Abstract
We investigated the mechanism of spontaneous cholesterol efflux induced by acyl-coenzyme A:cholesterol acyltransferase (ACAT) inhibition, and how an alteration of cholesterol metabolism in macrophages impacts on that in HepG2 cells. Oleic acid anilide (OAA), a known ACAT inhibitor reduced lipid storage substantially by promotion of cholesterol catabolism and repression of cholesteryl ester accumulation without further increase of cytotoxicity in acetylated low-density lipoprotein-loaded THP-1 macrophages. Analysis of expressed mRNA and protein revealed that cholesterol 7α-hydroxylase (CYP7A1), oxysterol 7α-hydroxylase (CYP7B1), and cholesterol 27-hydroxylase (CYP27) were highly induced by ACAT inhibition. The presence of a functional cytochrome P450 pathway was confirmed by quantification of the biliary cholesterol mass in cell monolayers and extracelluar medium. Notably, massively secreted biliary cholesterol from macrophages suppressed the expression of CYP7 proteins in a farnesoid X receptor (FXR)-dependent manner in HepG2 cells. The findings reported here provide new insight into mechanisms of spontaneous cholesterol efflux, and suggest that ACAT inhibition may stimulate cholesterol-catabolic (cytochrome P450) pathway in lesion-macrophages, in contrast, suppress it in hepatocyte via FXR induced by biliary cholesterol (BC).
Keywords: bile, cholesterol, cytochrome P-450 enzyme system, farnesoid X-activated receptor, oleoylanilide, sterol O-acyltransferase
Introduction
Macrophage foam cells, the hallmark of an early atherosclerotic lesion, results from unregulated uptake of modified low-density lipoprotein (LDL), such as acetylated LDL (acLDL), via the macrophage scavenger receptor A (MSRA) (Goldstein et al., 1979). This increased cholesterol influx activates ACAT-1, which is responsible for cholesterol esterification in macrophages (Buhman et al., 2000), and leads to formation of large amounts of intracellular cholesteryl ester (CE) (Cignarella et al., 2005). The only way for macrophages to maintain cholesterol homeostasis and to prevent cytotoxicity due to accumulation of cholesterol is for them somehow to efflux the excess cholesterol into the extracellular space, which is the first step of reverse cholesterol transport (von Eckardstein et al., 2001). Especially, spontaneous cholesterol efflux from macrophages may be significant within atherosclerotic lesions where the availability of specific subclasses of high-density lipoproteins (HDL) as lipid acceptors is limited (Cignarella et al., 2005), but the process of efflux is not well understood.
Unlike the negative effects of ACAT inhibitors on the formation of foam cells in rodent macrophages via accumulation of free cholesterol (FC) (Warner et al., 1995; Feng and Tabas, 2002), ACAT inhibition has been shown in many studies to repress the accumulation of total cholesterol (TC) in human macrophages by lowering the uptake of acLDL and facilitating FC efflux (Rodriguez et al., 1999; Rodriguez and Usher, 2002; Cignarella et al., 2005). Moreover, Cignarella et al. (2005) demonstrated that cholesterol efflux is not a simple consequence of the availability of FC.
The current study was designed to: (i) find novel factors involved in spontaneous cholesterol efflux stimulated by ACAT inhibition in acLDL-loaded macrophages; (ii) investigate the mechanism by which these factors are regulated; (iii) examine how an alteration of cholesterol metabolism in macrophages impacts on that in HepG2 cells.
Materials and Methods
Materials
Oleic acid anilide (OAA), a known ACAT inhibitor, was synthesized by one of the authors as described (Roth et al., 1992). [1-14C]Oleoyl-CoA (56 Ci/mol) and [4-14C]cholesterol (50 Ci/mol) were purchased from Amersham Biosciences (UK). The radioactivity of [1-14C]oleoyl-CoA, [4-14C]cholesterol, and related products was measured using a liquid scintillation counter (1450 Microbeta Trilux Wallac Oy, Turku, Finland). Blood was collected from normolipidemic volunteers with permission according to the Guidelines of Blood Donation Program for a Research of the Korea Red Cross Blood Center. LDL was isolated from the plasma by sequential ultracentrifugation (Hatch and Lees, 1968). AcLDL was prepared by repeated addition of acetic anhydride to LDL (Basu et al., 1976), and sterilized by filtration through a membrane with a pore size of 0.45 µm. The completeness of acetylation was assessed from electrophoretic mobility on agarose gels. AcLDL was stored at 4℃ and used within 1 month.
Cell cultures
Human acute monocytic leukemia THP-1 cells and HepG2 cells (American Type Tissue Culture Collection, Rockville, MO) were grown in RPMI-1640 medium (Gibco BRL, Carlsbad, CA) containing 10% FBS (Hyclone, Logan, UT) and DMEM (Gibco BRL) containing 10% FBS, respectively. THP-1 cells in suspension were plated in RPMI-1640 medium with 10% FBS and 50 ng/ml of PMA (Sigma, St. Louise, MO) for 3 days to become fully differentiated macrophages before use in experiments (Cheon et al., 2007). In the majority of experiments, macrophages were enriched with cholesterol by addition of acLDL (100 µg/ml) in RPMI-1640 medium containing 10% lipoprotein-deficient serum (LPDS) for 48 h.
Whole-cell cholesterol esterification assay
THP-I macrophages were pretreated overnight with or without OAA in complete RPMI-1640 medium, followed by incubation in serum-free RPMI-1640 medium containing 0.2 µCi/ml of [1-14C]oleoyl CoA-BSA (0.2%) complex and 100 µg/ml of acLDL with or without OAA for 18 h. The [1-14C]oleoyl CoA-BSA complex was prepared as described (Batetta et al., 2003). The cells were washed with PBS and extracted with hexane/ isopropyl alcohol (3:2, v/v). The extracts containing esterified products were isolated by thin-layer chromatography (Tabas et al., 1987). Whole-cell cholesterol esterification activity was assessed by determining the radioactivity of the cholesteryl [1-14C]oleate produced.
Parallel artificial membrane-permeation assay (PAMPA)
The permeability of OAA was measured by the parallel artificial membrane-permeation assay (PAMPA) (Kansy et al., 1998; Avdeef, 2005), which is based on the use of 96-well membrane filter-based plate (Millipore), in 5% DMSO/PBS, pH 7.4.
