) TFE (2,2,2-trifluoroethanol) and 30 mM SDS in 10 mM sodium phosphate buffer, pH 7.2, respectively at 25℃ using a 1 mm path length cell. The peptide concentrations were 50 µM. The mean residue ellipticity, [θ], is given in deg · cm2 · dmol-1: [θ] = [θ]obs (MRW/10lc), where [θ]obs is the ellipticity measured in millidegrees, MRW is the mean residue molecular weight of the peptide, c is the concentration of the sample in mg/ml, and l is the optical path length of the cell in cm (Lee et al., 2006).
Hemolytic activity
The hemolytic activity of the peptides was evaluated by determining the amount of released hemoglobin of a 4% suspension of fresh human red blood cells (RBC) at 414 nm (Blondelle et al., 1992). Human RBC were harvested and washed with PBS (35 mM phosphate buffer/0.15 M NaCl, pH 7.0) three times. One hundred microliters of human RBCs, diluted to 8% (v/v) in PBS, was seeded on 96-well plates, and then 100 µl of the peptide solution (from 100 to 0.39 µM) was added to each well. The plates were incubated for 1 h at 37℃. One hundred microliter aliquots were transferred to new 96-well plates, and hemolysis was measured by absorbance at 414 nm with an ELISA plate Reader (Molecular Devices Emax) (Sung et al., 2007). Zero and 100% hemolysis was determined in PBS and 0.1% Triton X-100, respectively (Jung et al., 2005). The hemolysis percentage was calculated by employing the following equation: Percentage hemolysis = [(Abs414nm in the peptide solution - Abs414 nm in PBS) / (Abs414 nm in 0.1% Triton X-100 - Abs414 nm in PBS)] × 100 (Jung et al., 2007a).
Calcein leakage measurement
Calcein-encapsulated large unilamellar vesicles (LUVs) composed of phosphatidylcholine and cholesterol (10:1, w/w) were prepared for calcein leakage measurement. The lipid components were mixed in chloroform by vortexing. The solvents were removed by evaporation for 2 h at 30℃ and then kept under argon gas for substitution. In succession, calcein entrapped LUVs were prepared by vortexing the dried lipids in dye buffer solution (70 mM calcein, 10 mM Tris, 150 mM NaCl and 0.1 mM EDTA, pH 7.4). The suspension was frozen and thawed in liquid nitrogen for ten cycles and extruded through polycarbonate filters (two stacked 100 nm pore size filters; Whatman, NJ) with LiposoFast (Avestin, Ottawa, Canada). Untrapped calcein was removed using a gel filtration composed of a Sephadex G-50 column (1.5 × 30 cm column), and a 10 mM sodium-phosphate buffer (containing 0.15 M NaCl, pH 7.4) was used as a filling liquid. Then, the LUVs treated with peptides were incubated for 2 min. The dye leakage was recorded at 490 nm excitation and 520 nm emission with a JASCO FP-6500 spectrofluorometer.
Results
Susceptibility of D-Ple towards a proteolysis
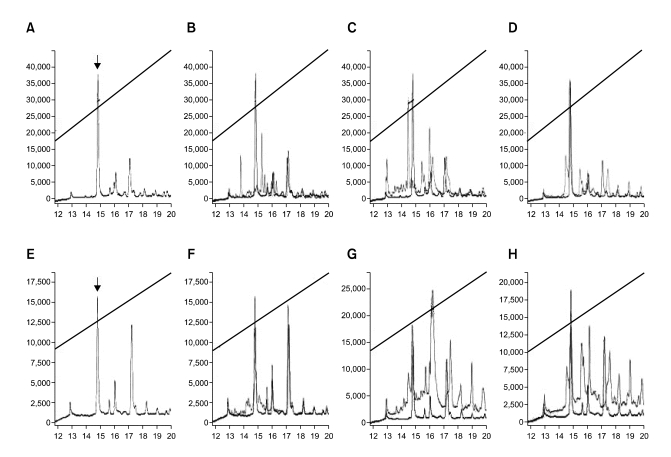
To investigate the susceptibility of D-Ple towards proteolysis, its resistance to the digestive action of proteases, such as alpha-chymotrypsin, endoproteinase Glu-C from Staphylococcus aureus V8 and trypsin, was examined as compared with that of L-Ple. The results by HPLC showed that the D-Ple remained intact completely even after 30 min of incubation with the enzymes, while the L-Ple was either extensively, partially or completely degraded (Figure 1). The result that D-Ple was stable in the proteases degrading of L-Ple indicated that D-amino acid substitutions could make peptides resistant to a proteolysis and thus D-Ple could exert its antimicrobial activity unimpaired. Also, this suggested that D-Ple or relevant derivatives might be potential candidates for clinical use.
