Abstract
Genetic polymorphisms may be linked to inter-individual differences in erythropoietin (EPO) resistance. We investigated the -511C/T polymorphism of the IL-1B gene and the I/D polymorphism of the ACE gene for any association with EPO resistance index (ERI) in maintenance hemodialysis patients (n = 167). Because EPO responsiveness is multi-factorial, we also included other possible influences (age, sex, time on dialysis, ACE inhibitor or angiotensin receptor blocker use, ferritin, transferrin saturation, intact PTH, high sensitivity C-reactive protein, albumin, Kt/V, and presence of diabetes mellitus) on ERI in our analyses. Multiple regression analysis showed significant association of the IL-1B-511CC and ACE DD polymorphisms with ERI (P = 0.038 and P = 0.004 in the recessive model, respectively). The combination (C) of alleles of two loci showed that C1 (I-T) was significantly associated with ERI in the co-dominant and recessive models (P = 0.005 and P = 0.0001, respectively). Subjects who did not carry C1 showed significantly decreased ERI (10.10 ± 5.15 IU/kg weight/g hemoglobin) compared to other study subjects (C1/C1 and C1/-; 12.97 ± 4.90 and 15.12 ± 7.43 IU/kg weight/g hemoglobin, respectively). Our study indicates that the IL-1B-511C/T and ACE I/D polymorphisms may be useful genetic markers of EPO requirement in hemodialysis patients. These findings might also provide a new perspective on therapeutic approaches to the treatment of end stage renal disease patients with anemia.
Keywords: end stage renal disease; erythropoietin; interleukin-1β; kidney failure, chronic; peptidyl-dipeptidase A; polymorphism, genetic
Introduction
Since the introduction of recombinant human erythropoietin (EPO) in 1986, it has been an effective treatment for renal anemia in the majority of patients with end-stage renal disease (ESRD) (Winearls et al., 1986; Schaefer et al., 1989). The most common cause of inadequate response to EPO is generally thought to be absolute or functional iron deficiency (Tarng et al., 1995). Various clinical conditions may also cause EPO resistance, including infections (Muirhead and Hodsman, 1990), chronic inflammation (Schardin, 1990), secondary hyperparathyroidism (Rao et al., 1993), and uremia.
Chronic inflammation can modify the process of erythropoiesis, probably via various inflammatory cytokines (Danielson, 1995; Cooper et al., 2003). Pro-inflammatory cytokines, which are under some degree of genetic control, may be associated with EPO resistance. Recent studies have shown that genetic polymorphisms in cytokine genes may influence the level of corresponding cytokines, which also play an important role in the pathogenesis of anemia (Maury et al., 2004). The IL-1 gene cluster on chromosome 2q contains 3 related genes within a 430-kb region, IL-1A, IL-1B, and IL-1RN, which encode the pro-infammatory cytokines IL-1α and IL-1β as well as their endogenous receptor antagonist IL-1ra (Dinarello, 1996). Moreover, the IL-1B-511C/T (rs1143627) single nucleotide polymorphism (SNP) has been associated with a variety of diseases in which inflammation plays an important role (Hurme et al., 1998). To date, however, there has been no published study on the relationship between IL-1B-511C/T polymorphism and EPO resistance in hemodialysis patients.
In 1990, the ACE gene polymorphism (rs1799752) characterized by the insertion or deletion of a 287-base pair fragment in the 17q23 chromosome was identified (Rigat et al., 1990). Mean angiotensin converting enzyme (ACE) levels in ACE DD carriers were approximately twice that found in ACE II genotype individuals (Rigat et al., 1990). ACE is a key enzyme in the production of angiotensin II; thus, ACE DD carriers show the highest ACE and angiotensin II levels (Rigat et al., 1990). Angiotensin II stimulates proliferation of early erythroid progenitors in vitro (Mrug et al., 1997). As a result, ACE DD individuals may display greater erythropoietic activity. Some investigators have observed an increase in EPO requirement in patients with the ACE II genotype undergoing continuous ambulatory peritoneal dialysis (CAPD) (Varagunam et al., 2003), but others have found no effect (Hatano et al., 2000).
It seems possible, therefore, that polymorphisms of the IL-1B and ACE genes play a role in the development of EPO resistance in ESRD patients. We evaluated the IL-1B-511C/T and ACE I/D polymorphisms in patients in hemodialysis to determine the association between various polymorphisms and EPO resistance. Because EPO responsiveness is multi-factorial, we also included other possible influences on EPO resistance in our analyses.
