Abstract
Previous studies have demonstrated that rottlerin, a specific PKCδ inhibitor, potentiates death receptormediated apoptosis through a cytochrome c-dependent or -independent pathway. However, its ability to regulate necrotic cell death, as well as the underlying mechanism, remains unknown. We found that in murine fibrosarcoma L929 cells, treatment with rottlerin protected the cells against TNF-induced necrosis, whereas it sensitized the cells to apoptosis induced by co-treatment with Hsp90 inhibitor geldanamycin and TNF, in a manner independent of its ability to inhibit PKC-δ. TNF treatment induced rapid accumulation of mitochondrial superoxide (O2-) through the Nox1 NADPH oxidase when cells undergo necrosis. Moreover, pretreatment with rottlerin failed to induce the GTP-bound form of small GTPase Rac1 by TNF treatment, and subsequently suppressed mitochondrial O2- production and poly(ADP-ribose) polymerase activation, thus inhibiting necrotic cell death. Therefore, our study suggests that Nox1 NADPH oxidase is a new molecular target for anti-necrotic activity of rottlerin upon death-receptor ligation.
Keywords: cell death, necrosis, NADPH oxidase 1, rottlerin, superoxidase, tumor necrosis factor-α
Introduction
TNF is a pleiotropic cytokine that mediates diverse biological responses ranging from inflammation to cell death (Fas et al., 2006). Although the mode of cell death induced by TNF appears to be apoptotic in most cellular systems, induction of necrosis, of the type characterized as necrotic programmed cell death, has also been observed in some types of cells, with or without signaling molecules, including caspases (Fiers et al., 1999). This indicates that, depending on the cellular context, the same stimulus can induce either apoptosis or necrosis. Recent findings have suggested that necrotic cell death occurs not only during pathological processes, but also during normal physiological processes such as tissue renewal, embryogenesis and immune responses (Mayhew et al., 1999; Murdoch et al., 1999). Importantly, despite numerous efforts, there has been little progress in increasing the efficiency of anticancer therapy by induction of apoptosis in tumor cells. The rate of apoptosis shows little correlation with suppression of clonogenic ability of cancer cells, which results in tumor recurrence. Some chemotherapeutic agents and ionizing radiation (IR) used in cancer therapy, along with apoptosis, can cause necrosis (Maurer et al., 1999; Olive et al., 1999). Therefore, further elucidation of pathways towards necrosis may not only uncover the fundamental mechanism of intracellular death signaling, but also provide a prospective strategy to increase efficiency of anticancer therapy.
Rottlerin has been used as a PKCδ-selective inhibitor in many different cellular systems. Subsequently, the alteration of biological events by rottlerin has been interpreted as a positive indication for the involvement of PKCδ in these events (Gschwendt et al., 1994). Several recent studies however, have implied that the effect of rottlerin is not caused by the direct inhibition of PKCδ. In one of these studies, rottlerin was reported to inhibit mitochondrial metabolism, which correlated with depletion of cellular ATP concentration and general inhibition of cellular processes (Soltoff, 2001). Of note, these recent observations have implicated the apoptosis-inducing effect of rottlerin on tumor cells, and synergistic sensitization of the cytotoxic effects of chemotherapeutic and apoptotic ligands (Tillman et al., 2003; Kurosu et al., 2007).
Although accumulating evidence suggests that rottlerin plays a PKCδ-independent role in modulating apoptotic cell death signaling, its effect or underlying mechanism in necrotic cell death has never been investigated. In this study, we showed that rottlerin effectively protected murine fibrosarcoma L929 cells against TNF-induced necrosis, whereas it sensitized the cells to apoptosis induced by co-treatment with Hsp90 inhibitor geldanamycin (GA) and TNF. It is of importance that rottlerin also inhibited mitochondrial superoxide (O2-) production, through suppressing Nox1 NADPH oxidase activity which led to the inhibition of TNF-induced necrosis.
Materials and Methods
Cell culture and infection of recombinant PKC adenovirus
Murine fibrosarcoma L929 cells and mouse embryonic fibroblast (MEF) cells were cultured in DMEM with 10% FBS, 2 mM glutamine, 100 U/ml penicillin/streptomycin. Adenovirus expression vectors for wild type and the dominant-negative type of PKCδ have been described previously (Ohba et al., 1998).
Determination of cell death
After cells were treated with the concentrations of reagents as described in the figure legends, cell death was quantified by trypan blue exclusion assay. For measurement of early apoptotic or necrotic/late apoptotic cell death, cells were stained with 10 µM FITC-labeled annexin V and PI in a Ca2+-enriched buffer (10 mM HEPES, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2), and analyzed by flow cytometry. Annexin V and PI emissions were detected in the FL1 and FL2 channels of a FACSCalibur flow cytometer, using emission filters of 488 and 532 nm, respectively.
