Abstract
Tropomyosin-related kinase A (TrkA) plays an important role in cell survival, differentiation, and apoptosis in various neuronal and nonneuronal cell types. Here we show that TrkA overexpression by the Tet-On system mimics NGF-mediated activation pathways in the absence of nerve growth factor (NGF) stimulation in U2OS cells. In addition, p53 upregulation upon DNA damage was inhibited by TrkA, and p21 was upregulated by TrkA in a p53-independent manner. TrkA overexpression caused cell death by interrupting cell cycle progression, and TrkA-induced cell death was diminished in the presence of its specific inhibitor GW441756. Interestingly, TrkA-mediated cell death was strongly related to γH2AX production and poly (ADP-ribose) polymerase cleavage in the absence of DNA damage inducer. In this study, we also reveal that γH2AX production by TrkA is blocked by TrkA kinase inhibitors K-252a and GW441756, and it is also significantly inhibited by JNK inhibitor SP600125. Moreover, reduction of cell viability by TrkA was strongly suppressed by SP600125 treatment, suggesting a critical role of JNK in TrkA-induced cell death. We also found that γH2AX and TrkA were colocalized in cytosol in the absence of DNA damage, and the nuclear localization of γH2AX induced by DNA damage was partly altered to cytosol by TrkA overexpression. Our results suggest that the abnormal cytosolic accumulation of γH2AX is implicated in TrkA-induced cell death in the absence of DNA damage.
Keywords: cell death; DNA damage; H2AFX protein, human; JNK mitogen-activated protein kinases; receptor, trkA
Introduction
Tropomyosin-related kinase A (TrkA) receptor tyrosine kinase is activated by binding to its specific ligand such as nerve growth factor (NGF) (Reichardt, 2006). NGF-mediated TrkA signaling can lead to the induction of cell survival, differentiation, or apoptosis, dependent on TrkA cellular location (Zhang et al., 2000; Saxena et al., 2005). In addition, the pleiotropic effects of TrkA appear to be determined by a cell type-specific manner in response to NGF. However, little has been reported about the mechanism and selective decision of TrkA-induced biological roles.
TrkA overexpression induces apoptosis via p53 activation in neuroblastoma cells (Lavoie et al., 2005), suggesting a potential role of TrkA in the DNA damage signaling pathways. DNA damage by doxorubicin and ionizing radiation induces autophosphorylation of ATM at Serine 1981 and subsequently activate multiple downstream targets such as p53, histone H2AX, Nbs1, Chk1, and Chk2 (Kurz et al., 2004; Kurz and Lees-Miller, 2004; Cho et al., 2005). As an early response to DNA damage, H2AX, a derivative of histone H2A, can be phosphorylated at Serine 139 by ATM, other PI-3 kinases such as ATR and DNA-PK (Takahashi and Ohnishi, 2005), and c-Jun NH2-terminal kinase (JNK) (Lu et al., 2006; Sluss and Davis, 2006). This phosphorylated H2AX is generally named γH2AX and detected by its phosphate-specific antibody. Accumulation of γH2AX at the DNA damage sites causes local foci formation in the nucleus, and many numbers of DNA damage proteins such as Mre11/RAD50/NBS1 complex, 53BP1, MDC1, and ATM are getting together in these nuclear foci for cellular response (Kurz and Lees-Miller, 2004).
A role for γH2AX has been demonstrated in DNA repair, cell cycle checkpoints, site-specific recombination, tumor suppression, and apoptosis upon DNA damage (Fernandez-Capetillo et al., 2004). In particular, γH2AX production can be blocked by the inhibitor of caspase-activated DNase (Rogakou et al., 2000), indicating that it is related to the induction of apoptotic cell death. On the contrary, γH2AX can be produced independent of DNA damage in a cell cycle-dependent manner in HeLa cells (Ichijima et al., 2005). In fact, H2AX-/- mouse embryonic fibroblasts exhibit growth defect (Celeste et al., 2002). Furthermore, γH2AX was largely produced in the outer root sheath and hair bulb during a hair cycle in the mouse skin in the absence of DNA double strand breaks, and its production was independent of ATM and DNA-PK (Koike et al., 2007). These results suggest other roles of γH2AX in normal cell proliferation of various cell types.
Using TrkA-inducible stable U2OS cell lines by the Tet-On system, we here first reveal that TrkA overexpression results in the accumulation of γH2AX in cytosol and cell death in the absence of either NGF stimulation or DNA damage inducer, suggesting a novel mechanism of TrkA-induced apoptotic cell death.
Materials and Methods
Materials
Doxorubicin, GW441756, propidium iodide, RNase A, and BSA were purchased from Sigma (St. Louis, MO). K-252a and JNK inhibitor II (SP600125) were from Calbiochem (San Diego, CA). Blasticidin, zeocin, pcDNA6-TR, and pcDNA4 (TO) were from Invitrogen (Calsbad, CA). Tetracycline was from Duchefa. 20% formaldehyde was from Tousimis. Aqueous mounting media was from Biomeda. Super signal west pico stable peroxide solution was from Pierce. Fugene-6 transfection reagent was from Roche. Antibodies used in this study were: TrkA (763) (Santa Cruz, CA), phospho-Trk (E6, Santa Cruz), ERK1 (Transduction Laboratories San Diego, CA), phospho-ERK [Cell Signaling Technology (Beverly, MA)], phospho-Tyr (Transduction Laboratories), ATM (5C2, Santa Cruz), phospho-Ser1981-ATM (Chemicon International, CA), p53 (DO1, Santa Cruz), p21 (Transduction Laboratories), β-Actin (Sigma), PARP (Transduction Laboratories), γH2AX (Upstate Biotechnology), secondary goat anti-mouse/rabbit HRP conjugate (Bio-Rad, Hercules, CA), anti-mouse FITC conjugate (Sigma), and anti-rabbit TRITC conjugate (Sigma).