Materials and Methods
Subjects
End-stage renal disease patients treated with maintenance hemodialysis from Kyung Hee University Medical Center were included in this study. All participants gave informed consent according to local ethics committee consent procedures. Those who satisfied the following criteria were recruited in our study population: (1) treatment with hemodialysis for three months or more; (2) age 18 years or above; and (3) injections of either EPO α or β for renal anemia. Exclusion criteria were: (1) symptoms and signs of bleeding less than two months before inclusion; (2) hypothyroidism (euthyrotic patients on thyroid hormone replacement therapy were included); (3) malignant disease; (4) hematologic disease; and (5) acute infectious disease. Relevant clinical data were evaluated, including age, primary cause of kidney disease, dry body weight, and use of ACE inhibitors or angiotensin receptor blocker (ARB).
Methods
Clinical data: The following clinical and laboratory data were obtained for three months prior to the time of the study, and average values were used for analysis: serum albumin, iron, total iron-binding capacity, ferritin, high sensitivity C-reactive protein (hs-CRP), Kt/V (monthly), and intact parathyroid hormone (PTH) levels (every three months). The precision of these laboratory findings was analyzed with 20 repeated measurements in 3 different levels of controls. The coefficient of variation was usually less than 5.0%. We measured hematocrit and hemoglobin (Hb) levels bimonthly and evaluated EPO responsiveness to the EPO therapy. The dose of EPO was titrated by 25% every two weeks in an attempt to maintain a target Hb level between 10 and 11 g/dl. Although there was a dose-response relationship between EPO and Hb, some patients required higher doses of EPO to reach a target Hb level. To quantitatively assess EPO responsiveness, an EPO resistance index (ERI) was calculated as weekly EPO dose per kg of body weight, divided by the Hb concentration (weekly EPO dose/kg weight/g Hb). We defined the weekly EPO dose as the average value over three months.
ELISA for ACE and IL-1β: To determine ACE and IL-1β levels, fasting blood samples were obtained between 08 : 00 and 10 : 00. Samples were centrifuged and stored at -80℃. Plasma ACE concentration was measured by ELISA (R&D systems, Inc., Minneapolis, MN) (Danilov et al., 1996). Intra- and inter-assay coefficients of variation were 3.7% and 5.9%, respectively. IL-1β concentrations were also assayed in plasma by ELISA according to the manufacturer's instructions (R&D systems, Inc., Minneapolis, MN). The intra- and inter-assay coefficients of variation were 6.7 and 8.9%, respectively.
DNA preparation and genotyping: Genomic DNA was isolated from peripheral blood samples. PCR-based genotyping of IL-1B-511 C/T and ACE I/D polymorphisms was carried out as previously described (di Giovine et al., 1992; Varagunam et al., 2003; Sharples et al., 2006). The following primers were used: IL-1B, 5'-TGGCATTGATCTGGTTCATC-3' and 5'-GTTTAGGAATCTTCCCACTT-3'; and ACE, 5'-CTGGAGACCACTCCCATCCTTTCT-3' and 5'-GATGTGGCCATCACATTCGTCAGAT-3'. The PCR products for IL-1B were digested with AvaI (NEB, Beverly, MA) at 37℃, analyzed by electrophoresis on 1.5% agarose gels, and visualized with ethidium bromide under UV light. Amplified ACE products were run on 2% agarose gels. AvaI digested the amplified products from the IL-1B-511C allele into two fragments, and products amplified from the IL-1B-511T allele remained undigested. The ACE I/D polymorphism was evident as a 490-bp fragment in the presence of the insertion (I allele) and as a 190-bp product in the absence of the insertion (D allele). Twenty percent of the subjects were randomly selected for DNA sequencing to confirm the accuracy of the analysis.
Statistical analysis: The SPSS statistical package, version 13.0 (SPSS; Chicago, IL), was used to compare all clinical and laboratory parameters. Differences between group means were tested using one-way ANOVA (with Tukey's post hoc test when ANOVA was significant), and differences in proportion were assessed by the chi-square test. All values are expressed as mean ± SD, with statistical significance defined as P < 0.05. Multiple regressions adjusted for age, sex, time on dialysis, ACE inhibitor or ARB use, ferritin, transferrin saturation, intact PTH, hs-CRP, albumin, Kt/V, and presence of diabetes mellitus were used for association analyses with ERI.