Western blot analysis
Upon treatment, cells were lysed in M2 buffer (20 mM Tris, pH 7.6, 0.5% NP-40, 250 mM NaCl, 3 mM EDTA, 3 mM EGTA, 2 mM DTT, 0.5 mM PMSF, 20 mM β-glycerol phosphate, 1 mM sodium vanadate, 1 µg/ml leupeptin). For Western blotting, 50 µg protein was visualized by enhanced chemiluminescence (ECL; Amersham).
Determination of mitochondria-derived O2- production
Production of mitochondria-derived O2- was measured using the O2--sensitive, hydroethidine analog, mitochondria-targeted probe, MitoSOX Red (Molecular Probes). Briefly, cells were incubated with MitoSOX Red (5 µM) for 20 min in KH buffer (pH 7.3, 15 mM NaHCO3, 5 mM KCl, 120 mM NaCl, 0.7 mM Na2HPO4/NaH2PO4), and the red fluorescence of ox-MitoSOX was imaged using an inverted laser scanning confocal microscope (LSM-510 META; Carl Zeiss) at λex 543 nm and λem 580 nm.
Cellular fractionation and NADPH oxidase activity
Upon treatment, cells were lysed in isotonic buffer (10 mM HEPES/KOH, pH 7.4, 250 mM sucrose, 1 mM EDTA, 1 mM DTT, 1 mM PMSF), and subjected to centrifugation at 3,500 g for 5 min to obtain a post-nuclear supernatant. The mitochondrial and plasma membrane fractions were obtained by centrifugation at 19,500 g for 40 min and 100,000 g for 60 min, respectively. The extracts from fractionated samples were subjected to NADPH oxidase activity, as described (Kim et al., 2007b).
Rac1 activation assay
The endogenous GTP-bound form of Rac1 was measured by Rac/CDC42 assay kit (Upstate Biotechnology), according to the manufacturer's instructions. Briefly, cells were harvested using 500 µl of lysis buffer (25 mM HEPES, pH 7.5, 150 mM NaCl, 1% NP-40, 10 mM MgCl2, 1 mM EDTA, 10% glycerol, 1 mM PMSF, 20 µg/ml leuptin). Cell lysates were centrifuged for 5 min at 3,000 × g, and the resulting supernatant were mixed with 100 µM of GTPγS and 10 µg of GST-PAK1-(67-150) conjugated to glutathione-agarose beads at 4℃ for 1 h. Beads were washed four times with lysis buffer and subjected to Western blotting. Rac1-GTP was visualized using an anti-Rac1 monoclonal antibody supplied with the kit (Upstate Biotechnology).
Statistical analysis
Data are expressed as the mean ± SE from at least three separate experiments performed in triplicate. Differences between groups were analyzed using Student's t test, and P < 0.05 was considered statistically significant, by using SPSS software (ver. 11.0).
Results and Discussion
Rottlerin protects murine fibrosarcoma L929 cells against TNF-induced necrotic cell death in a PKCδ-independent manner
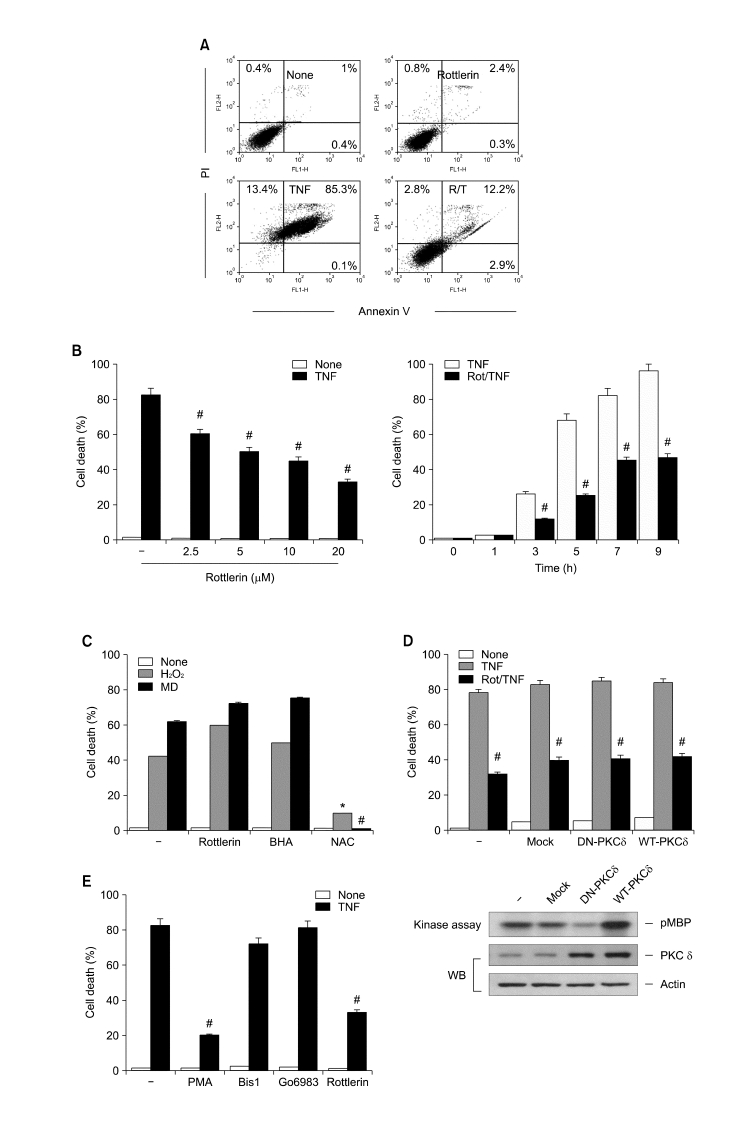
To explore the effect of rottlerin on TNF-induced necrosis, we decided to use murine fibrosarcoma L929 cells because of their known sensitivity to the necrotic action of TNF (Hehner et al., 1998). Therefore, they have been widely used to investigate the mechanism of necrotic signaling. First, we tested whether TNF induced non-apoptotic cell death. As expected, treatment of cells with TNF increased the number of necrotic and late-phase apoptotic cells, whereas very few cells were