Construction of TrkA cDNA to tetracycline-responsive vector
Human TrkA constructed to pLNCX5 vector, pLNCX5-TrkA, was obtained from Dr. David Kaplan (Stephens et al., 1994). Using pLNCX5-TrkA as a template, full-length TrkA cDNA was amplified by PCR with forward primer, 5'-gaattctCTGCGAGGCGGACGGCGC-3', and reverse primer, 5'-tctagaCTAGCCCAGGACATCCAGGTA-3'. The amplified TrkA cDNA was subcloned to pGEM-T Easy vector and confirmed by sequencing. The result showed that TrkA used here was an alternative spliced form of 6 amino acids (VSFSPV) in Exon 9 of human TrkA (Tacconelli et al., 2004). And then, pGEM-T Easy-TrkA was digested with EcoRI and XbaI and subcloned to EcoRI and XbaI digested pcDNA4 (TO)-2FLAG vector, which was generated from us for the convenient construction and expression of a FLAG-tagged protein. The pcDNA4(TO)-2FLAG-TrkA was used for the generation of cell lines which inducibly express TrkA in the presence of tetracycline.
Cell culture and TrkA-inducible stable cell line
The osteosarcoma cell line U2OS cells were cultured in medium A [DMEM (Gibco, Gibco-BRL, Gaithersburg, MD) containing tetracycline-screened 10% FBS (Gibco)], 100 U/ml of penicillin and 100 µg/ml of streptomycin) in a humidified 5% CO2 incubator at 37℃. To generate U2OS-TR cell line which expresses tetracycline repressor, U2OS cells were transfected with pcDNA6-TR plasmid using Fugene-6 transfection reagent and selected in medium A with 20 µg/ml of blasticidin. After one week, the cells were continuously selected with medium A containing 50 µg/ml of blasticidin and then maintained in medium A with 5 µg/ml of blasticidin. After testing the repressor activity from pcDNA6-TR in selected clones, we chose one cell line and named U2OS-TR-5, although it was not able to completely block TrkA expression in the absence of tetracycline. Thus, the U2OS-TR-5 cells were cotransfected with pcDNA4(TO)-2FLAG-TrkA and pcDNA6-TR to generate TrkA-inducible stable cell lines that could almost completely block TrkA expression in the absence of tetracycline. The cells were selected in medium A containing 5 µg/ml blasticidin and 100 µg/ml zeocin as described above. After picking up colonies, the cells were maintained in medium A with 2.5 µg/ml blasticidin and 50 µg/ml zeocin and then tested in the absence or presence of tetracycline. Finally, we generated TrkA-inducible clones (#8, #60, #67) that can induce TrkA expression in the presence of tetracycline, but not in the absence.
Western blot analysis
Whole cells were extracted with SDS sample buffer. After boiling for 5-10 min at 95℃, proteins were separated on 9% or 13.5% SDS-PAGE and transferred to nitrocellulose membrane. The blot was blocked for 1 h in blocking buffer [1X PBS containing 0.1% Tween-20 (1X PBST), 3% skim milk] and then incubated with primary antibody in blocking buffer by gently rocking overnight at 4℃. The blot was washed three times with 1X PBST for 15 min and incubated with HRP-conjugated secondary antibody in blocking buffer for 1-2 h at room temperature. After washing with 1X PBST, the blot was analyzed with super signal enhanced chemiluminescence (ECL) detection system (Pierce, Rockford, IL).
Cell cycle analysis using DNA histogram
U2OS-TrkA inducible stable cell lines were grown on 10 cm dish for 12 h in the absence or presence of tetracycline to induce TrkA overexpression and then untreated or treated with GW441756 as indicated. The floating cells in the medium were collected and the attached cells were trypsinized. Both cells were put together, washed with PBS, suspended in 0.5 ml of 0.1% glucose/PBS, and fixed with 5 ml of 70% cold ethanol for at least 2 h at 4℃. After washing with PBS, the cells were treated with RNase A (250 µg/ml) and propidium iodide (50 µg/ml) in PBS at 37℃ for 30-45 min. Cell cycle analysis was performed using fluorescence-activated cell sorter (FACS Calibur, Becton Dickinson) (Jung and Flemington, 2001).
Cell viability analysis using CCK-8 assay
U2OS-TrkA-67 cells were split on 24-well dish in the absence or presence of tetracycline and then treated either DMSO or SP600125 at 12 h after tetracycline treatment. After 24 h, cell counting kit-8 (CCK-8) assay (Dojindo) was performed as described previously (Liu and Hong, 2005). Cell viability was determined by measuring absorbance at OD485 nm using CHAMELEON microplate reader (Hidex).
