Abstract
Osteonecrosis of the femoral head (ONFH) is known as death of the cellular portion of the femoral head due to an interruption in the vascular supply. The underlying pathophysiology regarding bone cell death remains uncertain. Recently, several studies have shown that autoimmune disorders were related to the development of osteonecrosis. This study investigated the genetic effects of Interleukin 23 receptor (IL23R) polymorphisms regarding the risk of ONFH. Ten SNPs were selected and genotyped in 443 ONFH patients and 273 control subjects in order to perform the genetic association analysis. It was found that polymorphisms of the IL23R gene (rs4655686, rs1569922 and rs7539625) were significantly associated with an increased risk of ONFH (P values; 0.0198-0.0447, OR; 1.30-1.49). Particularly, a stratified analysis based on etiology (alcohol, steroid or idiopathic) showed that the associations between these polymorphisms and ONFH were most significant in idiopathic ONFH patients (P values; 0.0001-0.0150, OR; 1.45-2.17). These results suggest that IL23R polymorphisms may play an important role in the development of ONFH.
Keywords: femur head; interleukin-23; osteonecrosis; polymorphism, single nucleotide
Introduction
Osteonecrosis of the femoral head (ONFH) is a devastating disease that frequently leads to the progressive collapse of the femoral head, followed by degenerative arthritis of the hip joint. Non-traumatic ONFH has been associated with various factors such as corticosteroid usage, alcoholism, infections, marrow infiltrating diseases, coagulation defects, and some autoimmune diseases (Mont et al., 1998; Jones, 1999; Glueck et al., 2003; Boss et al., 2004; Childs, 2005). Among several confounding pathogenic mechanisms regarding ONFH, a vascular hypothesis appears to be the most persuasive, presuming that a decrease in the local blood flow in the femoral head plays a pivotal role in the pathogenesis of ONFH (de Camargo et al., 1984; Kerachian et al., 2006).
Particularly, some autoimmune disorders, including systemic lupus erythematosus (SLE), polymyalgia rheumatica (PMR), rheumatoid arthritis (RA), and inflammatory bowel disease (IBD), are related to the development of osteonecrosis (Migliaresi et al., 1994; Abu-Shakra et al., 2003; Boss et al., 2004; Tektonidou and Moutsopoulos, 2004; Lane, 2006). These diseases cause enough vasculature defects to cause a loss of microvascular flow to intraosseous tissue (Tektonidou and Moutsopoulos, 2004; Childs, 2005). Several studies reported that immunologic factors such as interleukins and TNFs might influence the development of osteonecrosis (Mont et al., 1998; Boss et al., 2004; Tektonidou and Moutsopoulos, 2004; Weitzmann and Pacifici, 2005; Sato and Takayanagi, 2006).
IL23 is a proinflammatory cytokine and consists of a p19 subunit and a p40 subunit of IL12. IL23R pairs with IL12R and both are necessary for IL23 signal pathway. IL23 regulates the activity of an immune response and promotes inflammation through the engagement of IL23R. These cytokines are primarily expressed in immune system cells such as a T cell, macrophage, and dendritic cell (Oppmann et al., 2000; Parham et al., 2002). Recently, it has been reported that IL23 deficient mice were resistant to collagen-induced arthritis (Murphy et al., 2003; Sanchez et al., 2007) and IL23 also inhibited osteoclastogenesis by RANKL (Receptor Activator for Nuclear Factor κB Ligand) through T cell activation (Kamiya et al., 2007).
Recently, many studies have reported that IL23R was related to inflammatory disorders. Several variations of the IL23R gene have been reported to influence the risk of a developing skin disorder called psoriasis (Capon et al., 2007; Cargill et al., 2007). Also, polymorphisms of the IL23R gene were associated with susceptibility to IBD and Crohn's disease (CD) in the genome wide association studies previously published (Cardon, 2006; Duerr et al., 2006; Raelson et al., 2007). In order to determine whether polymorphisms of the IL23R gene are associated with the susceptibility of ONFH, the genotype and allele frequencies between ONFH patients and controls were compared in this study.