stained exclusively with annexin V (Figure 1A). We next investigated the effect of rottlerin on TNFinduced necrosis. Pretreatment of L929 cells with rottlerin drastically abrogated TNF-induced necrosis in a dose- and time-dependent manner, as measured by flow cytometry and trypan blue exclusion assays (Figure 1A and B). Based on the ability of rottlerin to protect against TNF-induced necrosis, we evaluated whether it had a protective role against cell death caused by reactive oxygen species (ROS), such as H2O2 and menadione, which is caspase-independent (Sata et al., 1997; Troyano et al., 2003). Interestingly, pretreatment with rottelrin did not block necrosis, whereas the antioxidant N-acetlycystein clearly protected cells against cell death caused by H2O2 and menadione (Figure 1C). More importantly, pretreatment with butylated hydroxyanisole (BHA) a well known ROS scavenger that can enter mitochondria and inhibit TNF-induced necrosis (Vercammen et al., 1998), also did not block these exogenous sources of ROS-induced cell death (Figure 1C). These results suggest that, similar to BHA, rottlerin negatively and specifically regulates necrosis through the suppression of newly generated ROS in the mitochondria, upon death-receptor ligation.
Figure 1.
Rottlerin protects murine fibrosarcoma L929 cells against TNF-induced necrotic cell death in a PKC-δ-independent manner. (A) L929 cells were treated with 15 ng/ml TNF for 8 h in the presence or absence of 10 µM rottlerin, stained with FITC-labeled annexin V and PI, and analyzed by flow cytometry. (B) L929 cells were pretreated with rottlerin at the indicated concentrations (left) and times (right), and further treated with TNF for 8 h. Cell death was quantified by trypan blue exclusion assay. Each bar shows the mean ± SE of at least three independent experiments. #P < 0.05 compared with TNF-treated group. (C) After pretreatment with 10 µM rottlerin, 100 µM BHA and 10 mM NAC, L929 cells were treated with 500 µM H2O2 or 10 µM menadione (MD) for 12 h, and cell death was quantified as described in (B). *P < 0.05 compared with the H2O2-treated group. #P < 0.05 compared with the MD-treated group. (D) L929 cells were infected with wild type or dominant-negative type of PKC-δ adenovirus at an m.o.i. of 100. After 48 h incubation, cells were treated with TNF (15 ng/ml) for 8 h in the presence or absence of 10 µM rottlerin, and cell death was quantified as described in B. #P < 0.05 compared with the TNF-treated group. PKCδ activities in L929 cells infected with DN-PKCδ AdV or WT-PKCδ AdV were analyzed by immune complex kinase assay with [γ-32P] ATP and MBP as a substrate. Phosphorylation of MBP was assessed by SDS-PAGE and autoradiography. PKCδ or actin protein content was analyzed by Western blotting with anti-PKCδ or actin antibody. (E) After pretreatment with 50 nM PMA, 10 µM Bis1, 10 µM Go6983 or 10 µM rottlerin, L929 cells were treated with TNF (15 ng/ml) for 8 h, and cell death was quantified as described in (B). #P < 0.05 compared with the TNF-treated group.
