Abstract
Extracellular ATP (exATP) has been known to be a critical ligand regulating skeletal muscle differentiation and contractibility. ExATP synthesis was greatly increased with the high level of adenylate kinase 1 (AK1) and ATP synthase β during C2C12 myogenesis. The exATP synthesis was abolished by the knock-down of AK1 but not by that of ATP synthase β in C2C12 myotubes, suggesting that AK1 is required for exATP synthesis in myotubes. However, membrane-bound AK1β was not involved in exATP synthesis because its expression level was decreased during myogenesis in spite of its localization in the lipid rafts that contain various kinds of receptors and mediate cell signal transduction, cell migration, and differentiation. Interestingly, cytoplasmic AK1 was secreted from C2C12 myotubes but not from C2C12 myoblasts. Taken together all these data, we can conclude that AK1 secretion is required for the exATP generation in myotubes.
Keywords: adenosine triphosphate, adenylate kinase 1, TP synthase, membrane microdomains, muscle development
Introduction
Adenylate kinase (AK) is an enzyme that regulates adenine nucleotide metabolism by catalyzing the reaction: ATP + AMP ↔ 2ADP (Zeleznikar et al., 1990). In mammals, there are several AK isoforms with tissue-specific distributions and distinct subcellular localization (Van Rompay et al., 2000). Among these AK isoforms, AK1 is known to be a major AK isoform in the cytoplasm of skeletal muscle (Tanabe et al., 1993). AK1-null mice show delayed skeletal muscle relaxation due to abnormal accumulation of ADP in spite of normal muscle formation (Hancock et al., 2005). In addition, AK1 phosphotransfer might be necessary for communicating a signal between mitochondria and KATP channels at the plasma membrane (Carrasco et al., 2001). A membrane-associated AK1 isoform, referred to as AK1β, has recently been characterized (Collavin et al., 1999; Janssen et al., 2004). AK1β could be targeted to the plasma membrane because it contains a plausible myristoylation site at its N-terminus. AK1β might work as a membrane metabolic sensor because it mediates ATP-induced activation of KATP channels (Janssen et al., 2004).
Extracellular ATP (exATP) is a crucial mediator of chemosensory transduction in the central nervous system (Gourine et al., 2005), of asthmatic airway inflammation through the activation of dendrite cells (Idzko et al., 2007), and of skeletal muscle contractibility (Sandonà et al., 2005). There are three enzyme candidates for exATP generation; ectopic AK, ATP synthase, and nucleoside diphosphokinase. Indeed, ectopic AK is a major enzyme that maintains exATP levels in endothelial cells (Quillen et al., 2006; Yegutkin et al., 2001), epithelial cells (Donaldson et al., 2002; Picher and Boucher 2003), and keratinocytes (Burrell et al., 2005). In addition, ATP synthase, an enzyme that catalyzes the reaction, ADP + Pi → ATP, has been unambiguously localized to the cell surface of various cells (Arakaki et al., 2003; Martinez et al., 2003; Bae et al., 2004; Kim et al., 2004; Kim et al., 2006), which implies its involvement in exATP synthesis. However, the role of ATP synthase in exATP synthesis has been challenged because treatment with oligomycin, an ATP synthase inhibitor, does not change exATP synthesis in primary hepatocytes and HepG2 cells (Fabre et al., 2006). Here, we demonstrated that exATP synthesis was highly increased with high expression of AK1 and ATP synthase β during C2C12 myogenesis. The exATP synthesis was reduced by the down-regulation of AK1 but not by that of ATP synthase β, suggesting that AK1 is a major enzyme responsible for exATP synthesis in myotubes. Moreover, we show that cytoplasmic AK1 in myotubes was secreted to provide ectopic AK1 that is required for exATP synthesis.
Materials and Methods
Materials
Ap5A and oligomycin were purchased from Sigma (St. Louis, Mo). Anti-AK1 antibody was obtained from Novus Biologicals (Littleton, CO), anti-ATP synthase β antibody from Molecular Probes (Eugene, OR), anti-insulin receptor β, flotillin-1, and caveolin-1 antibodies from BD Biosciences (North Ryde, Australia), anti-β-actin, GAPDH, and caveolin-3 antibodies from Santa Cruz (Santa Cruz, CA), and anti-Myosin Heavy Chain antibody from Sigma. Amicon Ultra-15 (Ultracel-10K) was purchased from Millipore (Billerica, MA).
C2C12 muscle differentiation
C2C12 cells were purchased from ATCC, and were grown in DMEM supplemented with 1% penicillin/ streptomycin and 10% FBS (Welgene, Republic of Korea) in a 5% CO2 incubator at 37℃. One-day post-confluent C2C12 cells were differentiated into myotubes by incubating with DMEM supplemented with 2% horse serum and re-fed every 48 h.
Lipid raft isolation, immunoblotting, and immunofluorescence
Lipid rafts were isolated from C2C12 myoblasts and myotubes according to the method described by Kim et al. (2006). Immunoblotting and immunofluorescence were performed as described by Kim et al. (2004). Immunofluorescent signals were observed under an LSM 510 META confocal microscope (Carl ZEISS, Germany).
