Abstract
During a search for keratinocyte differentiation-related genes, we obtained a cDNA fragment from the 5'-untranslated region of a previously identified splicing variant of desmoglein 3 (Dg3). This transcript encodes a protein of 282 amino acids, which corresponds to the N-terminal truncated intracellular domain of Dg3 (ΔNDg3). Northern blot analysis detected a 4.6-kb transcript matching the predicted size of ΔNDg3 mRNA, and Western blot analysis with an antibody raised against the Dg3 C-terminus (H-145) detected a 31-kDa protein. Increased ΔNDg3 expression was observed in differentiating keratinocytes by RT-PCR and Western blot analysis, suggesting that ΔNDg3 is indeed a differentiation-related gene product. In immunohistochemical studies of normal and pathologic tissues, H-145 antibody detected the protein in the cytoplasm of suprabasal layer cells, whereas an antibody directed against the N-terminal region of Dg3 (AF1720) reacted with a membrane protein in the basal layer. In addition, ΔNDg3 transcript and protein were upregulated in psoriatic epidermis, and protein expression appeared to increase in epidermal tumors including Bowen's disease and squamous cell carcinoma. Moreover, overexpression of ΔNDg3 led to increased migration and weakening of cell adhesion. These results suggest that ΔNDg3 have a role in keratinocyte differentiation, and that may be related with tumorigenesis of epithelial origin.
Keywords: cell adhesion, cell differentiation, cell movement, desmoglein 3, epidermis, keratinocytes, skin neoplasms
Introduction
Cell-cell adhesion mediated by membrane structures such as adherens junctions and desmosomes plays an important role in development and in tissue integrity. Adhesion structures are composed of large proteins, including cadherins, catenins, and plakins, which are ultimately linked to the cytoskeleton (Kowalczyk et al., 1999). With the cooperation of these proteins, cytoskeletal elements such as actin and keratin filaments anchor to the plasma membrane and add strength to cell-cell adhesions, resulting in adherens junctions and desmosomes, respectively (Angst et al., 2001). At desmosomes, desmogleins (Dgs) and desmocollins (Dscs) are cadherin family proteins that form a bridge between two cells. The cytoplasmic portions of desmosomes contain the intracellular domains of Dgs and Dscs, as well as the linking proteins plakoglobin, plakophilins, and desmoplakins (Kowalczyk et al., 1999). Desmogleins and classical cadherins are homologous in amino acid sequence both in their extracellular domains and in their intracellular cadherin-specific regions. Their C-terminal subdomain contains a desmoglein-specific repeating amino acid motif, which is termed the intracellular repeat region. Whereas the classical cadherins localize to adherens junctions, the desmogleins are found in desmosomes (Angst et al., 2001). Two of the desmogleins, Dg1 and Dg3, are autoantigens in the autoimmune blistering skin diseases pemphigus foliaceus and pemphigus vulgaris, respectively (Stanley et al., 1982; Payne et al., 2004; Baroni et al., 2007).
Terminal differentiation of skin keratinocyte is a vertically directed multi-step process that is tightly controlled by the sequential expression of a variety of genes. To gain further insight into the molecular events involved in this process, we previously attempted to identify the differentially expressed genes using the techniques of suppression subtraction hybridization and cDNA microarray (Seo et al., 2004, 2005). Among the many candidates, we chose one transcript that has now been identified as a splicing variant of Dg3 (Genbank accession, BX538327). This transcript is identified originally from the human esophagus tumor cDNA, and thought to encode a hypothetical protein of 282 amino acids. However, the expression has not yet been determined in epidermis of human skin. Because it is predicted to encode a N-terminal truncated intracellular domain of Dg3, we named it as ΔNDg3, and investigated the expression and putative role in keratinocyte differentiation.
Results
Isolation of ΔNDg3, a novel gene associated with keratinocyte differentiation
We previously identified many of differentiation-related genes from primary skin keratinocytes cultured in vitro after treatment with calcium (Seo et al., 2004). We selected one differentially expressed clone that matched to the 5'-untranslated region of the cDNA BX538327, annotated as 'similar to desmoglein 3, differentially spliced'. This transcript is predicted to encode a hypothetical protein of 282 amino acids, which corresponds to the N-terminal truncated intracellular domain of Dg3. At this point, we designated it as ΔNDg3 (Figure 1A).