Results of recent studies have demonstrated that rottlerin sensitizes some cancer cells to death-receptor- or chemotherapeutic-agent-induced apoptosis in a manner independent of PKCδ activity (Tillman et al., 2003; Kurosu et al., 2007). To examine the possible involvement of PKCδ in the prevention of TNF-induced necrosis by rottlerin, we introduced adenovirus expressing wild-type (WT) and dominant-negative (DN) forms of PKCδ into L929 cells. WT-PKCδ and DN-PKCδ expression activated and inhibited PKCδ kinase activity, respectively, as measured by an in vitro kinase assay using myelin basic protein as a substrate (Figure 1D, lower panel). However, the overexpression of WT-PKCδ or DN-PKCδ did not influence the protective effect of rottlerin against TNF-induced necrosis (Figure 1D, upper panel). To further rule out the role of PKCδ in modulating sensitivity to TNF-induced necrosis, we investigated whether activating PKCδ with PMA restored the necrotic effect of TNF. However, pre-treating L929 cells with PMA before TNF exposure significantly abrogated necrosis. Moreover, pre-treatment with either bis-indolylmaleimide I or Go6983, both of which are general PKC inhibitors, also did not influence TNF-induced necrosis (Figure 1E). Thus, these results suggest that the anti-necrotic effect of rottlerin upon TNF treatment is not due to a specific PKCδ blockade.
Rottlerin sensitizes murine fibrosarcoma L929 cells against TNF plus GA-induced apoptotic cell death
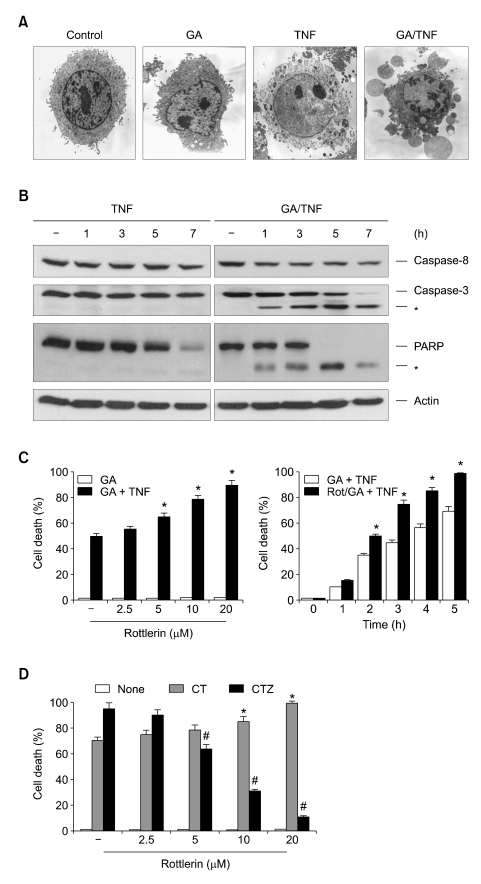
The above results clearly show the prevention of TNF-induced necrosis by rottlerin in L929 cells, even though several reports have demonstrated that rottlerin induces apoptosis (Tillman et al., 2003; Kurosu et al., 2007). This suggests that rottlerin may have an opposing regulatory role between apoptotic and necrotic modes of cell death upon death-receptor ligation. To address this issue in the same cell lines, we used the TNF plus geldanamycin (GA)-induced apoptotic model, since TNFR1-mediated necrosis can be reverted to apoptosis when cells are pretreated with an Hsp90 inhibitor (Vanden Berghe et al., 2003). As expected, cell death induced by TNF after pretreatment with GA was morphologically distinct from necrosis, and exhibited apoptotic features such as membrane blebbing, shrinkage of the cytoplasm and chromatin condensation (Figure 2A). This GA-induced shift from necrosis to apoptosis after TNF treatment was confirmed by the activation of caspases and the proteolytic cleavage of poly (ADP-ribose) polymerase (PARP), a typical caspase-3 substrate (Figure 2B, right panel). In contrast, none of these typically apoptotic events were detected in TNF-induced necrosis in the absence of GA (Figure 2B, left panel). Therefore, it is a useful model for investigating the pathway of necrosis and apoptosis upon cell death induced by TNF, alone and in combination with GA. Of interest, in contrast to its anti-necrotic effect, pretreatment with rottlerin sensitized the cells to apoptosis induced by TNF plus GA, and this sensitizing effect was observed 2 h after co-treatment with TNF and GA (Figure 2C). These results indicate that rottlerin has an opposing role between apoptotic and necrotic modes of TNF-induced cell death. To confirm these observations, we further examined the effect of rottlerin on the apoptotic or necrotic cell death by using a model of MEFs, since apoptotic or necrotic cell death in this MEF cells can be efficiently triggered in the absence or presence of Z-VAD-fmk, a membrane permeable pan-caspase inhibitor, followed by treatment of cells with TNF and cycloheximide (CHX) (Lin et al., 2004; Byun et al., 2006). Pre-treating MEF cells with rottlerin sensitized the cells to apoptosis induced by TNF/CHX, whereas it protected the cells against necrotic cell death induced by treatment with TNF/CHX under the caspase-inhibited condition (Figure 2D), indicating that the effect of rottlerin on TNF-induced necrotic cell death is not specific for L929 cells.