Materials and Methods
Subjects
The study population was comprised of 443 unrelated patients (366 men, 77 women; mean age: 49.7 ± 13.3) with ONFH and 273 (206 men, 67 women; mean age: 52.1 ± 10.6) unrelated control subjects consecutively enrolled at the Kyungpook National University Hospital (Daegu, Korea) from 2002 to 2006. Patients were diagnosed and subgrouped by criteria which were described previously (Kim et al., 2007). Briefly, patients were subgrouped, according to etiological factors, into osteonecrosis, idiopathic (181 cases), steroid-induced (56 cases), and alcohol-induced (206 cases) groups. The control subjects were defined by the following criteria: those having no hip pain and others whose anteroposterior and frog leg lateral pelvic radiographs did not show any lesions, or with a sclerotic margin or subchondral collapse consistent with ONFH. We excluded all persons related to patients from our control group. This study was approved by the Institutional Review Board of Kyungpook National University Hospital, and all subjects gave written informed consent.
Selection of SNP and genotyping
Genomic DNA was isolated from peripheral blood of each individual by using a FlexiGene DNA Kit (QIAGEN, Valencia, CA). A total of 10 polymorphic sites regarding IL23R were selected by considering their location, allele frequencies and relevance to diseases based on public databases (dbSNP; http://www.ncbi.nlm.nih.gov/SNP/, HAPMAP; http://www.hapmap.org/index.html.en). The genotype identification was performed by using a Taqman probe (Applied Biosystems, Foster city, CA), according to the manufacturer's instructions. Primer Express (Applied Biosystems) was used in order to design both the PCR primers and the MGB TaqMan probes. One allelic probe was labeled with the FAM dye and the other was labeled with fluorescent VIC dye. Detailed procedures regarding the PCR reaction for Taqman assay have been described previously (Kim et al., 2006). Fluorescence data files from each plate were collected and analyzed by using automated allele-calling software (SDS 2.2, Applied Biosystems).
Statistical analyses
Chi-square tests were used in order to determine whether individual variants were in intra-locus equilibrium in the samples (Hardy-Weinberg equilibrium). Logistic regression analyses were used to calculate the odds ratios (OR), 95% confidence intervals (CI) and corresponding P values of each SNP and haplotypes controlling for age and sex as covariates with three alternative models (codominant, dominant and recessive). The linkage disequilibrium (LD) between loci was measured by using the absolute value of Lewontin's D' (|D'|) (Hedrick, 1987). Haplotype structures and their frequencies were estimated from genotyped data within the LD block by using the Haploview 3.32 (http://www.broad.mit.edu/mpg/haploview/), which estimates haplotype by an accelerated EM algorithm similar to the partition ligation method (Qin et al., 2002). All analyses were two-tailed and a P-value of < 0.05 was considered to be statistically significant. Statistical analyses were performed by using SAS 9.1 (SAS Institute Inc., Cary, NC).