Figure 1.
Proteolytic resistance of D-Ple. From A to D and from E to H were treated with 5 µg various proteases to 3 µg L-Ple and D-Ple, respectively. B to D and F to H were an overlay with A and E, respectively, for comparison with the peptide itself. Incubation conditions were for 30 min at 37℃. The oblique line is the alteration of the concentration of acetonitrile. (A) only L-Ple, (B) L-Ple treated with trypsin, (C) L-Ple treated with α-chymotrypsin, (D) L-Ple treated with endoproteinase Glu-C from Staphylococcus aureus V8, (E) only D-Ple, (F) D-Ple treated with trypsin, (G) D-Ple treated with α-chymotrypsin, (H) D-Ple treated with endoproteinase Glu-C from Staphylococcus aureus V8.
Structure and antibacterial activity analysis of D-Ple compared with L-Ple
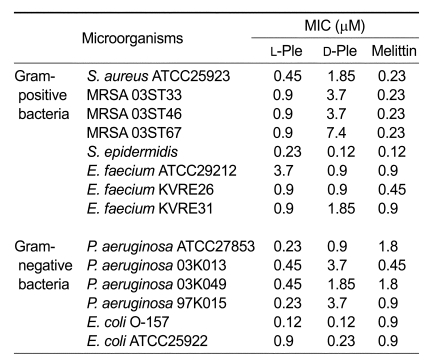
The antibacterial activity of D-Ple was compared with that of L-Ple against human pathogenic Gram-positive and Gram-negative bacteria (Table 1), and described in terms of minimum inhibitory concentration (MIC). In the current study, melittin was used as a positive control; melittin is the principal active component of bee venom and a powerful cytolytic antimicrobial peptide. D-Ple, in a MIC range of 0.12-7.4 µM, showed a noteworthy antibacterial activity against most strains examined, and it was potent compared with L-Ple and melittin. This result indicated that D-Ple has a remarkable potential as a therapeutic agent in the treatment of bacterial infectious diseases. As shown in the table, however, most bacteria tested were far more sensitive to L-Ple than D-Ple, and this result was conspicuous in the case of methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa in particular.
Table 1.
Antibacterial activity of enantiomers of pleurocidin.
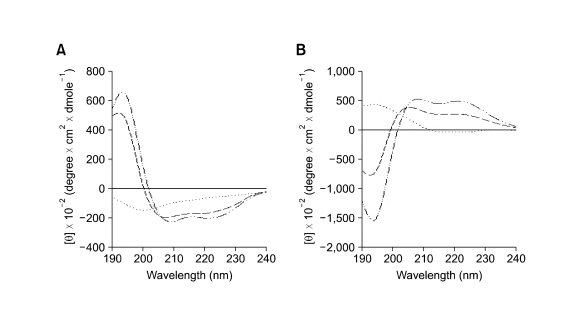
Since there were no differences in retention times using HPLC, this result suggested that the disparity of antibacterial activity between the two peptides was caused by other factors, such as structure. Thus, the secondary structure of the two peptides was investigated with CD spectroscopy. The results showed that not only L-Ple but also D-Ple had an α-helix structure with a mirror image, however, D-Ple revealed a more helical structure compared with L-Ple (Figure 2). This suggested that the degree of the helicity could be a major element in the discrepancy of the antibacterial activity.
Figure 2.
CD spectra of enantiomers of pleurocidin. (A) L-Ple, (B) D-Ple. (…, 10 mM sodium phosphate; ---, 50% TFE; -‥-, SDS).
Hemolytic activity of D-Ple
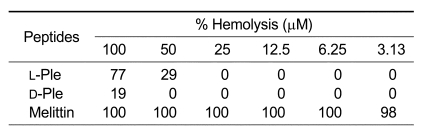
In due course, to investigate the hemolysis on mammalian erythrocytes, the hemolytic activity of the two peptides was examined on human RBCs at various peptide concentration levels (Table 2). As predicted, melittin, a potent lytic peptide used as a positive control, exhibited very strong hemolytic activity for most tested concentrations, and L-Ple showed a strong hemolysis at its highest concentration. However, D-Ple showed much lower hemolytic activity than L-Ple at the highest level, and, there was no hemolysis at other concentration levels.
Table 2.
Hemolytic activity of enantiomers of pleurocidin.