Figure 2.
Rottlerin sensitizes murine fibrosarcoma L929 cells against GA plus TNF-induced apoptosis. (A) L929 cells were pretreated with GA (1 µM) for 12 h, followed by treatment with TNF (15 ng/ml) for 5 h. Cells were immediately photographed under electron microscopy. (B) L929 cells were pretreated with or without GA (1 µM) for 12 h, followed by treatment with TNF for various times. Cell extracts were analyzed by SDS-PAGE and Western blotting with antibodies against caspase-8, caspase-3, PARP and actin. (C) L929 cells were pretreated with rottlerin at the indicated concentrations (left) and times (right), and further treated with GA (12 h) plus TNF (5 h). Cell death was quantified as described in Figure 1B. *P < 0.05 compared with the GA-plus-TNF-treated group. (D) Mouse embryonic fibroblast (MEF) cells were pretreated with 50 µM z-VAD-FMK for 30 min and then treated with 15 ng/ml TNF or 15 ng/ml TNF plus 10 µg/ml CHX in the absence of presence of rottlerin for various concentrations as indicated. Cell death was quantified as described in Figure 1B. Each bar shows mean ± SE of at least three independent experiments. *P < 0.05, when compared with CHX/TNF-treated group. #P < 0.05, when compared with TNF/CHX/z-VAD-FMK-treated group.
Rottlerin suppresses mitochondrial O2- production induced by TNF and subsequently blocks PARP activation
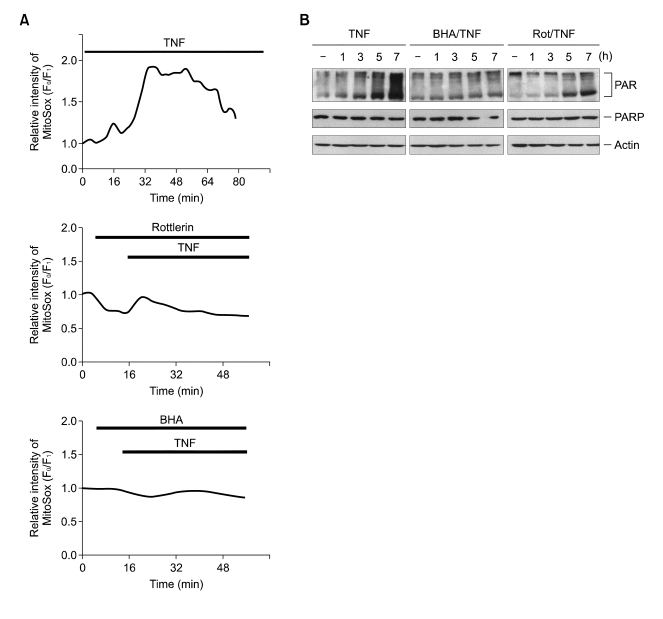
Caspase-independent necrotic cell death has been proposed to produce ROS which suggests mitochondrial involvement (Garg and Aggarwal, 2002). Given that the first oxygen reduction product generated in mitochondria is O2-, which can be converted to H2O2 (Hennet et al., 1993), we examined whether rottlerin interfered with TNFinduced mitochondrial O2- production by using a superoxide-sensitive mitochondria-targeted hydroethidine analog, MitoSOX Red (Robinson et al., 2006). MitoSOX Red oxidation, which indicated an increase in mitochondrial O2- production, was observed immediately after treatment with TNF, and reached a maximum after 30 min (Figure 3A, upper panel). Importantly, the TNF-induced MitoSOX Red oxidation signal was abolished in the presence of rottlerin or BHA (Figure 3A, middle and bottom panel). This indicates that the protective function of rottlerin against TNF-induced necrosis is derived from its ability to inhibit O2- production at the mitochondrial level. Previously, we have demonstrated that the ROS-dependent activation of PARP plays a role in TNF-induced necrosis (Byun et al., 2006). Overactivation of PARP after cellular injury consumes large amounts of NAD, which may cause massive ATP depletion in the effort to resynthesize NAD, and thus shift the mode of cell death toward necrosis (Los et al., 2002). Therefore, if rottlerin is able to protect against cell death via the inhibition of mitochondrial O2- production, then it should also impair PARP activation. Accordingly, immunoblotting with anti-PAR antibody, specific for poly (ADP-ribose) polymers, showed that treating L929 cells with TNF caused an increase in poly(ADP-ribosyl)-ation after 3 h, after which the level continued to gradually increase (Figure 3B, top panel). Similar to BHA, pre-treatment with rottlerin significantly inhibited these TNF-induced effects, which is consistent with our observation that rottlerin inhibited mitochondrial O2- production after TNF treatment.