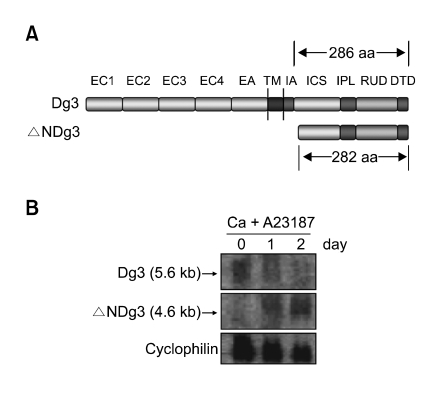
Figure 1.
(A) Overall structure of Dg3 and ΔNDg3. EC1-EC4, four extracellular cadherin-typical repeats; EA, extracellular anchor domain; TM, transmembrane domain; IA, intracellular anchor domain; ICS, intracellular cadherin-specific domain; IPL, proline-rich linker domain; RUD, repeating unit domain; DTD, desmoglein-specific terminal domain. ΔNDg3 contains slightly shorter ICS, IPL, RUD and DTD domains. (B) Northern blot analysis. HaCaT cells were treated with 1 µM A23187 and 0.3 mM calcium for the indicated time points. About 5.6 kb Dg3 mRNA and 4.6 kb ΔNDg3 mRNA were shown. Cyclophilin was detected as a loading control.
To investigate the relationship of this gene to Dg3, its mRNA was sized by Northern blot analysis. We made two different probes, one complementary to bases 1360-1619 of the Dg3 mRNA sequence (Genbank accession, NM_001944) and another recognizing a non-homologous sequence of ΔNDg3 (bases 866-1082 of the BX538327 mRNA). Using the model system in which immortalized keratinocytes HaCaT were induced to differentiate by calcium and ionophore A23187 (Fuchs, 1990; Zhao et al., 1992; Lee et al., 2005), we detected differential expression in RNAs. The sizes of the new gene product (4.6 kb) and of Dg3 (5.6 kb) corresponded to predictions from database sequences, and the clone's mRNA expression was upregulated in keratinocyte differentiation (Figure 1B).
Expression of ΔNDg3 in cultured keratinocytes and the epidermis
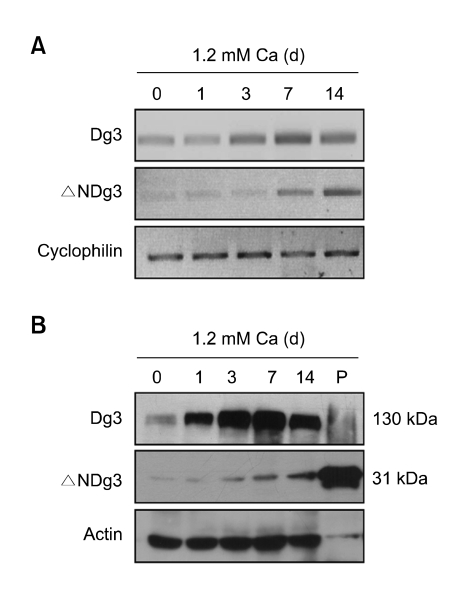
To further confirm the expression of ΔNDg3, we adopted another experimental model in which primary cultured human epidermal keratinocytes were differentiated by high calcium treatment. Consistent with previous data, RT-PCR showed that ΔNDg3 expression was markedly increased by calcium, in a time-dependent manner (Figure 2A). To determine the expression of ΔNDg3 at the protein level, we used two antibodies; one raised against the intracellular domain (residues 855-999, C-term) of Dg3 and one against the N-terminus (N-term) of Dg3. The N-term antibody could detect only Dg3, but the C-term antibody could bind to both Dg3 and ΔNDg3. Western blot analysis with C-term antibody showed the bands of Dg3 and ΔNDg3, with expected sizes (130 kDa and 31 kDa) respectively (Figure 2B).
Figure 2.
(A) RT-PCR analysis. Primary normal human epidermal keratinocytes were treated with high calcium at the indicated time points. Two µg of total RNAs were reverse transcribed with M-MLV reverse transcriptase and used for PCR amplification. (B) Western blot analysis. Cellular proteins were extracted from primary cultured keratinocytes, then separated on polyacrylamide gels. Blot was probed with C-term anti-Dg3 antibody. The positive control (P) for 31 kDa protein was prepared by transfection of pcDNA3.1-ΔNDg3 to HEK293 cells.