Fractionation of cytosol
Cells were harvested by scrapping and washed with TBS buffer [20 mM Tris (pH 7.5), 100 mM NaCl]. The cells were suspended with hypotonic buffer [25 mM Tris (pH 7.4), 1 mM MgCl2, 5 mM KCl] and incubated for 5 min on ice. After treatment with hypotonic buffer containing 1% NP-40 for 5 min on ice, the cells were centrifuged at 7,000 rpm for 5 min at 4℃. The supernatant was collected as a cytosolic fraction (Ransone, 1995).
Confocal immunofluorescence microscopy
Cells were grown on 6-well dish containing cover slide in the absence or presence of tetracycline and then untreated or treated with doxorubicin as indicated. After washing with PBS, cells were fixed with 3% formaldehyde/PBS for 45 min, permeabilized with 0.5% Triton X-100/PBS for 3-5 min, blocked with 1% BSA/PBS for 1 h, and then incubated with primary antibody for 1-2 h. After washing with PBS, cells were incubated with FITC- and TRITC-labelled secondary antibodies. The cells were washed with PBS and mounted with aqueous mounting media. Localization of protein was analyzed by confocal microscopy (Olympus FV-500).
Results
TrkA overexpression mimics NGF-mediated activation pathways
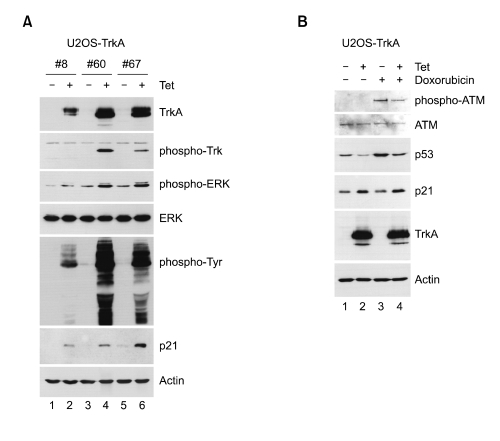
For the study of TrkA-mediated cellular responses, we generated TrkA-inducible stable cell lines in nonneuronal U2OS cells using a tetracycline repressor-regulated (Tet-On) expression system. Compared to other systems constitutively expressing proteins, the Tet-on system is very beneficial for the study of certain proteins necessary for their time-controlled expression. Among several tens of TrkA clones, we here characterized three TrkA-expressing clones at 12 h in the absence or presence of tetracycline by Western blot analysis. TrkA expression level was relatively low in #8 and high in #60 and #67 (Figure 1A, first panel). In response to NGF, TrkA become activated via its autophosphorylation and induces phosphorylation of downstream targets such as ERK and Akt (Reichardt, 2006). In addition, NGF has been known to induce the cyclin-dependent kinase inhibitor p21 (Decker, 1995; Yan and Ziff, 1995). Here, we show that in the absence of NGF stimulation, TrkA overexpression itself can lead to its autophosphorylation at tyrosine 490, phosphorylation of ERK, tyrosine phosphorylation of many cellular proteins, and upregulation of p21 (Figure 1A).
Figure 1.
Characterization of TrkA-inducible stable cell lines. (A) U2OS-TrkA (#8, #60, #67) cells were cultured for 12 h in the absence or presence of tetracycline. (B) U2OS-TrkA-60 cells were uninduced or induced with tetracycline for 12 h and untreated or treated with doxorubicin for 4 h. Proteins from the cells were extracted with SDS sample buffer and analyzed by Western blot with the indicated antibodies (A, B).
p21 is a representative transcriptional target of p53 which is activated upon DNA damage. Thus, we investigated whether TrkA is involved in DNA damage-mediated signal transduction. As shown in Figure 1B, ATM autophosphorylation at serine-1981 was observed in response to DNA double strand breaks by doxorubicin treatment (first panel), but not in only TrkA overexpression. In addition, p53 protein level was upregulated upon DNA damage as expected (third panel). Interestingly, DNA damage-induced p53 upregulation was significantly suppressed by TrkA overexpression (third panel, compare lanes 3 and 4), and p21 upregulation by TrkA was 53-independent (fourth panel), supporting the previous reports that p21 can be activated or upregulated by the MAPK pathway in a p53-independent manner (Bacus et al., 2001; Tang et al., 2002; Dangi et al., 2006). Taken together, our results suggest that TrkA can be activated when its protein level is abnormally high, and its pleiotropic effects such as cell death and differentiation can be studied in this system, even in the absence of NGF binding.