Subcellular fractionation
The plasma membrane was isolated according to Hubbard et al. (1983), with minor modifications. C2C12 myoblasts and myotubes were scraped after washing twice with TES buffer (20 mM Tris HCl, 1mM EDTA, 8.7% sucrose; pH 7.4), and were then homogenized in a TES buffer. The homogenized cells were centrifuged at 12,000 rpm in an SW55Ti rotor (Beckman) for 30 min. The pellet was resuspended with TES buffer, loaded on a 38.5% sucrose cushion, and centrifuged at 100,000 g in an SW55Ti rotor for 60 min at 4℃. The plasma membrane was collected from the top of the sucrose cushion, resuspended in TES buffer, and repelleted by centrifugation at 31,000 g for 60 min at 4℃.
Quantification of ATP by bioluminescent luciferase assay
Extracellular ATP was measured as described previously (Arakaki et al., 2003). C2C12 myoblasts and myotubes were washed three times with HEPES buffer (10 mM HEPES, pH 7.4, 150 mM NaCl), and were then incubated with 0.2 ml of HEPES buffer with 200 µM ADP, 20 mM Pi, and 2 mM MgCl2 at room temperature. After incubation, the extracellular media were collected and used for the determination of extracellular ATP content. ATP levels were measured by the bioluminescence assay according to the protocol provided with an ATP determination kit (Molecular Probes).
Down-regulation of ATP synthase and adenylate kinase
Control Si-RNA, Si-ATP synthase β, and Si-AK1 were purchased from Santa Cruz Biotechnology. Si-RNAs were transfected by electroporation according to the protocol of the electroporator MP-100 (Digital Bio, Republic of Korea).
Results
AK1 is required for exATP synthesis in myotubes
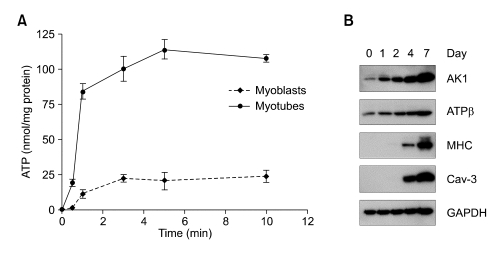
Because exATP is known to be required for C2C12 myogenesis (Ryten et al., 2002), it is tempting to speculate that exATP synthesis could be increased during skeletal muscle differentiation. In order to address the issue, exATP content was determined by bioluminescent luciferase assay after ADP, Pi, and MgCl2 had been administrated in C2C12 myoblasts and myotubes. In both cells, exATP content was highly increased and reached a plateau level at 1 min that was continuously maintained for longer time (Figure 1A). However, myotubes produced about four times more exATP than did myoblasts, indicating that myotubes have stronger exATP-synthesizing activity than myoblasts do. Since ectopic AK1 and ATP synthase are enzymes that are capable of synthesizing exATP from ADP and Pi, intracellular level of AK1 and ATP synthase might be increased during myogenesis. We investigated the expression level of AK1 and ATP synthase β by immunoblotting during C2C12 myogenesis. As shown in Figure 1B, the expression level of AK1 and ATP synthase β was strongly increased with myogenesis marker proteins such as caveolin-3 (Cav-3) (Ha and Pak, 2005) and myosin heavy chain (MHC) during C2C12 myogenesis, which indicates that these two enzymes could be involved in exATP synthesis.
Figure 1.
The increase of exATP synthesis is accompanied by high expression level of AK1 and ATP synthase β during myogenesis. (A) C2C12 myotubes were differentiated to myotubes for 3 days. After incubating myoblasts and myotubes with ADP (200 µM), Pi (20 mM), and MgCl2 (2 mM) for the indicated amounts of time, the ATP content was determined by bioluminescent luciferase assay. The ATP content was normalized by the protein concentration. (B) C2C12 myoblasts were differentiated to myotubes for the indicated amounts of time. The whole cell lysates were analyzed by immunoblotting with anti-AK1, ATP synthase β (ATPβ), caveolin-3 (Cav-3), myosin heavy chain (MHC), and GAPDH antibodies.
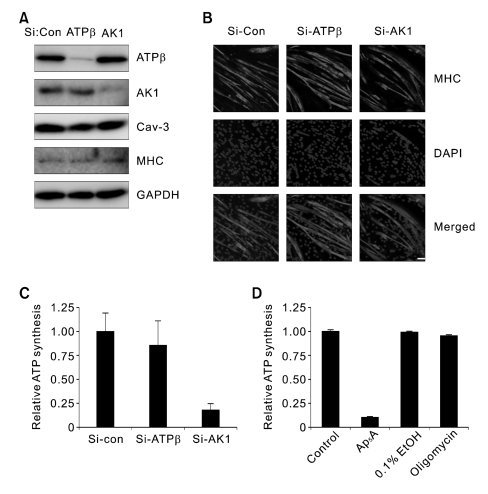
To determine the enzyme required for exATP synthesis in myotubes, small interference RNA (SiRNA) for AK1 or ATP synthase β was treated into C2C12 myoblasts that were further differentiated to myotubes for 3 days. In myotubes treated with SiRNA for AK1 or ATP synthase β, AK1 or ATP synthase β was down-regulated (Figure 2A). However, the expression level of myogenic marker proteins such as caveolin-3 (Cav-3) and myosin heavy chain (MHC) (Figure 2A) and the formation of multinuclear myotubes (Figure 2B) were not changed by the downregulation of AK1 or ATP synthase β during myogenesis, indicating that C2C12 myogenesis is not affected by the knock-down of AK1 or ATP synthase β. When exATP was measured after adding ADP, Pi, and MgCl2 in myotubes down-regulating AK1 or ATP synthase β, exATP content was greatly reduced by the down-regulation of AK1 but not by that of ATP synthase β (Figure 2C). In addition, exATP synthesis was abolished by AK1-specific inhibitor, Ap5A, but not by ATP synthase inhibitor, oligomycin (Figure 2D). Taken together, these data allow us to conclude that AK1 is responsible for exATP synthesis in C2C12 myotubes.