Results
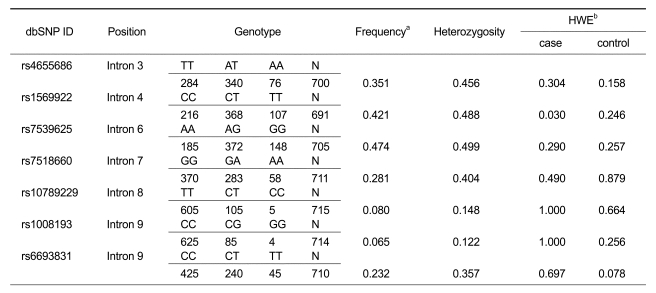
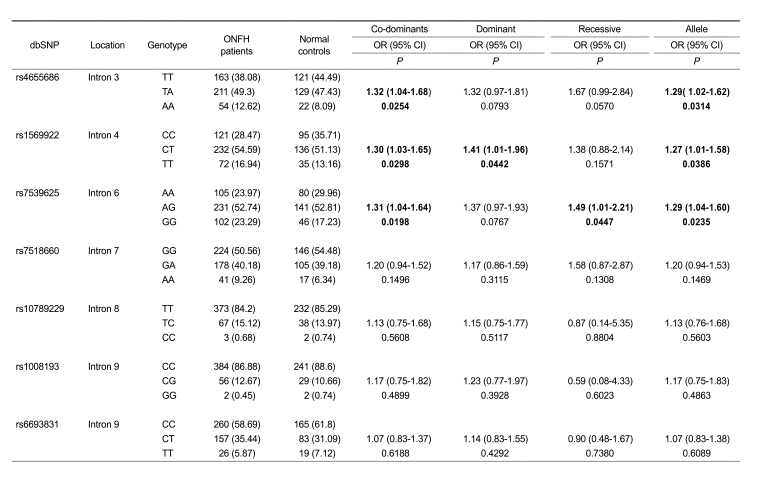
In order to investigate the association of IL23R gene polymorphisms with respect to ONFH, ten polymorphic sites in the IL23R gene were genotyped regarding 443 ONFH patients and 273 control subjects. Among the 10 SNPs genotyped, three SNP sites did not fulfill our criteria in a call rate (CR) > 0.90, minor allele frequency (MAF) > 0.05 and Hardy-Weinberg equilibrium (HWE) > 0.05. Seven SNP sites were in the HWE (P > 0.05) (Table 1). The P values of each polymorphism were obtained using logistic regression analyses by comparison between ONFH patients and the controls. It was found that rs4655686, rs1569922 and rs7539625 polymorphisms of the IL23R gene were significantly associated with the risk of ONFH in an alternative analysis model (P values; 0.0198-0.0447, OR; 1.30-1.49) (Table 2). These results suggested that the minor allele of rs4655686, rs1569922 and rs7539625 (A, T and G, respectively) contributes to an increase in the risk of ONFH.
Table 1.
Frequencies of IL23R gene polymorphisms in ONFH patients and normal controls.
aFrequencies of rare alleles. bP values of deviation from Hardy-Weinberg equilibrium among ONFH patients and control.
Table 2.
Association analyses of IL23R gene polymorphisms with the risk of ONFH.
Genotype distributions, odds ratio (OR; 95% confidence interval) and corresponding P values for logistic analyses of three alternative models and alleles are shown. Logistic regressions were applied in codominant (homozygote for major allele vs heterozygote vs homozygote for minor allele), dominant (homozygote for major allele vs heterozygote + homozygote for minor allele), recessive (homozygote for major allele + heterozygote vs homozygote for minor allele), and allele (major vs minor) models.
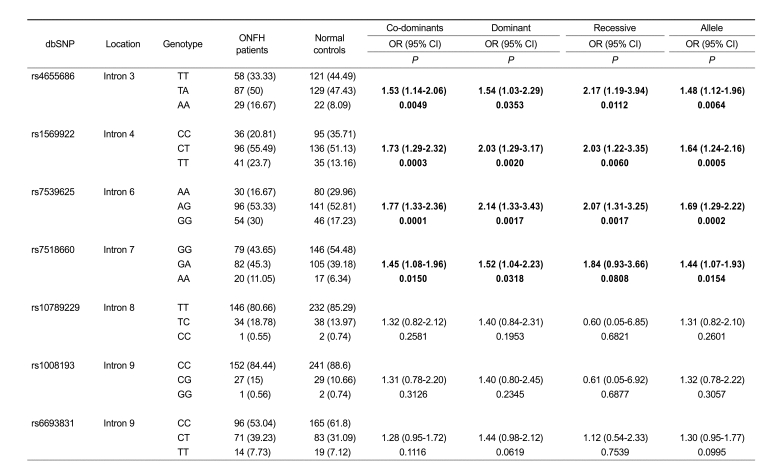
Further analysis based on pathological etiology (alcohol-, steroid- or idiopathic) showed that the genotypes of rs4655686, rs1569922, rs7539625 and rs7518660 of IL23R gene were specifically associated with the risk of ONFH in the idiopathic ONFH subgroup in all alternative models (P values; 0.0001-0.0150, OR; 1.45-2.17) (Table 3). These results suggested that the IL23R polymorphisms were possibly important risk factors in the idiopathic ONFH. However, there was no significant association with regard to alcohol- and steroid-induced subgroups (data not shown).
