Abstract
Reactive oxygen species (ROS) play a crucial role in acute lung injury. Tissue inflammation, the increased vascular permeability, and plasma exudation are cardinal features of acute lung injury. Angiopoietin-1 (Ang1) has potential therapeutic applications in preventing vascular leakage and also has beneficial effects in several inflammatory disorders. Recently developed COMP-Ang1 is more potent than native Ang1 in phosphorylating tyrosine kinase with immunoglobulin and EGF homology domain 2 receptor in endothelial cells. However, there are no data on effects and related molecular mechanisms of COMP-Ang1 on ROS-induced acute lung injury. We used hydrogen peroxide (H2O2)-inhaled mice to evaluate the effect of COMP-Ang1 on pulmonary inflammation, bronchial hyper-responsiveness, and vascular leakage in acute lung injury. The results have revealed that VEGF expression, the levels of IL-4, TNF-α, IL-1β, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in lungs, the levels of hypoxia-inducible factor-1α (HIF-1α) and NF-κB in nuclear protein extracts, phosphorylation of Akt, and vascular permeability were increased after inhalation of H2O2 and that the administration of COMP-Ang1 markedly reduced airway hyper-responsiveness, pulmonary inflammation, plasma extravasation, and the increases of cytokines, adhesion molecules, and VEGF in lungs treated with H2O2. We have also found that the activation of HIF-1α and NF-κB and the increase of phosphoinositide 3-kinase activity in lung tissues after H2O2 inhalation were decreased by the administration of COMP-Ang1. These results suggest that COMP-Ang1 ameliorates ROS-induced acute lung injury through attenuating vascular leakage and modulating inflammatory mediators.
Keywords: angiopoietin-1, capillary permeability, COMP-Ang1 fusion protein, hydrogen peroxide, lung, pneumonia, reactive oxygen species, vascular endothelial growth factor A
Introduction
Angiopoietin-1 (Ang1) has been identified as a secreted protein ligand of tyrosine kinase with immunoglobulin and EGF homology domain 2 (Tie2) (Yancopoulos et al., 2000). Tie2 is a member of the receptor tyrosine kinase family and expressed predominantly on vascular endothelial cells, early hematopoietic cells, and their embryonic precursors (Jones et al., 2001). Ang1- and Tie2-deficient mice have similar phenotypes characterized by embryonic lethality with severe vascular remodeling defects, insufficient vessel stabilization, and perturbed vascular maturation (Suri et al., 1996). In addition, Ang1 can counteract VEGF-induced side effects (Kim et al., 2001) while having an additive effect on vessel formation (Chae et al., 2000). These observations have suggested that Ang1 has potential therapeutic applications in inducing angiogenesis, enhancing endothelial cell survival, and preventing vascular leakage. Ang1 also reduces cytokine-induced expression of intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), E-selectin, and tissue factor, all of which are implicated in the activation of endothelial cells and leukocyte adhesion and migration in inflammation (Kim et al., 2001). However, production of Ang1 is hindered by aggregation and insolubility resulting from disulfide-linked higher-order structures. Recently, we have developed a soluble, stable, and potent Ang1 variant, COMP-Ang1 (Cho et al., 2004). To create this protein, the N-terminal portion of Ang1 was replaced with the short coiled-coil domain of cartilage oligomeric matrix protein (COMP). COMP-Ang1 is more potent than native Ang1 in phosphorylating the Tie2 receptor and Akt in primary cultured endothelial cells. Thus, COMP-Ang1 is an effective alternative to native Ang1 for therapeutic application in vivo.
Oxidative stress is caused by a large variety of free oxygen radicals (e.g. superoxide anion and hydroxyl radicals) and oxygen derivatives (e.g. hydrogen peroxide, hypochlorous acid, peroxy-nitrite, and ozone) known as reactive oxygen species (ROS) (Henricks and Nijkamp, 2001). ROS play a crucial role in the pathogenesis of various lung disorders such as asthma, chronic obstructive pulmonary diseases, idiopathic pulmonary fibrosis, acute lung injury, and acute respiratory distress syndrome (Henricks and Nijkamp, 2001). When lung tissues are exposed to oxidative stress, enhanced levels of ROS can induce a variety of deleterious effects thereby inducing pathophysiological conditions. ROS can lead to endothelial barrier dysfunction with subsequent increased permeability to fluids, macromolecules, and inflammatory cells (Henricks and Nijkamp, 2001). Very recently, we have reported that ROS increased VEGF levels closely correlated with vascular permeability and that administration of antioxidants markedly reduced the increased vascular permeability and VEGF levels, resulting in lung inflammation/injury in hydrogen peroxide (H2O2)-inhaled mice (Lee et al., 2006). However, no data are available on the role of COMP-Ang1 in ROS-induced acute lung injury.
In the present study, we have used a mouse model for H2O2-induced acute lung injury to investigate effects of COMP-Ang1 on ROS-induced acute lung injury. We have found that an Ang1 variant, COMP-Ang1 reduced the pathophysiological symptoms of acute lung injury in H2O2-inhaled mice. These findings suggest that COMP-Ang1 can be used as a therapeutic agent in acute lung injury.
Materials and Methods
Animals and experimental protocol
Female BALB/c mice, 8 to 10 wk of age and free of murine specific pathogens, were obtained from the Orientbio Inc. (Seongnam, Korea), were housed throughout the experiments in a laminar flow cabinet and maintained on standard laboratory chow ad libitum. All experimental animals used in this study were under a protocol approved by the Institutional Animal Care and Use Committee of the Chonbuk National University. The care and use of laboratory animals in this study were conformed to NIH guideline (National Institutes of Health, 1985). Mice were nebulized for 15 min with an aerosol of 3% (wt/vol) H2O2 (Junsei Chemical Co., Tokyo, Japan) in saline (or with saline as a control) using an ultrasonic nebulizer (NE-U12, Omron, Japan) as described previously (Lee et al., 2006). H2O2 was administered three times at 24-h intervals. Bronchoalveolar lavage (BAL) was performed at 12 h after the last inhalation. At the time of lavage, the mice (7 mice in each group) were sacrificed with an overdose of pentobarbital sodium (100 mg/kg of body wt, administered i.p.). The chest cavity was exposed to allow for expansion, after which the trachea was carefully intubated and the catheter was secured with ligatures. Prewarmed 0.9% NaCl solution was slowly infused into the lungs and withdrawn. The collected solutions were pooled and kept at 4℃. An aliquot of each pool was then centrifuged, and the supernatants were kept at -70℃ until use.
Administration of COMP-Ang1
We have produced COMP-Ang1 as previously described (Cho et al., 2004). COMP-Ang1 (0.5 or 1 mg/kg) suspended in PBS (vehicle control) was administered i.v. three times to each treated animal at 24-h intervals, beginning 2 h before the first inhalation with H2O2.