TrkA overexpression promotes cell death through cell cycle alteration
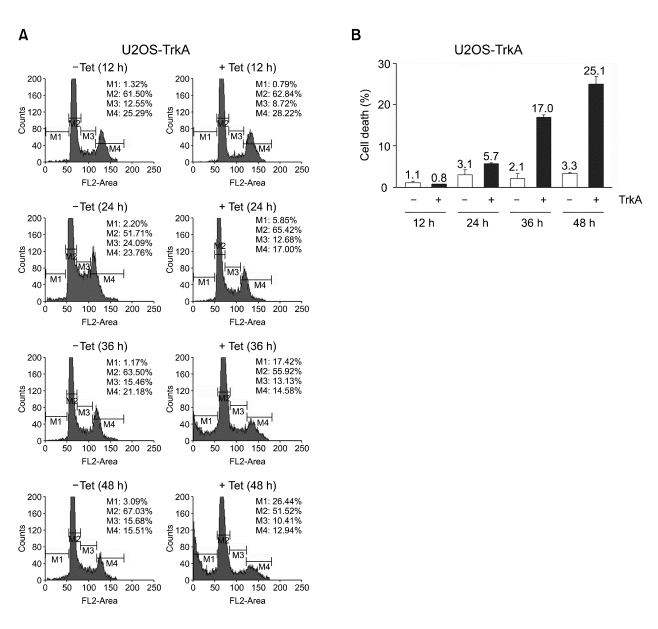
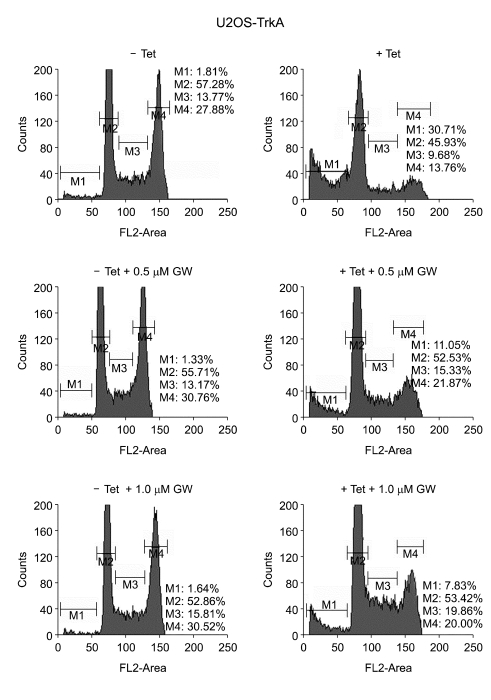
To investigate whether TrkA overexpression could affect cell cycle regulation and cell death, we performed cell cycle analysis in U2OS-TrkA-67 cells as described in "Materials and Methods". The results showed that cell cycle progression appeared to be normal in uninduced cells, however, it was significantly altered in TrkA-induced cells (Figure 2A). Notably, G1 to S phase transition between 12 h and 24 h was inhibited in TrkA-induced cells compared to the uninduced cells. While about 24% of uninduced cells appeared at S phase at 24 h, only 13% of TrkA-induced cells were with S phase, suggesting that TrkA is involved in suppression of G1 to S phase cell cycle progression. In Figure 1, we showed that TrkA overexpression induced p21, which is a key factor for cell cycle arrest (Erhardt and Pittman, 1998). This fact suggests that TrkA could inhibit cell cycle progression through, at least in part, p21 upregulation. In addition, cell death in uninduced cells has no change with time, whereas TrkA-induced cell death gradually increased in a time-dependent manner (Figure 2B). Other U2OS-TrkA clones also showed the similar results described above, and tetracycline itself had no effect on cell cycle progression in normal U2OS cells (data not shown). We further investigated the effect of GW441756, a TrkA-specific tyrosine kinase inhibitor, on TrkA-induced cell death. The result showed that GW441756 specifically blocked TrkA-induced cell death in a dose-dependent manner, but there was no effect in uninduced cells, indicating that TrkA tyrosine kinase activity is required for its cell death function (Figure 3).
Figure 2.
TrkA overexpression alters cell cycle and promotes cell death. U2OS-TrkA-67 cells were plated in duplicate in the absence or presence of tetracycline for the indicated times. Cell cycle distribution was analyzed by flow cytometry using propidium iodide stain. The representative DNA histogram was shown in (A). M1 gate (Sub-G1) includes cell death whereas M2, M3, and M4 indicate cells in G0-G1, S, and G2-M, respectively. The average percentage of cell death in duplicate (A) was shown in (B); bars, SD.
Figure 3.
Inhibition of TrkA-induced cell death by its specific inhibitor GW441756. U2OS-TrkA-67 cells were uninduced or induced with tetracycline for 12 h and untreated or treated with the indicated amount of GW441756 for 24 h followed by cell cycle analysis. M1 gate (Sub-G1) includes cell death whereas M2, M3, and M4 indicate cells in G0-G1, S, and G2-M, respectively.
Induction of γH2AX by TrkA overexpression
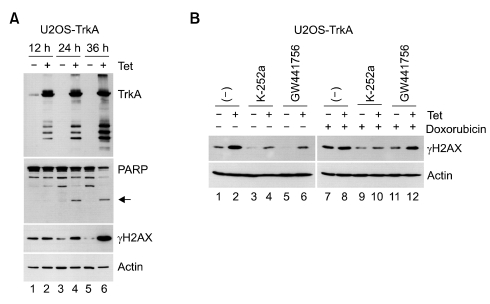
Next, we tried to investigate the mechanism on TrkA-induced cell death. Interestingly, γH2AX production and PARP cleavage, which have been known as indicators of DNA damage (Tanaka et al., 2006), were significantly induced at 36 h after tetracycline treatment (Figure 4A). Moreover, TrkA itself seemed to be cleaved at specific sites and also modified, showing accumulation of slow migrating TrkA proteins at the near top of gel (Figure 4A, first panel). These results suggest that not only γH2AX production and PARP cleavage but also modified TrkA production are related to TrkA-induced cell death.
Figure 4.