Table 3.
Association of IL23R gene polymorphisms between idiopathic ONFH patients and controls.
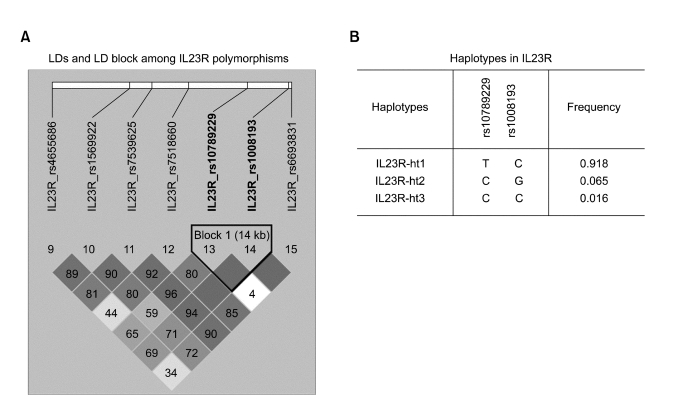
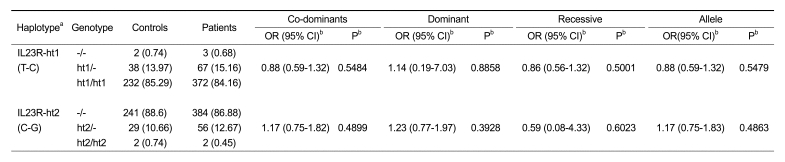
Since the LD pattern has been hypothesized to be highly structured as conserved blocks of sequence separated by hotspots of recombination, the final function of a conserved haplotype may be the result of interaction among polymorphisms within the block. LD coefficients (|D'|) between all SNP pairs were calculated, and complete LD (i.e. D' = 1) were found between the rs10789229 and rs1008193 (Figure 1A). Additionally, we calculated the haplotype frequencies with two loci (rs10789229, rs1008193) within the LD block, and tested for a haplotype association between the controls and ONFH patients. Two major haplotypes among three haplotypes explained more than 98% of the variation (Figure 1B). As shown in Table 4, haplotypes were not associated with susceptibility to ONFH. In addition, haplotype analysis in subgroup patients also showed no association with the risk of ONFH (data not shown).
Figure 1.
Linkage disequilibrium coefficients and haplotypes of IL23R (A) Linkage disequilibrium coefficients (|D'|) and LD block among IL23R polymorphisms. (B) Haplotypes of IL23R
Table 4.
Association of IL23R gene haplotypes with ONFH patients and normal controls.
Haplotypes: rs10789229 - rs1008193
Discussion
ONFH is a devastating disease that frequently leads to a progressive collapse of the femoral head, followed by degenerative arthritis of the hip joint. The precise pathophysiology of ONFH is not known, but it has been suggested that a common pathogenesis of ONFH involves an interruption of the circulation of blood to the anterior-superior-lateral region of the femoral head (Atsumi and Kuroki, 1992). A previous study reported that idiopathic ONFH coincided with an increased release of a VEGF and proinflammatory factors (Boss et al., 2004). These factors include the recruitment of endothe lial progenitor cells, macrophages, osteoclasts, fibroblasts, and osteoblasts, which, pervading throughout the necrotic areas, initiate the reparative processes. The bone effect resulting from systemic inflammation may lead to a variety of bone diseases including RA, osteoporosis and osteonecrosis (Migliaresi et al., 1994; Abu-Shakra et al., 2003; Lane, 2006). According to a previous report (Tektonidou and Moutsopoulos, 2004), immunologic factors may be related with pathogenesis of osteonecrosis.