Measurement of plasma exudation
To assess lung permeability, Evans blue dye (EBD) was dissolved in 0.9% saline at a final concentration of 5 mg/ml. Animals were weighed and injected with 20 mg/kg EBD in the tail vein. After 30 min, the animals were killed and their chests were opened. Normal saline containing 5 mM EDTA was perfused through the aorta until all venous fluid returning to the opened right atrium was clear. Lungs were removed and weighed wet. EBD was extracted in 2 ml formamide kept in a water bath at 60℃ for 3 h and the absorption of light at 620 nm was measured using a spectrophotometer (Eppendorf Biophotometer, Hamburg, Germany). The dye extracted was quantified by interpolation against a standard curve of dye concentration in the range of 0.01-10 µg/ml and is expressed as ng of dye/mg of wet lung.
Histology
At 12 h after the last inhalation, lungs were removed from the mice after sacrifice. Before the lungs were removed, the lungs and trachea were filled intratracheally with a fixative (0.8% formalin, 4% acetic acid) using a ligature around the trachea. Lung tissues were fixed with 10% (vol/vol) neutral buffered formalin. The specimens were dehydrated and embedded in paraffin. For histological examination, 4-µm sections of fixed embedded tissues were cut with a Leica model 2165 rotary microtome (Leica, Nussloch, Germany), placed on glass slides, deparaffinized, and stained sequentially with hematoxylin 2 and eosin-Y (Richard-Allan Scientific, Kalamazoo, MI).
Western blot analysis
Lung tissues were homogenized in the presence of protease inhibitors to obtain extracts of lung proteins. Protein concentrations were determined using Bradford reagent (Bio-Rad Laboratories Inc., Hercules, CA). Samples were loaded onto an SDS-PAGE gel. After electrophoresis, separated proteins were transferred to PVDF membranes (GE Healthcare, Little Chalfont, Buckinghamshire, U.K.) by the wet transfer method (250 mA, 90 min). Nonspecific sites were blocked with 5% non-fat milk in Tris-buffered saline Tween 20 (TBST; 25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% Tween 20) for 2 h, and the blots were then incubated with an anti-TNF-α Ab (R&D Systems, Inc. Minneapolis, MN), anti-IL-1β Ab (R&D Systems), anti-IL-4 Ab (Serotec Ltd, Oxford, United Kingdom), anti-ICAM-1 Ab (Santa Cruz Biotechnology, Santa Cruz, CA), anti-VCAM-1 Ab (Santa Cruz Biotechnology), anti-VEGF Ab (Santa Cruz Biotechnology), anti-Akt Ab (Cell Signaling Technology Inc., Beverly, MA), or anti-phosphorylated Akt (p-Akt) Ab (Cell Signaling Technology Inc.) overnight at 4℃. Anti-rabbit or anti-mouse HRP-conjugated IgG was used to detect binding of the Ab. The binding of the specific Ab was visualized by exposing to photographic film after treating with enhanced chemiluminescence system reagents (GE Healthcare).
Measurement of cytokines and VEGF in BAL fluids
Levels of IL-1β, TNF-α, IL-4, and VEGF were quantified in the supernatants of BAL fluids by enzyme immunoassays according to the manufacturer's protocols (IL-1β, TNF-α, and IL-4, Endogen, Inc., Woburn, MA; VEGF, R&D Systems, Inc.). The minimum detectable level of mouse IL-1β, TNF-α, IL-4, and VEGF assays were 3, 10, 5, and 3 pg/ml, respectively.
Cytosolic or nuclear protein extractions for analysis of hypoxia inducible factor (HIF)-1α, HIF-1β, and NF-κB p65
Lungs were removed and homogenized in 2 vol of buffer A (50 mM Tris-HCl, pH 7.5, 1 mM EDTA, 10% glycerol, 0.5 mM DTT, 5 mM MgCl2, and 1 mM PMSF) containing a protease inhibitor cocktail. The homogenates were centrifuged at 1,000 × g for 15 min at 4℃. The supernatants collected were incubated on ice for 10 min and centrifuged at 100,000 × g for 1 h at 4℃ to obtain cytosolic proteins for analysis of NF-κB p65. The pellets were washed twice in buffer A and resuspended in buffer B (1.3 M sucrose, 1.0 mM MgCl2, and 10 mM potassium phosphate buffer, pH 6.8) and pelleted at 1,000 × g for 15 min. The pellets were suspended in buffer B with a final sucrose concentration of 2.2 M and centrifuged at 100,000 × g for 1 h. The resulting pellets were washed once with a solution containing 0.25 M sucrose, 0.5 mM MgCl2, and 20 mM Tris-HCl, pH 7.2, and centrifuged at 1,000 × g for 10 min. The pellets were solubilized with a solution containing 50 mM Tris-HCl (pH 7.2), 0.3 M sucrose, 150 mM NaCl, 2 mM EDTA, 20% glycerol, 2% Triton X-100, 2 mM PMSF, and a protease inhibitor cocktail. The mixture was kept on ice for 1 h with gentle stirring and centrifuged at 12,000 × g for 30 min. The resulting supernatants were used as soluble nuclear proteins for analysis of HIF-1α, HIF-1β, and NF-κB p65. The levels of these proteins were analyzed by Western blotting using Ab against HIF-1α (Novus Biologicals Inc., Littleton, CO), HIF-1β (Novus Biologicals Inc.), or NF-κB p65 (Upstate Biotech, Lake Placid, NY) as described above.
Measurement of phosphoinositide 3-kinase (PI3K) enzyme activity in lung tissues
Whole lung tissues were homogenized in the presence of protease inhibitors to obtain extracts of lung proteins, as previously reported (Kwak et al., 2003). Protein concentrations were determined using Bradford reagent (Bio-Rad Laboratories Inc.). The amount of phosphatidyl inositol-3,4,5-triphosphate (PIP3) produced was quantified by a PIP3 competition enzyme immunoassay according to the manufacturer's protocol (Echelon, Inc., Salt Lake City, UT). The enzyme activity was expressed as pmol PIP3 produced by 1 ml of lung tissue extracts containing equal amounts of total protein.
Determination of airway responsiveness to methacholine
Airway responsiveness was assessed as a change in airway function after challenge with aerosolized methacholine via airways, as described elsewhere (Takeda et al., 1997). Anesthesia was achieved with 80 mg/kg of pentobarbital sodium injected i.p.. The trachea was then exposed through midcervical incision, tracheostomized, and an 18-gauge metal needle was inserted. Mice were connected to a computer-controlled small animal ventilator (flexi-Vent, SCIREQ, Montreal, Canada). The mouse was quasi-sinusoidally ventilated with nominal tidal volume of 10 ml/kg at a frequency of 150 breaths/min and a positive end-expiratory pressure of 2 cm H2O to achieve a mean lung volume close to that during spontaneous breathing. This was achieved by connecting the expiratory port of the ventilator to water column. Before methacholine challenge, an aerosol of saline was given to obtain baseline of airway responsiveness in each group. Methacholine aerosol was generated with an in-line nebulizer and administered directly through the ventilator. To determine the differences in airway response to methacholine, each mouse was challenged with methacholine aerosol in increasing concentrations (2.5 to 50 mg/ml in saline). After each methacholine challenge, the data of respiratory system resistance (Rrs) was continuously collected. Maximum values of Rrs were selected to express changes in airway function which was represented as a percentage change from baseline after saline aerosol.