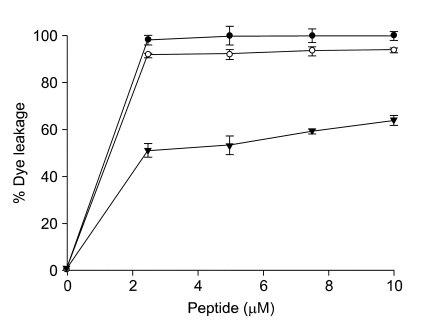
To confirm this result, we synthesized an artificial liposome composed of PC:cholesterol (10:1, w/w) and treated with the peptides. The result showed that both melittin and L-Ple exhibited a strong calcein leakage compared with D-Ple (Figure 3). This demonstrated that D-Ple did not make a pore on the membrane potently and could not damage human erythrocytes.
Figure 3.
Percent leakage of calcein from phosphatidyl cholin/cholesterol (10:1, w/w) LUVs, at pH 7.4, was measured 2 min after the addition of the peptides, and the error bars represented the standard deviation (S.D.) values for three independent experiments, performed in triplicate. (•, melittin; ○, L-Ple; ▾, D-Ple)
Discussion
Ple is one of the most noteworthy antimicrobial peptides studied in the past few years. It displays strong bacterial selectivity, showing potent antibacterial activity against human pathogenic Gram-positive and Gram-negative bacteria (Yang et al., 2006). In fact, several attempts have been made over recent years to advance novel broad-spectrum cationic antimicrobial peptides such as Ple into clinical use, but with limited success. The reasons for this failure are certainly diverse, but the key unresolved issues regarding toxicity and stability are the major causes of the lack of systemic application, towards which peptide therapeutic application holds the most potential (Marr et al., 2006). In particular, in order to overcome the obstacles of proteolysis, an enantiomer composed of all-D-amino acids was designed and the potential of D-Ple as a therapeutic agent was investigated.
First of all, a proteolytic resistance of D-Ple was examined through HPLC. When D-Ple had resistance to the digestive action of proteases, the peak of peptide could not be diminished. As was expected, the result showed the peak of D-Ple to be entire although that of L-Ple was diminished adequately (Figure 1). This indicated not only that D-Ple was synthesized perfectly but also that this enantiomer has remarkable therapeutic potential as a drug delivery system.
In order to investigate the possibility of a model for the design of potent peptidic therapeutic agents against bacterial infections, an antibacterial activity assay against human pathogenic bacteria was performed. Notably, most strains used were MRSA and P. aeruginosa. MRSA is an isolate of S. aureus that has acquired genes encoding antibiotic resistance to all penicillins, including methicillin and other narrow-spectrum β-lactamase-resistant penicillin antibiotics, and has recently become a major cause of hospital-acquired infections. Also, P. aeruginosa typically infects the pulmonary tract, urinary tract, burns, wounds, and also causes other blood infections, and it has been reported that one in ten hospital-acquired infections are from Pseudomonas (Todar et al.). The result showed that D-Ple had a potent antibacterial activity against these human pathogenic bacteria, although L-Ple exhibited a stronger activity (Table 1). As it was thought there was a specific thread of connections between the peptides and antibacterial activities, the factors influencing the results were considered. Above all, the same retention time of the two peptides demonstrated that the hydrophobicity of the peptides could not be a possible element of influence. Therefore, it was assumed that the structure would be a primary cause.
Accordingly, the secondary structure of the two peptides was investigated with CD analysis. In fact, D-amino acids have been researched and chemically engineered into peptides and proteins for various reasons. Notably, as stated above, D-amino acids have been extensively used to enhance the therapeutic index and in vivo stability of small antimicrobial peptides (Xie et al., 2005). In some cases, D-amino acids could be used as structural probes because of their known properties as α-helix or β-sheet breakers (Fairman et al., 1992; Krause et al., 2000) and all-D-peptide could exhibit a conformation that was the mirror image of that of the all-L-enantiomeric peptide (Bessalle et al., 1990). CD analysis showed that D-Ple as well as L-Ple had a typical α-helical structure and were mirror images (Figure 2). Surprisingly, however, D-Ple was more helical than L-Ple, and this led to the theory that the discrepancy of antibacterial activity could have originated from the different helicity.
Lastly, the hemolytic activity of D-Ple on human erythrocytes was investigated in order to estimate its applicability of treatment for humans. Contrary to L-Ple and melittin, D-Ple showed hardly any hemolysis (Table 2). In order to verify this result, a concerned experiment was performed with artificial liposome. Large unilamellar vesicles (LUVs) (PC/cholesterol; 10:1, w/w) were used as a model of mammalians' membrane. As was presumed, the result was very similar to the present hemolytic activity assay (Figure 3). This indicated that D-Ple, which showed a remarkable antibacterial activity with a very low hemolytic activity, could be used in clinical setting.
