Abstract
Epidemiological studies suggest that there is a beneficial effect of moderate ethanol consumption on the incidence of cardiovascular disease. Ethanol is metabolized to acetaldehyde, a two-carbon carbonyl compound that can react with nucleophiles to form covalent addition products. We have identified a biochemical modification produced by the reaction of acetaldehyde with protein-bound Amadori products. Amadori products typically arise from the nonenzymatic addition of reducing sugars (such as glucose) to protein amino groups and are the precursors to irreversibly bound, crosslinking moieties called advanced glycation endproducts, or AGEs. AGEs accumulate over time on plasma lipoproteins and vascular wall components and play an important role in the development of diabetes- and age-related cardiovascular disease. The attachment of acetaldehyde to a model Amadori product produces a chemically stabilized complex that cannot rearrange and progress to AGE formation. We tested the role of this reaction in preventing AGE formation in vivo by administering ethanol to diabetic rats, which normally exhibit increased AGE formation and high circulating levels of the hemoglobin Amadori product, HbA1c, and the hemoglobin AGE product, Hb-AGE. In this model study, diabetic rats fed an ethanol diet for 4 weeks showed a 52% decrease in Hb-AGE when compared with diabetic controls (P < 0.001). Circulating levels of HbA1c were unaffected by ethanol, pointing to the specificity of the acetaldehyde reaction for the post-Amadori, advanced glycation process. These data suggest a possible mechanism for the so-called “French paradox,” (the cardioprotection conferred by moderate ethanol ingestion) and may offer new strategies for inhibiting advanced glycation.
Keywords: Amadori product, atherosclerosis, diabetes, “French paradox”, low-density lipoprotein
Upon ingestion, alcohol (ethanol) is oxidatively metabolized by alcohol dehydrogenase to produce acetaldehyde (1). Acetaldehyde is chemically reactive, and its activity in modifying macromolecules has been hypothesized to contribute to certain of the organ toxicities associated with chronic ethanol abuse (2–4). At the biochemical level, there is only partial understanding of the structures of the covalently bound acetaldehyde adducts that may form in vivo (2, 5, 6).
Long-term ethanol consumption is known to lead to the formation of minor, electrophoretic variants of hemoglobin that migrate in the HbA1a-c region. There is evidence to indicate that these minor hemoglobin species arise by the covalent modification of primary amino groups, most likely via Schiff bases involving the acetaldehyde carbonyl (2, 5, 6). Only one class of these adducts appears to be reducible by sodium borohydride (a characteristic feature of Schiff bases)(2), and whereas 13C-NMR-based studies have provided evidence for the formation of a metastable, N-terminal 2-methylimidazolidinone derivative (7), the identity of the major classes of stable acetaldehyde-derived products that form on hemoglobin remains unknown.
In an effort to understand the association between acetaldehyde adducts and hemoglobin variants and the apparent nonreducibility of most acetaldehyde–hemoglobin bonds, we considered the possibility that secondary amines such as the Amadori product (AP) might be targets of acetaldehyde modification in vivo. A hemoglobin-bound AP is known to form by the nonenzymatic addition of glucose to the N terminus of the hemoglobin β-chain and accounts for the minor hemoglobin species HbA1c, levels of which can rise to >14% of total hemoglobin in patients with diabetes mellitus (8, 9).
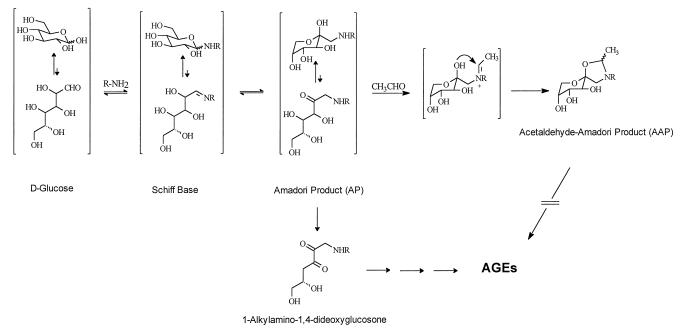
In contrast to the reaction of primary amines with acetaldehyde, which produces reversible Schiff base adducts, an acetaldehyde-derived Schiff base involving the secondary amine of the AP may be trapped by a ring closure reaction to form stable, bicyclic adduct(s) (Fig. 1). It is known that the open-chain form of the AP is free to rearrange and produce reactive diketone structures such as 1-alkylamino-1,4-dideoxyglucosone (10, 11). These diketones in turn are the precursors to a chemically more heterogenous group of adducts called advanced glycation endproducts or AGEs (10–12). AGEs form by a succession of chemical rearrangements that, over time, lead to heterocyclic structures that can exhibit yellow-brown color and the capacity to covalently crosslink proximate amino groups. AGEs progressively modify tissue proteins, and their formation on long-lived connective tissue and vascular wall components has been shown to account in part for the increase in cardiovascular disease that accompanies normal aging and that occurs at an accelerated rate in diabetes (10, 11, 13). AGEs also have been shown to modify an appreciable fraction of circulating low-density lipoprotein, thereby interfering with its normal uptake by high-affinity low-density lipoprotein receptors (14, 15). We considered the possibility that the addition of acetaldehyde to protein-bound APs could serve to “fix” the AP in a closed-chain form and inhibit the formation of open chain, carbonyl-containing adducts, thus preventing the successive rearrangement reactions that lead to crosslinking and pathogenic AGEs. We report herein in vitro and in vivo evidence for this reaction and discuss its potential for explaining the so-called “French paradox,” or the cardioprotective effect conferred by moderate ethanol ingestion.