Densitometric analyses and statistics
All immunoreactive and phosphorylative signals were analyzed by densitometric scanning (Gel Doc XR, Bio-Rad Laboratories Inc.). Data are expressed as mean ± SEM. Statistical comparisons were performed using one-way ANOVA followed by the Scheffe's test. Pearson's correlation was calculated to assess the correlation between data. Significant differences between two groups were determined using t test. Statistical significance was set at P < 0.05.
Results
Effect of COMP-Ang1 on VEGF protein expression in BAL fluids and lung tissues of H2O2-inhaled mice
Western blot analysis revealed that levels of VEGF protein in lung tissues were increased significantly at 12 h after the last inhalation of H2O2 compared with the levels in the control mice (Figure 1A and B). The increased VEGF levels were decreased significantly by the administration of COMP-Ang1. Consistent with the results obtained from the Western blot analysis, enzyme immunoassay revealed that levels of VEGF protein in BAL fluids were increased at 12 h after the last inhalation of H2O2 compared with the levels in the control mice (Figure 1C). The increased VEGF levels were decreased significantly by the administration of COMP-Ang1.
Figure 1.
Effect of COMP-Ang1 on VEGF protein expression in BAL fluids and in lung tissues and plasma exudation in H2O2-inhaled mice. Sampling was performed at 12 h after the last inhalation of H2O2 in saline-inhaled mice administered with drug vehicle (SAL + VEH), H2O2-inhaled mice administered with drug vehicle (H2O2 + VEH), H2O2-inhaled mice administered with COMP-Ang1 0.5 mg/kg (H2O2 + COMP-Ang1 0.5 mg/kg), or H2O2-inhaled mice administered with COMP-Ang1 1 mg/kg (H2O2 + COMP-Ang1 1 mg/kg). (A) Western blotting of VEGF in lung tissues. (B) Densitometric analyses are presented as the relative ratio of VEGF to actin. The relative ratio of VEGF in the lung tissues of SAL + VEH is arbitrarily presented as 1. (C) Enzyme immunoassay of VEGF in BAL fluids. (D) Measurement of plasma exudation using EBD. (E) Correlation between levels of VEGF protein in BAL fluids and plasma exudation. Bars represent mean ± SEM from 7 mice per group. #P < 0.05 versus SAL + VEH; *P < 0.05 versus H2O2 + VEH.
Effect of COMP-Ang1 on plasma extravasation and correlation between levels of VEGF protein in BAL fluids and plasma extravasation in H2O2-inhaled mice
The EBD assay showed that plasma extravasation was significantly increased at 12 h after the last inhalation of H2O2 compared with that in the control mice (Figure 1D). The increase in plasma extravasation was decreased significantly by the administration of COMP-Ang1. The levels of VEGF protein in BAL fluids correlated significantly with the levels of plasma extravasation (r = 0.588; P < 0.05) (Figure 1E).
Effect of COMP-Ang1 on HIF-1α expression in nuclear protein extracts from lung tissues of H2O2-inhaled mice
Western blot analysis showed that levels of HIF-1α protein in nuclear protein extracts from lung tissues were increased significantly at 12 h after the last inhalation of H2O2 compared with the levels in the control mice (Figure 2). The increased HIF-1α levels in nuclear protein extracts were significantly decreased by the administration of COMP-Ang1.
Figure 2.
Effect of COMP-Ang1 on HIF-1α and HIF-1β levels in nuclear protein extracts from lung tissues of H2O2-inhaled mice. (A) Western blotting of HIF-1α and HIF-1β in nuclear protein extracts from lung tissues. HIF-1α and HIF-1β expression were measured at 12 h after the last inhalation of H2O2 in saline-inhaled mice administered with drug vehicle (SAL + VEH), H2O2-inhaled mice administered with drug vehicle (H2O2 + VEH), H2O2-inhaled mice administered with COMP-Ang1 0.5 mg/kg (H2O2 + COMP-Ang1 0.5 mg/kg), or H2O2-inhaled mice administered with COMP-Ang1 1 mg/kg (H2O2 + COMP-Ang1 1 mg/kg). (B) Densitometric analyses are presented as the relative ratio of HIF-1α to HIF-1β. The relative ratio of HIF-1α in the lung tissues of SAL + VEH is arbitrarily presented as 1. Bars represent mean ± SEM from 7 mice per group. #P < 0.05 versus SAL + VEH; *P < 0.05 versus H2O2 + VEH.
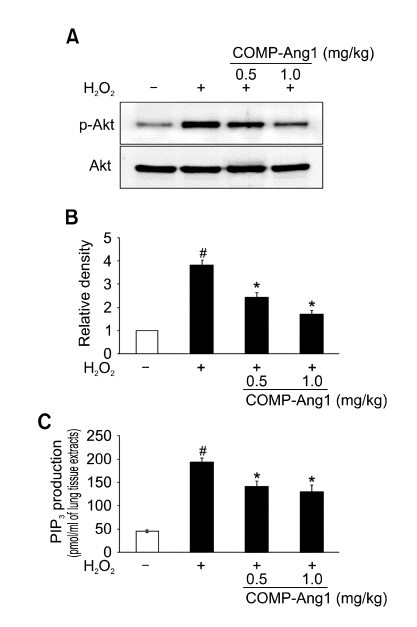
Effect of COMP-Ang1 on p-Akt and Akt protein expression and PI3K enzyme activity in lung tissues of H2O2-inhaled mice
We used Western blotting to evaluate the activation of Akt, p-Akt levels. Levels of p-Akt protein in the lung tissues were increased significantly at 12 h after H2O2 inhalation compared with the levels in the control mice (Figure 3A and B). However, no significant changes in total Akt protein levels were observed in any of the groups tested. The increased p-Akt but not Akt protein levels were significantly reduced by the administration of COMP-Ang1. In addition, we also measured PI3K enzyme activity using a PIP3 competition enzyme immunoassay. The PI3K activity in the lung tissues was increased significantly at 12 h after H2O2 inhalation compared with that in the control mice (Figure 3C). The increased PI3K activity in lung tissues at 12 h after H2O2 inhalation was significantly reduced by the administration of COMP-Ang1.
Figure 3.