Cytokines are recognized as being involved in the bone metabolism process. IL3, IL6, and IL11 may act to increase the proliferation as well as the differentiation of osteoclast precursors (Goldring and Goldring, 1996; Mont et al., 1998). A previous study had suggested that IL23, an IL6/IL12 cytokine member, was related to collagen-induced arthritis in mouse models and also inhibited osteoclast formation (Murphy et al., 2003; Kamiya et al., 2007). IL4 and IFN-γ, induced by IL23, have been shown to block osteoclastogenesis by means of inhibition of the transcription factors NFATc1 and c-Fos, and induction of TNF receptor-associated factor 6 (TRAF6) (Mundy, 1996; Takayanagi et al., 2002; Moreno et al., 2003; Kamel Mohamed et al., 2005; Gao et al., 2007). Therefore, these cytokines may represent a major target for the prevention of inflammation related bone diseases and promote bone metabolism. A genome wide association study has shown that the IL23R gene was associated with autoimmune diseases, including IBD, CD and psoriasis (Cardon, 2006; Duerr et al., 2006; Raelson et al., 2007). The association between IL23R and these diseases has been replicated in many other studies with different populations (Cargill et al., 2007; Cummings et al., 2007; Dubinsky et al., 2007; Glas et al., 2007; Rioux et al., 2007; Roberts et al., 2007). The recent findings of the association studies suggested that the IL-23/IL-23R cytokine receptor system is also involved in the pathogenesis of several diseases.
In this study, we have tried to determine the contribution of IL23R gene polymorphisms in regards to ONFH. We found that polymorphisms of the IL23R gene were significantly associated with ONFH in the Korean population. In particular, the genotypes of rs4655686, rs1569922, rs7539625 and rs7518660 of the IL23R gene were specifically associated with the risk of ONFH in the idiopathic subgroup (Table 3). These results suggested that inflammatory factors might be one of the important risk factors in regards to idiopathic ONFH. However, there were no significant associations shown in both alcohol- and steroid-induced subgroups. No association with ONFH in these subgroups could be attributable to different etiological characteristics of subgroup patients. Corticosteroid therapy and alcoholism, which can change fat metabolism, and accumulating cell stress, which causes occlusion of the blood flow, were major risk factors with respect to osteonecrosis (Gold and Cangemi, 1979; Asano et al., 2003). In addition, alcohol and steroids promote the generation of reactive oxygen species (ROS), resulting in oxidative stress. Therefore, it was generally thought that the effect of alcohol abuse or excessive steroid administration was stronger than genetic factors in regards to alcohol- and steroid-induced ONFH patients. On the other hand, genetic factors may be more strongly related to ONFH among idiopathic patients due to no obvious reasons having been identified.
Our study had several concerns. Firstly, since our hospital is a tertiary institution covering the Daegu-Kyungpook region, more severely ill patients are usually referred to our hospital, thus possibly resulting in selection bias. Secondly, patients in the early stage of osteonecrosis might have been included in the control group since only patients were identified by a MRI and we did not check the MRI results of the control group. The numbers of controls were less than numbers of cases since the age and sex-matched normal controls were difficult to obtain. Thirdly, the incidence of ONFH in Korea is relatively higher and sex-biased (male:female = 4:1) in comparison to other populations. The majority of osteonecrosis in Korea is either idiopathic or related to excessive alcohol use. Most male patients represent alcohol-induced cases, whereas female patients represent idiopathic or steroid-induced cases. These facts suggested that the etiology of ONFH may be quite complex and possibly attributable to gender and ethnic differences.