Figure 1.
The early stages of protein glycation showing the formation of the AP from the initial glucose-derived Schiff base adduct. The AP is known to undergo dehydration to a glucosone (diketone), which is the proximate intermediate in the reaction leading to AGE formation and protein crosslinking (7, 8). Acetaldehyde (CH3CHO) reacted with the AP to produce the five-membered AAP (only one stereoisomer is shown). Stabilization of the cyclic form of the AP by acetaldehyde prevents ring opening and the consequent formation of a diketone intermediate leading to AGE crosslinks.
MATERIALS AND METHODS
Synthesis of Nɛ-(1-deoxy-d-fructose-1-yl)-Nα-carboxybenzoyloxy-l-lysine.
Nɛ-(1-deoxy-d-fructose-1-yl)-Nα-carboxybenzoyloxy-l-lysine (CBZ-lysine-AP) was prepared as follows. A suspension of 3.6 g (0.02 mol) of anhydrous d-glucose and 0.2 g of sodium bisulfite in 6 ml of methanol and 3 ml of glycerol was refluxed for 30 min, followed by the addition of 7 mmol of Nα-CBZ-l-lysine and 0.8 ml of acetic acid (16). This solution was refluxed until most of the starting material was consumed, as assessed by thin layer chromatography performed on Silica Gel-60 glass plates using 4:1:1 (vol/vol/vol) n-butanol/acetic acid/water as irrigant. For product detection, the plates were sprayed with 0.2% ninhydrin in ethanol and heated at 100°C for ≈1 min. The AP was recovered from the silica gel by dissolution in methanol and addition of n-propanol with vigorous stirring. After standing for 3 h at room temperature, a yellowish oily material appeared. This upper layer was decanted and part of the oily material (yield: 65%) was subjected to preparative high HPLC. The chromatographic system consisted of a Waters 600E controller, a 60F pump, a 996 photodiode array detector, and a 717 plus autosampler (Millipore). A Primesphere 5 μm C18 HPLC column (Phenomenex, Belmont, CA) (250 × 21.2 mm) was equilibrated in buffer A (0.05% trifluoroacetic acid/H2O), and the HPLC was programmed to deliver a linear gradient of buffer B (100% methanol) over 40 min (buffer B = 0% at 0 min and 100% at 40 min) at a flow rate of 8 ml/min. Column eluant was monitored at λ = 254 nm, and the new peak appearing at 30 min was isolated, lyophilized, and analyzed by 1H-NMR and electrospray ionization mass spectrometry. 1H-NMR (270 MHz, D2O): δ 1.32 (m, 2 H), 1.62 (m, 4 H), 2.99 (t, 2 H, J = 7.6 Hz), 3.19 (s, 2 H), 3.62–3.93 (m, 6 H), 5.00 (d, 1H, J = 12.6 Hz), 5.08 (d, 1H, J = 12.5 Hz), 7.36 (m, 5 H); MS: m/z 443 (MH+).
Preparation and Characterization of CBZ-Lysine-AAP.
CBZ-lysine-AP (5 mg) was incubated together with acetaldehyde (4 or 20 equivalents) in 2 ml of 0.2 M phosphate buffer (pH 7.4). All incubations were performed under sterile conditions, at room temperature, and for up to 4 weeks. Parallel incubations were carried out in the absence of acetaldehyde. At intervals, aliquots of each incubation mixture were analyzed for the development of AGE-associated absorbance changes (17) and for immunoreactivity in a competitive ELISA employing two different AGE-specific antibodies: a polyclonal antibody raised to AGE-modified RNase A and a monoclonal antibody raised to AGE-modified keyhole limpet hemocyanin. It has been demonstrated previously that these antibodies do not recognize APs or other early glycation products but are reactive with different AGE epitopes that form in vivo, such as cypentodine and a covalent, imidazole-based arginine–lysine crosslink (18, 19). Acetaldehyde does not chemically modify these AGEs or otherwise alter the immunoreactivity of mature AGE epitopes (Y.A.-A. and R.B., unpublished observations). Competitive ELISA values were converted to AGE unit values by comparison to a standardized preparation of AGE-modified BSA as described (18).