Figure 2.
AK1 is required for exATP synthesis in myotubes. (A) Si-Control (Si-Con), Si-AK1, or Si-ATP synthase β (Si-ATPβ) was treated in myoblasts that were further differentiated for 3 days. The whole cell lysates were analyzed by immunoblotting with anti-ATP synthase β, AK1, Cav-3, and MHC antibodies. (B) Si-Con-, Si-ATPβ-, or Si-AK1-treated myotubes were analyzed by immunofluorescence with anti-MHC antibody. The myotubes were also stained with DAPI. The white bar indicates a length of 50 µm. (C) The exATP content was measured in the myotubes down-regulating AK1 or ATP synthase β after the cells were incubated with ADP (200 µM), Pi (20 mM), and MgCl2 (2 mM) for 1 min. ATP content was normalized by the protein concentration. (D) Myotubes that had been differentiated for three days were pretreated with 100 µM Ap5A or 20 µg/ml oligomycin for 30 min, and the exATP content was measured after the cells had been incubated with ADP (200 µM), Pi (20 mM), and MgCl2 (2 mM) for 1 min. Ethanol was used as a vehicle for oligomycin. The ATP content was normalized by the protein concentration.
AK1β is localized in sarcolemma lipid rafts in myoblasts
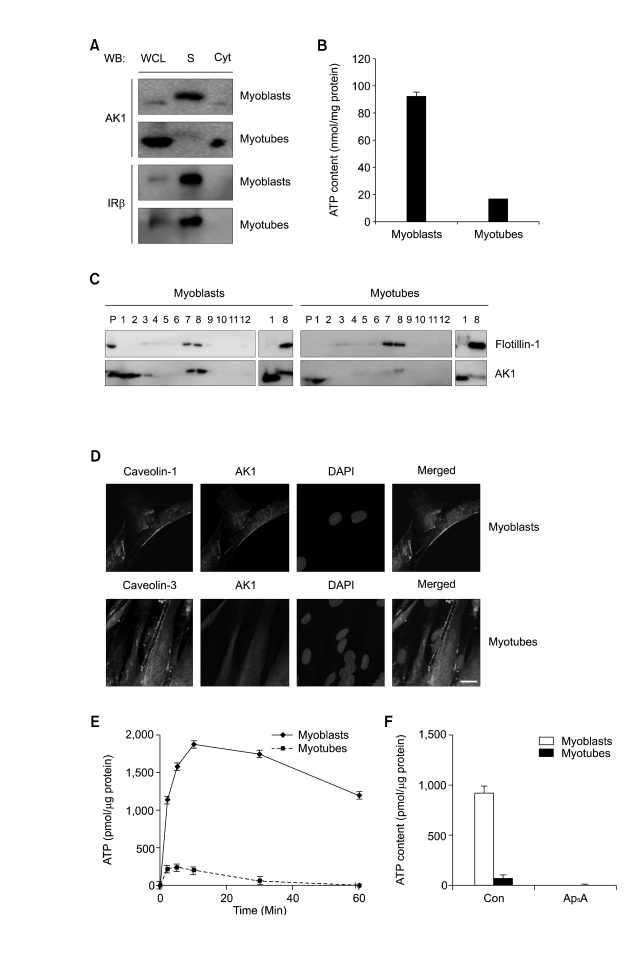
AK1-induced exATP synthesis could be explained by the presence of membrane-associated AK1β in myotubes. In order to identify the membrane-bound AK1β, we monitored AK1β from the plasma membrane (= sarcolemma) of myoblasts and myotubes by immunoblotting with anti-AK1 antibody. AK1β with higher molecular weight appeared in the sarcolemma fraction of myoblasts, but not in that of myotubes (Figure 3A). In order to further confirm AK1β localization in the sarcolemma, we assayed ATP-synthesizing activity in the sarcolemma isolated from myoblasts and myotubes by measuring ATP content after incubating the purified sarcolemma with ADP, Pi, and MgCl2. Figure 3B shows that ATP-synthesizing activity was four times higher in the sarcolemma of myoblasts than in those of myotubes, coincident with the reduced level of AK1β in the sarcolemma of the myotubes (Figure 3A).
Figure 3.