[4-14C]cholesterol efflux assay
The cholesterol efflux assay was performed essentially as described (Feng and Tabas, 2002) with slight modification. Briefly, 1 mg of acLDL was incubated with 10 µCi of [4-14C]cholesterol for 30 min at 37℃, and then 10 ml of RPMI-1640 medium was added. The THP-1 macrophages were incubated in this medium for 48 h with or without OAA, washed three times with PBS, and then incubated in RPMI-1640 medium containing 0.2% fatty acid-free BSA (Sigma, St. Louis, MO) overnight. After equilibration of the cholesterol pool, the cells were washed with PBS and incubated in RPMI-1640 medium containing 0.2% BSA with or without OAA. Efflux incubations were performed for up to 24 h in 6-well plates.
Quantification of intracellular and secreted cholesterol and biliary cholesterol
To quantify intracellular storage of cholesterol and non-cholesterol 3-α-hydroxysteroid (biliary cholesterol), macrophages were harvested after incubation for 48 h in RPMI-1640 medium with or without 100 µg/ml of acLDL or oleic acid anilide (OAA). For quantification of secreted sterols, the cells were washed extensively with PBS and incubated for an additional 24 h in RPMI-1640 medium with or without OAA. The medium was collected and centrifuged at 13,000 g for 30 min at 4℃ to remove detached cells and cell debris. A portion of the cells was assayed for protein using the Bio-Rad DC protein assay kit, and the volume of cell suspension containing 1 mg of protein and the corresponding medium were analyzed for mass of steroids. TC and FC were quantified by an enzymatic-spectrophotometric method (Wako) after extraction with hexane/isopropyl alcohol (3:2, v/v) (Bligh and Dyer, 1959), and CE mass was calculated from the difference between the measurements. The mass of 3-α-hydroxysteroid (3αHS) was quantified also by an enzymatic-spectrophotometric method (DCLchem) after extraction with hexane/isopropyl alcohol (3:2, v/v) (Bligh and Dyer, 1959), and the mass of biliary cholesterol (BC) was calculated by subtraction of the mass of FC from the mass of 3αHS. Neutral lipids deposited in the cells were visualized by staining with oil red O as described (Rong et al., 2005).
Real-time quantitative reverse transcription-polymerase chain reaction
Real-time quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis was performed to determine the expression of genes involved in cholesterol metabolism and mobilization in THP-1 macrophages, encoding for apoE (BC003557), ABCA1 (AF165281), ABCG1 (BC029158), CYP7A1 (X56088), CYP7B1 (AF127090), CYP27 (M62401) with a rotor-gene 3000 (Corbett Research). The cells were incubated for 48 h with or without OAA as indicated, in the presence of 100 µg/ml of acLDL. The following sets of primers and probes were used (forward and reverse, respectively) in qRT-PCR: β-actin, 5'-agc-gcg-gct-aca-gct-tca-3' and 5'-cat-ttg-cgg-tgg-acg-atg-3'; apoE, 5'-cgc-ctg-gtg-cag-tac-cg-3' and 5'-tga-ttg-tcg-ctg-ggc-aca-g-3'; ABCA1, 5'-cta-gga-tgg-caa-tca-tgg-tc-3' and 5'-aac-tgc-aac-gtc-cac-tac-tg-3'; ABCG1, 5'-gga-aga-tgt-agg-cag-att-gg-3' and 5'-aat-gtc-tgc-atg-gct-cag-tg-3'; CYP7A1, 5'-cag-aag-caa-tga-aag-cag-cta-ctg-3' and 5'-tgt-att-cac-aaa-tgc-ttg-aat-tta-tat-tta-3'; CYP7B1, 5'-gct-tcc-tta-tct-tgg-agt-gg-3' and 5'-gag-ctg-cag-aat-gga-tac-ag-3'; CYP27, 5'-cct-gtt-cga-gaa-acg-cat-tg-3' and 5'-tcc-ttt-gag-agg-tgg-tac-ag-3.
Statistical analysis
Results are given as mean ± S.D. Statistical analysis was done using Student's t-test. A value of P < 0.05 was accepted as statistically significant. The experiments were repeated three times (separate cell preparations) unless noted otherwise.
Results
OAA (Figure 1A) inhibited ACAT activity in THP-1 macrophages with an IC50 value of 15.2 µM (Figure 1B), which is a much higher value than that from an in vitro assay (hACAT1, IC50 = 140 nM) (Cho et al., 2003). OAA showed a medium permeability in the parallel artificial membrane-permeation assay (PAMPA) with a Log Pe value of - 5.18 ± 0.02. As the result, only 3 µmol of OAA was shown to be able to cross the biological membrane from 100 µmol of OAA in the donor compartment. Therefore, the reason why OAA exhibits a relatively lower ACAT inhibition activity in the cell system could be explained by the poor membrane permeability. But there is no doubt that OAA inhibits CE formation in acLDL-loaded macrophages.
Figure 1.
The ACAT inhibitor OAA promotes spontaneous cholesterol efflux from THP-1, cultured macrophages. (A) The structure of OAA. Whole-cell ACAT activity (B) and cholesterol efflux (C) in THP-1, cultured macrophages were determined. ACAT activity was measured by determining the incorporation of [14C]oleoyl-CoA into labeled cholesteryl oleate. The values shown are expressed as the percentage inhibition of ACAT activity in control incubations. The cholesterol efflux are expressed as the percentage of total cellular [14C]cholesterol. *P < 0.05, **P < 0.01, ***P < 0.001 versus acLDL-loaded cells.
The extent of cytoxicity was assessed by measuring the release of lactate dehydrogenase (LDH) into the extracellular medium with an LDH assay kit (Takara) or the formation of MTT formazan (Liu and Hong, 2005). As the result, acLDL loading decreased cell viability by about 20%, while the addition of OAA to the medium containing acLDL did not cause decrease in cell viability (data not shown).