Results
Subject characteristics
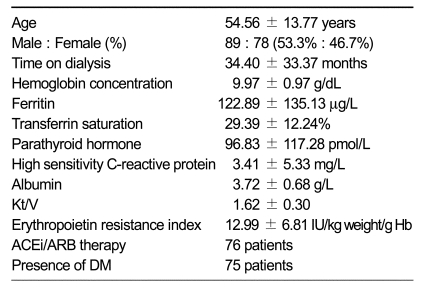
Of the 167 hemodialysis patients included in this study, 89 were male (53.3%). Table 1 shows clinical profiles of participants. Mean age was 54.56 ± 13.77 years (range, 23-88 years) and mean time on dialysis was 34.40 ± 33.37 months (range, 3-139 months). The mean Hb concentration, ferritin, intact PTH, hs-CRP, and ERI of the subjects were 9.97 ± 0.97 g/dL, 122.89 ± 135.13 µg/L, 96.83 ± 117.28 pmol/L, 3.41 ± 5.33 mg/L and 12.99 ± 6.81 IU/kg weight/g Hb, respectively. The enter method of multiple regression analysis was used to detect those clinical variables with the most influence on EPO responsiveness. Based on this analysis, no significant association with ERI level was detected (data not shown).
Table 1.
Demographic and clinical characteristics of participants.
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; DM, diabetes mellitus.
Genotype distributions of ACE I/D and IL-1B-511C/T polymorphism
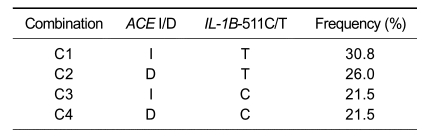
Genotype distributions of the two loci studied are shown in Table 2. The distributions of the ACE II, ACE ID, and ACE DD genotypes among patients were 25.1% (n = 42), 54.5% (n = 91), and 20.4% (n = 34), respectively. The distributions of IL-1B-511C/T genotypes were 21.6% in the IL-1B-511CC group (n = 36), 43.1% in the IL-1B-511CT group (n = 72), and 35.3% in the IL-1B-511TT group (n = 59). In this study the ACE I and IL-1B-511C polymorphisms had allele frequencies of 52.4% and 43.1%, respectively. The possible combinations of alleles of two loci studied were C1 (I-T), C2 (D-T), C3 (I-C), and C4 (D-C). The frequencies of the observed combinations are presented in Table 3.
Table 2.
Frequencies of the ACE I/D and IL-1B-511C/T polymorphisms.
n, number of patients.
Table 3.
Combinations of alleles of the ACE I/D, IL-1B-511C/T polymorphisms and their frequencies.
C, combination.
Association with ERI
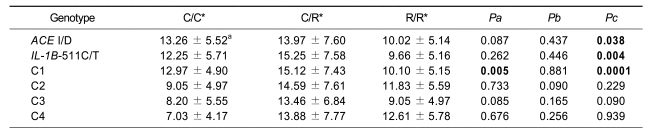
Associations between ACE I/D and IL-1B-511C/T polymorphisms and ERI were analysed using multiple regressions, adjusting for age, sex, time on dialysis, ACE inhibitor or ARB use, ferritin, transferrin saturation, intact PTH, hs-CRP, albumin, Kt/V, and presence of diabetes mellitus. Two SNPs (ACE DD, IL-1B-511CC) showed significant association with ERI (P = 0.038 and P = 0.004 in the recessive model, respectively). Patients with the ACE DD genotype (10.02 ± 5.14 IU/kg weight/g Hb) had lower ERI compared with those without (ACE I/I, 13.26 ± 5.52 IU/kg weight/g Hb; ACE I/D, 13.97 ± 7.60 IU/kg weight/g Hb). ERI values were also lower in IL-1B-511CC individuals (9.66 ± 5.16 IU/kg weight/g Hb) than in IL-1B-511CT (15.25 ± 7.58 IU/kg weight/g Hb) and IL-1B-511TT (12.25 ± 5.71 IU/kg weight/g Hb) genotype patients.
Four combinations of alleles were constructed using the two SNPs (Table 4). We found a significant association between C1 (I-T) and ERI values in both co-dominant and recessive models (P = 0.005 and P = 0.0001, respectively); e.g., individuals who did not carry C1 showed significantly decreased ERI (10.10 ± 5.15 IU/kg weight/g Hb) compared to those with C1/C1 and C1/-(12.97 ± 4.90 and 15.12 ± 7.43 IU/kg weight/g Hb, respectively). Other combinations (C2, C3, and C4) were not associated with ERI.