AK1β was localized in the lipid rafts of myoblasts and diminished during myogenesis. (A) Myoblasts and 3-days-differentiated myotubes were fractionated into cytoplasm and sarcolemma (= plasma membrane of skeletal muscle). AK1 and insulin receptor β(IRβ were analyzed by immunoblotting. WCL, whole cell lysates; S, sarcolemma Cyt, cytoplasm. (B) ATP content was measured after incubating sarcolemma with ADP (200 µM), Pi (20 mM), and MgCl2 (2 mM) for 1 min. The ATP content was normalized by the protein concentration of sarcolemma. (C) Lipid rafts were isolated from myoblasts and myotubes, based on detergent insolubility and low density (Brown et al., 1992). After sucrose gradient ultracentrifugation, each fraction was analyzed by immunoblotting with anti-flotillin-1 and AK1 antibodies. Fraction number 1, and 2 represent non-raft fractions whereas fraction number 7, and 8 do raft fractions. P indicates pellet. Non-raft fraction (fraction number 1) and raft fraction (fraction number 8) were also analyzed by immunoblotting for side-by-side comparison of AK1 and AK1β with different molecular weights. (D) Co-localization of AK1 and Caveolin-1 in myoblasts and AK1 and Caveolin-3 in 3-days-differentiated myotubes was determined by immunofluorescence. The cells were also stained with DAPI. The white bar indicates a length of 10 µm. (E) ATP content was measured after incubating the lipid rafts of myoblasts or myotubes with ADP (200 µM), Pi (20 mM), and MgCl2 (2 mM) for the indicated amounts of time. The ATP content was normalized by the protein concentration of the lipid rafts. (F) The lipid rafts from myoblasts or 3-days-differentated myotubes were preincubated with Ap5A (100 µM) for 30 min, and were then incubated with ADP (200 µM), Pi (20 mM), and MgCl2 (2 mM) for 1 min before the measurement of ATP content. The ATP content was normalized by the protein concentration of the lipid rafts.
Lipid-modified proteins with fatty acids have been known to be concentrated in the lipid rafts that mediate different cellular events such as cell signal transduction, cell migration, and differentiation. Thus, AK1β with a plausible myristoylation site might be localized in lipid rafts that have distinct biochemical properties of detergent insolubility and low density due to their cholesterol (Brown and Rose 1992). In order to address this issue, we isolated detergent-resistant lipid rafts from myoblasts and myotubes. As shown in Figure 3C, AK1β with higher molecular weight was predominantly found in the lipid raft fractions of myoblasts, but not in those of myotubes, which indicates that AK1β is a lipid raft protein of myoblasts. Furthermore, AK1 localization in the lipid rafts was re-confirmed by immunofluorescence. In myoblasts, sarcolemma-bound AK1β was co-localized with caveolin-1, a lipid raft marker protein in myoblasts. In myotubes, however, AK1 was not co-localized with caveolin-3, a myotube-specific lipid raft protein (Figure 3D).
Next, we assayed the ATP-synthesizing activities from lipid rafts of myoblasts and myotubes by measuring ATP content after incubating lipid rafts with ADP, Pi and MgCl2. Figure 3E shows that lipid rafts from myoblasts had eight times higher ATP synthesizing activity than did those of myotubes. Since ATP synthesis in myoblast lipid rafts was completely abolished by Ap5A, an AK1-specific inhibitor (Figure 3F), the ATP synthesis reflects AK1 enzymatic activity.
AK1 is secreted for the synthesis of exATP in myotubes
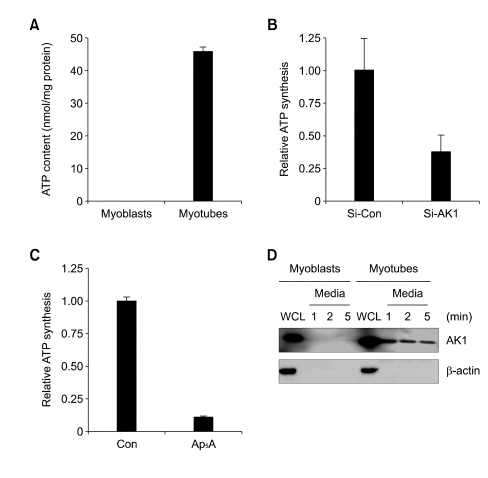
Because AK1β was not found in the sarcolemma of myotubes (Figure 3A), exATP synthesis in myotubes might require the secretion of cytoplasmic AK1. In order to identify the secreted AK1, we measured ATP-synthesizing activity in extracellular fluids from myoblasts and myotubes. Figure 4A shows that strong ATP-synthesizing activity was observed in extracellular fluid of myotubes, but not in that of myoblasts. Interestingly, ATP-synthesizing activity was decreased from extracellular fluid of myotubes down-regulating AK1 or treated with Ap5A (Figure 4B and C), which suggests that AK1 is an ATPsynthesizing enzyme that is present in extracellular fluid of myotubes. Next, we confirmed the presence of AK1 from extracellular fluid of myotubes by immunoblotting. Figure 4D shows that AK1 was secreted into extracellular fluid as fast as 1 min after the incubation of myotubes with serum-free media, but was not secreted into extracellular fluid of myoblasts. The AK1 secretion was very specific, because there was no β-actin from medium at any time points, indicating that the cells were not damaged by incubating with serum-free media. All these data suggest that exATP synthesis requires the secretion of AK1 in myotubes.