Decrement of CE mass dominates the negative effect of the accumulation of FC
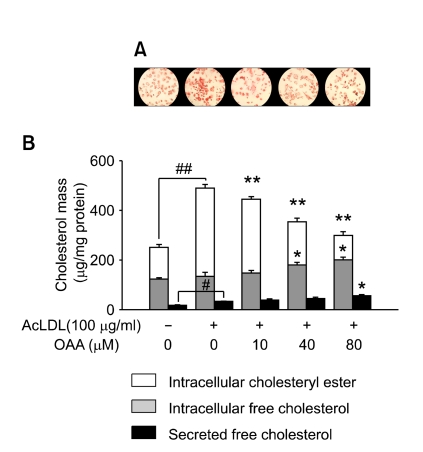
The results from the staining of the cells with oil red O showed that acLDL-loading led to massive cell formation in THP-1 macrophages while the addition of OAA appeared to deplete storage lipid from the cells in a dose-dependent manner (Figure 2A). Next, we measured the cholesterol mass to investigate the effect of ACAT inhibition on intracellular CE and FC accumulation, and FC secretion to the medium. As shown in Figure 2B, acLDL-loading increased cellular CE mass by 2.7-fold (354 ± 15 µg/mg versus 128 ± 5 µg/mg of cell protein, P < 0.001) and free cholesterol secretion about 1.9-fold (33 ± 2 µg/mg versus 17 ± 2 µg/mg of cell protein, P < 0.01), but did not cause change in the cellular content of FC significantly (135 ± 16 µg/mg versus 122 ± 6 µg/mg of cell protein). OAA reduced CE mass significantly in acLDL-loaded cells in a dose-dependent manner. The 80% decrease of ACAT activity by addition of 80 µM OAA in the cells (Figure 1B) resulted in a substantial decrease of CE formation to a level lower than that for the control cells (99 ± 16 µg/mg of cell protein, P < 0.001) (Figure 2B), but increased slightly the accumulation of FC in the cell monolayer, by 1.5-fold (200 ± 12 µg/mg versus 135 ± 16 µg/mg of cell protein, P < 0.05), and secretion of FC into the extracellular space, by 1.2-fold (56 ± 5 µg/mg versus 33 ± 2 µg/mg of cell protein, P < 0.05). A moderate increase of FC efflux is not sufficient to explain a marked reduction of CE accumulation. Thus, it is speculated that FC might have been secreted after conversion into other molecules. Interestingly, the calculated ratio of efflux of cholesterol to total cholesterol derived from exogenous acLDL was increased from 7% to 80% during ACAT inhibition; i.e. it resulted mainly from reduction of the TC mass and not from an increase of FC efflux. Nevertheless, this observation was quite different from that of the [14C]cholesterol efflux experiment where cholesterol efflux was increased from 3.2% to 16.3% by ACAT inhibition (P < 0.01) (Figure 1C). It was concluded that the cholesterol mass data and the [14C]cholesterol efflux data might not be correlated simply, because [14C]cholesterol might have been secreted simultaneously, unlike acLDL, which was processed in various cellular organelles.
Figure 2.
CE accumulation in acLDL-loaded macrophages is repressed during ACAT inhibition, which dominates FC accumulation. To quantify intracellular storage of cholesterol, THP-1 macrophages were incubated for 48 h in RPMI medium containing 10% LPDS with or without 100 µg/ml of acLDL and the indicated concentrations of OAA. For quantification of secreted cholesterol, the cells were washed extensively and incubated for a further 24 h without acLDL. (A) Cells were fixed with 4% paraformaldehyde and stained with oil red O. Magnification 100 ×. (B) Cholesterol mass of hexane/isopropyl alcohol (3:2, v/v) extracts from the cells and media. #P < 0.01, ##P < 0.001 versus control cells; *P < 0.05, **P < 0.001 versus acLDL-loaded cells.
ACAT inhibition triggers changes in expression of genes involved in cholesterol catabolism and mobilization
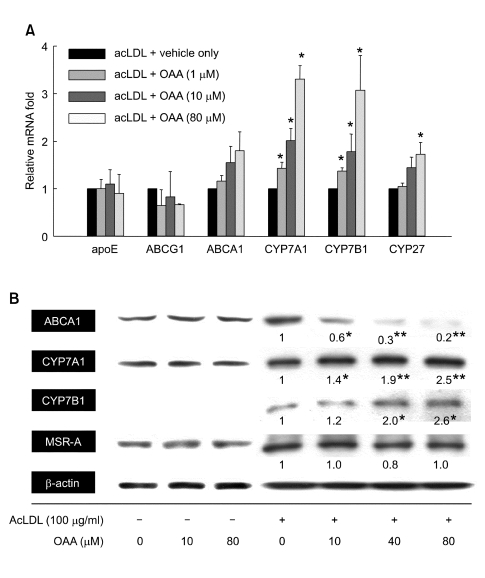
Quantitative real-time PCR analysis was conducted to investigate the levels of the relevant mRNA of various genes after incubation for 48 h with 100 µg/ml of acLDL plus the concentration of OAA indicated. As shown in Figure 3A, the levels of Apoe and Abcg1 were unaffected by acLDL plus OAA compared with acLDL alone, which is not consistent with the results of previous studies (Cignarella et al., 2005). In contrast, the mRNA level of cytochrome P450 family genes Cyp7a1, Cyp7b1, and Cyp27 were increased in a dose-dependent manner. Especially, Cyp7a1 and Cyp7b1 were highly induced by 3.3-fold and 3.1-fold, respectively (P < 0.05), in the presence of 80 µM OAA, while Cyp27 was induced by 1.7-fold (P < 0.05). The mRNA levels of Abca1 were not significantly induced.
Figure 3.
ACAT inhibition regulates the expression of genes involved in cholesterol catabolism and mobilization in cultured macrophages. Cells were incubated for 48 h with acLDL and the indicated concentration of OAA. (A) The levels of mRNA were measured by qRT-PCR and normalized by β-actin. *P < 0.05 versus acLDL-loaded cells. (B) Each protein expression level was analyzed by Western blot. The intensity of the detected bands was compared using a Calibrated Densitometer GS800 (Bio-Rad) with Quantity One software (version 4.4.0) and demonstrated as a average of three independent experiment. The intensity of the β-actin band was used as an internal control. *P < 0.05, **P < 0.01 versus acLDL-loaded cells.