Table 4.
Regression analysis of ERI in hemodialysis patients according to the ACE I/D, IL-1B-511C/T polymorphisms and combinations of alleles of two loci.
ERI, erythropoietin resistance index; C, combination
*C/C, C/R and R/R represent homozygotes for the common allele, heterozygotes and homozygotes for the rarer allele, respectively.
aMean ± standard deviation of ERI (IU/kg weight/g Hb).
Pa, Pb and Pc are P values of codominant (minor allele homozygotes vs. heterozygotes vs. major allele homozygotes), dominant (minor allele homozygotes plus heterozygotes vs. major allele homozygotes) and recessive (minor allele homozygotes vs. heterozygotes plus major allele homozygotes) models for multiple regression analysis controlling for age, sex, time on dialysis, ACE inhibitor or ARB use, ferritin, transferrin saturation, intact PTH, hs-CRP, albumin, Kt/V and presence of diabetes mellitus as covariates.
ACE and IL-1β level
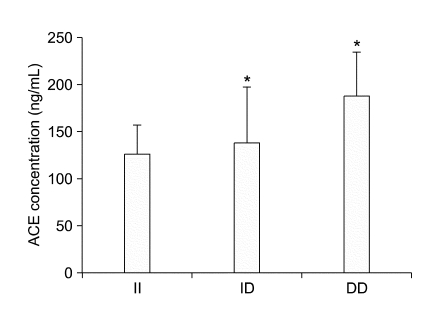
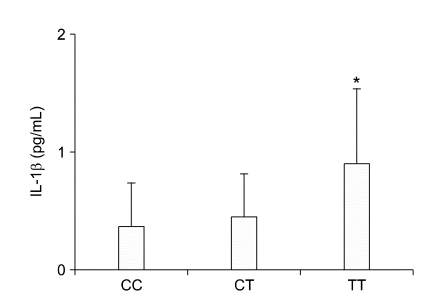
We measured ACE and IL-1β levels directly to show the biological functions of the two SNPs in this study. Seventy-six patients in this study were taking ACE inhibitors or ARB, drugs known to affect ACE or angiotensin II levels. For this reason, we evaluated ACE levels only ninety-one patients (ACE II, n = 24; ACE ID, n = 46; ACE DD, n = 21) who were not taking ACE inhibitors or ARB. The ACE I/D polymorphism had a significant effect on on plasma ACE levels (Figure 1). Patients with the ACE DD genotype had the highest plasma ACE levels compared to patients with ACE ID or II genotypes (187.2 ± 46.2, 137.1 ± 59.5, 125.0 ± 29.66 ng/ml, respectively; P=0.0005). Patients with the IL-1B-511TT genotype also showed a significantly higher mean IL-1β level (0.9 ± 0.6 pg/mL), compared to those with the IL-1B-511CC genotype (0.3 ± 0.3 pg/mL; P = 0.02) (Figure 2).
Figure 1.
Association of plasma ACE level with the ACE I/D polymorphism. Plasma ACE levels determined by sandwich ELISA (Data are means ± SD. *P <0.05, versus the II group).
Figure 2.
Association of plasma IL-1β levels with the IL-1B-511C/T polymorphism. Plasma IL-1β levels determined by sandwich ELISA (Data are means ± SD. *P < 0.05, versus CC group).
Discussion
EPO production is markedly lower in patients with ESRD, resulting in the development of renal anemia. The use of EPO in ESRD patients results in a significant increase in Hb concentration and improvements in quality of life for the majority of patients. Approximately 5-10% of ESRD patients receiving EPO, however, appear to be resistant to this drug (Priyadarshi and Shapiro, 2006).
In the current study we found that this variability was linked in part to genetic polymorphisms. Patients with the ACE DD genotype had significantly lower ERI values compared to those with ACE II or ACE ID, independent of other traditional factors. ACE is a key enzyme in circulatory homeostasis, where it catalyzes the conversion of angiotensin I to angiotensin II. The ACE I/D polymorphism affects plasma and tissue levels of ACE activity, and individuals with the ACE DD genotype show the highest levels of this enzyme (Rigat et al., 1990). We also evaluated plasma ACE levels in ninety-one patients who were not taking ACE inhibitors or ARB. There was a significant effect of ACE I/D polymorphism on plasma ACE levels. This study argues for a role of ACE I/D polymorphism in renal anemia, in which the ACE DD genotype lowers the EPO requirement due to the relatively high level of angiotensin II, an important stimulus of erythropoiesis. Previous work by Hantano et al. (2000) on hemodialysis patients found no significant effect of the ACE I/D polymorphism on EPO requirement. Our results are, however, in agreement with those from two recently published studies that included CAPD patients and demonstrated that the ACE II/ID genotypes seem to be associated with suboptimal EPO response (Varagunam et al., 2003; Sharples et al., 2006).