Figure 4.
The extracellular fluid of myotubes has a strong AK1 activity and contains the secreted form of AK1. (A) Myoblasts and 3-days-differentiated myotubes were incubated with HEPES buffer for 1 min. The buffer was collected and incubated with ADP (200 µM) and MgCl2 (2 mM) for 1 min before the measurement of ATP content. It should be noted that only ADP was added into extracellular fluid in order to measure just the activity of AK1 that reacts 2ADP ↔ ATP + AMP. The ATP content was normalized by the protein concentration of the whole cell lysates. (B, C). Myotubes that had been differentiated for three days were downregulated by Si-AK1 (B) or pretreated with Ap5A (100 µM) for 30 min (C), and were then incubated with HEPES buffer for 1 min. The buffer was collected and reacted with ADP (200 µM) and MgCl2 (2 mM) for 1 min before the ATP content was measured. The ATP content was normalized by the protein concentration of whole cell lysates. (D) Myoblasts and 3-days-differentiated myotubes were incubated with serum-free media for the indicated amounts of time.The media were collected, and then concentrated by using Amicon Ultra. Whole cell lysates (WCL) and media were analyzed by immunoblooting with anti-AK1 and β-actin antibodies.
Discussion
Since exATP was found to be an extracellular messenger in 1972, the concept of purinergic signaling has been proposed with "purinergic nerves" (Burnstock 1972). Since exATP has a function in neurotransmission, it has been known to be a mediator of mechanosensory transduction, vasodilation, and cell proliferation, differentiation, and death (Burnstock 2006). ExATP binds two types of purinergic receptors, P2X and P2Y. P2X receptors are ligand-gated ion channels and have 7 subtypes. P2Y receptors are G protein-coupled receptors with 8 subtypes (Abbracchio et al., 2003). In skeletal muscle, exATP acts as an autocrine or paracrine via P2X(4) to induce Ca2+ entry for subsequent contraction after released during muscle contraction (Sandonà et al., 2005). P2X(5) receptor activation by exATP was required for muscle differentiation. When C2C12 cells are treated with apyrase, an exATP-degrading enzyme or a P2X receptor inhibitor, periodate-oxidized adenosine 5'-triphosphate, their differentiation is blocked (Araya et al., 2004). It suggests that calcium signaling by purinergic receptor is required for myogenesis. In addition, exATP stimulates glucose uptake through both P2X and P2Y receptor to provide energy in skeletal muscle cells (Kim et al., 2002).
ExATP has several roles on muscle development and functions. However, the source of exATP in skeletal muscle is still not clear. It is generally accepted that exATP might be released from cells exposed to different stimuli such as shear stress (Yamamoto et al., 2003) and hypoxia (Buttigieg and Nurse 2004). ATP has been known to be released from contracting skeletal muscle (Sandonà et al., 2005). Its release mechanisms from intact cell include exocytosis, transporters, and stretch-activated channels (Bodin and Burnstock 2001; Lazarowski et al., 2003). In addition, ATP can be released into extracellular space from damaged cells and during tissue injury. In addition to ATP release, exATP might be generated from extracellular ADP. Since platelets might release ADP (Gordon, 1986), exATP could be synthesized by AK reaction with extracellular ADP as a substrate. However, the mechanisms of ATP or ADP release have never been investigated in skeletal muscle.
When endogenous exATP concentration was measured from 3-days differentiated myotubes in the absence of ADP and Pi, the exATP content was reached to about 350 pmole/mg protein at 2 min (data not shown), indicating that small amount of ATP is secreted from myotubes. However, exATP concentration was reached to more than 110 nmole/ mg protein in the presence of ADP and Pi (Figure 1A), demonstrating that cell surface or media of C2C12 myotubes contain some enzymes synthesizing exATP from ADP or ADP + Pi. Thus, we can distinguish the newly-synthesized exATP from the secreted ATP by incubating cells with ADP and Pi. Several research groups have demonstrated that exATP regulates skeletal muscle differentiation via activation of a P2X(5) receptor on satellite cells (Araya et al., 2004; Ryten et al., 2002). ExATP-induced myogenesis is prevented by the treatment of P2X(5) receptor inhibitor, periodate-oxidized adenosine 5'-triphosphate as well as apyrase, ATP-degrading enzyme, showing that exATP is a critical inducer of myogenesis (Araya et al., 2004; Ryten et al., 2002). Endogenous secreted exATP concentration was not dramatically reduced after AK1 down-regulation that did not affect myogenesis (Figure 2A and B). ExATP concentration was about 320 pmole/mg protein in Si-Con-treated myotubes, and about 170 pmole/mg protein in Si-AK1-treated myotubes (data not shown), indicating that AK1 down-regulation does not affect ATP secretion. Thus, AK1-dependent exATP generation might not be necessary for skeletal muscle differentiation because AK1-independent ATP secretion might supply enough amount of exATP to induce skeletal muscle differentiation.