Western blot analysis was performed to determine whether ACAT inhibition caused a change in the post-transcriptional process, and whether quantitative mRNA levels were correlated with protein levels. OAA itself did not influence the expression of any genes tested in THP-1 macrophages. The protein level of ABCA1, the mRNA expression of which tends to increase, was decreased substantially by ACAT inhibition in acLDL-loaded macrophages (Figure 3B). This result is in agreement with that of an earlier study, which demonstrated that ACAT inhibition induced the degradation of ABCA1 protein due to membrane stiffening effect (Feng and Tabas, 2002). The protein expressions of CYP7A1 and CYP7B1 were induced by 80 µM OAA treatment (2.5-fold, P < 0.01 and 2.6-fold, P < 0.05, respectively) in a very similar way to transcriptional regulation (Figure 3B). Translation of MSRA remains unchanged suggesting that ACAT inhibition does not affect the acLDL uptake into the cells.
ACAT inhibition promotes cholesterol catabolism into BC
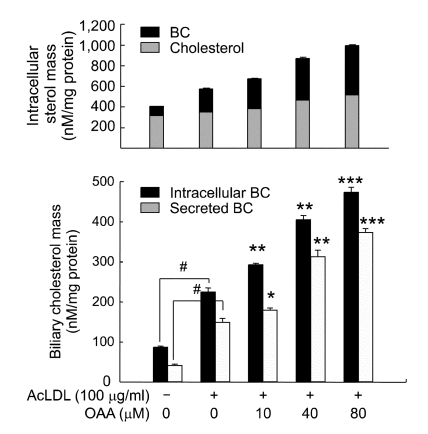
To investigate whether ACAT inhibition increased functional CYP7A1 and CYP7B1, and stimulated cholesterol catabolism into BC, the products of the cytochrome P450 pathway, we quantified the mass of intracellular and secreted BC using an enzymatic-spectrophotometric method (Figure 4). We observed that acLDL-loading induced formation of BC (225 ± 10 nmol/mg versus 87 ± 3 nmol/mg of cell protein, P < 0.01) that was more intensified during ACAT inhibition. The intracellular mass of BC was increased in proportion to ACAT inhibition. While FC was secreted by 20~30% of intracelluar FC (Figure 2B), BC was secreted easily from cells to the medium, 70~80% of intracellular BC. These novel effects of ACAT inhibition may explain the reduction of lipid accumulation in THP-1 macrophages loaded with acLDL.
Figure 4.
ACAT inhibition increases the mass of intracellular and secreted BC in cultured macrophages. The effect of ACAT inhibition on the production of BC was measured in the macrophages treated as described for Figure 2. (A) Intracellular cholesterol mass and biliary cholesterol mass are compared in the same graph. (B) The intracellular and secreted BC mass were analyzed and calculated as described in Materials and Methods. #P < 0.01 versus control cells; *P < 0.05, **P < 0.01, ***P < 0.001 versus acLDL-loaded cells.
BC secreted from macrophages controls the gene expression in an FXR-dependent manner in HepG2 cells
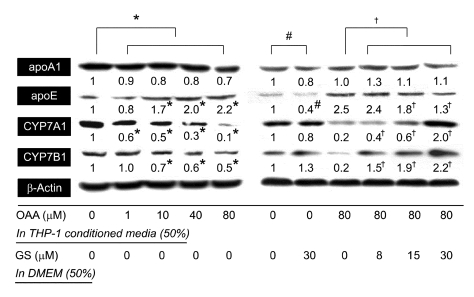
In liver cells, BC could be a ligand of FXR (Nishimaki-Mogami et al., 2004), which promotes apoE expression (Mak et al., 2002) and represses the expression of apoA1 and the enzymes that catalyze bile acid synthesis, including CYP7A1 (Goodwin et al., 2000) and CYP7B1 (Stedman et al., 2006). Guggulsterone (GS) is a plant sterolfrom the Commiphora mukul tree and has been widely used to treat hyperlipidemia in humans (Urizar and Moore, 2003). It is well established that GS can act as an FXR antagonist and decrease expression of FXR target genes (Urizar et al., 2002; Wu et al., 2002). It has also been demonstrated that the hepatic lipid-lowering effect of GS was mediated through FXR using FXR knockout mice (Urizar et al., 2002). To address the question as to whether BC secreted from macrophages could modulate the FXR pathway in HepG2 cells, the cells were incubated with 50% THP-1 macrophage conditioned medium (TMCM), which confirmed the presence of BC (Figure 4). The concentration of BC in TMCM was increased by 2.5 fold with 80% inhibition of ACAT activity (149 ± 10 nM/mg versus 373 ± 10 nM/mg of cell protein, P < 0.001). OAA itself did not influence the expression of any gene tested in HepG2 cells (data not shown), like the THP-1 macrophages. As shown in Figure 5, among the tested FXR-mediated genes, CYP7A1, CYP7B1, and apoE were regulated in proportion to the amount of BC included in TMCM. The expression of CYP7A1 and CYP7B1 was decreased by 75% and 50% with maximal concentration of BC in TMCM (Figure 4). In contrast, apoE expression was increased 3-fold. On the same concentration of BC, the FXR pathway seems to be inactivated by GS in a dose- dependent manner, and the expression of CYP7A1, CYP7B1, and ApoE were restored.
Figure 5.
BC excreted from macrophages regulates the expressional level of apoE, CYP7A1 and CYP7B1 in an FXR-dependent manner in HepG2 cells. The HepG2 cells (5 × 106) were incubated with 50% TMCM, derived from Figure 4, and 50% DMEM containing 10% LPDS with or without the indicated concentration of GS for 48 h. Each level of protein expression was analyzed by Western blot. The intensity of the detected bands is demonstrated as an average of three independent experiments. #,*,†P < 0.01 versus the indicated control.
ACAT inhibition exhibits different regulation of cytochrome P450 gene expression between macrophages and HepG2 cells
Next, we investigated the direct effects of ACAT inhibition and the combinational effect of ACAT inhibition and TMCM treatment on HepG2 cells. Interestingly, we observed that the expression of CYP7A1 and CYP7B1 was mildly repressed by acLDL treatment, which is sustained by same expression level during ACAT inhibition and that TMCM treatment repressed those gene expressions (Figure 6). This result was distinct with that in macrophages, suggesting quite different regulation of CYP pathway between lesion-macrophages and HepG2 cells (Figure 6).
Figure 6.
