Abstract
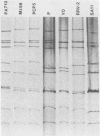
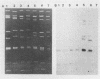
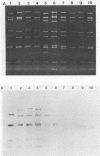
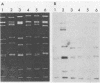
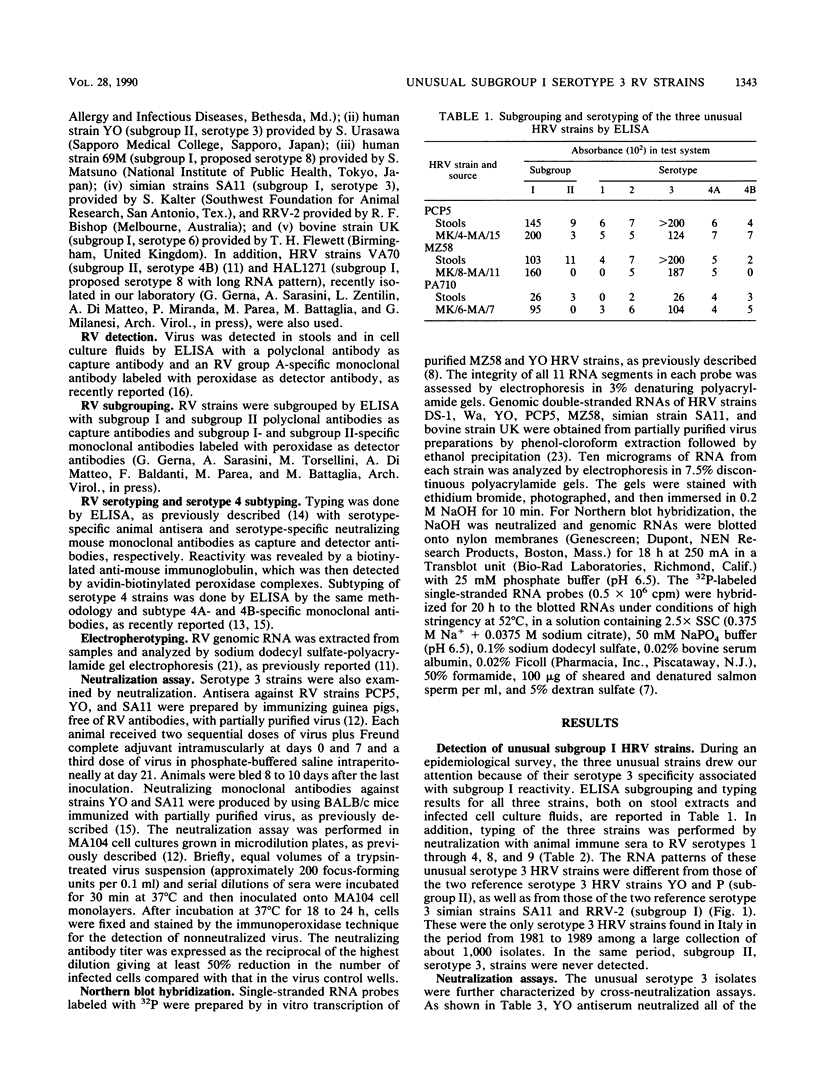
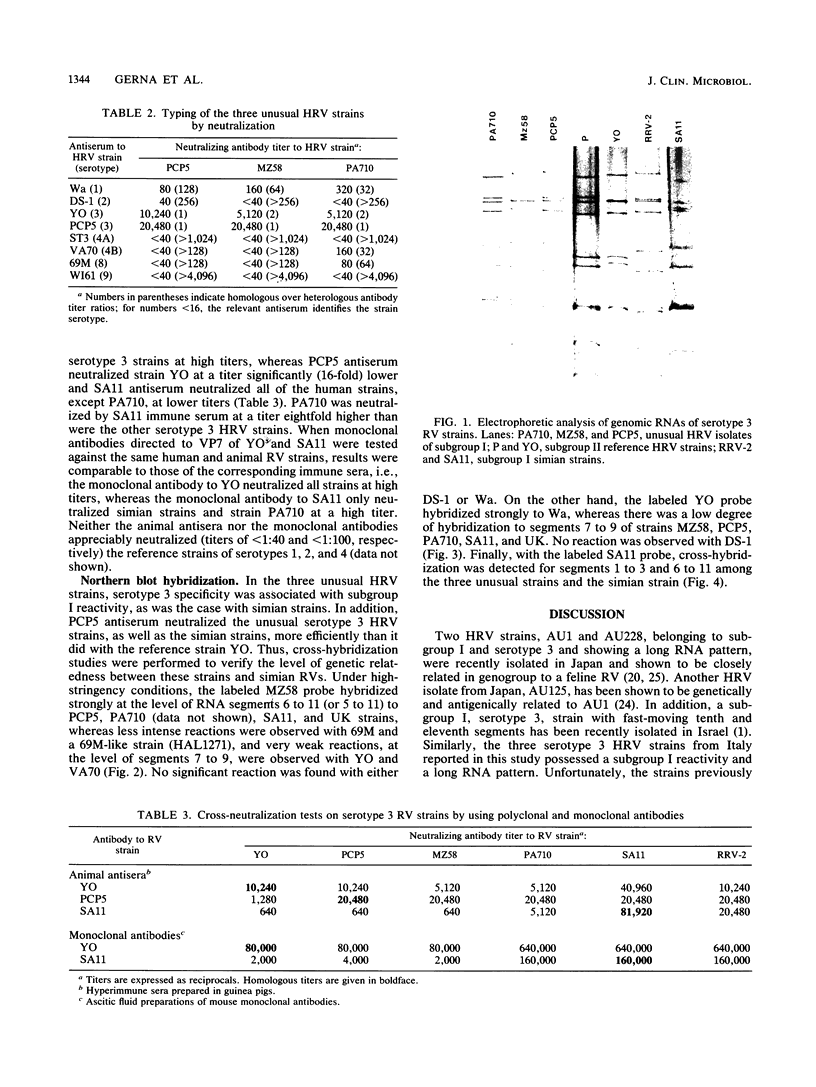
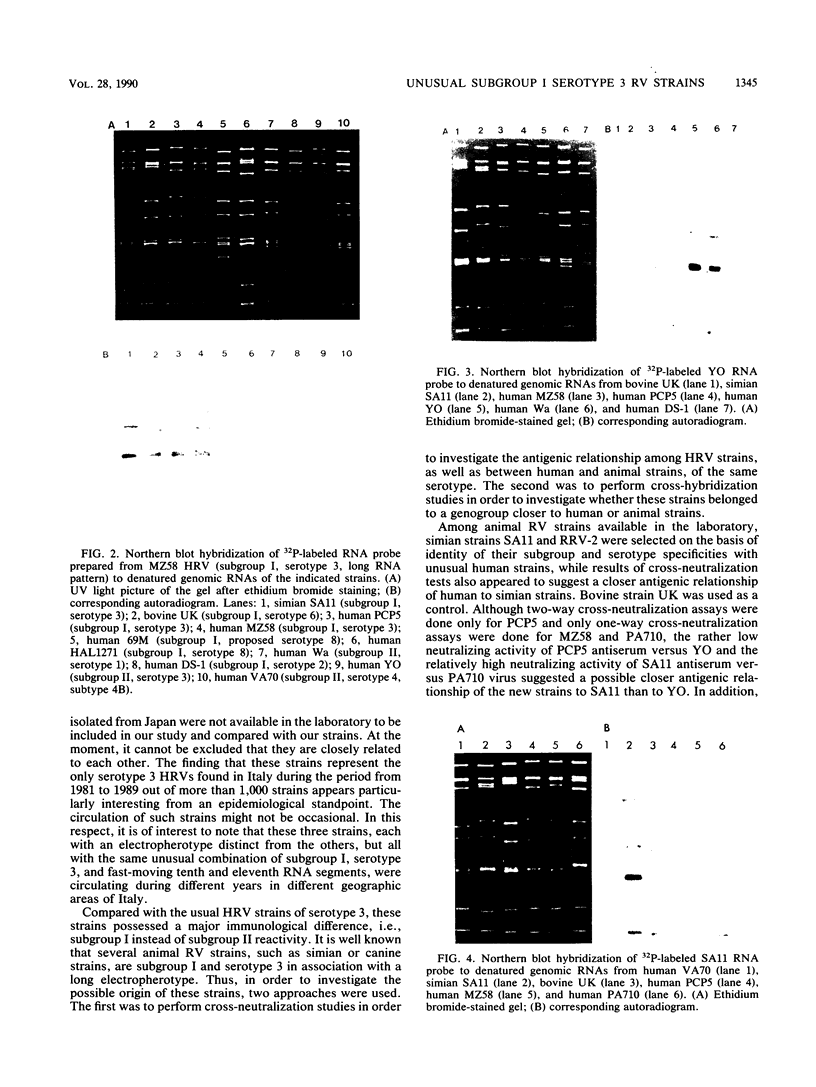
During an epidemiological study on human rotavirus (HRV) infections in Italy, three subgroup I strains not associated with serotype 2 reactivity were detected. All three strains were serotype 3, each with a distinct RNA pattern showing fast-moving tenth and eleventh segments (long electropherotype). Following successful adaptation to growth in cell cultures, the serotype 3 strains (MZ58, PCP5, and PA710) were further characterized by neutralization and by RNA-RNA (Northern blot) hybridization. Antiserum to reference HRV strain YO (subgroup II, serotype 3), as well as a monoclonal antibody to VP7 of YO neutralized, at comparable titers, the homologous virus, the three unusual HRV strains, and two reference simian strains (SA11 and RRV-2, both subgroup I, serotype 3), whereas SA11 antiserum and a monoclonal antibody to VP7 of SA11 neutralized simian strains more efficiently. However, antiserum to PCP5 neutralized the three unusual isolates and the simian strains at significantly higher titers than it did with reference strain YO. With 32P-labeled RNA from MZ58 as a probe, a high degree of homology was detected by Northern blot hybridization with strains PCP5, PA710, SA11, and UK (bovine rotavirus) at the level of several segments and with strain YO only at the level of genes 7 to 9. Conversely, labeled RNA of strain YO hybridized extensively with Wa (subgroup II, serotype 1 HRV strain) but only at the level of genes 7 to 9 with MZ58, PCP5, PA710, SA11, and UK. Finally, the labeled SA11 probe hybridized at the level of RNA segments 1 to 3 and 6 to 11 to the three unusual strains. These findings suggest that the unusual subgroup I, serotype 3, strains isolated from humans are more likely to be animal rotaviruses rather than natural reassortants between different HRV strains.