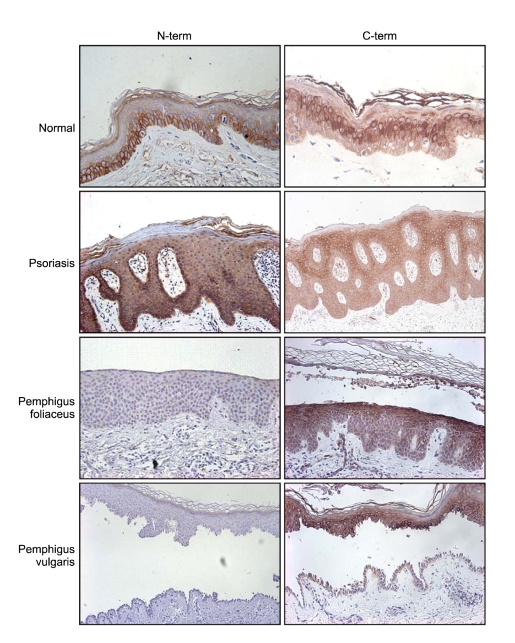
Immunostaining with the C-term antibody identified the presence of ΔNDg3 protein at the spinous layer in normal epidermis, increasing in accordance with differentiation to the granular layer (Figure 3). In contrast, experiments with the Dg3 N-term antibody showed Dg3 expression primarily at the basal layer in normal epidermis, indicating different localization of the two homologs. Additionally, the N-term antibody stained along cell membranes, while the C-term antibody reacted more strongly in cytoplasmic regions. In psoriasis, staining with the C-term antibody showed the same pattern of expression as seen in in situ hybridization. We also examined immunostaining in two bullous diseases, pemphigus foliaceus, in which separation occurs in the subcorneal layer, and in pemphigus vulgaris, separating at the suprabasal layer. We did not see Dg3 expression in these samples, but confirmed ΔNDg3 expression from the suprabasal layer to subcorneal layer in the epidermis (Figure 3).
Figure 3.
Immunohistochemical staining. Paraffin-embedded tissue sections of skin specimens were stained with N-term and/or C-term anti-Dg3 antibodies. The N-term antibody stains primarily along the cell membrane of basal layer, while the C-term antibody stains the cytoplasm of suprabasal layer.
ΔNDg3 supports epithelial cell migration and weakens keratinocyte intercellular adhesion in epithelial cells
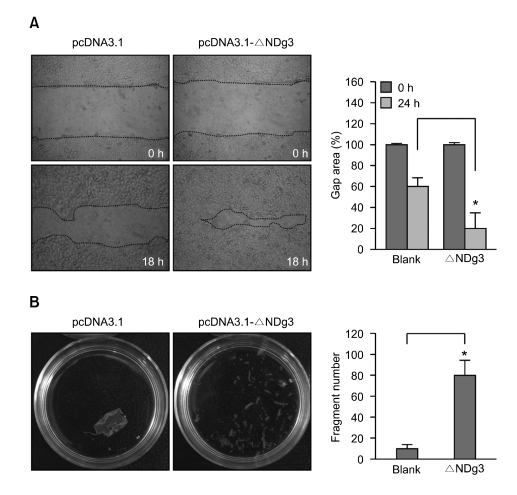
Its expression from the suprabasal to the subcorneal layer in the epidermis suggested that ΔNDg3 could play a specialized role in the migration of keratinocytes. If adhesion between keratinocytes is too strong, they may not be able to move upward in the epidermis. To determine whether ΔNDg3 expression affected cell migration, we transfected HaCaT cells with ΔNDg3 expression vector, then performed in vitro wound-healing assay. Compared to vector control, HaCaT overexpressing ΔNDg3 showed markedly enhanced migratory behavior (Figure 4A).
Figure 4.
ΔNDg3 regulates migration of epithelial cells. (A) HaCaT cells were transfected with control vector (pcDNA3.1) or κNDg3 expressing construct (pcDNA3.1-ΔNDg3). Scratch wounds were made using a pipette tip, then cells were incubated for 18 h. A cell-free area was photographed and measured by image analyzer. Data are represented as percent control and SEM, measured from three independent experiments (*P < 0.05). (B) Control and ΔNDg3 transfectants were grown to confluence. Monolayers were separated from culture dishes via incubation with dispase. Dissociation assay results were quantified by counting the number of total particles. Data represents the average and SEM from three independent experiments (*P < 0.05).