Figure 3.
Rottlerin suppresses mitochondrial O2- production induced by TNF, and subsequently block PARP activation. (A) L929 cells were treated with TNF (15 ng/ml) for various times as indicated in the presence or absence of rottlerin (10 µM) and BHA (100 µM). Mitochondrial O2- production was determined using superoxide-sensitive MitoSOX Red. (B) L929 cells were treated with TNF in the presence or absence of 100 µM BHA and 10 µM rottlerin. Cell extracts were analyzed by SDS-PAGE and Western blotting with antibodies against PAR, PARP and actin.
Rottlerin suppresses TNF-induced Nox1 activation in murine fibrosarcoma L929 cells
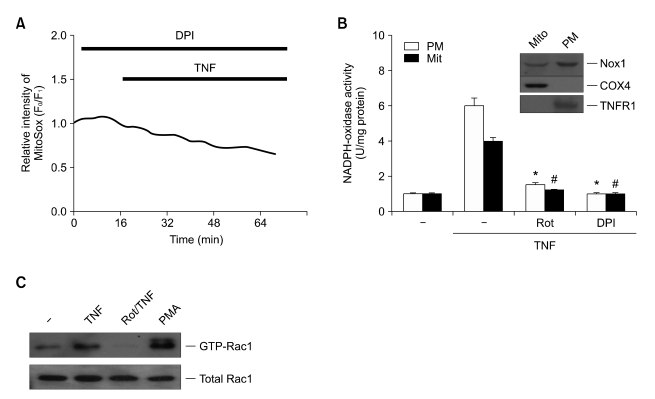
The data from the above study provide evidence that the protective mechanism of rottlerin against TNF-induced necrosis involves decreased mitochondrial O2- production. Another critical issue remaining to be further investigated is the molecular targeting of rottlerin to inhibit mitochondrial O2- production. The Nox family of NADPH oxidase has been implicated as a major source of ROS (Lambeth, 2004; Kim et al., 2005, 2007). To test whether the ROS generated by NADPH oxidase play a role, we pretreated L929 cells with diphenylene iodonium (DPI), a well-established NADPH oxidase inhibitor in the presence of TNF, and measured mitochondrial O2- production. Pretreatment with DPI completely abrogated mitochondrial O2- production in TNF-exposed L929 cells (Figure 4A), which suggests that activation of NADPH oxidase by TNF treatment plays a role in mitochondrial O2- production. More importantly, a recent study has indicated that Nox1 is the major NADPH oxidase responsible for TNF-induced O2- production in non-phagocytic cells (Kim et al., 2007b). Similarly, we were able to detect expression of Nox1, but not Nox2 by Western blotting of L929 cells (data not shown). Thus, Nox1 may be the most likely source of NADPH oxidase activity under conditions of TNF-induced necrosis in L929 cells.
Figure 4.
Mitochondrial Nox1 NADPH oxidase is expressed in murine fibrosarcoma L929 cells, and rottlerin inhibits Nox1 activity through down-regulation of GTP-bound Rac1. (A) L929 cells were treated with TNF for various times in the presence of 10 µM DPI, and mitochondrial O2- production was determined as described for Figure 3A. (B) Mitochondrial and plasma membrane fractions of L929 cells were obtained, and lysates were analyzed by SDS-PAGE, followed by Western blotting with antibodies against Nox1, COX-4 and TNFR1 (upper panel). After treatment with TNF in the presence of rottlerin (10 µM), BHA (100 µM) and DPI (10 µM), NADPH oxidase assays were conducted. (C) L929 cells were transfected with the Xp-tagged Nox1, NOXO1 and NOXA1 expression plasmids, and treated with PMA (50 nM) and/or TNF (15 ng/ml) for 30 min, in the presence or absence of rottlerin (10 µM). The PAK1 pull-down complex was analyzed by SDS-PAGE and Western blotting with antibodies against Rac1.