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboudy Y., Shif I., Zilberstein I., Gotlieb-Stematsky T. Use of polyclonal and monoclonal antibodies and analysis of viral RNA in the detection of unusual group A human rotaviruses. J Med Virol. 1988 Jul;25(3):351–359. doi: 10.1002/jmv.1890250312. [DOI] [PubMed] [Google Scholar]
- Ahmed M. U., Taniguchi K., Kobayashi N., Urasawa T., Wakasugi F., Islam M., Shaikh H., Urasawa S. Characterization by enzyme-linked immunosorbent assay using subgroup- and serotype-specific monoclonal antibodies of human rotavirus obtained from diarrheic patients in Bangladesh. J Clin Microbiol. 1989 Jul;27(7):1678–1681. doi: 10.1128/jcm.27.7.1678-1681.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M. J., Unicomb L. E., Bishop R. F. Cultivation and characterization of human rotaviruses with "super short" RNA patterns. J Clin Microbiol. 1987 Jan;25(1):183–185. doi: 10.1128/jcm.25.1.183-185.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beards G. M., Desselberger U., Flewett T. H. Temporal and geographical distributions of human rotavirus serotypes, 1983 to 1988. J Clin Microbiol. 1989 Dec;27(12):2827–2833. doi: 10.1128/jcm.27.12.2827-2833.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson B. S., Unicomb L. E., Pitson G. A., Bishop R. F. Simple and specific enzyme immunoassay using monoclonal antibodies for serotyping human rotaviruses. J Clin Microbiol. 1987 Mar;25(3):509–515. doi: 10.1128/jcm.25.3.509-515.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., Hoshino Y., Boeggeman E., Purcell R., Chanock R. M., Kapikian A. Z. Genetic relatedness among animal rotaviruses. Arch Virol. 1986;87(3-4):273–285. doi: 10.1007/BF01315305. [DOI] [PubMed] [Google Scholar]
- Flores J., Midthun K., Hoshino Y., Green K., Gorziglia M., Kapikian A. Z., Chanock R. M. Conservation of the fourth gene among rotaviruses recovered from asymptomatic newborn infants and its possible role in attenuation. J Virol. 1986 Dec;60(3):972–979. doi: 10.1128/jvi.60.3.972-979.