TrkA overexpression induces γH2AX production and PARP cleavage. (A) U2OS-TrkA-67 cells were uninduced or induced with tetracycline for the indicated times followed by Western blot analysis. The arrow in the second panel indicates the cleaved product of PARP. (B) U2OS-TrkA-67 cells were uninduced or induced with tetracycline for 24 h and then untreated or treated with K-252a (0.5 µgM), GW441756 (0.5 µgM), or doxorubicin (1 µg/ml) for 4 h followed by Western blot analysis.
Since TrkA requires its kinase activity for the induction of cell death, we investigated the effect of TrkA kinase inhibitors K-252a and GW441756 on TrkA-induced γH2AX production. The results showed that TrkA ability to induce γH2AX production was significantly downregulated by both K-252a and GW441756 in the absence of DNA damage inducer (Figure 4B, lanes 1-6). In addition, it was also suppressed by K-252a during DNA damage by doxorubicin treatment, but not by GW441756 (Figure 4B, lanes 7-12). DNA damage activates PI-3 kinase families (ATM, ATR, and DNA-PK) and JNK kinase, which play a critical role in γH2AX production in response to DNA damage. Thus, our results suggest that K-252a, but not GW441756, might inhibit other kinases, which are involved in γH2AX production upon DNA damage, as well as TrkA. Indeed, K-252a is used not only as a TrkA inhibitor but also acts as a general serine/threonine kinase inhibitor.
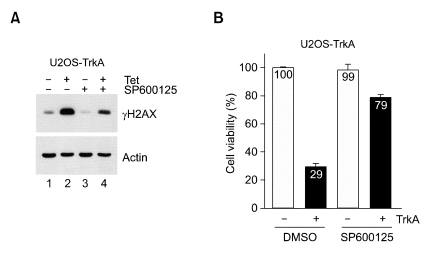
Inhibition of TrkA-induced γH2AX production by JNK inhibitor
As shown in Figure 1B, ATM, one of major PI-3 kinases, did not seem to be activated by TrkA overexpression in the absence of DNA damage, suggesting that ATM does not function in γH2AX production by TrkA. Two other PI-3 kinases, ATR and DNA-PK, are also unlikely to produce γH2AX because they are mostly responsive to some different types of DNA damage signaling. Rather, we investigated the role of JNK in γH2AX production by TrkA overexpression using JNK inhibitor SP600125. Very interestingly, γH2AX production by TrkA was significantly inhibited by SP600125, suggesting that H2AX is predominantly phosphorylated at serine 139 by JNK during TrkA-induced cell death (Figure 5A). Furthermore, γH2AX reduction by JNK inhibitor suppressed TrkA-mediated cell death, resulting in increase of cell viability in TrkA-induced cells (Figure 5B). Our results suggest that γH2AX production by JNK is strongly associated with TrkA-induced cell death in the absence of DNA damage inducer.
Figure 5.
Effect of JNK inhibitor SP600125 on γH2AX production and cell viability in TrkA-induced cells. (A) U2OS-TrkA-67 cells were uninduced or induced with tetracycline for 12 h and then treated with either DMSO (lanes 1 and 2) or 2 µg/ml of SP600125 (lanes 3 and 4) for 24 h followed by Western blot analysis. (B) U2OS-TrkA-67 cells were split on 24-well dish in the absence or presence of tetracycline for 12 h and treated with either DMSO or 2 µg/ml of SP600125 for 24 h. Cell viability was determined by CCK-8 assay as described in "Materials and Methods".
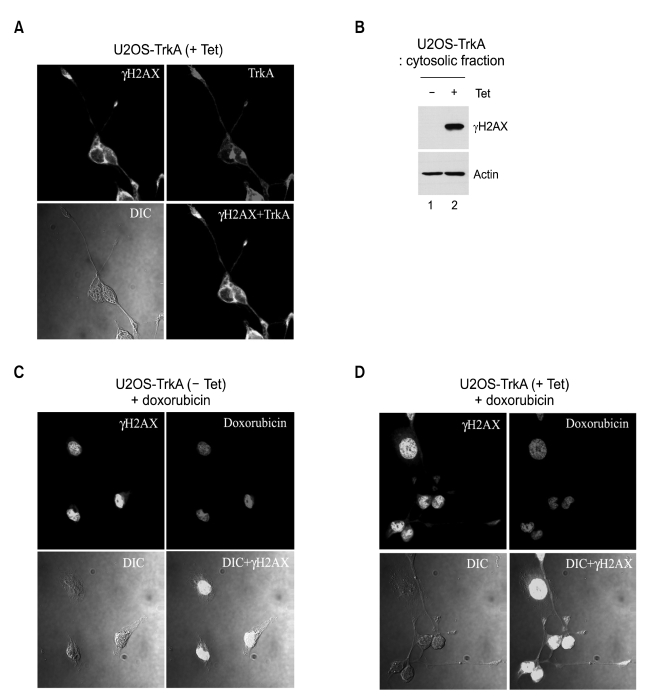
Interaction between TrkA and γH2AX in the cytosol of TrkA-induced cells
The intracellular location of γH2AX in TrkA-induced cells has been observed by the imaging analysis using confocal immunofluorescence microscopy. Unexpectedly, γH2AX was predominantly accumulated in cytosol rather than nucleus in TrkA-induced cells (Figure 6A, γH2AX panel). Especially, it was also localized at the terminal region extended from cell body. Moreover, γH2AX was colocalzed with TrkA in cytosol (Figure 6A, γH2AX+TrkA panel). We further proved this by detecting γH2AX production in the cytosolic fraction of TrkA-induced cells, but not uninduced cells (Figure 6B). Interestingly, nonneuronal U2OS cells appeared to be changed to neuron-like phenotype when TrkA was overexpressed (Figure 6A and D). However, uninduced U2OS cells showed a typical adherent morphology of epithelial cell as shown in Figure 6C (DIC image). We further characterized the molecular basis about morphological change of nonneuronal TrkA-expressing U2OS cells (unpublished data).