ONFH is one of the most common diseases of the hip in Korea, responsible for more than half of the underlying causes of total hip arthroplasty. Therefore, early diagnosis of the disease using several noted genetic markers will provide beneficial information regarding individual susceptibility to ONFH and may also help high-risk individuals to take precautions against further aggravating symptoms. In conclusion, the present study suggests, for the first time, to our knowledge, that the polymorphisms of the IL23R gene are likely to be associated with the risk of ONFH, at least in the Korean population. Further replication studies in a large cohort would be needed to confirm the suggested association.
Acknowledgements
This work was supported by a grant from the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (Project No.: A010252), and intramural grants from the Korea National Institute of Health, Korea Center for Disease Control, Republic of Korea.
Abbreviations
- CI
confidence interval
- IL23R
interleukin 23 receptor
- LD
linkage disequilibrium
- ON
osteonecrosis
- ONFH
osteonecrosis of the femoral head
- OR
odd ratio
- SNP
single nucleotide polymorphism
References
- 1.Abu-Shakra M, Buskila D, Shoenfeld Y. Osteonecrosis in patients with SLE. Clin Rev Allergy Immunol. 2003;25:13–24. doi: 10.1385/CRIAI:25:1:13. [DOI] [PubMed] [Google Scholar]
- 2.Asano T, Takahashi KA, Fujioka M, Inoue S, Satomi Y, Nishino H, Tanaka T, Hirota Y, Takaoka K, Nakajima S, Kubo T. Genetic analysis of steroid-induced osteonecrosis of the femoral head. J Orthop Sci. 2003;8:329–333. doi: 10.1007/s10776-003-0646-7. [DOI] [PubMed] [Google Scholar]
- 3.Atsumi T, Kuroki Y. Role of impairment of blood supply of the femoral head in the pathogenesis of idiopathic osteonecrosis. Clin Orthop Relat Res. 1992;277:22–30. [PubMed] [Google Scholar]
- 4.Boss JH, Misselevich I, Bejar J, Norman D, Zinman C, Reis DN. Experimentally gained insight - based proposal apropos the treatment of osteonecrosis of the femoral head. Med Hypotheses. 2004;62:958–965. doi: 10.1016/j.mehy.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 5.Capon F, Di Meglio P, Szaub J, Prescott NJ, Dunster C, Baumber L, Timms K, Gutin A, Abkevic V, Burden AD, et al. Sequence variants in the genes for the interleukin-23 receptor (IL23R) and its ligand (IL12B) confer protection against psoriasis. Hum Genet. 2007;122:201–206. doi: 10.1007/s00439-007-0397-0. [DOI] [PubMed] [Google Scholar]
- 6.Cardon LR. Genetics. Delivering new disease genes. Science. 2006;314:1403–1405. doi: 10.1126/science.1136668. [DOI] [PubMed] [Google Scholar]
- 7.Cargill M, Schrodi SJ, Chang M, Garcia VE, Brandon R, Callis KP, Matsunami N, Ardlie KG, Civello D, Catanese JJ, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007;80:273–290. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Childs SG. Osteonecrosis: death of bone cells. Orthop Nurs. 2005;24:295–301. doi: 10.1097/00006416-200507000-00012. quiz 302-3. [DOI] [PubMed] [Google Scholar]
- 9.Cummings JR, Ahmad T, Geremia A, Beckly J, Cooney R, Hancock L, Pathan S, Guo C, Cardon LR, Jewell DP. Contribution of the novel inflammatory bowel disease gene IL23R to disease susceptibility and phenotype. Inflamm Bowel Dis. 2007;13:1063–1068. doi: 10.1002/ibd.20180. [DOI] [PubMed] [Google Scholar]
- 10.de Camargo FP, de Godoy RM, Jr, Tovo R. Angiography in Perthes' disease. Clin Orthop Relat Res. 1984;191:216–220. [PubMed] [Google Scholar]