ATP synthase is expressed on the surfaces of mammalian cells (Arakaki et al., 2003; Bae et al., 2004; Kim et al., 2004; Kim et al., 2006; Martinez et al., 2003), and treatment with ATP synthase inhibitors (oligomycin and efrapeptin) and anti-ATP synthase antibody decreases exATP generation in HUVECs (Arakaki et al., 2003; Moser et al., 2001; Quillen et al., 2006), which indicates that the surface ATP synthase might be required for exATP synthesis. In contrast, oligomycin treatment does not affect exATP synthesis in primary hepatocytes and HepG2 (Fabre et al., 2006). The discrepancy in ectopic ATP synthase-mediated exATP production could be unambiguously resolved by an experiment using RNA silencing of ATP synthase. Here, we demonstrated that exATP synthesis was largely decreased by the down-regulation of AK1, but not by that of ATP synthase in C2C12 myotubes. Thus, we can conclude that exATP synthesis requires AK1 but not ATP synthase (Figure 2C).
AK1 has two isotypes; cytoplasmic AK1 and membrane-bound AK1β in various mammalian cells (Collavin et al., 1999; Janssen et al., 2004; Notari et al., 2003; Van Rompay et al., 2000). During C2C12 myogenesis, the cytoplasmic AK1 was highly increased and secreted to extracellular fluid, whereas membrane-bound AK1β disappeared (Figure 3A and 4D). The disappearance of AK1β could explain the decreased activity of KATP channels in myotubes (Kubo, 1991) because AMP-induced activation of KATP channels is mediated by AK1β in the presence of ATP (Janssen et al., 2004). Since AK1-dependent exATP synthesis is highly increased in myotubes, it is tempting to speculate that the exATP might be required for myogenesis, which has been reported to be strongly induced by exposure to exATP (Ryten et al., 2002). However, the RNA silencing of AK1 did not change myogenesis, which indicates that the exATP generated by AK1 could be necessary for other muscular functions such as muscle contraction and relaxation, as AK1-disrupted mice show delayed muscle relaxation (Hancock et al., 2005). In addition, AK1, which is secreted to extracellular fluid, might regulate the extracellular adenine nucleotide pool to control cell signaling through purinergic receptors in skeletal muscle.
AK activity is detected in nasal submucosal gland secretions but its presence and its secretion mechanism has never been challenged (Donaldson et al., 2002). AK1 might be localized in cytoplasm due to the lack of secretory signal sequence. Many cellular proteins have been reported to be secreted from various kinds of cells without a consensus secretory signal sequence. For example, the leaderless proteins such as heat shock protein 70 (Hsp70) (Mambula and Calderwood 2006) and IL-1β (Andrei et al., 1999) are secreted into extracellular fluid by a nonclassical pathway involving lysosomal endosomes and ABC transporter after cellular exposure to heat shock. An aminoacyl-tRNA synthetase-interacting multifunctional protein-1, also called p43, is constitutively secreted without signal peptide sequence via an unknown mechanism (Ko et al., 2001). As like Hsp70, IL-1β, and p43, another leaderless AK1 might be secreted from myotubes by a nonclassical pathway or an unknown mechanism.
Acknowledgments
This work was supported by grants awarded to Y.-G. Ko from the Korea Research Foundation (KRF-2006-C00407), KOSEF (R01-2004-000-10765-0), and the Top Brand Project of the Korea Basic Science Institute. This work was also partially supported by a Korea University Grant (to Y.-G. Ko).
Abbreviations
- AK1
adenylate kinase 1
- Ap5A
P1,P5-di(adenosine-5')penaphosphate
- exATP
extracellular ATP
References
- 1.Abbracchio MP, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Miras-Portugal MT, King BF, Gachet C, Jacobson KA, Weisman GA, Burnstock G. Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol Sci. 2003;24:52–55. doi: 10.1016/S0165-6147(02)00038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrei C, Dazzi C, Lotti L, Torrisi MR, Chimini G, Rubartelli A. The secretory route of the leaderless protein interleukin 1beta involves exocytosis of endolysosome-related vesicles. Mol Biol Cell. 1999;10:1463–1475. doi: 10.1091/mbc.10.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arakaki N, Nagao T, Niki R, Toyofuku A, Tanaka H, Kuramoto Y, Emoto Y, Shibata H, Magota K, Higuti T. Possible role of cell surface H+-ATP synthase in the extracellular ATP synthesis and proliferation of human umbilical vein endothelial cells. Mol Cancer Res. 2003;1:931–939. [PubMed] [Google Scholar]