To confirm the role of ΔNDg3 in weakening keratinocyte intercellular adhesion, we examined the adhesive strength of cells overexpressing ΔNDg3, using an enzymatic and mechanical dissociation assay. HaCaT cell monolayers were lifted from culture dishes using dispase, a recombinant protease that cleaves cell-matrix, but not cell-cell adhesion molecules (Calautti et al., 1998; Huen et al., 2002). Under shear stress, both vector control and ΔNDg3-transfected cell sheets dissociated into fragments. However, ΔNDg3-transfected cell sheet fragmented more than control, supporting the idea that ΔNDg3 has impact for weakening intercellular adhesion (Figure 4B).
ΔNDg3 is associated with plakoglobin
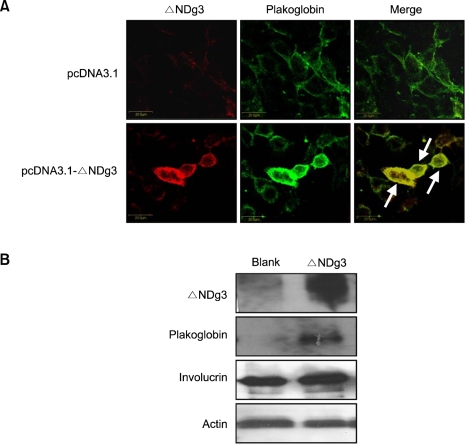
As a mechanism for weakening intercellular junction, we hypothesized that ΔNDg3 might recruit and compete for plakoglobin, which is necessary for Dg3 to connect to keratin intermediate filaments. Using ΔNDg3 overexpressing keratinocytes, we demonstrated that plakoglobin could co-localize with ΔNDg3. Interestingly, plakoglobin increased its expression in those cells, as evidenced by immunocytochemistry and immunoblotting, and its localization changed from membranes to cytoplasm with ΔNDg3 overexpression (Figure 5). These results suggest that ΔNDg3 could be involved in the regulation of plakoglobin, although such an interaction remains uncharacterized.
Figure 5.
Plakoglobin and ΔNDg3 expression in HaCaT cells. (A) Cells were transfected with control vector or ΔNDg3 expressing vector. Cells were double-stained with C-term anti-Dg3 antibody and anti-plakoglobin antibody. Plakoglobin colocalizes with ΔNDg3 in merged cells (arrows). (B) Cells were transfected with ΔNDg3 overexpressing vector, then protein levels for ΔNDg3, plakoglobin and involucrin were detected by Western blot. Actin was used as a loading control.
Expression of ΔNDg3 in several skin diseases and cancer tissues
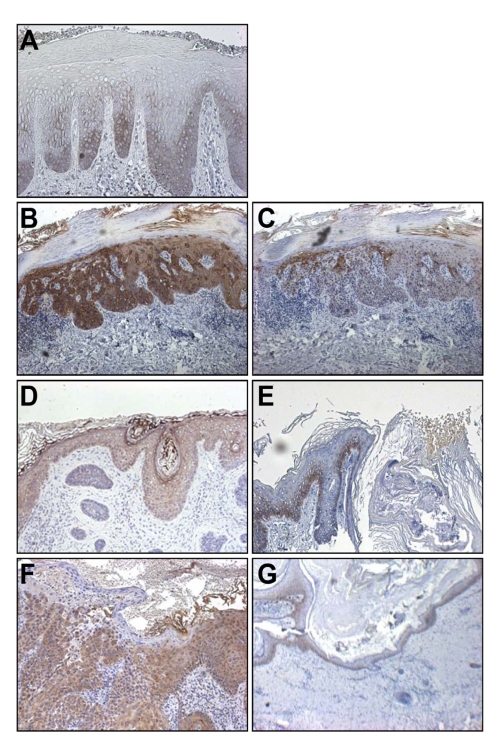
Next, we examined ΔNDg3 expression in diseases known to have alterations in keratinocyte migration using immunohistochemistry. Psoriasis is known to show abnormal differentiation of keratinocytes, with enhanced migration. In contrast, lichen simplex chronicus (LSC) is a disease characterized by a thickened epidermis due to delayed migration of individual keratinocytes from repeated scratching (Bernardin et al., 2006; Lotti et al., 2008). As expected, LSC tissue showed positive staining in the basal cells and in a few suprabasal layers (Figure 6A). In psoriasis, however, strong expression was observed in the cytoplasm of keratinocytes through almost the entire thickness of the epidermis (Figure 3).