- 11.Dubinsky MC, Wang D, Picornell Y, Wrobel I, Katzir L, Quiros A, Dutridge D, Wahbeh G, Silber G, Bahar R, et al. IL-23 receptor (IL-23R) gene protects against pediatric Crohn's disease. Inflamm Bowel Dis. 2007;13:511–515. doi: 10.1002/ibd.20126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao Y, Grassi F, Ryan MR, Terauchi M, Page K, Yang X, Weitzmann MN, Pacifici R. IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J Clin Invest. 2007;117:122–132. doi: 10.1172/JCI30074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glas J, Seiderer J, Wetzke M, Konrad A, Torok HP, Schmechel S, Tonenchi L, Grassl C, Dambacher J, Pfennig S, et al. rs1004819 is the main disease-associated IL23R variant in German Crohn's disease patients: combined analysis of IL23R, CARD15, and OCTN1/2 variants. PLoS ONE. 2007;2:e819. doi: 10.1371/journal.pone.0000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glueck CJ, Freiberg RA, Wang P. Role of thrombosis in osteonecrosis. Curr Hematol Rep. 2003;2:417–422. [PubMed] [Google Scholar]
- 16.Gold EW, Cangemi PJ. Incidence and pathogenesis of alcohol-induced osteonecrosis of the femoral head. Clin Orthop Relat Res. 1979;143:222–226. [PubMed] [Google Scholar]
- 17.Goldring SR, Goldring MB. Cytokines and skeletal physiology. Clin Orthop Relat Res. 1996;324:13–23. doi: 10.1097/00003086-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Hedrick PW. Gametic disequilibrium measures: proceed with caution. Genetics. 1987;117:331–341. doi: 10.1093/genetics/117.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones JP., Jr Coagulopathies and osteonecrosis. Acta Orthop Belg. 1999;65(Suppl 1):5–8. [PubMed] [Google Scholar]
- 20.Kamel Mohamed SG, Sugiyama E, Shinoda K, Hounoki H, Taki H, Maruyama M, Miyahara T, Kobayashi M. Interleukin-4 inhibits RANKL-induced expression of NFATc1 and c-Fos: a possible mechanism for downregulation of osteoclastogenesis. Biochem Biophys Res Commun. 2005;329:839–845. doi: 10.1016/j.bbrc.2005.02.049. [DOI] [PubMed] [Google Scholar]
- 21.Kamiya S, Nakamura C, Fukawa T, Ono K, Ohwaki T, Yoshimoto T, Wada S. Effects of IL-23 and IL-27 on osteoblasts and osteoclasts: inhibitory effects on osteoclast differentiation. J Bone Miner Metab. 2007;25:277–285. doi: 10.1007/s00774-007-0766-8. [DOI] [PubMed] [Google Scholar]
- 22.Kerachian M, Harvey E, Cournoyer D, Chow T, Séguin C. Avascular Necrosis of the Femoral Head: Vascular Hypotheses. Endothelium. 2006;13:237–244. doi: 10.1080/10623320600904211. [DOI] [PubMed] [Google Scholar]
- 23.Kim DJ, Park BL, Koh JM, Kim GS, Kim LH, Cheong HS, Shin HD, Hong JM, Kim TH, Park EK, Kim SY. Methionine synthase reductase polymorphisms are associated with serum osteocalcin levels in postmenopausal women. Exp Mol Med. 2006;38:519–524. doi: 10.1038/emm.2006.61. [DOI] [PubMed] [Google Scholar]
- 24.Kim TH, Hong JM, Park EK, Kim SY. Peroxisome Proliferator-activated Receptor-gamma Gene Polymorphisms are not associated with Osteonecrosis of the Femoral Head in the Korean Population. Mol Cells. 2007;24:388–393. [PubMed] [Google Scholar]
- 25.Lane NE. Therapy Insight: osteoporosis and osteonecrosis in systemic lupus erythematosus. Nat Clin Pract Rheumatol. 2006;2:562–569. doi: 10.1038/ncprheum0298. [DOI] [PubMed] [Google Scholar]
- 26.Migliaresi S, Picillo U, Ambrosone L, Di Palma G, Mallozzi M, Tesone ER, Tirri G. Avascular osteonecrosis in patients with SLE: relation to corticosteroid therapy and anticardiolipin antibodies. Lupus. 1994;3:37–41. doi: 10.1177/096120339400300108. [DOI] [PubMed] [Google Scholar]