- 4.Araya R, Riquelme MA, Brandan E, Saez JC. The formation of skeletal muscle myotubes requires functional membrane receptors activated by extracellular ATP. Brain Res Brain Res Rev. 2004;47:174–188. doi: 10.1016/j.brainresrev.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Bae TJ, Kim MS, Kim JW, Kim BW, Choo HJ, Lee JW, Kim KB, Lee CS, Kim JH, Chang SY, Kang CY, Lee SW, Ko YG. Lipid raft proteome reveals ATP synthase complex in the cell surface. Proteomics. 2004;4:3536–3548. doi: 10.1002/pmic.200400952. [DOI] [PubMed] [Google Scholar]
- 6.Bodin P, Burnstock G. Purinergic signalling: ATP release. Neurochem Res. 2001;26:959–969. doi: 10.1023/a:1012388618693. [DOI] [PubMed] [Google Scholar]
- 7.Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 8.Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- 9.Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev. 2006;58:58–86. doi: 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- 10.Burrell HE, Wlodarski B, Foster BJ, Buckley KA, Sharpe GR, Quayle JM, Simpson AW, Gallagher JA. Human keratinocytes release ATP and utilize three mechanisms for nucleotide interconversion at the cell surface. J Biol Chem. 2005;280:29667–29676. doi: 10.1074/jbc.M505381200. [DOI] [PubMed] [Google Scholar]
- 11.Buttigieg J, Nurse CA. Detection of hypoxia-evoked ATP release from chemoreceptor cells of the rat carotid body. Biochem Biophys Res Commun. 2004;322:82–87. doi: 10.1016/j.bbrc.2004.07.081. [DOI] [PubMed] [Google Scholar]
- 12.Carrasco AJ, Dzeja PP, Alekseev AE, Pucar D, Zingman LV, Abraham MR, Hodgson D, Bienengraeber M, Puceat M, Janssen E, Wieringa B, Terzic A. Adenylate kinase phosphotransfer communicates cellular energetic signals to ATP-sensitive potassium channels. Proc Natl Acad Sci USA. 2001;98:7623–7628. doi: 10.1073/pnas.121038198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collavin L, Lazarevic D, Utrera R, Marzinotto S, Monte M, Schneider C. wt p53 dependent expression of a membraneassociated isoform of adenylate kinase. Oncogene. 1999;18:5879–5888. doi: 10.1038/sj.onc.1202970. [DOI] [PubMed] [Google Scholar]
- 14.Donaldson SH, Picher M, Boucher RC. Secreted and cell-associated adenylate kinase and nucleoside diphosphokinase contribute to extracellular nucleotide metabolism on human airway surfaces. Am J Respir Cell Mol Biol. 2002;26:209–215. doi: 10.1165/ajrcmb.26.2.4650. [DOI] [PubMed] [Google Scholar]
- 15.Fabre AC, Vantourout P, Champagne E, Tercé F, Rolland C, Perret B, Collet X, Barbaras R, Martinez LO. Cell surface adenylate kinase activity regulates the F(1)-ATPase/P2Y (13)-mediated HDL endocytosis pathway on human hepatocytes. Cell Mol Life Sci. 2006;63:2829–2837. doi: 10.1007/s00018-006-6325-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon JL. Extracellular ATP: effects, sources and fate. Biochem J. 1986;233:309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005;436:108–111. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- 18.Ha H, Pak Y. Modulation of the caveolin-3 and Akt status in caveolae by insulin resistance in H9c2 cardiomyoblasts. Exp Mol Med. 2005;37:169–178. doi: 10.1038/emm.2005.23. [DOI] [PubMed] [Google Scholar]
- 19.Hancock CR, Janssen E, Terjung RL. Skeletal muscle contractile performance and ADP accumulation in adenylate kinase-deficient mice. Am J Physiol Cell Physiol. 2005;288:C1287–C1297. doi: 10.1152/ajpcell.00567.2004. [DOI] [PubMed] [Google Scholar]
- 20.Hubbard AL, Wall DA, Ma A. Isolation of rat hepatocyte plasma membranes. I. Presence of the three major domains. J Cell Biol. 1983;96:217–229. doi: 10.1083/jcb.96.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MA, Muskens F, Hoogsteden HC, Luttmann W, Ferrari D, Di Virgilio F, Virchow JC, Jr, Lambrecht BN. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med. 2007;13:913–919. doi: 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- 22.Janssen E, Kuiper J, Hodgson D, Zingman LV, Alekseev AE, Terzic A, Wieringa B. Two structurally distinct and spatially compartmentalized adenylate kinases are expressed from the AK1 gene in mouse brain. Mol Cell Biochem. 2004;256-257:59–72. doi: 10.1023/b:mcbi.0000009859.15267.db. [DOI] [PubMed] [Google Scholar]
- 23.Kim BW, Choo HJ, Lee JW, Kim JH, Ko YG. Extracellular ATP is generated by ATP synthase complex in adipocyte lipid rafts. Exp Mol Med. 2004;36:476–485. doi: 10.1038/emm.2004.60. [DOI] [PubMed] [Google Scholar]
- 24.Kim KB, Lee JW, Lee CS, Kim BW, Choo HJ, Jung SY, Chi SG, Yoon YS, Yoon G, Ko YG. Oxidation-reduction respiratory chains and ATP synthase complex are localizedin detergent-resistant lipid rafts. Proteomics. 2006;6:2444–2453. doi: 10.1002/pmic.200500574. [DOI] [PubMed] [Google Scholar]