Figure 6.
(A) ΔNDg3 expression in lichen simplex chronicus (LSC), a skin disease related with delayed migration of keratinocytes. Skin specimens were stained with C-term anti-Dg3 antibody. (B-G) ΔNDg3 expression in skin cancers. Specimens were stained with C-term anti-Dg3 antibody (B, D, F) and N-term anti-Dg3 antibody (C, E, G). (B, C) Bowen's disease, (D, E) basal cell carcinoma (BCC), (F, G) squamous cell carcinoma (SCC).
A fundamental characteristic of cancer cells is altered adhesion, both to other cells and to the extracellular matrix. The possibility of a role for ΔNDg3 in keratinocyte migration in the epidermis led us to wonder whether its expression might be altered in cancers originating from keratinocytes. We examined ΔNDg3 expression in epidermal cancers, including Bowen's disease, basal cell carcinoma (BCC), and squamous cell carcinoma (SCC) by tissue immunohistochemistry. The expression of ΔNDg3 was upregulated in cancer cells of Bowen's disease and SCC, while the N-term anti-Dg3 antibody stained primarily the basal layer keratinocytes and did not stain the cancer mass (Figure 6B-G). This results support the view that upregulation of ΔNDg3 may have impact for skin cancer development, and that it might be a new target for cancer therapy.
Discussion
Although many genes required for keratinocyte differentiation have been discovered, it is not sufficient to understand the sophisticated molecular mechanism underlying this process. In this study, we identified a differentially expressed human gene product in a calcium-induced keratinocyte differentiation model. We designated this transcript, which was previously cloned and identified as the product of alternative splicing of the desmoglein 3 gene, as ΔNDg3. The results presented here highlight a previously unrecognized role of ΔNDg3 in support of epithelial cell migration in the keratinocyte differentiation process. Specifically, we found that ΔNDg3 is expressed at comparatively high levels in differentiated epidermal keratinocytes; forced ΔNDg3 expression enhances migration of keratinocytes, but decreases intercellular adhesion in epidermal sheets; ΔNDg3 expression in cancer tissues is upregulated relative to normal tissues; and ΔNDg3 may act in concert with plakoglobin to influence cell adhesion and migration during differentiation.
The identification of ΔNDg3 and its pattern of expression may help clarify why Dg3 staining was observed in all epidermal layers, using antibodies raised against multiple epitopes of Dg3 rather than against a specific epitope. These antibodies could detect both Dg3 and ΔNDg3, although earlier explanations proposed cross-reactivity between Dg3 and other desmogleins or desmocollins (Suhrbier and Garrod, 1986; Holton et al., 1990). In efforts to elucidate the processing of Dg3, the presence of ΔNDg3 could obfuscate our understanding of events in the cytoplasm, provided antibodies reacting with ΔNDg3 are not carefully excluded. For example, antibodies raised against the IA domain of Dg3, not present in ΔNDg3, could be chosen for studies of Dg3 processing inside cells.
The deduced amino acid sequence from the human ΔNDg3 cDNA predicted a protein of 282 amino acids with a calculated molecular mass of 31 kDa. The protein contained plakoglobin-binding sites like other desmoglein family members, but no E-cadherin binding domain. In contrast to Dg3 expression in membrane of the basal layer, ΔNDg3 was found to be cytoplasmically expressed in the suprabasal layer of the epidermis. Because of its expression pattern, we hypothesized that ΔNDg3 play a role in the migration of keratinocytes during differentiation. In our experiments, keratinocytes showed markedly enhanced migratory behavior and weakened intercellular adhesion after transfection with ΔNDg3. We speculate that this effect may be, in part, due to the competitive binding of ΔNDg3 to the Dg3 binding partners. Based on the previous data and its domain structure, we postulated that one possible binding partner for ΔNDg3 is plakoglobin. Plakoglobin may contribute to desmosome assembly through its interaction with the intracellular region of desmocollins and desmogleins (Mathur et al., 1994; Yin and Green, 2004; Stokes, 2007). In our experiments, overexpression of ΔNDg3 resulted in broken focal cell adhesions and the movement of plakoglobin from the membrane to the cytoplasm, despite increasing plakoglobin protein levels. These results suggest that ΔNDg3 may disturb desmosome assembly by depleting the free intracellular desmosome components, thereby contribute to the weakening of cell-cell interaction. The precise mechanism remains to be investigated further.