The products of the reaction between CBZ-lysine-AP and acetaldehyde were analyzed by reversed-phase HPLC employing a Primesphere 5 μm C18 HPLC column and the buffer system described above. The flow rate was 8 ml/min, and the column eluant was monitored at λ 254 nm. The new peak appearing at 30 min was isolated, lyophilized, and analyzed by 1H-NMR and mass spectroscopy. 1H-NMR spectra were recorded in D2O on a JEOL 270 instrument. 1H-NMR (270 MHz, D2O): δ 1.10 (d, 1.2 H, J = 5.8, CH3), 1.25 (d, 1.8 H, J = 5.8, CH3), 1.33 (m, 2 H), 1.65 (m, 4 H), 2.97 (t, 2 H, J = 7.6 Hz), 3.21 (m, 2 H), 3.60–4.05 (m, 6 H), 5.00 (d, 1H, J = 12.6 Hz), 5.08 (d, 1H, J = 12.5 Hz), 5.27 (m, 1H, OCHN) 7.36 (m, 5 H).
Animals.
Outbred male Wistar rats (weighing 150–175 g) first were acclimated for 1 week to one of two calorically matched liquid diets (BioServ Ethanol Diet or Bioserv Caloric Control Diet; Bioserv, Frenchtown, NJ) (20–22). Half of the rats in each group then were treated with streptozotocin (65 mg/kg) to induce diabetes mellitus, and half were reserved as the nondiabetic group. Diabetes was confirmed one week later in the streptozotocin-treated rats by blood glucose determination (Mean ± SD = 440 ± 38 mg/dl). The rats then were grouped as follows (n = 9–10 per group): group 1: diabetic, control diet; group 2: diabetic, ethanol diet; group 3: nondiabetic, control diet; and group 4: nondiabetic, ethanol diet. Blood glucose determinations again at 1 month showed no difference between the mean blood glucose levels in group 1 vs. group 2 (P = NS) or group 3 vs. group 4 (P = NS, Student’s t test).
Blood, HbA1c, and Hb-AGE Measurements.
Whole blood was collected into heparinized tubes, and the red blood cells were washed three times with PBS. Hemolysates were prepared, and the lipids were removed by resuspending the cells in 3 ml of distilled H2O and 2 ml of toluene. Hemoglobin was further purified by a standard procedure (23), and its concentration was determined with Drabkin’s reagent (Sigma). The quantity of HbA1c (HbAo modified by a glucose-derived AP) was determined by using a standardized chromatography method (Sigma) that retains glycoadducts and does not distinguish between APs and acetaldehyde-modified APs (AAPs) (2, 24, 25). AGE-modified hemoglobin (Hb-AGE) was measured by an AGE-specific ELISA as described (26). Chronic ethanol ingestion can lead to an anemia (27), which can confound the quantitation of minor hemoglobin species (28). Of importance, there were no significant differences in total hemoglobin values between the experimental groups, indicating that an ethanol-induced anemia did not occur under the experimental conditions of this study.
Hemoglobin Analysis.
Separation of the various hemoglobin species was achieved by a high-resolution chromatographic technique employing a PolyCAT A (Poly LC, Columbia, MD) cation exchange column (200 × 4.6 mm). A binary solvent gradient consisting of buffer A (35 mM Bistris, 16.85 mM ammonium acetate, 90 mM sodium acetate, and 1.5 mM potassium cyanide, buffered to pH 6.8 with acetic acid) and buffer B (35 mM Bistris, 3 mM ammonium acetate, and 1.5 mM potassium cyanide, buffered to pH 6.5 with acetic acid) was delivered at 3 ml/min as follows: 0–3 min, isocratically at A:B (22:78); 3–30 min, linear gradient from A:B (22:78) to A:B (50:50); 30–40 min, linear gradient from A:B (50:50) to A:B (0:100); and 40–45 min, linear gradient from A:B (0:100) to A:B (22:78). Hemoglobin elution was monitored by absorbance at 415 nm.
The globin chains present in isolated fractions (HbA0, HbA1c, and HbA-AAP) were separated and analyzed by reversed-phase HPLC/mass spectrometry as follows: LC-electrospray ionization (ESI) samples were run on a C4 column (Vydac, 250 × 10 mm; Sigma) employing the ABI pump and Rheodyne valve with a 20 μl loop at a flow of 25 μl/min. A binary solvent gradient consisting of 0.05% trifluoroacetic acid in H2O (solvent A) and CH3CN (solvent B) was delivered as follows: 0–40 min, linear gradient from A:B (95:5) to A:B (15:85); 45–50 min, linear gradient from A:B (15:85) to A:B (95:5).
Mass Spectrometry.
ESI mass spectra were obtained on a Quattrotriple quadrupole instrument employing an ABI model 140B syringe pump (H2O/CH3CN at a flow of 15 μl/min) and a Rheodyne model 7125 valve with a 10 μl loop. The probe was a Micromass Megaflow ESI probe using nitrogen for the nebulizer/drying gas. This is a “soft” ionization technique that produces minimal fragmentation under these experimental conditions (29).
RESULTS
To obtain evidence for a reaction between acetaldehyde and APs, we first established an experimental model system to study the progression of advanced glycation reactions in vitro. We prepared as a model substrate CBZ-lysine-AP (16). The α-amino function of the lysine is blocked by the CBZ moiety, leaving only the ɛ-amino group available for the rearrangement reactions and the covalent crosslinking that occurs during the more advanced stages of the glycation process. The CBZ group also serves as a convenient UV-active “tag” so that newly formed adducts can be identified for further structural characterization.