Based on the ability of rottlerin to suppress TNF-induced mitochondrial O2- production, it is important to know, for instance, whether Nox1 is expressed in the mitochondria of L929 cells and if so, does rottlerin affect its activity. To address these issues, we used cell fractionation experiments. As expected, the expression of COX-4 unlike that of TNFR1, occurred principally in the mitochondrial fraction, whereas no signal was detected in the plasma membrane fraction (Figure 4B, top panel). In the same lysates, we found that expression of Nox1 occurred in both the mitochondrial and plasma membrane fractions. Furthermore, we also observed that the NADPH oxidase activity in the mitochondrial fraction was increased significantly by TNF treatment, compared to similar activity in the plasma membrane fraction (Figure 4B, bottom panel). This suggests that both mitochondrial and plasma membrane Nox1 are involved in ROS production under necrotic conditions. Notably, treatment with rottlerin, and to a similar extent DPI, which has been shown to suppress mitochondrial O2- production (Figure 3A, middle panel), completely inhibited TNF-induced NADPH oxidase activation (Figure 4B, bottom panel). These results suggest that the Nox1 activity correlated well with O2- production, as well as necrosis, and that rottlerin inhibits mitochondrial ROS production through Nox1, an important factor driving cells toward necrotic death. Although recent study has demonstrated that Nox1 at the level of receptor signaling complex is involved in TNF-induced necrotic cell death (Kim et al., 2007b), the mitochondrial involvement in this process has not yet excluded. Recent results including ours showed that BHA efficiently suppressed not only mitochondrial O2- production (Figure 3A) but also necrosis (Lin et al., 2004; Byun et al., 2006; Kim et al., 2007b) upon TNF treatment. Therefore, we speculated that the mitochondrial Nox1 is also responsible for ROS production and necrosis by TNF. This conjecture was further supported by the findings of both the potency of DPI to block the mitochondrial O2- production, and Nox1 expression in the mitochondrial fraction of L929 cells. However, treatment with rottlerin suppresses the Nox1 activities from both mitochondrial and plasma membrane fractions (Figure 4B), suggesting that rottlerin functions in interfering the Nox1 activities from both sources. Therefore, further studies will be required to elucidate the involvement of exact cellular compartments in Nox1 activation during the necrotic conditions.
Although we have established that the Nox1 is a critical target for anti-necrotic effect of rottlerin in this study, it is still unclear how rottlerin functions to suppress Nox1 activity. Recent reports have indicated that activation of Rac1 by GTP loading functions as a major trigger for activating the Nox1 system (Cheng et al., 2006; Miyano et al., 2006). The Rac1-GTP directly binds to regulatory protein NOXA1 (Nox activator 1) and thus facilitates membrane recruitment of this protein in the presence of NOXO1 (Nox organizer1) (Banfi et al., 2003; Takeya et al., 2003). Therefore, the GTP-bound form of the small GTPase Rac1 plays a crucial role in Nox1 activation. We next examined the mechanism whereby rottlerin affect the content of endogenous Rac1-GTP, which is required for the formation of active Nox1 complex. Since optimal reconstitution of Nox1 activity requires the co-expression of the NOXO1 and NOXA1 (Ueyama et al., 2006), a pull-down assay was performed using the Rac1-binding domain of protein kinase PAK in Nox1, NOXO1 and NOXA1 coexpressed cells. This binding domain interacts exclusively with the GTP form of Rac1 and CDC42. Consistent with previous data, Rac1 activation assay detected the basal activity of endogenous Rac1 (GTP bound) in unstimulated cells after these were simultaneously transfected with the plasmids of Nox1 and its regulatory subunits, and the treatment of TNF increased the level of activated endogenous Rac1 (Figure 4C). Notably, rottlerin pretreatment failed to induce the GTP-bound form of Rac1 in extracts from TNF-treated cells, despite the fact that comparable amounts of total Rac1 were expressed in each sample. As a control, PMA increased the content of GTP-bound Rac1. Thus these data suggest that rottlerin suppresses the Nox1 activity through affecting the endogenous level of Rac1-GTP in L929 cells. However it is still uncertain whether Rac1-mediated Nox1 activation is occurred in mitochondria and/or plasma membrane. Future studies using isolated components will be able to address this issue properly.
Taken together, our results indicate that ROS generated through activation of Nox1 plays a critical role in TNF-induced necrotic cell death. In addition, rottlerin inhibits Nox1 activity through the down-regulation of GTP-bound Rac1, and subsequent suppression of mitochondrial O2- production, therefore inhibiting necrotic cell death after ligation of death receptors such as TNFR.
Acknowledgments
This work was supported by the Korea Science and Engineering Foundation through the Infection Signaling Network Research Center (R13-2007-020-01002-0) at Chungnam National University, and a grant R01-2005-000-10240-0 from the Basic Research Program of the Korea Science & Engineering Foundation.