Two disorders showing significant increases in epidermal thickness (acanthosis) from different mechanisms revealed different patterns of ΔNDg3 expression. Lichen simplex chronicus (LSC) from delayed keratinocyte migration was associated with downregulation of ΔNDg3, but psoriasis, with increased keratinocyte migration, showed upregulation of ΔNDg3. These results further hint a role for ΔNDg3 in keratinocyte migration.
As previously recognized, alterations in epithelial cell interactions have impact on carcinogenesis, tumor invasion, and metastasis. For example, reduced synthesis of epithelial junctional proteins was frequently observed in dedifferentiation, tumorigenesis, and metastasis. Thus, it is generally accepted that loss of adhesive molecules and of adhesion structures is implicated in the development of an invasive phenotype, and predicts a poor prognosis for patients (Trosko et al., 2004; Kartenbeck et al., 2005). In this study, the expression of ΔNDg3 is highly increased in SCC, but not in BCC, suggesting that ΔNDg3 may have a relation with tumor cell invasion. A better understanding of invasion patterns in many skin diseases are needed to explain the effects of ΔNDg3 on cancer development.
In conclusion, we identified a new gene, ΔNDg3, and characterized its structure and expression in cultured human keratinocytes, normal epidermis, and various skin disorders, including psoriasis and several epithelial cancers. Our results suggest that ΔNDg3 is involved in the differentiation and migration of cells in epithelia. It may have a novel function in epidermal differentiation and in related processes, and thus serve as a potential target in skin cancer and other disease states.
Methods
Cell culture and transfection
All skin samples were obtained under the written informed consent of donors, in accordance with the ethical committee approval process of Chungnam National University Hospital. Skin specimens were briefly sterilized in 70% ethanol, minced, and then treated with dispase for overnight at 4℃. The epidermis was separated and placed in a solution containing 0.05% trypsin and 0.02% EDTA (Gibco BRL, Rockville, MD) for 15 min at 37℃. After vigorous pipetting, cells were pelleted and resuspended in keratinocyte-serum free medium (K-SFM) supplemented with bovine pituitary extract and recombinant human EGF (Gibco BRL). Primary keratinocytes were differentiated by addition of 1.2 mM calcium. Immortalized human keratinocytes HaCaT and HEK293 cells were cultured in DMEM supplemented with 10% FBS (Gibco BRL). For differentiation of HaCaT, cells were cultured with 1 µM A23187 and 0.3 mM calcium for the indicated time points. For transfection, cells were incubated with FuGENE6 (Roche Applied Science, Indianapolis, IN) according to the manufacturer's instructions.
Northern blot analysis
Total RNAs were prepared using an Easy blue RNA isolation kit (Intron, Daejeon, Korea). For Northern blot analysis, 20 µg of total RNAs were electrophoresed in 1% agarose gels containing formaldehyde and transferred onto Hybond-N+ membranes (Amersham, Buckinghamshire, UK). Blots were prehybridized for 2 h at 42℃, then hybridized overnight at 42℃ in the same solution containing 32P-labeled probe (specific activity is ~1 × 109 cpm/µg). Probes were prepared by PCR amplification of cDNA with following primer sets: Dg3, 5'-CAAAGCTGCCTCAAATGTCA and 5'-TGCAAACTGCATCTTTTTCG; ΔNDg3, 5'-TGTTTCCTCTTTGGTTTCCA and 5'-GAGGGCATATAAACGGGTGA.
Western blot analysis
Cell extracts were prepared using Pro-prep protein extraction solution (Intron). Protein samples were run on 7-12% SDS-polyacrylamide gel, transferred onto nitrocellulose membrane and incubated with appropriate antibodies. To detect ΔNDg3, the antibody raised against C-term (855-999 amino acids) of human Dg3 (H-145, Santa Cruz Biotechnologies, Santa Cruz, CA) was used. Blots were then incubated with peroxidase-conjugated secondary antibody and developed by enhanced chemiluminescence (Amersham). Anti-plakoglobin and anti-involucrin antibodies were purchased from Santa Cruz Biotechnologies.