ACAT inhibition does not affect the expression level of CYP7A1 and CYP7B1 in HepG2 cells. Cells were incubated for 48 h with or without acLDL, the indicated concentration of OAA and/or 50% TMCM. Each protein expression level was analyzed by Western blot. The intensity of the detected bands is demonstrated as an average of three independent experiments. #P < 0.05, ##P < 0.01 versus control cells; *P < 0.05 versus acLDL-loaded cells.
Discussion
The first part of this study showed that OAA effectively reduced cholesterol accumulation in THP-1 macrophages by inhibiting CE formation without increased cytotoxicity compared with acLDL alone. Also, the fluctuation of intracellular CE decrease is much bigger than that of secreted FC increase. To better understand about cholesterol flux as a consequence of ACAT inhibition and to investigate, if any, novel factors involved in spontaneous cholesterol efflux in human THP-1 macrophages, we performed a microarray experiment using GenePlorer TwinChip Human-8K (Digital genomicsTM, Seoul, Korea). Analyzed levels of the expressed mRNA of genes related to lipid catabolism and mobilization, such as CYP7B1 and apoC1, were induced by 2-fold during even mild ACAT inhibition (40%) (data not shown). This result led us to focus on the catabolic pathway to BC in acLDL-loaded macrophages during ACAT inhibition. Similarly, we discovered that CYP7A1, CYP7B1, and CYP27 were highly expressed during ACAT inhibition. Our data showed for the first time that ACAT inhibition activated the cytochrome P450 pathway in acLDL-loaded macrophages, and thus the cells were rendered resistant to accumulation of cholesterol by increased catabolism to BC, which is immediately secreted out of the extracellular space.
Cytochrome P450 pathway is accomplished via two pathways, the classic pathway and the alternative pathway, where CYP7A1 and CYP7B1 function as rate-limiting enzymes, respectively (Nishimaki-Mogami et al., 2004). In mammals, the CYP7A1 pathway accounts for the majority of cholesterol that is metabolized and removed from the body (Siperstein et al., 1952), and predominantly causes the formation of cholate and chenodeoxycholate (CDCA) (Li et al., 1990). Also, the CYP7B1 pathway contributes considerably to the total bile acid mass in humans and leads predominantly to the formation of CDCA (Axelson and Sjövall, 1990). These CYP7 proteins have been shown to be liver-specific enzymes (Russell, 1999), and have been believed not to function in non-hepatic cells under normal conditions (Zhang et al., 1995). Notably, avasimibe, a known ACAT inhibitor, increased the expression of CYP7A1 and bile acid synthesis in rat hepatocytes (Post et al., 1999). Transgenic expression of CYP7A1 in McArdle rat hepatoma cells (Labonte et al., 2000) and in the livers of mice (Miyake et al., 2002) could prevent massive accumulation of cholesterol. Most importantly, RAW264.7 macrophages, which express rat CYP7A1 stably, displayed a complete resistance to accumulation of cholesterol via both increased metabolism and efflux of cholesterol with no adverse effect on cell growth or viability (Moore and Davis, 2002). These studies support the concept that the cytochrome P450 pathway might be critical in the maintenance of cholesterol homeostasis in lesion-macrophages as well as in hepatocytes. In this research, we found that the intracellular mass of BC was increased by 3-fold with only acLDL-loading (Figure 4). The result demonstrated that macrophages have a functional cytochrome P450 pathway as a defense mechanism against the cholesterol accumulation. It is generally accepted that Cyp7a1 is regulated by LXRα in the hepatocytes, although the action of LXRα in the macrophages has not been fully elucidated. LXRα signaling might be activated by oxysterol transformed from cholesterol during ACAT inhibition. It is not clear whether oxysterol is generated simply by an intracellular oxidative mechanism concurrent with a general increase of the cellular cholesterol level or by a more specific manner when ACAT is inhibited in macrophages. It is certain, however, that inhibition of ACAT enhances the pool of free cholesterol available for conversion into oxysterol (Post et al., 1999). Notably, 27-hydroxycholesterol has been identified as a ligand of LXR in cholesterol-loaded, monocyte-derived macrophages (Fu et al., 2001). In this study, we observed that ACAT inhibition up-regulated CYP27 expression mildly but significantly. Thus, it is acceptable that at least 27-hydroxycholesterol among the various oxysterols might have a role as a ligand for LXRα.
Interestingly, CDCA, a major end-product of the cytochrome P450 pathway, is the most potent physiological ligand of FXR, a negative regulator of bile acid synthesis (Chiang et al., 2000) and excretion (Stedman et al., 2006). Activation of FXR result in decreased expression of CYP7A1 (Goodwin et al., 2000), CYP7B1 (Stedman et al., 2006), and apoA-1 (Claudel et al., 2002), and increased expression of apoE (Mak et al., 2002). FXR deletion in cholestasis model mice improved cholestatic liver disorders by increased excretion of bile acid from the body (Stedman et al., 2006). In this study, it has shown that FXR down-regulates the multidrug resistance-associated proteins 1 and 4, which are postulated to act as alternative basolateral bile acid efflux transporters, and ABCG5 and ABCG8, which is a major pathway for cholesterol elimination. The results implicated that FXR antagonism possibly has a significantly increased capacity to export bile acids out of the hepatocyte back into the circulation and ability to excrete cholesterol into bile. Also, FXR deficiency in Ldlr-/- mice resulted in a reduction in size of atherosclerotic lesions in the aorta, mainly via a decreased level of plasma LDL cholesterol, and a decrease of the accumulation of neutral lipid in peritoneal macrophages (Zhang et al., 2006). There have been many conflicting results, depending on the experimental animal model in research areas related to atherosclerosis, which might originate from different mechanisms of cholesterol metabolism between species. It has been reported that rodents have a very hydrophilic bile acid pool, which is less potent in activation of FXR; thus, the LXRα could function as an important regulator of CYP7A1 in mice (Chiang et al., 2001). In contrast, CYP7A1 expression was down-regulated by a high-cholesterol diet in African green monkeys (Rudel et al., 1994) and in rabbits (Xu et al., 2003), since the inhibitory effect of FXR may override the stimulatory effect of LXRα. There are another regulator of bile acid synthesis, named steroid and xenobiotic receptor pregnane X receptor (PXR), which induces human cytochrome P4503A4 in drug metabolism and represses CYP7A1 in bile acid synthesis in the liver. PXR is activated by a large number of endogenous and exogenous chemicals including naturally occurring steroids, antibiotics, antimycotics, bile acids, and the herbal antidepressant St. John's wort (Kliewer et al., 2002). It is possible that various steroids released from acLDL might stimulate PXR, which down-regulated CYP proteins in HepG2 cells. These results led us to propose that the disappointing results of ACAT inhibitors, avasimibe (Tardif et al., 2004) and pactimibe (Nicholls et al., 2006) shown in several clinical studies might result from activation of FXR, owing to the elevated pool of ligand for FXR; as consequence, cholesterol could not be excreted from the body. In this study, we discovered that BC secreted from acLDL-loaded macrophages during ACAT inhibition behaved as an FXR activator and regulated the expression of apoE, CYP7A1, and CYP7B1, and that these effects were abolished by the FXR antagonist, GS. Therefore, it is potential that ACAT inhibition promotes secretion of BC from macrophages but represses bile acid synthesis in hepatocytes via the activation of FXR as presented in Figure 7. Nishimaki-Mogami et al. (2004) demonstrated that some BC, which is metabolized further than 27 hydroxylation in the classic pathway of bile acid synthesis, exhibited activity for FXR comparable to that of CDCA; conversely, early intermediates in the bile acid synthesis pathway, such as 7α-hydroxycholesterol and 27-hydroxycholesterol, showed no activity. Thus, we could consider that cholesterol was metabolized at least further than 27-hydroxylation in macrophages during ACAT inhibition, which is why the small change of absolute values of BC in the TMCM could activate the FXR pathway of HepG2 cells dramatically.