Effect of COMP-Ang1 on p-Akt and Akt protein expression and PI3K enzyme activity in lung tissues. (A) Western blotting of p-Akt and Akt in lung tissues. Expression of p-Akt and Akt protein were determined at 12 h after the last inhalation of H2O2 in saline-inhaled mice administered with drug vehicle (SAL + VEH), H2O2-inhaled mice administered with drug vehicle (H2O2 + VEH), H2O2-inhaled mice administered with COMP-Ang1 0.5 mg/kg (H2O2 + COMP-Ang1 0.5 mg/kg), or H2O2-inhaled mice administered with COMP-Ang1 1 mg/kg (H2O2 + COMP-Ang1 1 mg/kg). (B) Densitometric analyses are presented as the relative ratio of p-Akt to Akt. The relative ratio of p-Akt in the lung tissues of SAL + VEH is arbitrarily presented as 1. (C) PIP3 generation by PI3Ks in lung tissue extracts. Bars represent mean ± SEM from 7 mice per group. #P < 0.05 versus SAL + VEH; *P < 0.05 versus H2O2 + VEH.
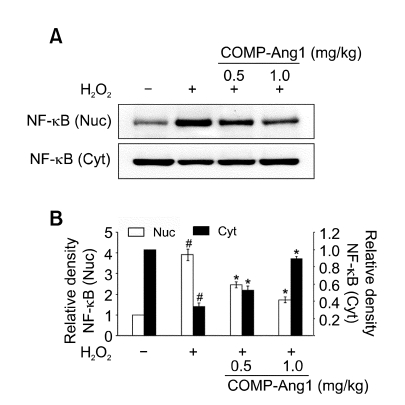
Effect of COMP-Ang1 on NF-κB p65 protein levels in lung tissues of H2O2-inhaled mice
Western blot analysis showed that levels of NF-κB p65 in nuclear protein extracts from lung tissues were increased at 12 h after H2O2 inhalation compared with the levels in the control mice (Figure 4). The increased NF-κB p65 levels in nuclear protein extracts were significantly decreased by the administration of COMP-Ang1. In contrast, the levels of NF-κB p65 protein in cytosolic protein fractions from lung tissues were decreased at 12 h after H2O2 inhalation as compared with the levels in the control mice. The decreased NF-κB p65 protein levels in cytosolic protein fractions from lung tissues were substantially increased by the administration of COMP-Ang1.
Figure 4.
Effect of COMP-Ang1 on NF-κB p65 protein levels in lung tissues. (A) Western blotting of NF-κB p65 in nuclear (Nuc) and cytosolic (Cyt) protein extracts from lung tissues. NF-κBp65 protein levels were determined at 12 h after the last inhalation of H2O2 in saline-inhaled mice administered with drug vehicle (SAL + VEH), H2O2-inhaled mice administered with drug vehicle (H2O2 + VEH), H2O2-inhaled mice administered with COMP-Ang1 0.5 mg/kg (H2O2 + COMP-Ang1 0.5 mg/kg), or H2O2-inhaled mice administered with COMP-Ang1 1 mg/kg (H2O2 + COMP-Ang1 1 mg/kg). (B) Densitometric analyses are presented as the relative ratio of NF-κBp65 in H2O2 + VEH, H2O2 + COMP-Ang1 0.5 mg/kg, or H2O2 + COMP-Ang1 1 mg/kg to those in SAL + VEH. The relative ratio of p-Akt in the lung tissues of SAL + VEH is arbitrarily presented as 1. Bars represent mean ± SEM from 7 mice per group. #P < 0.05 versus SAL + VEH; *P < 0.05 versus H2O2 + VEH.
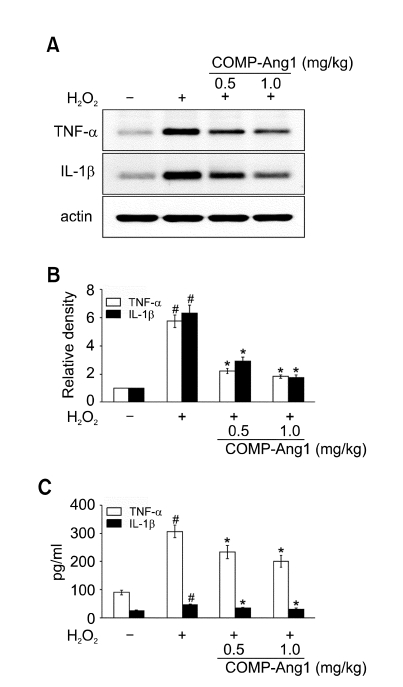
Effect of COMP-Ang1 on TNF-α and IL-1β protein expression in BAL fluids and lung tissues of H2O2-inhaled mice
Western blot analysis showed that TNF-α and IL-1β protein levels in lung tissues were increased significantly at 12 h after the last inhalation of H2O2 compared with the levels in the control mice (Figure 5A and B). The increased TNF-α and IL-1β protein levels were significantly reduced by the administration of COMP-Ang1. Consistent with the results obtained from the Western blot analysis, enzyme immunoassays revealed that levels of TNF-α and IL-1β protein in BAL fluids were increased at 12 h after the last inhalation of H2O2 compared with the levels in the control mice (Figure 5C). The increased TNF-α and IL-1β levels were decreased significantly by the administration of COMP-Ang1.
Figure 5.
Effect of COMP-Ang1 on expression of TNF-α and IL-1β protein in BAL fluids and in lung tissues and plasma exudation in H2O2-inhaled mice. Sampling was performed at 12 h after the last inhalation of H2O2 in saline-inhaled mice administered with drug vehicle (SAL + VEH), H2O2-inhaled mice administered with drug vehicle (H2O2 + VEH), H2O2-inhaled mice administered with COMP-Ang1 0.5 mg/kg (H2O2 + COMP-Ang1 0.5 mg/kg), or H2O2-inhaled mice administered with COMP-Ang1 1 mg/kg (H2O2 + COMP-Ang1 1 mg/kg). (A) Western blotting of TNF-α and IL-1β in lung tissues. (B) Densitometric analyses are presented as the relative ratio of each molecule to actin. The relative ratio of each molecule in the lung tissues of SAL+VEH is arbitrarily presented as 1. (C) Enzyme immunoassay of TNF-α and IL-1β in BAL fluids. Bars represent mean ± SEM from 7 mice per group. #P < 0.05 versus SAL + VEH; *P < 0.05 versus H2O2 + VEH.
Effect of COMP-Ang1 on IL-4 protein levels in BAL fluids and lung tissues of H2O2-inhaled mice
Western blot analysis showed that IL-4 protein levels in lung tissues were increased significantly at 12 h after the last inhalation of H2O2 compared with the levels in the control mice (Figure 6A and B). The increased IL-4 protein levels were significantly reduced by the administration of COMP-Ang1. Consistent with these results, an enzyme immunoassay revealed that levels of IL-4 protein in BAL fluids were increased at 12 h after the last inhalation of H2O2 compared with the levels in the control mice (Figure 6C). The increased IL-4 levels were decreased significantly by the administration of COMP-Ang1.
Figure 6.