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., Myslinski J., Kalica A. R., Greenberg H. B., Wyatt R. G., Kapikian A. Z., Chanock R. M. In vitro transcription of two human rotaviruses. J Virol. 1982 Sep;43(3):1032–1037. doi: 10.1128/jvi.43.3.1032-1037.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., Perez-Schael I., Boeggeman E., White L., Perez M., Purcell R., Hoshino Y., Midthun K., Chanock R. M., Kapikian A. Z. Genetic relatedness among human rotaviruses. J Med Virol. 1985 Oct;17(2):135–143. doi: 10.1002/jmv.1890170206. [DOI] [PubMed] [Google Scholar]
- Flores J., Perez I., White L., Perez M., Kalica A. R., Marquina R., Wyatt R. G., Kapikian A. Z., Chanock R. M. Genetic relatedness among human rotaviruses as determined by RNA hybridization. Infect Immun. 1982 Aug;37(2):648–655. doi: 10.1128/iai.37.2.648-655.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerna G., Arista S., Passarani N., Sarasini A., Battaglia M. Electropherotype heterogeneity within serotypes of human rotavirus strains circulating in Italy. Brief report. Arch Virol. 1987;95(1-2):129–135. doi: 10.1007/BF01311340. [DOI] [PubMed] [Google Scholar]
- Gerna G., Battaglia M., Milenesi G., Passarani N., Percivalle E., Cattaneo E. Serotyping of cell culture-adapted subgroup 2 human rotavirus strains by neutralization. Infect Immun. 1984 Feb;43(2):722–729. doi: 10.1128/iai.43.2.722-729.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerna G., Passarani N., Uricomb L. E., Parea M., Sarasini A., Battaglia M., Bishop R. F. Solid-phase immune electron microscopy and enzyme-linked immunosorbent assay for typing of human rotavirus strains by using polyclonal and monoclonal antibodies: a comparative study. J Infect Dis. 1989 Feb;159(2):335–339. doi: 10.1093/infdis/159.2.335. [DOI] [PubMed] [Google Scholar]