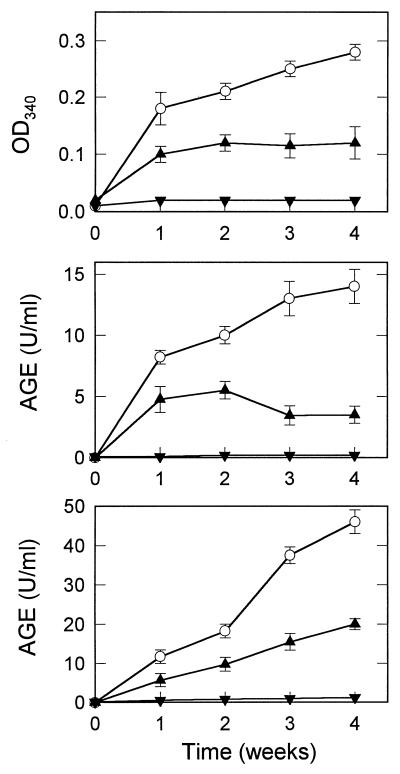
By visual inspection, the incubation mixture containing CBZ-lysine-AP alone gradually became yellow-brown in color, whereas the solution containing CBZ-lysine-AP plus acetaldehyde remained clear and unpigmented. This was taken as evidence that CBZ-lysine-AP was transformed over time to more advanced (and pigmented) AGEs and that the development of AGEs was inhibited by a reaction involving acetaldehyde. Aliquots of each incubation mixture were analyzed at weekly intervals for AGE-associated absorbance changes (OD340) and for immunoreactivity against two different antibodies shown previously to recognize AGE epitopes that form both in vitro and in vivo (18). The addition of acetaldehyde to CBZ-lysine-AP thus was found to inhibit both AGE-associated absorbance changes and AGE epitope formation (Fig. 2).
Figure 2.
Purified CBZ-lysine AP was incubated for up to 4 weeks in the absence (○) or the presence of 4 (▴) or 20 (▾) equivalents of acetaldehyde (12). At intervals, aliquots of each incubation mixture were analyzed for the development of AGE-associated absorbance changes (Top) and for immunoreactivity in independent competitive ELISAs employing two different AGE-specific antibodies: a polyclonal antibody raised to AGE-modified RNase A (Middle), and a monoclonal antibody raise to AGE-modified KLH (Bottom). The values displayed show the mean ± SD of triplicate measurements.
In vivo, the damaging effects of AGEs occur largely by the advanced glycation of proteins (10–15). We next studied the effect of acetaldehyde in a second model system that utilized serum albumin, a protein substrate that is known to undergo advanced glycation in vivo (30). For these studies, serum albumin (50 mg/ml) was first preincubated with 0.1 M glucose for 48 hours (pH 7.4, 37°C) so as to accelerate the early phases of the glycation reaction and ensure AP formation. After dialysis to remove unreacted glucose, aliquots of this albumin preparation (now “preloaded” with covalently attached APs) were incubated in the presence or the absence of acetaldehyde, and the advanced glycation process was allowed to proceed for an additional 2 weeks. When assessed by AGE-ELISA, the content of immunoreactive AGEs present in the preparation of glycated albumin that included acetaldehyde was decreased by 71.4 ± 7.2% when compared with the preparation of glycated albumin incubated without acetaldehyde (data not shown).
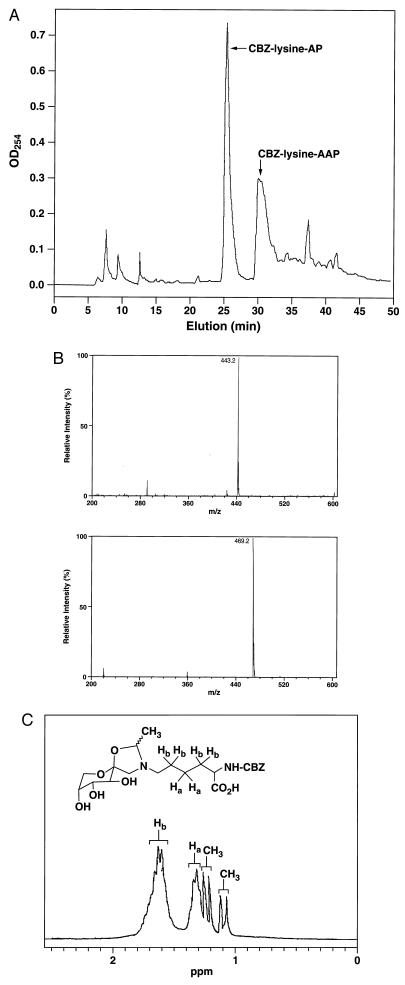
HPLC analysis of the CBZ-lysine-AP incubation mixtures led to the identification of a major, CBZ-lysine containing product present only when acetaldehyde was included in the incubation mixture (Fig. 3A). Eluent fractions corresponding to the elution time of this peak were pooled, lyophilized, and characterized by ESI spectrocscopy and 1H-NMR. The mass spectrum displayed a molecular ion at m/z = 469.2 (MH+), which is consistent with the mass of a CBZ-lysine-AP (m/z = 443.2) modified by the addition of an acetaldehyde moiety (CH3CHO-H2O = 26 Da) (Fig. 3B). The 1H-NMR spectrum of the purified compound showed, in addition to the expected CBZ-lysine-AP resonances, the appearance of two sets of methyl groups at δ 1.10 and 1.25 ppm, each appearing as a doublet (J = 5.8 Hz) because of coupling with the vicinal methine proton (OCHN) (Fig. 3C). These resonances are consistent with the formation of stereoisomers at the acetaldehyde carbon located between the O and the N of the newly formed five- membered ring. These data verify the specific reaction of acetaldehyde with APs to form a cyclic AAP structures of the type proposed in Fig. 1. Once formed, AAPs do not progress to AGE formation, as assessed by absorbance and immunochemical criteria.