Cell migration assay
Cell migration assay was performed according to the previously reported method with slight modifications (Kim et al., 2007). HaCaT cells were seeded in six-well plates and transfected with pcDNA3.1-ΔNDg3 or empty vector using FuGENE6. To rule out the proliferative effect, cells were pretreated with 10 µg/ml mitomycin C (Sigma, St. Louis, MO) for 2 h. A cell-free area was introduced by scraping the monolayer with a pipette tip. After incubation, cells were photographed by using an inverted phase-contrast microscope, and gap areas were measured using Image-Pro Plus software (MediaCybernetics, Silver Spring, MD).
Dispase-based dissociation assay
The dispase-based dissociation assay was modified from a published protocol (Calkins et al., 2006). HaCaT cells were seeded in triplicate onto 60-mm dishes. At about 40~50% confluency, cells were transfected with pcDNA3.1-ΔNDg3 and mock vector. After reaching confluency, culture dish were washed twice in PBS and then incubated in 4.8 Unit of dispase (Roche Applied Science) for 30 min. Released monolayers were carefully washed with PBS and transferred to 15 ml tubes. Enough PBS was added for a final volume of 5 ml. Tubes were subjected to 50 inversions. Fragments were counted using a dissecting microscope. Statistical analysis was performed on the average of three separate experiments. Statistical significance (t test) was defined as P < 0.05.
Immunofluorescence
HaCaT cells were grown on coverslips. After transfection, cells were fixed and permeabilized with 4% paraformaldehyde for 15 min at room temperature, and 0.5% tritonX-100 for 1 min. Cell were then incubated with appropriate antibodies for overnight at room temperature, then incubated with FITC- and TRITC-conjugated secondary antibodies, and visualized under the fluorescent microscope.
Acknowledgements
This study was supported by a grant of the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (01-PJ3-PG6-01GN12-0001). Sohn KC was supported by Brain Korea 21 Research Fellowship from the Korea Ministry of Education and Human Resources.
Abbreviations
- Dg3
desmoglein 3
- ΔNDg3
N-terminal truncated intracellular domain of Dg3
References
- 1.Angst BD, Marcozzi C, Magee AI. The cadherin superfamily: diversity in form and function. J Cell Sci. 2001;114:629–641. doi: 10.1242/jcs.114.4.629. [DOI] [PubMed] [Google Scholar]
- 2.Baroni A, Lanza A, Cirillo N, Brunetti G, Ruocco E, Ruocco V. Vesicular and bullous disorders: pemphigus. Dermatol Clin. 2007;25:597–603. doi: 10.1016/j.det.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Bernardin RM, Altman CE, Meffert JJ. What is your diagnosis? Lichen simplex chronicus. Cutis. 2006;78:96, 101–102. [PubMed] [Google Scholar]
- 4.Calautti E, Cabodi S, Stein PL, Hatzfeld M, Kedersha N, Dotto GP. Tyrosine phosphorylation and src family kinases control keratinocyte cell-cell adhesion. J Cell Biol. 1998;141:1449–1465. doi: 10.1083/jcb.141.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calkins CC, Setzer SV, Jennings JM, Summers S, Tsunoda K, Amagai M, Kowalczyk AP. Desmoglein endocytosis and desmosome disassembly are coordinated responses to pemphigus autoantibodies. J Biol Chem. 2006;281:7623–7634. doi: 10.1074/jbc.M512447200. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs E. Epidermal differentiation: the bare essentials. J Cell Biol. 1990;111:2807–2814. doi: 10.1083/jcb.111.6.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holton JL, Kenny TP, Legan PK, Collins JE, Keen JN, Sharma R, Garrod DR. Desmosomal glycoproteins 2 and 3 (desmocollins) show N-terminal similarity to calcium-dependent cell-cell adhesion molecules. J Cell Sci. 1990;97:239–246. doi: 10.1242/jcs.97.2.239. [DOI] [PubMed] [Google Scholar]