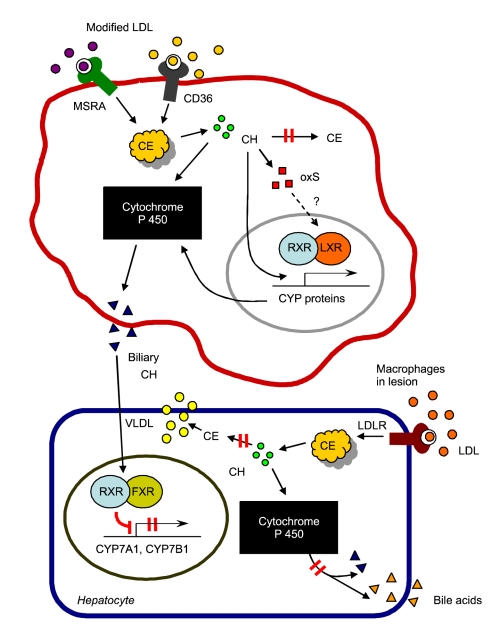
Figure 7.
Schematic presentation of hypothesis proposed in this paper. In lesion-macrophages, unregulated cholesterol influx from modified LDL activates ACAT-1 and leads to formation of large amounts of intracellular CE. Inhibition of ACAT enhances the pool of free cholesterol available for conversion into oxysterol, thus LXRα signaling might be activated by oxysterol. ACAT inhibition induce cytochrome P450 proteins in acLDL-loaded macrophages, and thus the cells were rendered resistant to accumulation of cholesterol by increased catabolism to BC, which is immediately secreted out of the extracellular space. In liver cells, BC could be a ligand of FXR, which represses the expression of the enzymes involved in bile acid synthesis, including CYP7A1 and CYP7B1. Finally, ACAT inhibition could repress bile acid synthesis in hepatocytes and cholesterol excretion from the body via the activation of FXR. CH: cholesterol, CE: cholesteryl ester, oxS: oxysterol, BC: biliary cholesterol.
Our results also imply that the combination therapy of an ACAT inhibitor and an FXR antagonist in vivo may prove to have clinical benefit in the treatment of atherosclerosis by decreasing the accumulation of cholesterol in lesion-macrophages through enhancing the efflux of BC, and by facilitating the excretion of cholesterol out of the body.
Acknowledgments
This study was supported by a grant from the KRIBB Research Initiative Program, and a grant from the Korea Science and Engineering Foundation (KOSEF) funded by the Ministry of Education, Science and Technology (MEST) (No. R01-2008-000-10960-0).
Abbreviations
- ACA
T, acyl-coenzyme A: cholesterol acyltransferase
- BC
biliary cholesterol
- FXR
farnesoid X receptor
- GS
guggulsterone
- OAA
oleic acid anilide
- TMCM
THP-1 macrophage conditioned medium
References
- 1.Avdeef A. The rise of PAMPA. Expert Opin Drug Metab Toxicol. 2005;1:325–342. doi: 10.1517/17425255.1.2.325. [DOI] [PubMed] [Google Scholar]
- 2.Axelson M, Sjövall J. Potential bile acid precursors in plasma-possible indicators of biosynthetic pathways to cholic and chenodeoxycholicacids in man. J Steroid Biochem. 1990;36:631–640. doi: 10.1016/0022-4731(90)90182-r. [DOI] [PubMed] [Google Scholar]
- 3.Basu SK, Goldstein JL, Anderson RGW, Brown MS. Degradation of cationized low density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc Natl Acad Sci USA. 1976;73:3178–3182. doi: 10.1073/pnas.73.9.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batetta B, Mulas MF, Sanna F, Putzolu M, Bonatesta RR, Gasperi-Campani A, Roncuzzi L, Baiocchi D, Dessi S. Role of cholesterol ester pathway in the control of cell cycle in human aortic smooth muscle cells. FASEB J. 2003;17:746–748. doi: 10.1096/fj.02-0396fje. [DOI] [PubMed] [Google Scholar]
- 5.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 6.Buhman KF, Accad M, Farese RV. Mammalian acyl-CoA: cholesterol acyltransferases. Biochim Biophys Acta. 2000;1529:142–154. doi: 10.1016/s1388-1981(00)00144-x. [DOI] [PubMed] [Google Scholar]
- 7.Cheon H, Woo YS, Lee JY, Kim HS, Kim HJ, Cho S, Won NH, Sohn J. Signaling pathway for 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced TNF-alpha production in differentiated THP-1 human macrophages. Exp Mol Med. 2007;39:524–534. doi: 10.1038/emm.2007.58. [DOI] [PubMed] [Google Scholar]
- 8.Chiang JY, Kimmel R, Weinberger C, Stroup D. Farnesoid X receptor responds to bile acids and represses cholesterol 7-hydroxylase gene (CYP7A1) transcription. J Biol Chem. 2000;275:10918–10924. doi: 10.1074/jbc.275.15.10918. [DOI] [PubMed] [Google Scholar]
- 9.Chiang JY, Kimmel R, Stroup D. Regulation of cholesterol 7α-hydroxylase gene (CYP7A1) transcription by the liver orphan receptor (LXRα) Gene. 2001;262:257–265. doi: 10.1016/s0378-1119(00)00518-7. [DOI] [PubMed] [Google Scholar]
- 10.Cho KH, An S, Lee WS, Paik YK, Kim YK, Jeong TS. Mass-production of human ACAT-1 and ACAT-2 to screen isoform-specific inhibitor: A different substrate specificity and inhibitory regulation. Biochem Biophys Res Commun. 2003;309:864–872. doi: 10.1016/j.bbrc.2003.08.077. [DOI] [PubMed] [Google Scholar]
- 11.Cignarella A, Engel T, von Eckardstein A, Kratz M, Lorkowski S, Lueken A, Assmann G, Cullen P. Pharmacological regulation of cholesterol efflux in human monocyte-derived macrophages in the absence of exogenous cholesterol acceptors. Atherosclerosis. 2005;179:229–236. doi: 10.1016/j.atherosclerosis.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Claudel T, Sturm E, Duez H, Torra IP, Sirvent A, Kosykh V, Fruchart JC, Dallongeville J, Hum DW, Kuipers F, Staels B. Bile acid-activated nuclear receptor FXR suppresses apolipoprotein A-I transcription via a negative FXR response element. J Clin Invest. 2002;109:961–971. doi: 10.1172/JCI14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng B, Tabas I. ABCA1-mediated cholesterol efflux is defective in free cholesterol-loaded macrophages. Mechanism involves enhanced ABCA1 degradation in a process requiring full NPC1 activity. J Biol Chem. 2002;277:43271–43280. doi: 10.1074/jbc.M207532200. [DOI] [PubMed] [Google Scholar]