Effect of COMP-Ang1 on IL-4 protein expression in BAL fluids and in lung tissues and plasma exudation in H2O2-inhaled mice. Sampling was performed at 12 h after the last inhalation of H2O2 in saline-inhaled mice administered with drug vehicle (SAL + VEH), H2O2-inhaled mice administered with drug vehicle (H2O2 + VEH), H2O2-inhaled mice administered with COMP-Ang1 0.5 mg/kg (H2O2 + COMP-Ang1 0.5 mg/kg), or H2O2-inhaled mice administered with COMP-Ang1 1 mg/kg (H2O2 + COMP-Ang1 1 mg/kg). (A) Western blotting of IL-4 in lung tissues. (B) Densitometric analyses are presented as the relative ratio of IL-4 to actin. The relative ratio of IL-4 in the lung tissues of SAL + VEH is arbitrarily presented as 1. (C) Enzyme immunoassay of IL-4 in BAL fluids. Bars represent mean ± SEM from 7 mice per group. #P < 0.05 versus SAL + VEH; *P < 0.05 versus H2O2 + VEH.
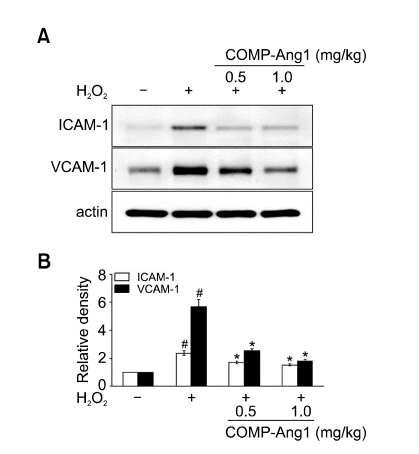
Effect of COMP-Ang1 on ICAM-1 and VCAM-1 protein expression in lung tissues of H2O2-inhaled mice
Western blot analysis showed that ICAM-1 and VCAM-1 protein levels in lung tissues were increased significantly at 12 h after the last inhalation of H2O2 compared with the levels in the control mice (Figure 7). The increased ICAM-1 and VCAM-1 protein levels were significantly reduced by the administration of COMP-Ang1.
Figure 7.
Effect of COMP-Ang1 on expression of ICAM-1 and VCAM-1 protein in lung tissues and plasma exudation in H2O2-inhaled mice. Sampling was performed at 12 h after the last inhalation of H2O2 in saline-inhaled mice administered with drug vehicle (SAL + VEH), H2O2-inhaled mice administered with drug vehicle (H2O2 + VEH), H2O2-inhaled mice administered with COMP-Ang1 0.5 mg/kg (H2O2 + COMP-Ang1 0.5 mg/kg), or H2O2-inhaled mice administered with COMP-Ang1 1 mg/kg (H2O2 + COMP-Ang1 1 mg/kg). (A) Western blotting of ICAM-1 and VCAM-1 in lung tissues. (B) Densitometric analyses are presented as the relative ratio of each molecule to actin. The relative ratio of each molecule in the lung tissues of SAL + VEH is arbitrarily presented as 1. Bars represent mean ± SEM from 7 mice per group. #P < 0.05 versus SAL + VEH; *P < 0.05 versus H2O2 + VEH.
Effect of COMP-Ang1 on pathological changes of H2O2-inhaled mice
Histologic analyses of the lung tissues revealed that numerous inflammatory cells infiltrated around the bronchioles and mucus and debris had accumulated in the lumen of bronchioles at 12 h after the last inhalation of H2O2 (Figure 8B) as compared to the control (Figure 8A). Mice treated with COMP-Ang1 (Figure 8C) showed marked reductions in the infiltration of inflammatory cells in the peribronchiolar region and the amount of mucus and debris in the airway lumen.
Figure 8.
Effect of COMP-Ang1 on pathologic changes in lung tissues. Sampling was performed at 12 h after the last inhalation in saline-inhaled mice administered with drug vehicle (A), H2O2-inhaled mice administered with drug vehicle (B), or H2O2-inhaled mice administered COMP-Ang1 1 mg/kg (C). Bars indicate 50 µm.
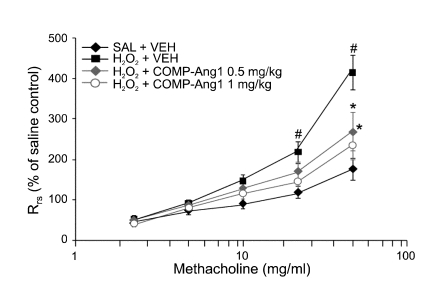
Effect of COMP-Ang1 on airway hyper-responsiveness
Airway responsiveness was assessed as a percent increase of Rrs in response to increasing doses of methacholine. In H2O2-inhaled mice, the dose-response curve of percent Rrs shifted to the left compared with that of control mice (Figure 9). In addition, the percent Rrs produced by methacholine administration (at doses from 25 mg/ml to 50 mg/ml) increased significantly in H2O2-inhaled mice compared with the controls. H2O2-inhaled mice treated with COMP-Ang1 showed a dose-response curve of percent Rrs that shifted to the right compared with that of untreated mice. These results indicate that COMP-Ang1 treatment reduces H2O2-induced airway hyper-responsiveness.
Figure 9.
Effect of COMP-Ang1 on airway responsiveness in H2O2-inhaled mice. Airway responsiveness was measured at 12 h after the last challenge in saline-inhaled mice administered with drug vehicle (SAL + VEH), H2O2-inhaled mice administered with drug vehicle (H2O2 + VEH), H2O2-inhaled mice administered with COMP-Ang1 0.5 mg/kg (H2O2 + COMP-Ang1 0.5 mg/kg), or H2O2-inhaled mice administered with COMP-Ang1 1 mg/kg (H2O2 + COMP-Ang1 1 mg/kg). Bars represent mean ± SEM from 7 mice per group. #P < 0.05 versus SAL + VEH; *P < 0.05 versus H2O2 + VEH.
Discussion
Despite recent advances in mechanical ventilation and a better understanding of the underlying inflammatory pathophysiology of acute lung injury, there are few effective treatments for this devastating illness. Oxidative stress has been shown to play critical roles in lung inflammation, including vascular leakage, which is a hallmark of acute lung injury (MacNee, 2001). Moreover, angiogenic growth factors are shown to play a role in the pathophysiology of a murine model of acute lung injury (Karmpaliotis et al., 2002). Our present study with the H2O2-induced murine model of acute lung injury revealed that COMP-Ang1, which is more potent than native Ang1 in phosphorylating the Tie2 receptor in endothelial cells (Cho et al., 2004), reduced H2O2-induced pulmonary inflammation, increased secretion of IL-4, TNF-α, IL-1β, ICAM-1, VCAM-1, and VEGF in lungs, airway hyper-responsiveness to methacholine, and increased vascular permeability. These findings suggest that COMP-Ang1 can attenuate the pathophysiological features of ROS-induced lung injury in mice.