- 25.Kim MS, Lee J, Ha J, Kim SS, Kong Y, Cho YH, Baik HH, Kang I. ATP stimulates glucose transport through activation of P2 purinergic receptors in C(2)C(12) skeletal muscle cells. Arch Biochem Biophys. 2002;401:205–214. doi: 10.1016/S0003-9861(02)00056-5. [DOI] [PubMed] [Google Scholar]
- 26.Ko YG, Park H, Kim T, Lee JW, Park SG, Seol W, Kim JE, Lee WH, Kim SH, Park JE, Kim S. A cofactor of tRNA synthetase, p43, is secreted to up-regulate proinflammatory genes. J Biol Chem. 2001;276:23028–23033. doi: 10.1074/jbc.M101544200. [DOI] [PubMed] [Google Scholar]
- 27.Kubo Y. Comparison of initial stages of muscle differentiation in rat and mouse myoblastic and mouse mesodermal stem cell lines. J Physiol. 1991;442:743–759. doi: 10.1113/jphysiol.1991.sp018817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X and P2Y-receptor activating molecules. Mol Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- 29.Mambula SS, Calderwood SK. Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J Immunol. 2006;177:7849–7857. doi: 10.4049/jimmunol.177.11.7849. [DOI] [PubMed] [Google Scholar]
- 30.Martinez LO, Jacquet S, Esteve JP, Rolland C, Cabezón E, Champagne E, Pineau T, Georgeaud V, Walker JE, Térce F, Collet X, Perret B, Barbaras R. Ectopic beta-chain of ATP synthase is an apolipoprotein A-I receptor in hepatic HDL endocytosis. Nature. 2003;421:75–79. doi: 10.1038/nature01250. [DOI] [PubMed] [Google Scholar]
- 31.Moser TL, Kenan DJ, Ashley TA, Roy JA, Goodman MD, Misra UK, Cheek DJ, Pizzo SV. Endothelial cell surface F1-F0 ATP synthase is active in ATP synthesis and is inhibited by angiostatin. Proc Natl Acad Sci USA. 2001;98:6656–6661. doi: 10.1073/pnas.131067798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Notari L, Morelli A, Pepe IM. Studies on adenylate kinase isoform bound to disk membranes of the rod outer segment of bovine retina. Photochem Photobiol Sci. 2003;2:1299–1302. doi: 10.1039/b306774m. [DOI] [PubMed] [Google Scholar]
- 33.Picher M, Boucher RC. Human airway ecto-adenylate kinase. A mechanism to propagate ATP signaling on airway surfaces. J Biol Chem. 2003;278:11256–11264. doi: 10.1074/jbc.M208071200. [DOI] [PubMed] [Google Scholar]
- 34.Quillen EE, Haslam GC, Samra HS, Amani-Taleshi D, Knight JA, Wyatt DE, Bishop SC, Colvert KK, Richter ML, Kitos PA. Ectoadenylate kinase and plasma membrane ATP synthase activities of human vascular endothelial cells. J Biol Chem. 2006;281:20728–20737. doi: 10.1074/jbc.M513042200. [DOI] [PubMed] [Google Scholar]
- 35.Ryten M, Dunn PM, Neary JT, Burnstock G. ATP regulates the differentiation of mammalian skeletal muscle by activation of a P2X5 receptor on satellite cells. J Cell Biol. 2002;158:345–355. doi: 10.1083/jcb.200202025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandonà D, Danieli-Betto D, Germinario E, Biral D, Martinello T, Lioy A, Tarricone E, Gastaldello S, Betto R. The T-tubule membrane ATP-operated P2X4 receptor influences contractility of skeletal muscle. Faseb J. 2005;19:1184–1186. doi: 10.1096/fj.04-3333fje. [DOI] [PubMed] [Google Scholar]
- 37.Tanabe T, Yamada M, Noma T, Kajii T, Nakazawa A. Tissuespecific and developmentally regulated expression of the genes encoding adenylate kinase isozymes.(Tokyo) J Biochem. 1993;113:200–207. doi: 10.1093/oxfordjournals.jbchem.a124026. [DOI] [PubMed] [Google Scholar]
- 38.Van Rompay AR, Johansson M, Karlsson A. Phosphorylation of nucleosides and nucleoside analogs by mammalian nucleoside monophosphate kinases. Pharmacol Ther. 2000;87:189–198. doi: 10.1016/s0163-7258(00)00048-6. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto K, Sokabe T, Ohura N, Nakatsuka H, Kamiya A, Ando J. ndogenously released ATP mediates shear stress-induced Ca2+ influx into pulmonary artery endothelial cells. Am J Physiol Heart Circ Physiol. 2003;285:H793–H803. doi: 10.1152/ajpheart.01155.2002. [DOI] [PubMed] [Google Scholar]
- 40.Yegutkin GG, Henttinen T, Jalkanen S. Extracellular ATP formation on vascular endothelial cells is mediated by ecto-nucleotide kinase activities via phosphotransfer reactions. Faseb J. 2001;15:251–260. doi: 10.1096/fj.00-0268com. [DOI] [PubMed] [Google Scholar]
- 41.Zeleznikar RJ, Heyman RA, Graeff RM, Walseth TF, Dawis SM, Butz EA, Goldberg ND. Evidence for compartmentalized adenylate kinase catalysis serving a high energy phosphoryl transfer function in rat skeletal muscle. J Biol Chem. 1990;265:300–311. [PubMed] [Google Scholar]