- 14.Fu X, Menke JG, Chen Y, Zhou G, MacNaul KL, Wright SD, Sparrow CP, Lund EG. 27-Hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol-loaded cells. J Biol Chem. 2001;42:38378–38387. doi: 10.1074/jbc.M105805200. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein JL, Ho YK, Basu SK, Brown MS. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci USA. 1979;76:333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, Maloney PR, Willson TM, Kliewer SA. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 17.Hatch FT, Lees RS. Practical methods for plasma lipoprotein analysis. Adv Lipid Res. 1968;6:1–68. [PubMed] [Google Scholar]
- 18.Kansy M, Senner F, Gubernator K. Physicochemical high throughput screening: parallel artificial membrane permeability assay in the description of passive absorption processes. J Med Chem. 1998;41:1007–1010. doi: 10.1021/jm970530e. [DOI] [PubMed] [Google Scholar]
- 19.Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev. 2002;23:687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- 20.Labonte ED, Li Q, Agellon LB. Expression of cholesterol 7alpha-hydroxylase restores bile acid synthesis in McArdle RH7777 cells. Arch Biochem Biophys. 2000;381:273–277. doi: 10.1006/abbi.2000.1985. [DOI] [PubMed] [Google Scholar]
- 21.Li YC, Wang DP, Chiang JY. Regulation of cholesterol 7 alpha-hydroxylase in the liver. Cloning, sequencing, and regulation of cholesterol 7 alpha-hydroxylase mRNA. J Biol Chem. 1990;265:12012–12019. [PubMed] [Google Scholar]
- 22.Liu ML, Hong ST. Early phase of amyloid beta42-induced cytotoxicity in neuronal cells is associated with vacuole formation and enhancement of exocytosis. Exp Mol Med. 2005;37:559–566. doi: 10.1038/emm.2005.69. [DOI] [PubMed] [Google Scholar]
- 23.Mak PA, Kast-Woelbern HR, Anisfeld AM, Edwards PA. Identification of PLTP as an LXR target gene and apoE as an FXR target gene reveals overlapping targets for the two nuclear receptors. J Lipid Res. 2002;43:2037–2041. doi: 10.1194/jlr.c200014-jlr200. [DOI] [PubMed] [Google Scholar]
- 24.Miyake JH, Duong-Polk XT, Taylor JM, Du EZ, Castellani LW, Lusis AJ, Davis RA. Transgenic expression of cholesterol-7a-hydroxylase prevents atherosclerosis in C57BL/6J mice. Arterioscler Thromb Vasc Biol. 2002;22:121–126. doi: 10.1161/hq0102.102588. [DOI] [PubMed] [Google Scholar]
- 25.Moore GL, Davis RA. Expression of cholesterol-7alpha-hydroxylase in murine macrophages prevents cholesterol loading by acetyl-LDL. J Lipid Res. 2002;43:629–635. [PubMed] [Google Scholar]
- 26.Nicholls SJ, Sipahi I, Schoenhagen P, Wisniewski L, Churchill T, Crowe T, Goormastic M, Wolski K, Tuzcu EM, Nissen SE, ACTIVATE Investigators Intravascular ultrasound assessment of novel antiatherosclerotic therapies: Rationale and design of the acyl- CoA:cholesterol acyltransferase intravascular atherosclerosis treatment evaluation (ACTIVATE) study. Am Heart J. 2006;152:67–74. doi: 10.1016/j.ahj.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 27.Nishimaki-Mogami T, Une M, Fujino T, Sato Y, Tamehiro N, Kawahara Y, Shudo K, Inoue K. Identification of intermediates in the bile acid synthetic pathway as ligands for the farnesoid X receptor. J Lipid Res. 2004;45:1538–1545. doi: 10.1194/jlr.M400102-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Post SM, Zoeteweij JP, Bos MH, de Wit EC, Havinga R, Kuipers F, Princen HM. Acyl-coenzyme A:cholesterol acyltransferase inhibitor, avasimibe, stimulates bile acid synthesis and cholesterol 7alpha-hydroxylase in cultured rat hepatocytes and in vivo in the rat. Hepatology. 1999;30:491–500. doi: 10.1002/hep.510300230. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez A, Bachorik PS, Wee SB. Novel effects of the acyl-coenzyme A:Cholesterol acyltransferase inhibitor 58-035 on foam cell development in primary human monocyte-derived macrophages. Arterioscler Thromb Vasc Biol. 1999;19:2199–2206. doi: 10.1161/01.atv.19.9.2199. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez A, Usher DC. Anti-atherogenic effects of the acyl-CoA:cholesterol acyltransferase inhibitor, avasimibe (CI-1011), in cultured primary human macrophages. Atherosclerosis. 2002;161:45–54. doi: 10.1016/s0021-9150(01)00620-7. [DOI] [PubMed] [Google Scholar]