- Gerna G., Sarasini A., Coulson B. S., Parea M., Torsellini M., Arbustini E., Battaglia M. Comparative sensitivities of solid-phase immune electron microscopy and enzyme-linked immunosorbent assay for serotyping of human rotavirus strains with neutralizing monoclonal antibodies. J Clin Microbiol. 1988 Jul;26(7):1383–1387. doi: 10.1128/jcm.26.7.1383-1387.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerna G., Sarasini A., Di Matteo A., Parea M., Torsellini M., Battaglia M. Rapid detection of human rotavirus strains in stools by single-sandwich enzyme-linked immunosorbent assay systems using monoclonal antibodies. J Virol Methods. 1989 Apr-May;24(1-2):43–56. doi: 10.1016/0166-0934(89)90006-2. [DOI] [PubMed] [Google Scholar]
- Gerna G., Sarasini A., di Matteo A., Parea M., Orsolini P., Battaglia M. Identification of two subtypes of serotype 4 human rotavirus by using VP7-specific neutralizing monoclonal antibodies. J Clin Microbiol. 1988 Jul;26(7):1388–1392. doi: 10.1128/jcm.26.7.1388-1392.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H., McAuliffe V., Valdesuso J., Wyatt R., Flores J., Kalica A., Hoshino Y., Singh N. Serological analysis of the subgroup protein of rotavirus, using monoclonal antibodies. Infect Immun. 1983 Jan;39(1):91–99. doi: 10.1128/iai.39.1.91-99.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Greenberg H. B., Flores J., Kapikian A. Z. Serotypic similarity and diversity of rotaviruses of mammalian and avian origin as studied by plaque-reduction neutralization. J Infect Dis. 1984 May;149(5):694–702. doi: 10.1093/infdis/149.5.694. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., Greenberg H. B., Espejo R. T., Flores J., Wyatt R. G., Kapikian A. Z., Chanock R. M. Distinctive ribonucleic acid patterns of human rotavirus subgroups 1 and 2. Infect Immun. 1981 Sep;33(3):958–961. doi: 10.1128/iai.33.3.958-961.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaoka S., Nakagomi T., Fukuhara N., Hoshino Y., Suzuki H., Nakagomi O., Kapikian A. Z., Ebina T., Konno T., Ishida N. Serologic characteristics of a human rotavirus isolate, AU-1, which has a "long" RNA pattern and subgroup I specificity. J Med Virol. 1987 Dec;23(4):351–357. doi: 10.1002/jmv.1890230407. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsuno S., Hasegawa A., Mukoyama A., Inouye S. A candidate for a new serotype of human rotavirus. J Virol. 