Figure 6.
Cytosolic accumulation of γH2AX by TrkA. (A) U2OS-TrkA-67 cells were induced with tetracycline for 16 h. γH2AX was stained with FITC-labelled green and TrkA was stained with TRITC-labelled red and analyzed by confocal immunofluorescence microscopy. γH2AX+TrkA panel shows merged image of γH2AX and TrkA panels. (B) Western blot was performed in cytosolic fractions of uninduced and TrkA-induced U2OS cells. (C, D) U2OS-TrkA-67 cells were uninduced (C) or induced (D) with tetracycline for 12 h and treated with doxorubicin (1 µg/ml) for 4 h. γH2AX was stained with FITC-labelled green and doxorubicin was imaged in TRITC visible region by confocal immunofluorescence microscopy. DIC+γH2AX panel shows merged image of DIC and γH2AX panels.
It has been well reported that γH2AX is accumulated in nucleus upon DNA damage and forms local foci at the damage sites. In consistence with this, DNA damage exclusively induced γH2AX-mediated nuclear foci in uninduced cells (Figure 6C). Here, doxorubicin staining indicates nucleus position similar to DAPI staining. However, γH2AX could be also induced in cytosol as well as nucleus upon DNA damage when TrkA was overexpressed (Figure 6D). Taken together, our results suggest that γH2AX could be a novel mediator of the TrkA-induced cell death, although it still remains to figure out how it participates in TrkA-induced cell death.
Discussion
TrkA plays an important role in cell survival, differentiation, and apoptosis. Since TrkA is a neurotrophin receptor tyrosine kinase, most studies about TrkA were accomplished in neuronal cells in response to NGF. However, little is known about TrkA-induced biological effects in the absence of NGF. In this study, using TrkA-inducible stable cell lines by the Tet-On system in nonneuronal U2OS cells, we found that in the absence of NGF stimulation, TrkA overexpression caused ERK phosphorylation and tyrosine phosphorylation of cellular proteins, mimicking NGF-mediated activation pathway (Figure 1A). Moreover, it resulted in a significant cell death (Figure 2) and morphological change of nonneuronal U2OS cells to neuron-like phenotype such as neurite outgrowth (Figure 6). These results suggest that TrkA could also exhibit pleiotropic effects in nonneuronal cells when its protein level is high, even in the absence of its ligand binding.
We also showed that TrkA-induced cell death was largely associated with γH2AX production and PARP cleavage, indicating an apoptotic cell death by TrkA (Figure 4A). γH2AX has been well reported to be induced in nucleus upon DNA damage in a cell cycle-dependent manner as a downstream target of ATM (MacPhail et al., 2003; Ichijima et al., 2005; Takahashi and Ohnishi, 2005). However, TrkA-induced γH2AX production did not seem to be related to DNA damage-induced ATM activation pathway because there was no autophosphorylation of ATM at serine 1981 in TrkA-induced cells (Figure 1B). According to some reports, γH2AX production is also independent of DNA damage signals. It can be produced in a cell cycle-dependent manner in HeLa cells (Ichijima et al., 2005). Furthermore, it can control cell proliferation under the normal cell growth condition. Indeed, H2AX-deficient cells reveal severe growth defect (Celeste et al., 2002). In addition, a recent report showed that γH2AX is accumulated in mouse skin cells during a hair cycle under no DNA damage and this γH2AX production was independent of ATM and DNA-PK (Koike et al., 2007), indicating that γH2AX can be produced by other kinases in the absence of DNA damage signal and play a crucial role in cell growth control. Besides three major PI-3 kinases (ATM, ATR, and DNA-PK) responding to γH2AX production, JNK MAPKs are also directly responsible for production of γH2AX involving in cellular apoptosis (Lu et al., 2006; Sluss and Davis, 2006). In our study, γH2AX production was significantly inhibited by JNK inhibition and it caused suppression of TrkA-induced cell death (Figure 5). These results suggest that TrkA might participate in γH2AX production via JNK signaling. In many cells, TrkA activation by NGF or NGF withdrawal largely induced JNK pathway (Aloyz et al., 1998; Jezierski et al., 2001).
Why is γH2AX largely induced in cytosol upon TrkA overexpression and how does this lead to cell death? Through TrkA signaling, H2AX could be phosphorylated at serine 139 by JNK and other unknown kinases. Although the reason is not clear at present, our results suggest that γH2AX cytosolic accumulation is involved in the choice of cell death in TrkA-induced cells. The nuclear γH2AX accumulation has been suggested as inducer of caspase-activated DNase (CAD) in apoptosis (Lu et al., 2006; Sluss and Davis, 2006), but the cytosolic induction of γH2AX leading to cell death as shown in this study is elusive. According to a very recent report, histone H1.2 can be translocated to mitochodria and associated with Bak, leading to apoptotic cell death, in the bleomycin-treated human squamous carcinoma cells (Okamura et al., 2007). Similarly, γH2AX accumulated in cytosol in TrkA-induced cells might be associated with some pro-apoptotic factors and cause cell death when TrkA is overexpressed. Although further experiments are required to prove how the cytosolic accumulation of γH2AX leads to cell death, at least we report here a novel finding that cytosolic induction of γH2AX is related with an apoptotic signal following the TrkA overexpression in nonneuronal U2OS cells.