In summary, the potential of D-Ple as a peptidic therapeutic agent was investigated and it was suggested that one possible reason for it exerting a different antibacterial activity was the discrepant structure. Actually, D-Ple itself had strong antibacterial activity with a much lower hemolytic effect in comparison with melittin, L-Ple and other well-known antibiotics, such as ampicillin, vancomycin, cefotaxime, chloramphenicol and kanamycin. Thus, when the problem of the structure of D-Ple is improved by peptide engineering, this peptide may help form a leading model for developing new and novel therapeutic agents.
Acknowledgements
This work was supported by the Marine and Extreme Genome Research Center Program of the Ministry of Land, Transportation and Maritime Affairs, Republic of Korea.
Abbreviations
- LUVs
large unilamellar vesicles
- PC
phosphatidyl choline
- Ple
pleurocidin
References
- 1.Bessalle R, Kapitkovsky A, Gorea A, Shalit I, Fridkin M. All-D-magainin: chirality, antimicrobial activity and proteolytic resistance. FEBS Lett. 1990;274:151–155. doi: 10.1016/0014-5793(90)81351-n. [DOI] [PubMed] [Google Scholar]
- 2.Blondelle SE, Houghten RA. Design of model amphipathic peptides having potent antimicrobial activities. Biochemistry. 1992;31:12688–12694. doi: 10.1021/bi00165a020. [DOI] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing, fifteenth informational supplement, approved standard MS100-S15. Wayne, Pa: CLSI; 2005. [Google Scholar]
- 4.Cole AM, Weis P, Diamond G. Isolation and characterization of pleurocidin, an antimicrobial peptide in the skin secretions of winter flounder. J Biol Chem. 1997;272:12008–12013. doi: 10.1074/jbc.272.18.12008. [DOI] [PubMed] [Google Scholar]
- 5.DeGrado WF, Kézdy FJ, Kaiser ET. Design, synthesis and characterization of a cytotoxic peptide with melittin-like activity. J Am Chem Soc. 1981;103:679–681. [Google Scholar]
- 6.Fairman R, Anthony-Cahill SJ, DeGrado WF. The helix-forming propensity of D-alanine in a right-handed.alpha.-helix. J Am Chem Soc. 1992;114:5458–5459. [Google Scholar]
- 7.Ganz T, Selsted ME, Lehrer RI. Defensins. Eur J Haematol. 1990;44:1–8. doi: 10.1111/j.1600-0609.1990.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 8.Habermann E. Bee and wasp venoms. Science. 1972;177:314–322. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- 9.Hong SY, Oh JE, Lee KH. Effect of D-amino acid substitution on the stability, the secondary structure, and the activity of membrane-active peptide. Biochem Pharmacol. 1999;58:1775–1780. doi: 10.1016/s0006-2952(99)00259-2. [DOI] [PubMed] [Google Scholar]
- 10.Jung HJ, Choi KS, Lee DG. Synergistic killing effects of synthetic peptide P20 and cefotaxime on methicillin-resistant nosocomial isolates Staphylococcus aureus. J Microbiol Biotechnol. 2005;15:1039–1046. [Google Scholar]
- 11.Jung HJ, Park Y, Sung WS, Suh BK, Lee J, Hahm KS, Lee DG. Fungicidal effect of pleurocidin by membrane-active mechanism and design of enantiomeric analogue for proteolytic resistance. Biochim Biophys Acta-Biomemb. 2007a;1768:1400–1405. doi: 10.1016/j.bbamem.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 12.Jung HJ, Seu YB, Lee DG. Candicidal action of resveratrol isolated from grapes on human pathogenic yeast C. albicans. J Microbiol Biotechnol. 2007b;17:1324–1329. [PubMed] [Google Scholar]
- 13.Jungblut P, Thiede B. Protein identification from 2-DE gels by MALDI mass spectrometry. Mass Spectrom Rev. 1997;16:145–162. doi: 10.1002/(SICI)1098-2787(1997)16:3<145::AID-MAS2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 14.Kaiser ET. In: Prediction of protein structure and the principles of protein conformation. Fasman G. D., editor. New York: Plenum press; 1988. pp. 761–775. [Google Scholar]