1985 May;54(2):623–624. doi: 10.1128/jvi.54.2.623-624.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagomi O., Nakagomi T., Hoshino Y., Flores J., Kapikian A. Z. Genetic analysis of a human rotavirus that belongs to subgroup I but has an RNA pattern typical of subgroup II human rotaviruses. J Clin Microbiol. 1987 Jul;25(7):1159–1164. doi: 10.1128/jcm.25.7.1159-1164.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagomi O., Oyamada H., Kuroki S., Kobayashi Y., Ohshima A., Nakagomi T. Molecular identification of a novel human rotavirus in relation to subgroup and electropherotype of genomic RNA. J Med Virol. 1989 Jul;28(3):163–168. doi: 10.1002/jmv.1890280311. [DOI] [PubMed] [Google Scholar]
- Nakagomi T., Nakagomi O. RNA-RNA hybridization identifies a human rotavirus that is genetically related to feline rotavirus. J Virol. 1989 Mar;63(3):1431–1434. doi: 10.1128/jvi.63.3.1431-1434.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Blavat G. Identification of the two rotavirus genes determining neutralization specificities. J Virol. 1986 Jan;57(1):376–378. doi: 10.1128/jvi.57.1.376-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Morita Y., Greenberg H. B., Urasawa S. Direct serotyping of human rotavirus in stools by an enzyme-linked immunosorbent assay using serotype 1-, 2-, 3-, and 4-specific monoclonal antibodies to VP7. J Infect Dis. 1987 Jun;155(6):1159–1166. doi: 10.1093/infdis/155.6.1159. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Urasawa S. Reactivity patterns to human rotavirus strains of a monoclonal antibody against VP2, a component of the inner capsid of rotavirus. Brief report. Arch Virol. 1986;87(1-2):135–141. doi: 10.1007/BF01310550. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Urasawa S., Yasuhara T. Production of subgroup-specific monoclonal antibodies against human rotaviruses and their application to an enzyme-linked immunosorbent assay for subgroup determination. J Med Virol. 1984;14(2):115–125. doi: 10.1002/jmv.1890140205. [DOI] [PubMed] [Google Scholar]