Inflammation is one of the major independent predictors of resistance to EPO therapy, and pro-inflammatory cytokines have been shown to inhibit erythropoiesis (Means and Krantz, 1991, 1993). For example, IL-1β suppresses the colony formation of bone marrow erythroid progenitors and inhibits EPO production (Jelkmann, 1998). In addition, pro-inflammatory cytokines may negatively influence iron utilization, thereby interfering with Hb synthesis (Nemeth et al., 2004). These data also suggest that the pro-inflammatory cytokine IL-1β, which is under some degree of genetic control, might be associated with EPO resistance. Recent research indicated that the IL-1B-511C/T polymorphism was associated with several inflammatory diseases (Hurme et al., 1998; Maury et al., 2004). Maury et al. (2004) reported that the occurrence of anemia in patients with AA amyloidosis was associated with the -511T allele of the IL-1B gene and high circulating levels of IL-1β. We found in this study that the IL-1B-511CC genotype was significantly associated with lower ERI values in hemodialysis patients.
This study was limited by its small sample size and single-center focus. Compared with those of more heterogeneous populations, Korean genotype frequencies are relatively even and similar regardless of center, making it reasonable to generalize the results of a single-center study to the greater population. To clarify the exact impact of polymorphisms in the IL-1B and ACE genes on EPO resistance in hemodialysis patients, a family-based association study or a longitudinal study including appropriately adjusted clinical variables would be helpful. There are several additional potential limitations of this study. We did not measure angiotensin II levels, and it is unclear from our study whether angiotensin II mediates the association between ACE genotype and erythropoietic response. In addition, we did not measure EPO levels, another possible explanation for differences in EPO resistance.
In summary, we found that the IL-1B-511CC genotype is associated with lower ERI values in hemodialysis patients. Furthermore, the ACE I/D polymorphism has predictive value when determining EPO responsiveness; the ACE DD genotype may be associated with lower ERI values. Anemia and resistance to recombinant EPO contribute to the excess morbidity and mortality associated with ESRD. Moreover, these genetic variations might contribute to patient-specific anemia risk profiling in ESRD in the future.
Acknowledgements
This work was supported in part by a grant from Kyung Hee University, Seoul, Korea (2005).
Abbreviations
- ACE
angiotensin converting enzyme
- EPO
erythropoietin
- ERI
erythropoietin resistance index
- ESRD
end stage renal disease
- Hb
hemoglobin
- hs-CRP
high sensitivity C-reactive protein
- iPTH
intact parathyroid hormone
- TIBC
total iron binding capacity
References
- 1.Cooper AC, Mikhail A, Lethbridge MW, Kemeny DM, Macdougall IC. Increased expression of erythropoiesis inhibiting cytokines (IFN-gamma, TNF-alpha, IL-10, and IL-13) by T cells in patients exhibiting a poor response to erythropoietin therapy. J Am Soc Nephrol. 2003;14:1776–1784. doi: 10.1097/01.asn.0000071514.36428.61. [DOI] [PubMed] [Google Scholar]
- 2.Danielson B. R-HuEPO hyporesponsiveness-who and why? Nephrol Dial Transplant. 1995;10(Suppl 2):69–73. doi: 10.1093/ndt/10.supp2.69. [DOI] [PubMed] [Google Scholar]
- 3.Danilov S, Savoie F, Lenoir B, Jeunemaitre X, Azizi M, Tarnow L, Alhenc-Gelas F. Development of enzyme-linked immunoassays for human angiotensin I converting enzyme suitable for large-scale studies. J Hypertens. 1996;14:719–727. doi: 10.1097/00004872-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 4.di Giovine FS, Takhsh E, Blakemore AI, Duff GW. Single base polymorphism at -511 in the human interleukin-1 beta gene (IL-1 beta) Hum Mol Genet. 1992;1:450. doi: 10.1093/hmg/1.6.450. [DOI] [PubMed] [Google Scholar]