Acknowledgments
We thank Dr. David Kaplan for providing pLNCX-TrkA plasmid. We also thank Dr. Young-Sool Hah and Ji Hye Kim for technical assistance. This work was supported by Korea Research Foundation(KRF-2006-005-J04203) and MRC program of MOST/KOSEF (R13-2005-012-01-003-0).
Abbreviations
- ATM
ataxia telangiectasia mutated
- CCK-8
cell counting kit-8
- NGF
nerve growth factor
- PARP
poly(ADP-ribose) polymerase
- PI
propidium iodide
- PI-3 kinase
phosphatidylinositol 3-kinase
- Tet
tetracycline
- TrkA
tropomyosin-related kinase A
References
- 1.Aloyz RS, Bamji SX, Pozniak CD, Toma JG, Atwal J, Kaplan DR, Miller FD. P53 is essential for developmental neuron death as regulated by the TrkA and p75 neurotrophin receptors. J Cell Biol. 1998;143:1691–1703. doi: 10.1083/jcb.143.6.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacus SS, Gudkov AV, Lowe M, Lyass L, Yung Y, Komarov AP, Keyomarsi K, Yarden Y, Seger R. Taxol-induced apoptosis depends on MAP kinase pathways (ERK and p38) and is independent of p53. Oncogene. 2001;20:147–155. doi: 10.1038/sj.onc.1204062. [DOI] [PubMed] [Google Scholar]
- 3.Celeste A, Petersen S, Romanienko PJ, Fernandez-Capetillo O, Chen HT, Sedelnikova OA, Reina-San-Martin B, Coppola V, Meffre E, Difilippantonio MJ, Redon C, Pilch DR, Olaru A, Eckhaus M, Camerini-Otero RD, Tessarollo L, Livak F, Manova K, Bonner WM, Nussenzweig MC, Nussenzweig A. Genomic instability in mice lacking histone H2AX. Science. 2002;296:922–927. doi: 10.1126/science.1069398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho ES, Lee SB, Bae IH, Lee YS, Lee SJ, Um HD. Ionizing radiation induces blockade of c-jun N-terminal kinase-dependent cell death pathway in a manner correlated with p21Cip/Waf1 induction in primary cultured normal human fibroblasts. Exp Mol Med. 2005;37:282–289. doi: 10.1038/emm.2005.38. [DOI] [PubMed] [Google Scholar]
- 5.Dangi S, Chen FM, Shapiro P. Activation of extracellular signal-regulated kinase (ERK) in G2 phase delays mitotic entry through p21CIP1. Cell prolif. 2006;39:261–279. doi: 10.1111/j.1365-2184.2006.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Decker SJ. Nerve growth factor-induced growth arrest and induction of p21Cip1/WAF1 in NIH-3T3 cells expressing TrkA. J Biol Chem. 1995;270:30841–30844. doi: 10.1074/jbc.270.52.30841. [DOI] [PubMed] [Google Scholar]
- 7.Erhardt JA, Pittman RN. p21WAF1 induces permanent growth arrest and enhances differentiation, but does not alter apoptosis in PC12 cells. Oncogene. 1998;16:443–451. doi: 10.1038/sj.onc.1201577. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A. H2AX: the histone guardian of the genome. DNA Repair. 2004;3:959–967. doi: 10.1016/j.dnarep.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 9.Ichijima Y, Sakasai R, Okita N, Asahina K, Mizutani S, Teraoka H. Phosphorylation of histone H2AX at M phase in human cells without DNA damage response. Biochem Biophys Res Commun. 2005;336:807–812. doi: 10.1016/j.bbrc.2005.08.164. [DOI] [PubMed] [Google Scholar]
- 10.Jezierski MK, Strum AK, Scarborough MM, Sohrabji F. NGF stimulation increase JNK2 phosphorylation and reduces caspase-3 activity in the olfactory bulb of estrogen-replaced animals. Endocrinology. 2001;142:2401–2404. doi: 10.1210/endo.142.6.8316. [DOI] [PubMed] [Google Scholar]
- 11.Jung EJ, Flemington EK. Transfection-mediated cell synchronization: acceleration of G1-S phase transition by gamma irradiation. BioTechniques. 2001;31:1026, 1028, 1031–1034. doi: 10.2144/01315st02. [DOI] [PubMed] [Google Scholar]
- 12.Koike M, Mashino M, Sugasawa J, Koike A. Dynamic change of histone H2AX phosphorylation independent of ATM and DNA-PK in mouse skin in situ. Biochem Biophys Res Commun. 2007;363:1009–1012. doi: 10.1016/j.bbrc.2007.09.080. [DOI] [PubMed] [Google Scholar]
- 13.Kurz EU, Douglas P, Lees-Miller SP. Doxorubicin activates ATM-dependent phosphorylation of multiple downstream targets in part through the generation of reactive oxygen species. J Biol Chem. 2004;279:53272–53281. doi: 10.1074/jbc.M406879200. [DOI] [PubMed] [Google Scholar]