Recently, we have reported that an increase in vascular permeability in a murine model of airway inflammation is associated with bronchial inflammation and airway hyper-responsiveness (Lee, et al., 2002b). In addition, a previous study has demonstrated that Ang1 decreases plasma leakage by reducing number and size of endothelial gaps in airway inflammation of mice (Baffert et al., 2006). In this study, we have found that amounts of plasma extravasation were greatly enhanced after the H2O2 inhalation in the murine model of acute lung injury. Interestingly, administration of COMP-Ang1 reduced the lung inflammation, airway hyperresponsiveness, and vascular leakage. These results suggest that administration of COMP-Ang1 decreases the pulmonary inflammation and bronchial hyper-responsiveness in ROS-induced acute lung injury by inhibiting vascular permeability. Increased vascular permeability causes leakage of intravascular components, including plasma extravasation. Although the pathogenesis of acute lung injury induced by plasma extravasation is not clearly defined, plasma protein leakage has been implicated to play a role in the induction of a thickened, engorged, and edematous airway wall, resulting in the airway luminal narrowing. Exudation of plasma proteins into the airways correlates with bronchial hyper-reactivity (Van de Graaf et al., 1991). It is also possible that the plasma exudates may readily pass the inflamed mucosa and reach the airway lumen through leaky epithelium, thus compromising epithelial integrity and reducing ciliary function and mucus clearance (Persson, 1996).
Though some investigators have described a role for Ang1 in promoting chemotaxis and migration of leukocytes in vitro (Feistritzer et al., 2004; Lemieux et al., 2005), Ang1 has also been shown to reduce leukocyte trafficking in inflammation through the modulation of various inflammatory mediators (Kim et al., 2001; Witzenbichler et al., 2005). Cytokines, adhesion molecules, and VEGF are involved in the chemotactic responses for leukocytes (Montefort and Holgate, 1991; Moser et al., 1992; Seder et al., 1992; Lee et al., 2002b). Moreover, cytokine-inducible leukocyte-endothelial adhesion molecules are important players for the recruitment and migration of leukocytes to the sites of inflammation (Montefort and Holgate, 1991). Expression of ICAM-1 or VCAM-1 is regulated by cytokines such as TNF-α, IL-1β, and IL-4 (Osborn et al., 1989; Hirata et al., 1998). In addition, IL-4 is shown to induce lung inflammation by promoting T-helper type 2 cell differentiation, IgE synthesis and its receptor up-regulation, and airway hyper-secretion beyond VCAM-1 expression (Moser et al., 1992; Seder et al., 1992). In the present study, we have found that levels of IL-4, adhesion molecules (ICAM-1 and VCAM-1), TNF-α, IL-1β, and VEGF in lungs were increased after H2O2 inhalation and that administration of COMP-Ang1 inhibited the increase in these protein levels. Decrease in the expression of ICAM-1 and VCAM-1 proteins may be partly due to the reduced TNF-α, IL-1β, and IL-4 levels in the lungs. Hence, these results strongly indicate that COMP-Ang1 modulates inflammatory cell migration by reducing expression of ICAM-1 and VCAM-1 and possibly also by suppressing expression of TNF-α, IL-1β, and IL-4. Taken together, these observations suggest that COMP-Ang1 can decrease airway inflammation/injury by reducing chemotactic effects on leukocytes as well as by inhibiting the increase of vascular permeability in ROS-induced acute lung injury.
ROS are shown to induce VEGF expression in vitro and in vivo (Kuroki et al., 1996). VEGF increases vascular permeability, resulting in the leakage of plasma proteins into the extravascular space, which leads to edema and profound alterations in the extracellular matrix. Previous studies have shown that overproduction of VEGF is associated with an augmentation of vascular permeability and plasma exudation in a murine model of pulmonary inflammation, which induces overproduction of ROS in the airways (Lee et al., 2005, 2006). In this study, we have found that expression of VEGF protein was upregulated and vascular permeability was also increased and that there was a close correlation between VEGF levels and vascular permeability in H2O2-induced acute lung injury. Administration of COMP-Ang1 to H2O2-inhaled mice reduced the increased vascular permeability and VEGF levels in the lungs. These results suggest that oxidative stress is associated with the upregulation of VEGF expression and that treatment of COMP-Ang1 decreases the vascular permeability by inhibiting the upregulation of VEGF expression. Activation of hypoxia-responsive genes including VEGF is mediated by HIF-1, a heterodimeric basic helix-loop-helix-PAS domain transcription factor (Semenza, 1997). HIF-1 is composed of two subunits, HIF-1α and HIF-1β. Whereas the β-subunit protein is constitutively present, the stability of the α-subunit and its transcriptional activity are precisely controlled by the intracellular oxygen concentration (Ivan et al., 2001). Determination of HIF-1α protein level in nuclear extracts has revealed that this protein level was substantially increased in our present H2O2-inhaled animal model, indicating that HIF-1 is activated. Several reports have shown that increase of PI3K/Akt activity can activate the HIF pathway (Li et al., 2003; Mottet et al., 2003). Li et al. (2003) have also reported that activation of Akt turns on HIF-1α independently of hypoxia. In addition, ROS have been shown to stabilize HIF-1α during hypoxia and/or non-hypoxia (Chandel et al., 2000). We have previously demonstrated that HIF-1α protein levels increased after H2O2 inhalation were decreased significantly by administration of PI3K inhibitors and that an HIF-1α inhibitor significantly reduced the increase of VEGF expression in lungs of H2O2-inhaled mice (Lee et al., 2006). In addition, administration of antioxidants, L-2-oxothiazolidine-4-carboxylic acid and α-lipoic acid, markedly attenuates augmentation of PI3K activity, nuclear HIF-1α levels, and VEGF levels in a murine model of ROS-induced lung injury (Lee et al., 2005, 2006). Consistent with the previous findings (Lee et al., 2006), present results have revealed that Akt phosphorylation and PI3K activity in lung tissues and HIF-1α protein levels in nuclear extracts were increased after H2O2 inhalation and that the administration of COMP-Ang1 attenuated the augmentation of PI3K activity and nuclear HIF-1α levels. These findings suggest that COMP-Ang1 modulates HIF-1α action through PI3K/Akt pathway, thereby decreasing VEGF expression and vascular permeability increased in ROS-induced murine model.