Figure 3.
(A) Reversed-phase HPLC chromatogram showing the elution of the CBZ-lysine-AP and the CBZ-lysine-AAP. Column eluant was monitored at 254 nm to detect all CBZ-containing products. The yield of CBZ-lysine-AAP was 32% and was determined by integration of the eluant peaks. (B) ESI mass spectrum of the CBZ-lysine-AP starting material (Upper), and that of the purified CBZ-lysine-AAP showing the predicted, molecular ion of 469.2 [MH]+ (Lower). (C) Partial 1H-NMR spectrum (D2O; 270 MHz) of the purified, CBZ-lysine-AAP showing new resonances of two methyl groups derived from the condensation of acetaldehyde and AP, at 1.10 and 1.25 ppm. The ratio of the proposed stereoisomers was 2:3, based on the integration of the methyl groups. The other resonances shown (Ha and Hb) correspond to two and four protons, respectively, that arise from methylene protons of the lysine backbone.
In vivo, acetaldehyde is produced from ethanol by the activity of constitutive and inducible alcohol dehydrogenase enzymes (1). We hypothesized that the elevation in circulating acetaldehyde levels that occurs on ethanol ingestion might result in a decrease in the formation and subsequent accumulation of AGE-modified proteins in the body. Because steady-state levels of APs are typically low under normal, nonhyperglycemic conditions (8–11, 13), we considered that the AGE-inhibiting activity of alcohol consumption might best be evaluated by studying animals with experimentally induced diabetes. The chronic hyperglycemia of diabetes is known to produce measurable increases in the amount of covalently bound APs and corresponding increases in the accumulation of AGEs on numerous plasma and tissue proteins (8–11, 13–15).
We evaluated AGE formation in groups of diabetic and nondiabetic rats fed either a control or ethanol-supplemented diet (20–22). Additionally, half of the rats were treated with streptozotocin to ablate pancreatic beta cells, thereby inducing persistent hyperglycemia and diabetes mellitus, whereas half were reserved as a nondiabetic, control group. One month later, blood samples were obtained for measurement of total hemoglobin, HbA1c (the hemoglobin AP), and Hb-AGE (the hemoglobin-AGE product) (23, 26).
Table 1 shows that Hb-AGE levels were significantly lower in diabetic rats fed a high-ethanol diet than in diabetic rats fed the control diet. Circulating HbA1c levels did not differ between the ethanol-fed diabetic group and the control-fed diabetic group, indicating that the decrease in AGE levels produced by ethanol consumption was not the result of a decrease in the formation of total HbA1c, the Hb-AGE precursor. Because HbA1c levels reflect ambient glycemia over the preceding 3- to 4-week period (i.e., the time period required to reach equilibrium levels of the hemoglobin AP) (24, 25), these data also confirm that the two experimental groups experienced the same degree of hyperglycemia. Overall, these results indicate that ethanol ingestion inhibits advanced glycation in vivo at a stage after AP formation and are consistent with the reaction of acetaldehyde with the AP to prevent further progression to AGE formation.
Table 1.
Hemoglobin glycation analysis
| Group | Hb-AGE, units AGE/mg Hb | HbA1c, % Hb | Total Hb, mg/dl |
|---|---|---|---|
| 1 | 3.07 ± 0.24* | 4.21 ± 0.14 | 14.6 ± 0.3 |
| 2 | 1.46 ± 0.20* | 3.95 ± 0.16 | 14.1 ± 0.3 |
| 3 | 1.65 ± 0.13 | 2.43 ± 0.23 | 13.9 ± 0.5 |
| 4 | 1.54 ± 0.16 | 2.64 ± 0.28 | 14.7 ± 0.4 |
Hemoblobin glycation analysis in the experimental rat groups shown (n = 9–10 per group). Hb-AGE was measured by specific ELISA, HbA1c was assessed by a standard clinical assay (Sigma), and total hemoglobin was determined by Drabkin’s method (18). ∗, P < 0.001 for group 1 versus group 2. P not significant for group 1 versus group 2 with respect to HbA1c and total Hb comparisons and for all group 3 group 4 comparisons (Student’s t test). Group 1, diabetic, control diet; group 2, diabetic, ethanol diet; group 3, nondiabetic, control diet; group 4, nondiabetic ethanol diet.