- 14.Kurz EU, Lees-Miller SP. DNA damage-induced activation of ATM and ATM-dependent signaling pathways. DNA Repair. 2004;3:889–900. doi: 10.1016/j.dnarep.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 15.Lavoie JF, Lesauteur L, Kohn J, Wong J, Furtoss O, Thiele CJ, Miller FD, Kaplan DR. TrkA induces apoptosis of neuroblastoma cells and does so via a p53-dependent mechanism. J Biol Chem. 2005;280:29199–29207. doi: 10.1074/jbc.M502364200. [DOI] [PubMed] [Google Scholar]
- 16.Liu M-L, Hong S-T. Early phase of amyloid b42-induced cytotoxicity in neuronal cells is associated with vacuole formation and enhancement of exocytosis. Exp Mol Med. 2005;37:559–566. doi: 10.1038/emm.2005.69. [DOI] [PubMed] [Google Scholar]
- 17.Lu C, Zhu F, Cho YY, Tang F, Zykova T, Ma WY, Bode AM, Dong Z. Cell apoptosis: requirement of H2AX in DNA ladder formation, but not for the activation of caspase-3. Mol Cell. 2006;23:121–132. doi: 10.1016/j.molcel.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacPhail SH, Banath JP, Yu Y, Chu E, Olive PL. Cell cycle-dependent expression of phosphorylated histone H2AX: reduced expression in unirradiated but not X-irradiated G1-phase cells. Radiat Res. 2003;159:759–767. doi: 10.1667/rr3003. [DOI] [PubMed] [Google Scholar]
- 19.Okamura H, Yoshida K, Amorim BR, Haneji T. Histone H1.2 is translocated to mitochondria and associates with bak in bleomycin-induced apoptotic cells. J Cell Biochem. 2008;103:1488–1496. doi: 10.1002/jcb.21537. [DOI] [PubMed] [Google Scholar]
- 20.Ransone LJ. Detection of protein-protein interactions by coimmunoprecipitation and dimerization. Methods Enzymol. 1995;254:491–497. doi: 10.1016/0076-6879(95)54034-2. [DOI] [PubMed] [Google Scholar]
- 21.Reichardt LF. Neurotrophin-regulated signalling pathways. Phil Trans R Soc B. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogakou EP, Nieves-Neira W, Boon C, Pommier Y, Bonner WM. Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at serine 139. J Biol Chem. 2000;275:9390–9395. doi: 10.1074/jbc.275.13.9390. [DOI] [PubMed] [Google Scholar]
- 23.Saxena S, Bucci C, Weis J, Kruttgen A. The small GTPase Rab7 controls the endosomal trafficking and neuritogenic signaling of the nerve growth factor receptor TrkA. J Neurosci. 2005;25:10930–10940. doi: 10.1523/JNEUROSCI.2029-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sluss HK, Davis RJ. H2AX is a target of the JNK signaling pathway that is required for apoptotic DNA fragmentation. Mol Cell. 2006;23:152–153. doi: 10.1016/j.molcel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Stephens RM, Loeb DM, Copeland TD, Pawson T, Greene LA, Kaplan DR. Trk receptors use redundant signal transduction pathways involving SHC and PLC-gamma 1 to mediate NGF responses. Neuron. 1994;12:691–705. doi: 10.1016/0896-6273(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 26.Tacconelli A, Farina AR, Cappabianca L, Desantis G, Tessitore A, Vetuschi A, Sferra R, Rucci N, Argenti B, Screpanti I, Gulino A, Mackay AR. TrkA alternative splicing: a regulated tumor-promoting switch in human neuroblastoma. Cancer Cell. 2004;6:347–360. doi: 10.1016/j.ccr.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi A, Ohnishi T. Does gammaH2AX foci formation depend on the presence of DNA double strand breaks? Cancer Lett. 2005;229:171–179. doi: 10.1016/j.canlet.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka T, Kurose A, Huang X, Dai W, Darzynkiewicz Z. ATM activation and histone H2AX phosphorylation as indicators of DNA damage by DNA topoisomerase I inhibitor topotecan and during apoptosis. Cell Prolif. 2006;39:49–60. doi: 10.1111/j.1365-2184.2006.00364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang D, Wu D, Hirao A, Lahti JM, Liu L, Mazza B, Kidd VJ, Mak TW, Ingram AJ. ERK activation mediates cell cycle arrest and apoptosis after DNA damage independently of p53. J Biol Chem. 2002;277:12710–12717. doi: 10.1074/jbc.M111598200. [DOI] [PubMed] [Google Scholar]
- 30.Yan GZ, Ziff EB. NGF regulates the PC12 cell cycle machinery through specific inhibition of the Cdk kinases and induction of cyclin D1. J Neurosci. 1995;15:6200–6212. doi: 10.1523/JNEUROSCI.15-09-06200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Moheban DB, Conway BR, Bhattacharyya A, Segal RA. Cell surface Trk receptors mediate NGF-induced survival while internalized receptors regulate NGF-induced differentiation. J Neurosci. 2000;20:5671–5678. doi: 10.1523/JNEUROSCI.20-15-05671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]