- 8.Huen AC, Park JK, Godsel LM, Chen X, Bannon LJ, Amargo EV, Hudson TY, Mongiu AK, Leigh IM, Kelsell DP, Gumbiner BM, Green KJ. Intermediate filament-membrane attachments function synergistically with actin-dependent contacts to regulate intercellular adhesive strength. J Cell Biol. 2002;159:1005–1017. doi: 10.1083/jcb.200206098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kartenbeck J, Haselmann U, Gassler N. Synthesis of junctional proteins in metastasizing colon cancer cells. Eur J Cell Biol. 2005;84:417–430. doi: 10.1016/j.ejcb.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Kim HS, Kim HJ, Park KG, Kim YN, Kwon TK, Park JY, Lee KU, Kim JG, Lee IK. Alpha-lipoic acid inhibits matrix metalloproteinase-9 expression by inhibiting NF-κB transcriptional activity. Exp Mol Med. 2007;39:106–113. doi: 10.1038/emm.2007.12. [DOI] [PubMed] [Google Scholar]
- 11.Kowalczyk AP, Bornslaeger EA, Norvell SM, Palka HL, Green KJ. Desmosomes: intercellular adhesive junctions specialized for attachment of intermediate filaments. Int Rev Cytol. 1999;185:237–302. doi: 10.1016/s0074-7696(08)60153-9. [DOI] [PubMed] [Google Scholar]
- 12.Lee WH, Jang S, Lee JS, Lee Y, Seo EY, You KH, Lee SC, Nam KI, Kim JM, Kee SH, Yang JM, Seo YJ, Park JK, Kim CD, Lee JH. Molecular cloning and expression of human keratinocyte proline-rich protein (hKPRP), an epidermal marker isolated from calcium-induced differentiating keratinocytes. J Invest Dermatol. 2005;125:995–1000. doi: 10.1111/j.0022-202X.2005.23887.x. [DOI] [PubMed] [Google Scholar]
- 13.Lotti T, Buggiani G, Prignano F. Prurigo nodularis and lichen simplex chronicus. Dermatol Ther. 2008;21:42–46. doi: 10.1111/j.1529-8019.2008.00168.x. [DOI] [PubMed] [Google Scholar]
- 14.Mathur M, Goodwin L, Cowin P. Interactions of the cytoplasmic domain of the desmosomal cadherin Dsg1 with plakoglobin. J Biol Chem. 1994;269:14075–14080. [PubMed] [Google Scholar]
- 15.Payne AS, Hanakawa Y, Amagai M, Stanley JR. Desmosomes and disease: pemphigus and bullous impetigo. Curr Opin Cell Biol. 2004;16:536–543. doi: 10.1016/j.ceb.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Seo EY, Lee WH, Piao YJ, Kim KH, Lee KM, Ahn KS, Yang JM, Seo YJ, Kim CD, Park JK, Lee JH. Identification of calcium-inducible genes in primary keratinocytes using suppression-subtractive hybridization. Exp Dermatol. 2004;13:163–169. doi: 10.1111/j.0906-6705.2004.0144.x. [DOI] [PubMed] [Google Scholar]
- 17.Seo EY, Namkung JH, Lee KM, Lee WH, Im M, Kee SH, Park GT, Yang JM, Seo YJ, Park JK, Kim CD, Lee JH. Analysis of calcium-inducible genes in keratinocytes using suppression subtractive hybridization and cDNA microarray. Genomics. 2005;86:528–538. doi: 10.1016/j.ygeno.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Stanley JR, Yaar M, Hawley-Nelson P, Katz SI. Pemphigus antibodies identify a cell surface glycoprotein synthesized by human and mouse keratinocytes. J Clin Invest. 1982;70:281–288. doi: 10.1172/JCI110615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stokes DL. Desmosomes from a structural perspective. Curr Opin Cell Biol. 2007;19:565–571. doi: 10.1016/j.ceb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suhrbier A, Garrod D. An investigation of the molecular components of desmosomes in epithelial cells of five vertebrates. J Cell Sci. 1986;81:223–242. doi: 10.1242/jcs.81.1.223. [DOI] [PubMed] [Google Scholar]
- 21.Trosko JE, Chang CC, Upham BL, Tai MH. Ignored hallmarks of carcinogenesis: stem cells and cell-cell communication. Ann NY Acad Sci. 2004;1028:192–201. doi: 10.1196/annals.1322.023. [DOI] [PubMed] [Google Scholar]
- 22.Yin T, Green KJ. Regulation of desmosome assembly and adhesion. Semin Cell Dev Biol. 2004;15:665–677. doi: 10.1016/j.semcdb.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Y, Sudol M, Hanafusa H, Krueger J. Increased tyrosine kinase activity of c-Src during calcium-induced keratinocyte differentiation. Proc Natl Acad Sci USA. 1992;89:8298–8302. doi: 10.1073/pnas.89.17.8298. [DOI] [PMC free article] [PubMed] [Google Scholar]