No statistically significant difference in hemoglobin glycation was noted between the ethanol-treated, nondiabetic group when compared with the nonethanol-treated, nondiabetic group. Nondiabetic animals show a lower level of protein glycation than diabetic animals as measured by currently available immunoassays (13, 18, 26). Appreciable decreases in AGEs might become evident with longer term ethanol administration or by studying AGE accumulation on tissue proteins such as basement membrane collagen that have a longer in vivo half-life than red cell hemoglobin and thus the potential to accumulate a higher degree of AGE modification (13, 31).
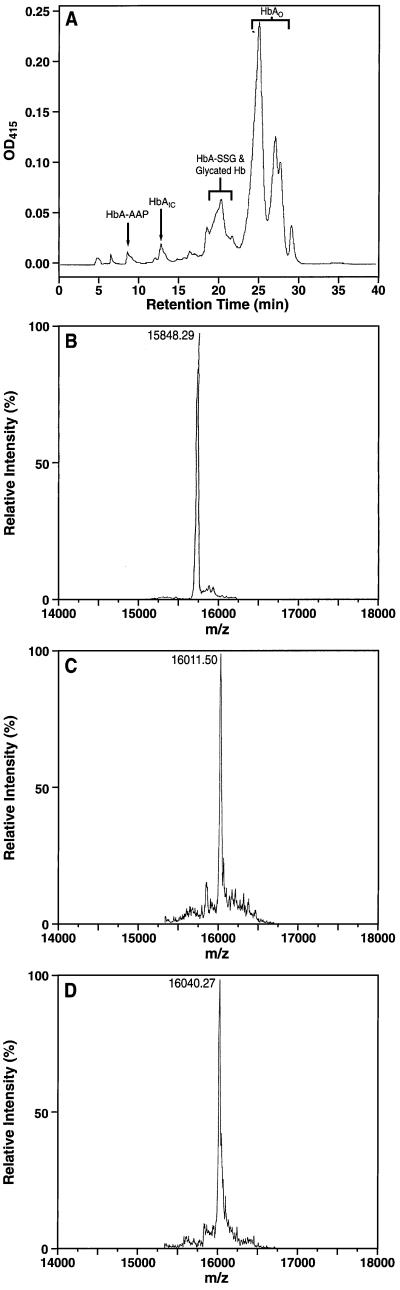
The results of analyses for glycation-modified hemoglobin in the diabetic animals are consistent with an inhibitory effect of acetaldehyde on AGE formation in vivo. We next sought to obtain direct, chemical evidence for the in vivo formation of AAPs by employing a sensitive HPLC-ESI technique that differentiates between hemoglobin-bound APs (APs) and hemoglobin-bound AAPs. Hemolysates obtained from the red cells of diabetic, ethanol-treated rats first were fractionated over a PolyCAT A cation-exchange HPLC column using a stepwise salt and pH gradient (Fig. 4A). This method resolves several hemoglobin species, and these fractions were analyzed further for their mass composition by LC-ESI (Fig. 4 B–D). Two fractions obtained from the peak corresponding to HbAo (unmodified hemoglobin) yielded molecular ions that were consistent with the masses of the native α- and β-globin polypeptide chains (m/z = 15198.72 ± 2.04 and 15848.29 ± 1.25 Da, respectively). The characteristic hemoglobin glycation product HbA1c is produced by the nonenzymatic attachment of glucose to the N terminus of the β-chain, and modifications at this site are associated with a more rapid elution of hemoglobin in cation exchange chromatography (8, 9). We therefore focused our LC-ESI analysis on the β-chains present within the chromatographically faster eluting hemoglobin fractions. The mass spectrum of the globin β-chain present within the fraction corresponding to HbA1c (peak at 13 min, Fig. 4A) displayed a molecular ion of 16011.50 ± 2.35 Da. This mass is consistent with that of a native globin β-chain modified covalently by an AP formed by the nonenzymatic condensation of glucose [AP (162 Da) = glucose (180 Da) − H2O (18 Da)]. Of significance, LC-ESI analysis of a chromatographically “faster” eluting hemoglobin fraction (8.5 min peak, Fig. 4A) showed the same, unmodified α-globin chain (m/z = 15198.42 ± 1.52) together with that of a β-globin chain with a mass of 16040.27 ± 2.93 Da. This particular fraction was demonstrable only in hemolysates of blood from diabetic, alcohol-treated rats, and the observed mass is within experimental error of the calculated mass of a β-globin chain containing an AP modified additionally by acetaldehyde [AAP (192 Da) = AP (162 Da] + acetaldehyde (44 Da) − H20 (18 Da)].
Figure 4.
(A) HPLC (PolyCAT A) separation of the hemoglobin components obtained from the erythrocytes of diabetic rats fed an alcohol-supplemented diet. Each distinct fraction was collected, dialyzed extensively, and subjected to LC-ESI to achieve globin-chain separation as described in ref. 22. The fractions corresponding to unmodified hemoglobin (HbAo), and shown to contain the hemoglobin AP (HbA1c) and a hemoglobin AP modified additionally by acetaldehyde (HbA-AAP) are labeled. Biochemical and LC-ESI analysis have identified the 20-min region to contain hemoglobin species modified by glutathione (HbA-SSG) and by glycation at sites other than the N terminus (Y.A.-A., unpublished results). (B) ESI mass spectrum obtained by LC-ESI analysis of the globin β-chain from the native (unmodified) HbAo fraction. (C) ESI mass spectrum of the glycated β-chain obtained from the peak corresponding to HbA1c. (D) ESI mass spectrum of a chromatographically “faster” eluting fraction (8 min, Fig. 4A) showing the predicted mass of a hemoglobin AP modified additionally by acetaldehyde (HbA-AAP).