NF-κB is expressed in most cell types and is considered a pivotal transcription factor in inflammatory processes (Barnes and Karin, 1997). NF-κB is activated by a wide variety of cellular stress and stimuli and is very sensitive to oxidants (Barnes and Karin, 1997). ROS have been directly implicated as second messengers in the activation of NF-κB, based upon the ability of oxidants to activate NF-κB by oxidation of its cysteine sulfhydryl group or by ubiquitination and proteolysis of IκB (Rahman and MacNee, 1998). The activated NF-κB subsequently translocates into the nuclei where it regulates expression of a variety of inflammatory genes such as cytokines, chemokines, adhesion molecules, and growth factors. In addition, NF-κB is involved in the regulation of VEGF expression (Tong et al., 2006). Several studies have demonstrated that NF-κB activation by ROS increases expression of VEGF and VEGF receptor (Chua et al., 1998; Gonzalez-Pacheco et al., 2006). In our present H2O2-induced acute lung injury model, NF-κB levels in nuclear protein extracts were substantially increased, showing that NF-κB is activated and administration of COMP-Ang1 suppressed NF-κB activation induced by H2O2-inhalation. These findings are consistent with previous reports that Ang1/Tie2 signaling inhibits upregulation of NF-κB activity (Hughes et al., 2003; Lee et al., 2007). Together, we suggest that one likely mechanism of H2O2-mediated increase in vascular permeability is upregulation of VEGF gene expression by NF-κB activation and that COMP-Ang1-mediated reduction of vascular permeability and VEGF expression is partly due to inhibition of NF-κB activation.
In the present study, we have examined the effect of COMP-Ang1 in a murine model of H2O2-induced acute lung injury/inflammation including vascular permeability. Our data have revealed that COMP-Ang1 ameliorates H2O2-induced pathophysiological features such as acute lung inflammation, vascular leakage, and airway hyper-responsiveness via modulating the expression of various cytokines and VEGF beyond its own effect of enhancing vascular integrity. We have also demonstrated that the effects of COMP-Ang1 on ROS-induced acute lung injury may be at least partly through the regulation of activation of HIF-1α and NF-κB. Therefore, this study provides a rationale that use of COMP-Ang1 can be a recommendable therapeutic strategy for the treatment of oxidative stress-induced lung disorders.
Acknowledgment
We thank professor Mie-Jae Im for critical readings of the manuscript. This work was supported by a grant of the Korea Science and Engineering Foundation (KOSEF) through the National Research Lab. Program funded by the Ministry of Science and Technology (R0A-2005-000-10052-0(2007)), by Korea Research Foundation Grant funded by Korea Government (MOEHRD, Basic Research Promotion Fund) (KRF-2005-201-E00014), by a grant of the Korea Health 21 R&D project, Ministry of Health and Welfare, Republic of Korea (A060169) (to Y. C. L.), and also by a grant from the Korea Health 21 R&D Project (0412-CR03-0704-0001) (to S. J. P.).
Abbreviations
- Ang1
angiopoietin-1
- BAL
bronchoalveolar lavage
- COMP
cartilage oligomeric matrix protein
- EBD
Evans blue dye
- HIF
hypoxia inducible factor
- ICAM-1
intercellular adhesion molecule-1
- p-Akt
phosphorylated Akt
- PI3K
phosphoinositide 3-kinase
- PIP3
phosphatidyl inositol-3,4,5-triphosphate
- ROS
reactive oxygen species
- Rrs
respiratory system resistance
- Tie2
tyrosine kinase with immunoglobulin and epidermal growth factor homology domain 2
- VCAM-1
vascular cell adhesion molecule-1
References
- 1.Baffert F, Le T, Thurston G, McDonald DM. Angiopoietin-1 decreases plasma leakage by reducing number and size of endothelial gaps in venules. Am J Physiol Heart Circ Physiol. 2006;290:H107–H118. doi: 10.1152/ajpheart.00542.2005. [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ, Karin M. Nuclear factor-κB - a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 3.Chae JK, Kim I, Lim ST, Chung MJ, Kim WH, Kim HG, Ko JK, Koh GY. Coadministration of angiopoietin-1 and vascular endothelial growth factor enhances collateral vascularization. Arterioscler Thromb Vasc Biol. 2000;20:2573–2578. doi: 10.1161/01.atv.20.12.2573. [DOI] [PubMed] [Google Scholar]
- 4.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1α during hypoxia. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 5.Cho CH, Kammerer RA, Lee HJ, Steinmetz MO, Ryu YS, Lee SH, Yasunaga K, Kim KT, Kim I, Choi HH, Kim W, Kim SH, Park SK, Lee GM, Koh GY. COMP-Ang1: a designed angiopoietin-1 variant with nonleaky angiogenic activity. Proc Natl Acad Sci USA. 2004;101:5547–5552. doi: 10.1073/pnas.0307574101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chua CC, Hamdy RC, Chua BH. Upregulation of vascular endothelial growth factor by H2O2 in rat heart endothelial cells. Free Radic Biol Med. 1998;25:891–897. doi: 10.1016/s0891-5849(98)00115-4. [DOI] [PubMed] [Google Scholar]
- 7.Feistritzer C, Mosheimer BA, Sturn DH, Bijuklic K, Patsch JR, Wiedermann CJ. Expression and function of the angiopoietin receptor Tie-2 in human eosinophils. J Allergy Clin Immunol. 2004;114:1077–1084. doi: 10.1016/j.jaci.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Pacheco FR, Deudero JJ, Castellanos MC, Castilla MA, Alvarez-Arroyo MV, Yague S, Caramelo C. Mechanisms of endothelial response to oxidative aggression: protective role of autologous VEGF and induction of VEGFR2 by H2O2. Am J Physiol Heart Circ Physiol. 2006;291:H1395–H1401. doi: 10.1152/ajpheart.01277.2005. [DOI] [PubMed] [Google Scholar]
- 9.Henricks PA, Nijkamp FP. Reactive oxygen species as mediators in asthma. Pulm Pharmacol Ther. 2001;14:409–420. doi: 10.1006/pupt.2001.0319. [DOI] [PubMed] [Google Scholar]
- 10.Hirata N, Kohrogi H, Iwagoe H, Goto E, Hamamoto J, Fujii K, Yamaguchi T, Kawano O, Ando M. Allergen exposure induces the expression of endothelial adhesion molecules in passively sensitized human bronchus: time course and the role of cytokines. Am J Respir Cell Mol Biol. 1998;18:12–20. doi: 10.1165/ajrcmb.18.1.2704. [DOI] [PubMed] [Google Scholar]
- 11.Hughes DP, Marron MB, Brindle NP. The anti-inflammatory endothelial tyrosine kinase Tie2 interacts with a novel nuclear factor-kB inhibitor ABIN-2. Circ Res. 2003;92:630–636. doi: 10.1161/01.RES.0000063422.38690.DC. [DOI] [PubMed] [Google Scholar]