- 5.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 6.Hatano M, Yoshida T, Mimuro T, Kimata N, Tsuchiya K, Sanaka T, Nihei H. The effects of ACE inhibitor treatment and ACE gene polymorphism on erythropoiesis in chronic hemodialysis patients. Nippon Jinzo Gakkai Shi. 2000;42:632–639. [PubMed] [Google Scholar]
- 7.Hurme M, Lahdenpohja N, Santtila S. Gene polymorphisms of interleukins 1 and 10 in infectious and autoimmune diseases. Ann Med. 1998;30:469–473. doi: 10.3109/07853899809002488. [DOI] [PubMed] [Google Scholar]
- 8.Jelkmann W. Proinflammatory cytokines lowering erythropoietin production. J Interferon Cytokine Res. 1998;18:555–559. doi: 10.1089/jir.1998.18.555. [DOI] [PubMed] [Google Scholar]
- 9.Maury CP, Liljestrom M, Laiho K, Tiitinen S, Kaarela K, Hurme M. Anaemia of chronic disease in AA amyloidosis is associated with allele 2 of the interleukin-1beta-511 promoter gene and raised levels of interleukin-1beta and interleukin-18. J Intern Med. 2004;256:145–152. doi: 10.1111/j.1365-2796.2004.01353.x. [DOI] [PubMed] [Google Scholar]
- 10.Means RT, Jr, Krantz SB. Inhibition of human erythroid colony-forming units by gamma interferon can be corrected by recombinant human erythropoietin. Blood. 1991;78:2564–2567. [PubMed] [Google Scholar]
- 11.Means RT, Jr, Krantz SB. Inhibition of human erythroid colony-forming units by tumor necrosis factor requires beta interferon. J Clin Invest. 1993;91:416–419. doi: 10.1172/JCI116216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mrug M, Stopka T, Julian BA, Prchal JF, Prchal JT. Angiotensin II stimulates proliferation of normal early erythroid progenitors. J Clin Invest. 1997;100:2310–2314. doi: 10.1172/JCI119769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muirhead N, Hodsman AB. Occult infection and resistance of anaemia to rHuEpo therapy in renal failure. Nephrol Dial Transplant. 1990;5:232–234. doi: 10.1093/ndt/5.3.232. [DOI] [PubMed] [Google Scholar]
- 14.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Priyadarshi A, Shapiro JI. Erythropoietin resistance in the treatment of the anemia of chronic renal failure. Semin Dial. 2006;19:273–278. doi: 10.1111/j.1525-139X.2006.00172.x. [DOI] [PubMed] [Google Scholar]
- 16.Rao DS, Shih MS, Mohini R. Effect of serum parathyroid hormone and bone marrow fibrosis on the response to erythropoietin in uremia. N Engl J Med. 1993;328:171–175. doi: 10.1056/NEJM199301213280304. [DOI] [PubMed] [Google Scholar]
- 17.Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaefer RM, Horl WH, Massry SG. Treatment of renal anemia with recombinant human erythropoietin. Am J Nephrol. 1989;9:353–362. doi: 10.1159/000167996. [DOI] [PubMed] [Google Scholar]
- 19.Schardin KE. Case management of the anemic patient. Epoetin alfa: focus on inflammation and infection. Anna J. 1990;17:468–469. [PubMed] [Google Scholar]
- 20.Sharples EJ, Varagunam M, Sinnott PJ, McCloskey DJ, Raftery MJ, Yaqoob MM. The effect of proinflammatory cytokine gene and angiotensin-converting enzyme polymorphisms on erythropoietin requirements in patients on continuous ambulatory peritoneal dialysis. Perit Dial Int. 2006;26:64–68. [PubMed] [Google Scholar]
- 21.Tarng DC, Chen TW, Huang TP. Iron metabolism indices for early prediction of the response and resistance to erythropoietin therapy in maintenance hemodialysis patients. Am J Nephrol. 1995;15:230–237. doi: 10.1159/000168837. [DOI] [PubMed] [Google Scholar]
- 22.Varagunam M, McCloskey DJ, Sinnott PJ, Raftery MJ, Yaqoob MM. Angiotensin-converting enzyme gene polymorphism and erythropoietin requirement. Perit Dial Int. 2003;23:111–115. [PubMed] [Google Scholar]
- 23.Winearls CG, Oliver DO, Pippard MJ, Reid C, Downing MR, Cotes PM. Effect of human erythropoietin derived from recombinant DNA on the anaemia of patients maintained by chronic haemodialysis. Lancet. 1986;2:1175–1178. doi: 10.1016/s0140-6736(86)92192-6. [DOI] [PubMed] [Google Scholar]