DISCUSSION
We have elucidated the structure of a posttranslational modification produced by the reaction of the ethanol metabolite, acetaldehyde, with glucose-derived APs. The reaction between acetaldehyde and the AP stabilizes its cyclic form and prevents further progression to AGE formation, which requires ring opening to an open-chain structure. The pro-atherogenic effect of mature AGEs has been attributed to a number of mechanisms. Within the vessel wall, AGEs accumulate in situ on long-lived, structural proteins such as basement membrane collagen (13, 32, 33), and their crosslinking properties contribute to vascular rigidity (31). AGE modification of circulating plasma proteins increases their tendency to be trapped within the walls of blood vessels. AGEs exert a number of toxic effects on endothelial cells, and they can act as chemoattractants to promote macrophage migration into the vessel wall and activation of cytokine responses (34). The lipid and lipoprotein components of low-density lipoprotein also have been identified as substrates for advanced glycation reactions, and the presence of AGEs on apolipoprotein B has been shown to interfere with its normal uptake by high-affinity, tissue low-density lipoprotein receptors. This can serve to delay the clearance of low-density lipoprotein from plasma and to promote its deposition into vascular wall plaque (14, 15).
The cardioprotection conferred by mild-to-moderate alcohol consumption, the so-called “French paradox” (35–39), has been attributed various factors, such as the presence of antioxidant substances in alcohol-containing beverages (40). The present data indicate that a likely mechanism for the beneficial effect of moderate alcohol ingestion on cardiovascular mortality is the direct chemical modification and stabilization of APs by alcohol-derived acetaldehyde, thereby preventing progression to AGE formation.
Acknowledgments
We thank Dr. Kirk Manogue for his comments on the manuscript. Thiswork was supported by grants from the National Institutes of Health (DK19655-15) and the American Heart Association (970131).
ABBREVIATIONS
- AGE
advanced glycation endproduct
- AP
Amadori product
- AAP
acetaldehyde-modified AP
- CBZ-lysine
Nα-carbobenzoyloxy-lysine
- ESI
electrospray ionization
References
- 1.Eriksson C J P. Science. 1980;207:1383–1384. doi: 10.1126/science.7355298. [DOI] [PubMed] [Google Scholar]
- 2.Stevens V J, Fantl W J, Newman C B, Sims R V, Cerami A, Peterson C M. J Clin Invest. 1981;67:361–369. doi: 10.1172/JCI110043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorrell M F, Tuma D J. Alcohol Clin Exp Res. 1985;9:306–309. doi: 10.1111/j.1530-0277.1985.tb05549.x. [DOI] [PubMed] [Google Scholar]
- 4.Lin R C, Smith R S, Lumeng L. J Clin Invest. 1988;81:615–619. doi: 10.1172/JCI113362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gross M D, Hays R, Gapstur S M, Chaussee M, Potter J D. Alcohol Alcohol. 1994;29:31–41. [PubMed] [Google Scholar]
- 6.Nguyen L B, Peterson C M. Diabetes Res. 1986;3:249–253. [PubMed] [Google Scholar]
- 7.San George R C, Hoberman H D. J Biol Chem. 1986;261:6811–6821. [PubMed] [Google Scholar]
- 8.Koenig R J, Peterson C M, Jones R L, Saudek C, Lehrman M, Cerami A. N Engl J Med. 1976;295:417–420. doi: 10.1056/NEJM197608192950804. [DOI] [PubMed] [Google Scholar]
- 9.Koenig R J, Blobstein S H, Cerami A. J Biol Chem. 1977;252:2992–2997. [PubMed] [Google Scholar]
- 10.Njoroge F G, Monnier V. Prog Clin Biol Res. 1988;304:85–107. [PubMed] [Google Scholar]
- 11.Ledl F, Schleicher E. Angew Chem. 1990;6:565–594. [Google Scholar]
- 12.Chen H-J C, Cerami A. J Carbohydrate Chem. 1993;12:731–742. [Google Scholar]
- 13.Bucala R, Cerami A, Vlassara H. Diabetes Reviews. 1995;3:258–268. [Google Scholar]