- 12.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG. HIF-1α targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 13.Jones N, Iljin K, Dumont DJ, Alitalo K. Tie receptors: new modulators of angiogenic and lymphangiogenic responses. Nat Rev Mol Cell Biol. 2001;2:257–267. doi: 10.1038/35067005. [DOI] [PubMed] [Google Scholar]
- 14.Karmpaliotis D, Kosmidou I, Ingenito EP, Hong K, Malhotra A, Sunday ME, Haley KJ. Angiogenic growth factors in the pathophysiology of a murine model of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2002;283:L585–L595. doi: 10.1152/ajplung.00048.2002. [DOI] [PubMed] [Google Scholar]
- 15.Kim I, Moon SO, Park SK, Chae SW, Koh GY. Angiopoietin-1 reduces VEGF-stimulated leukocyte adhesion to endothelial cells by reducing ICAM-1, VCAM-1, and E-selectin expression. Circ Res. 2001;89:477–479. doi: 10.1161/hh1801.097034. [DOI] [PubMed] [Google Scholar]
- 16.Kuroki M, Voest EE, Amano S, Beerepoot LV, Takashima S, Tolentino M, Kim RY, Rohan RM, Colby KA, Yeo KT, Adamis AP. Reactive oxygen intermediates increase vascular endothelial growth factor expression in vitro and in vivo. J Clin Invest. 1996;98:1667–1675. doi: 10.1172/JCI118962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwak YG, Song CH, Yi HK, Hwang PH, Kim JS, Lee KS, Lee YC. Involvement of PTEN in airway hyperresponsiveness and inflammation in bronchial asthma. J Clin Invest. 2003;111:1083–1092. doi: 10.1172/JCI16440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee KS, Park HS, Park SJ, Kim SR, Min KH, Jin SM, Park KH, Kim UH, Kim CY, Lee YC. A prodrug of cysteine, L-2-oxothiazolidine-4-carboxylic acid, regulates vascular permeability by reducing vascular endothelial growth factor expression in asthma. Mol Pharmacol. 2005;68:1281–1290. doi: 10.1124/mol.105.016055. [DOI] [PubMed] [Google Scholar]
- 19.Lee KS, Kim SR, Park SJ, Park HS, Min KH, Lee MH, Jin SM, Jin GY, Yoo WH, Lee YC. Hydrogen peroxide induces vascular permeability via regulation of vascular endothelial growth factor. Am J Respir Cell Mol Biol. 2006;35:190–197. doi: 10.1165/rcmb.2005-0482OC. [DOI] [PubMed] [Google Scholar]
- 20.Lee S, Kim W, Moon SO, Sung MJ, Kim DH, Kang KP, Jang KY, Lee SY, Park BH, Koh GY, Park SK. Renoprotective effect of COMP-angiopoietin-1 in db/db mice with type 2 diabetes. Nephrol Dial Transplant. 2007;22:396–408. doi: 10.1093/ndt/gfl598. [DOI] [PubMed] [Google Scholar]
- 21.Lee TH, Avraham H, Lee SH, Avraham S. Vascular endothelial growth factor modulates neutrophil transendothelial migration via up-regulation of interleukin-8 in human brain microvascular endothelial cells. J Biol Chem. 2002;277:10445–10451. doi: 10.1074/jbc.M107348200. [DOI] [PubMed] [Google Scholar]
- 22.Lee YC, Kwak YG, Song CH. Contribution of vascular endothelial growth factor to airway hyperresponsivenessand inflammation in a murine model of toluene diisocyanate-induced asthma. J Immunol. 2002;168:3595–3600. doi: 10.4049/jimmunol.168.7.3595. [DOI] [PubMed] [Google Scholar]
- 23.Lemieux C, Maliba R, Favier J, Théorêt JF, Merhi Y, Sirois MG. Angiopoietins can directly activate endothelial cells and neutrophils to promote proinflammatory responses. Blood. 2005;105:1523–1530. doi: 10.1182/blood-2004-09-3531. [DOI] [PubMed] [Google Scholar]
- 24.Li YM, Zhou BP, Deng J, Pan Y, Hay N, Hung M. A hypoxia independent hypoxia-inducible factor-1 activation pathway induced by phosphatidylinositol-3 kinase/Akt in HER2 overexpressing cells. Cancer Res. 2003;65:3257–3263. doi: 10.1158/0008-5472.CAN-04-1284. [DOI] [PubMed] [Google Scholar]
- 25.MacNee W. Oxidative stress and lung inflammation in airways disease. Eur J Pharmacol. 2001;429:195–207. doi: 10.1016/s0014-2999(01)01320-6. [DOI] [PubMed] [Google Scholar]
- 26.Montefort S, Holgate ST. Adhesion molecules and their role in inflammation. Respir Med. 1991;85:91–99. doi: 10.1016/s0954-6111(06)80284-2. [DOI] [PubMed] [Google Scholar]
- 27.Moser R, Fehr J, Bruijnzeel PL. IL-4 controls the selective endothelium-driven transmigration of eosinophils from allergic individuals. J Immunol. 1992;149:1432–1438. [PubMed] [Google Scholar]
- 28.Mottet D, Dumont V, Deccache Y, Demazy C, Ninane N, Raes M, Michiels C. Regulation of hypoxia-inducible factor-1alpha protein level during hypoxic conditions by the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3beta pathway in HepG2 cells. J Biol Chem. 2003;278:31277–31285. doi: 10.1074/jbc.M300763200. [DOI] [PubMed] [Google Scholar]
- 29.National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Washington, D.C: U. S. Government Printing Office; 1985. NIH publication No. 86-23. [Google Scholar]
- 30.Osborn L, Hession C, Tizard R, Vassallo C, Luhowskyj S, Chi-Rosso G, Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989;59:1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- 31.Persson CG. Epithelial cells: barrier functions and shedding-restitution mechanisms. Am J Respir Crit Care Med. 1996;153:S9–S10. doi: 10.1164/ajrccm/153.6_Pt_2.S9. [DOI] [PubMed] [Google Scholar]
- 32.Rahman I, MacNee W. Role of transcription factors in inflammatory lung diseases. Thorax. 1998;53:601–612. doi: 10.1136/thx.53.7.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seder RA, Paul WE, Davis MM, Fazekas de St Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med. 1992;176:1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1997;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- 35.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 36.Takeda K, Hamelmann E, Joetham A, Shultz LD, Larsen GL, Irvin CG, Gelfand EW. Development of eosinophilic airway inflammation and airway hyperresponsiveness in mast cell-deficient mice. J Exp Med. 1997;186:449–454. doi: 10.1084/jem.186.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tong Q, Zheng L, Lin L, Li B, Wang D, Huang C, Li D. VEGF is upregulated by hypoxia-induced mitogenic factor via the PI-3K/Akt-NF-kappaB signaling pathway. Respir Res. 2006;7:37. doi: 10.1186/1465-9921-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van de Graaf EA, Out TA, Roos CM, Jansen HM. Respiratory membrane permeability and bronchial hyperreactivity in patients with stable asthma. Effects of therapy with inhaled steroids. Am Rev Respir Dis. 1991;143:362–368. doi: 10.1164/ajrccm/143.2.362. [DOI] [PubMed] [Google Scholar]
- 39.Witzenbichler B, Westermann D, Knueppel S, Schultheiss HP, Tschope C. Protective role of angiopoietin-1 in endotoxic shock. Circulation. 2005;111:97–105. doi: 10.1161/01.CIR.0000151287.08202.8E. [DOI] [PubMed] [Google Scholar]
- 40.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]