- 15.Krause E, Bienert M, Schmieder P, Wenschuh H. The helix-destabilizing propensity scale of D-amino acids: the influence of side chain steric effects. J Am Chem Soc. 2000;122:4865–4870. [Google Scholar]
- 16.Lee MK, Kim HK, Lee TY, Hahm KS, Kim KL. Structure-activity relationships of anti-HIV-1 peptides with disulfide linkage between D- and L-cysteine at positions i and i+3, respectively, derived from HIV-1 gp41 C-peptide. Exp Mol Med. 2006;38:18–26. doi: 10.1038/emm.2006.3. [DOI] [PubMed] [Google Scholar]
- 17.Marchini D, Giordano PC, Amons R, Bernini LF, Dallai R. Purification and primary structure of ceratotoxin A and B, two antibacterial peptides from the female reproductive accessory glands of the medfly Ceratitis capitata (insecta:Diptera) Insect Biochem Mol Biol. 1993;23:591–598. doi: 10.1016/0965-1748(93)90032-n. [DOI] [PubMed] [Google Scholar]
- 18.Marr AK, Gooderham WJ, Hancock REW. Antibacterial peptides for therapeutic use: obstacles and realistic outlook. Curr Opin Pharmacol. 2006;6:468–472. doi: 10.1016/j.coph.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Merrifield B. Solid phase synthesis. Science. 1986;232:341–347. doi: 10.1126/science.3961484. [DOI] [PubMed] [Google Scholar]
- 20.Mor A, Nguyen VH, Delfour A, Migliore-Samour D, Nicolas P. Isolation, amino acid sequence, and synthesis of dermaseptin, a novel antimicrobial peptide of amphibian skin. Biochemistry. 1991;30:8824–8830. doi: 10.1021/bi00100a014. [DOI] [PubMed] [Google Scholar]
- 21.Okada M, Natori S. Primary structure of sarcotoxin I, an antibacterial protein induced in the hemolymph of Sarcophaga peregrina (flesh fly) larvae. J Biol Chem. 1985;260:7174–7177. [PubMed] [Google Scholar]
- 22.Park KH, Kim JS, Lee YR, Moon YJ, Hur H, Choi YH, Kim CH, Kim UH, Song EK, Yoo WH, Lee CS, Kim BS, Lee SH, Ryu PY, Han MK. Low-density lipoprotein protects Vibrio vulnificus-induced lethality through blocking lipopolysaccharide action. Exp Mol Med. 2007;39:673–678. doi: 10.1038/emm.2007.73. [DOI] [PubMed] [Google Scholar]
- 23.Saint N, Cadiou H, Bessin Y, Molle G. Antibacterial peptide pleurocidin forms ion channels in planar lipid bilayers. Biochim Biophys Acta. 2002;1564:359–364. doi: 10.1016/s0005-2736(02)00470-4. [DOI] [PubMed] [Google Scholar]
- 24.Steiner H, Hultmark D, Engström A, Bennich H, Boman HG. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature. 1981;292:246–248. doi: 10.1038/292246a0. [DOI] [PubMed] [Google Scholar]
- 25.Sung WS, Jung HJ, Lee IS, Kim HS, Lee DG. Antimicrobial effect of furaneol against human pathogenic bacteria and fungi. J Microbiol Biotechnol. 2006;16:349–354. [Google Scholar]
- 26.Sung WS, Lee IS, Lee DG. Damage to the cytoplasmic membrane and cell death caused by lycopene in Candida albicans. J Microbiol Biotechnol. 2007;17:1797–1804. [PubMed] [Google Scholar]
- 27.Todar . Online textbook of bacteriology. [Google Scholar]
- 28.Xie C, Prahl A, Ericksen B, Wu Z, Zeng P, Li X, Lu WY, Lubkowski J, Lu W. Reconstruction of the conserved beta-bulge in mammalian defensins using D-amino acids. J Biol Chem. 2005;280:32921–32929. doi: 10.1074/jbc.M503084200. [DOI] [PubMed] [Google Scholar]
- 29.Yang JY, Shin SY, Lim SS, Hahm KS, Kim Y. Structure and bacterial cell selectivity of a fish-derived antimicrobial peptide, Pleurocidin. J Microbiol Biotechnol. 2006;16:880–888. [Google Scholar]
- 30.Yoshida K, Mukai Y, Niidome T, Takashi C, Tokunaga Y, Hatakeyama T, Aoyagi H. Interaction of pleurocidin and its analogs with phospholipid membrane and their antibacterial activity. J Peptide Res. 2001;57:119–126. doi: 10.1034/j.1399-3011.2001.00802.x. [DOI] [PubMed] [Google Scholar]
- 31.Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci USA. 1987;84:5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]