- 14.Bucala R, Makita Z, Vega G, Grundy S, Koschinsky T, Cerami A, Vlassara H. Proc Natl Acad Sci USA. 1994;91:9441–9445. doi: 10.1073/pnas.91.20.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bucala R, Mitchell R, Arnold K, Innerarity T, Vlassara H, Cerami A. J Biol Chem. 1995;270:10828–10832. doi: 10.1074/jbc.270.18.10828. [DOI] [PubMed] [Google Scholar]
- 16.Mossine V V, Glinsky G V, Barnes C L, Feather M S. Carbohydr Res. 1995;266:5–14. doi: 10.1016/0008-6215(94)00256-f. [DOI] [PubMed] [Google Scholar]
- 17.Monnier V, Cerami A. Science. 1981;211:491–493. doi: 10.1126/science.6779377. [DOI] [PubMed] [Google Scholar]
- 18.Makita Z, Vlassara H, Cerami A, Bucala R. J Biol Chem. 1992;267:5133–5138. [PubMed] [Google Scholar]
- 19.Zhang X, Ulrich P. Tetrahedron Lett. 1996;37:4667–4670. [Google Scholar]
- 20.Lieber C S. Trans Assoc Am Physicians. 1963;76:289. [Google Scholar]
- 21.Hagman M, Eriksson T, Kitson K E. Alcohol Clin Exp Res. 1993;17:299–303. doi: 10.1111/j.1530-0277.1993.tb00767.x. [DOI] [PubMed] [Google Scholar]
- 22.Okamoto Y, Murayama T, Ogata M. Folia Psychiatr Neurobiol. 1979;33:111–121. doi: 10.1111/j.1440-1819.1979.tb00180.x. [DOI] [PubMed] [Google Scholar]
- 23.Makita Z, Nakayama H, Taneda S, Kato M, Kuroda Y, Aoki S, Misawa K, Nakagawa S. Diabetologia. 1991;34:40–45. doi: 10.1007/BF00404023. [DOI] [PubMed] [Google Scholar]
- 24.Haney D N, Bunn H F. Proc Natl Acad Sci USA. 1976;73:3534–3538. doi: 10.1073/pnas.73.10.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevens V J, Vlassara H, Abati A, Cerami A. J Biol Chem. 1977;252:2998. [PubMed] [Google Scholar]
- 26.Makita Z, Vlassara H, Rayfield E, Cartwright K, Friedman E, Rodby R, Cerami A, Bucala R. Science. 1992;258:651–653. doi: 10.1126/science.1411574. [DOI] [PubMed] [Google Scholar]
- 27.Savage D, Lindenbaum J. Medicine. 1986;65:322–338. doi: 10.1097/00005792-198609000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Panzer S, Kronik G, Lechner K, Bettelheim P, Neumann E, Dudczak R. Blood. 1982;59:1348–1350. [PubMed] [Google Scholar]
- 29.Banks J F, Jr, Quinn J P, Whitehouse C M. Anal Chem. 1994;66:3688–3695. doi: 10.1021/ac00093a024. [DOI] [PubMed] [Google Scholar]
- 30.Hayase F, Nagaraj R H, Miyata S, Njoroge F G, Monnier V M. J Biol Chem. 1989;264:3758–3764. [PubMed] [Google Scholar]
- 31.Huijberts M S, Wolffenbuttel B H, Boudier H A, Crijns F R, Kruseman A C, Poitevin P, Levy B I. J Clin Invest. 1993;92:1407–1411. doi: 10.1172/JCI116716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura Y, Horii Y, Nishino T, Shiiki H, Sakaguchi Y, Kagoshima T, Dohi K, Makita Z, Vlassara H, Bucala R. Am J Pathol. 1993;143:1649–1656. [PMC free article] [PubMed] [Google Scholar]
- 33.Kume S, Takeya M, Mori T, Araki N, Suzuki H, Horiuchi S, Kodama T, Miyauchi Y, Takahashi K. Am J Pathol. 1995;147:654–667. [PMC free article] [PubMed] [Google Scholar]
- 34.Esposito C, Gerlach H, Brett J, Stern D, Vlassara H. J Exp Med. 1989;170:1387–1407. doi: 10.1084/jem.170.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Backwelder W C, Yano K, Rhoads G G, Kagan A, Gordon T, Palesch Y. Am J Med. 1980;68:164–169. doi: 10.1016/0002-9343(80)90350-2. [DOI] [PubMed] [Google Scholar]
- 36.Stampfer M J, Colditz G A, Willet W G, Speizer F E, Hennekens C H. N Engl J Med. 1988;319:267–273. doi: 10.1056/NEJM198808043190503. [DOI] [PubMed] [Google Scholar]
- 37.de Labry L O, Glynn R J, Levenson M R, Hermos J A, LoCastro J S, Vokonas P S. J Stud Alcohol. 1992;53:25–32. doi: 10.15288/jsa.1992.53.25. [DOI] [PubMed] [Google Scholar]
- 38.Klatsky A L, Armstrong M A, Friedman G D. Am J Cardiol. 1990;66:1237–1242. doi: 10.1016/0002-9149(90)91107-h. [DOI] [PubMed] [Google Scholar]
- 39.Miller G J, Beckles G L A, Maude G H, Carson D C. Int J Epidemiol. 1990;19:923–930. doi: 10.1093/ije/19.4.923. [DOI] [PubMed] [Google Scholar]
- 40.Klatsky A L, Armstrong M A, Friedman G D. Am J Cardiol. 1997;80:16–20. doi: 10.1016/s0002-9149(97)00388-3. [DOI] [PubMed] [Google Scholar]