- 27.Mont MA, Jones LC, Einhorn TA, Hungerford DS, Reddi AH. Osteonecrosis of the femoral head. Potential treatment with growth and differentiation factors. Clin Orthop Relat Res. 1998;355(Suppl):S314–S335. [PubMed] [Google Scholar]
- 28.Moreno JL, Kaczmarek M, Keegan AD, Tondravi M. IL-4 suppresses osteoclast development and mature osteoclast function by a STAT6-dependent mechanism: irreversible inhibition of the differentiation program activated by RANKL. Blood. 2003;102:1078–1086. doi: 10.1182/blood-2002-11-3437. [DOI] [PubMed] [Google Scholar]
- 29.Mundy GR. Regulation of bone formation by bone morphogenetic proteins and other growth factors. Clin Orthop Relat Res. 1996;324:24–28. doi: 10.1097/00003086-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 32.Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, Pflanz S, Zhang R, Singh KP, Vega F, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 33.Qin ZS, Niu T, Liu JS. Partition-ligation-expectation-nmaximization algorithm for haplotype inference with single-nucleotide polymorphisms. Am J Hum Genet. 2002;71:1242–1247. doi: 10.1086/344207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raelson JV, Little RD, Ruether A, Fournier H, Paquin B, Van Eerdewegh P, Bradley WE, Croteau P, Nguyen-Huu Q, Segal J, et al. Genome-wide association study for Crohn's disease in the Quebec Founder Population identifies multiple validated disease loci. Proc Natl Acad Sci U S A. 2007;104:14747–14752. doi: 10.1073/pnas.0706645104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rioux JD, Xavier RJ, Taylor KD, Silverberg MS, Goyette P, Huett A, Green T, Kuballa P, Barmada MM, Datta LW, et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet. 2007;39:596–604. doi: 10.1038/ng2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts RL, Gearry RB, Hollis-Moffatt JE, Miller AL, Reid J, Abkevich V, Timms KM, Gutin A, Lanchbury JS, Merriman TR, et al. IL23R R381Q and ATG16L1 T300A Are Strongly Associated With Crohn's Disease in a Study of New Zealand Caucasians With Inflammatory Bowel Disease. Am J Gastroenterol. 2007;102:2754–2761. doi: 10.1111/j.1572-0241.2007.01525.x. [DOI] [PubMed] [Google Scholar]
- 37.Sanchez E, Rueda B, Callejas JL, Sabio JM, Ortego-Centeno N, Jimenez-Alonso J, Lopez-Nevot MA, Martin J. Analysis of interleukin-23 receptor (IL23R) gene polymorphisms in systemic lupus erythematosus. Tissue Antigens. 2007;70:233–237. doi: 10.1111/j.1399-0039.2007.00881.x. [DOI] [PubMed] [Google Scholar]
- 38.Sato K, Takayanagi H. Osteoclasts, rheumatoid arthritis, and osteoimmunology. Curr Opin Rheumatol. 2006;18:419–426. doi: 10.1097/01.bor.0000231912.24740.a5. [DOI] [PubMed] [Google Scholar]
- 39.Takayanagi H, Kim S, Taniguchi T. Signaling crosstalk between RANKL and interferons in osteoclast differentiation. Arthritis Res. 2002;4(Suppl 3):S227–S232. doi: 10.1186/ar581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tektonidou MG, Moutsopoulos HM. Immunologic factors in the pathogenesis of osteonecrosis. Orthop Clin North Am. 2004;35:259–263. doi: 10.1016/j.ocl.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Weitzmann MN, Pacifici R. The role of T lymphocytes in bone metabolism. Immunol Rev. 2005;208:154–168. doi: 10.1111/j.0105-2896.2005.00324.x. [DOI] [PubMed] [Google Scholar]