- 31.Rong JX, Kusunoki J, Oelkers P, Sturley SL, Fisher EA. Acyl-Coenzyme A (CoA):cholesterol acyltransferase inhibition in rat and human aortic smooth muscle cells is nontoxic and retards foam cell formation. Arterioscler Thromb Vasc Biol. 2005;25:122–127. doi: 10.1161/01.ATV.0000148202.49842.3b. [DOI] [PubMed] [Google Scholar]
- 32.Roth BD, Blankley CJ, Hoefle ML, Holmes A, Roark WH, Trivedi BK, Essenburg AD, Kieft KA, Krause BR, Stanfield RL. Inhibitors of acyl-CoA:cholesterol acyltransferase. 1. Identification and structure-activity relationships of a novel series of fatty acid anilide hypocholesterolemic agents. J Med Chem. 1992;35:1609–1617. doi: 10.1021/jm00087a016. [DOI] [PubMed] [Google Scholar]
- 33.Rudel L, Deckelman C, Wilson M, Scobey M, Anderson R. Dietarycholesterol and down-regulation of cholesterol 7α-hydroxylase and cholesterol absorption in African green monkeys. J Clin Invest. 1994;93:2463–2472. doi: 10.1172/JCI117255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russell DW. Nuclear orphan receptors control cholesterol catabolism. Cell. 1999;97:539–542. doi: 10.1016/s0092-8674(00)80763-1. [DOI] [PubMed] [Google Scholar]
- 35.Siperstein MD, Jayko ME, Chaikoff IL, Dauben WG. Nature of the metabolic products of 14C-cholesterol excreted in bile and feces. Proc Soc Exp Biol Med. 1952;81:720–724. doi: 10.3181/00379727-81-19999. [DOI] [PubMed] [Google Scholar]
- 36.Stedman C, Liddle C, Coulter S, Sonoda J, Alvarez JG, Evans RM, Downes M. Benefit of farnesoid X receptor inhibition in obstructive cholestasis. Proc Natl Acad Sci USA. 2006;103:11323–11328. doi: 10.1073/pnas.0604772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tabas I, Boykow GC, Tall AR. Foam cell-forming J774 macrophages have markedly elevated acyl coenzyme A:cholesterol acyl transferase activity compared with mouse peritoneal macrophages in the presence of low density lipoprotein (LDL) despite similar LDL receptor activity. J Clin Invest. 1987;79:418–426. doi: 10.1172/JCI112828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tardif JC, Gregoire J, L'Allier PL, Anderson TJ, Bertrand O, Reeves F, Title LM, Alfonso F, Schampaert E, Hassan A, McLain R, Pressler ML, Ibrahim R, Lesperance J, Blue J, Heinonen T, Rodes-Cabau J, Avasimibe and Progression of Lesions on UltraSound (A-PLUS) Investigators Effects of the acyl coenzyme A:cholesterol acyltransferase inhibitor avasimibe on human atherosclerotic lesions. Circulation. 2004;110:3372–3377. doi: 10.1161/01.CIR.0000147777.12010.EF. [DOI] [PubMed] [Google Scholar]
- 39.Urizar NL, Liverman AB, Dodds DT, Silva FV, Ordentlich P, Yan Y, Gonzalez FJ, Heyman RA, Mangelsdorf DJ, Moore DD. A natural product that lowers cholesterol as an antagonist ligand for FXR. Science. 2002;296:1703–1706. doi: 10.1126/science.1072891. [DOI] [PubMed] [Google Scholar]
- 40.Urizar NL, Moore DD. GUGULIPID: a natural cholesterol-lowering agent. Annu Rev Nutr. 2003;23:303–313. doi: 10.1146/annurev.nutr.23.011702.073102. [DOI] [PubMed] [Google Scholar]
- 41.Von Eckardstein A, Nofer JR, Assmann G. High density lipoproteins and arteriosclerosis. Role of cholesterol efflux and reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 2001;21:13–27. doi: 10.1161/01.atv.21.1.13. [DOI] [PubMed] [Google Scholar]
- 42.Warner GJ, Stoudt G, Bamberger M, Johnson WJ, Rothblat GH. Cell toxicity induced by inhibition of acyl coenzyme A: cholesterol acyltransferaseand accumulation of unesterified cholesterol. J Biol Chem. 1995;270:5772–5778. doi: 10.1074/jbc.270.11.5772. [DOI] [PubMed] [Google Scholar]
- 43.Wu J, Xia C, Meier J, Li S, Hu X, Lala DS. The hypolipidemic natural product guggulsterone acts as an antagonist of the bile acid receptor. Mol Endocrinol. 2002;16:1590–1597. doi: 10.1210/mend.16.7.0894. [DOI] [PubMed] [Google Scholar]
- 44.Xu G, Li H, Pan LX, Shang Q, Honda A, Ananthanarayanan M, Erickson SK, Shneider BL, Shefer S, Bollineni J, Forman BM, Matsuzaki Y, Suchy FJ, Tint GS, Salen G. FXR-mediated down-regulation of CYP7A1 dominates LXRα in long-term cholesterol-fed NZW rabbits. J Lipid Res. 2003;44:1956–1962. doi: 10.1194/jlr.M300182-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.Zhang J, Larsson O, Sjövall J. 7α-Hydroxylation of 25-hydroxycholesterol and 27-hydroxycholesterol in human fibroblasts. Biochim Biophys Acta. 1995;1256:353–359. doi: 10.1016/0005-2760(95)00045-e. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Wang X, Vales C, Lee FY, Lee H, Lusis AJ, Edwards PA. FXR Deficiency Causes Reduced Atherosclerosis in Ldlr-/- Mice. Arterioscler Thromb Vasc Biol. 2006;26:2316–2321. doi: 10.1161/01.ATV.0000235697.35431.05. [DOI] [PubMed] [Google Scholar]