Abbreviations
- BHA
butylated hydroxyanisole
- DN
dominant negative
- DPI
diphenylene iodomium
- GA
geldanamycin
- IR
ionizing radiation
- O2-
superoxide
- PARP
poly(ADP-ribose) polymerase
- ROS
reactive oxygen species
- WT
wild type
References
- 1.Bánfi B, Clark RA, Steger K, Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem. 2003;278:3510–3513. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- 2.Byun HS, Park KA, Won M, Yang KJ, Shin S, Piao L, Kwak JY, Lee ZW, Park J, Seok JH, Liu ZG, Hur GM. Phorbol 12-myristate 13-acetate protects against tumor necrosis factor (TNF)-induced necrotic cell death by modulating the recruitment of TNF receptor 1-associated death domain and receptor-interacting protein into the TNF receptor 1 signaling complex: Implication for the regulatory role of protein kinase C. Mol Pharmacol. 2006;70:1099–1108. doi: 10.1124/mol.106.025452. [DOI] [PubMed] [Google Scholar]
- 3.Cheng G, Diebold BA, Hughes Y, Lambeth JD. Nox1-dependent reactive oxygen generation is regulated by Rac1. J Biol Chem. 2006;281:17718–17726. doi: 10.1074/jbc.M512751200. [DOI] [PubMed] [Google Scholar]
- 4.Fas SC, Fritzsching B, Suri-Payer E, Krammer PH. Death receptor signaling and its function in the immune system. Curr Dir Autoimmun. 2006;9:1–17. doi: 10.1159/000090767. [DOI] [PubMed] [Google Scholar]
- 5.Fiers W, Beyaert R, Declercq W, Vandenabeele P. More than one way to die: apoptosis, necrosis and reactive oxygen damage. Oncogene. 1999;18:7719–7730. doi: 10.1038/sj.onc.1203249. [DOI] [PubMed] [Google Scholar]
- 6.Garg AK, Aggarwal BB. Reactive oxygen intermediates in TNF signaling. Mol Immunol. 2002;39:509–517. doi: 10.1016/s0161-5890(02)00207-9. [DOI] [PubMed] [Google Scholar]
- 7.Gschwendt M, Müller HJ, Kielbassa K, Zang R, Kittstein W, Rincke G, Marks F. Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Commun. 1994;199:93–98. doi: 10.1006/bbrc.1994.1199. [DOI] [PubMed] [Google Scholar]
- 8.Hehner SP, Hofmann TG, Ratter F, Dumont A, Droge W, Schmitz ML. Tumor necrosis factor-alpha-induced cell killing and activation of transcription factor NF-kappaB are uncoupled in L929 cells. J Biol Chem. 1998;273:18117–18121. doi: 10.1074/jbc.273.29.18117. [DOI] [PubMed] [Google Scholar]
- 9.Hennet T, Richter C, Peterhans E. Tumour necrosis factor-alpha induces superoxide anion generation in mitochondria of L929 cells. Biochem J. 1993;289:587–592. doi: 10.1042/bj2890587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JS, Kim JG, Jeon CY, Won HY, Moon MY, Seo JY, Kim JI, Kim J, Lee JY, Choi SY, Park J, Yoon Park JH, Ha KS, Kim PH, Park JB. Downstream components of RhoA required for signal pathway of superoxide formation during phagocytosis of serum opsonized zymosans in macrophages. Exp Mol Med. 2005;37:575–587. doi: 10.1038/emm.2005.71. [DOI] [PubMed] [Google Scholar]
- 11.Kim SY, Moon JH, Lee HG, Kim SU, Lee YB. ATP released from beta-amyloid-stimulated microglia induces reactive oxygen species production in an autocrine fashion. Exp Mol Med. 2007a;39:820–827. doi: 10.1038/emm.2007.89. [DOI] [PubMed] [Google Scholar]
- 12.Kim YS, Morgan MJ, Choksi S, Liu ZG. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol Cell. 2007b;26:675–687. doi: 10.1016/j.molcel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 13.Kurosu T, Tsuji K, Kida A, Koyama T, Yamamoto M, Miura O. Rottlerin synergistically enhances imatinib-induced apoptosis of BCR/ABL-expressing cells through its mitochondrial uncoupling effect independent of protein kinase C-delta. Oncogene. 2007;26:2975–2987. doi: 10.1038/sj.onc.1210117. [DOI] [PubMed] [Google Scholar]
- 14.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 15.Lin Y, Choksi S, Shen HM, Yang QF, Hur GM, Kim YS, Tran JH, Nedospasov SA, Liu ZG. Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. J Biol Chem. 2004;279:10822–10828. doi: 10.1074/jbc.M313141200. [DOI] [PubMed] [Google Scholar]
- 16.Los M, Mozoluk M, Ferrari D, Stepczynska A, Stroh C, Renz A, Herceg Z, Wang ZQ, Schulze-Osthoff K. Activation and caspase-mediated inhibition of PARP: a molecular switch between fibroblast necrosis and apoptosis in death receptor signaling. Mol Biol Cell. 2002;13:978–988. doi: 10.1091/mbc.01-05-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maurer BJ, Metelitsa LS, Seeger RC, Cabot MC, Reynolds CP. Increase of ceramide and induction of mixed apoptosis/ necrosis by N-(4-hydroxyphenyl)-retinamide in neuroblastoma cell lines. J Natl Cancer Inst. 1999;91:1138–1146. doi: 10.1093/jnci/91.13.1138. [DOI] [PubMed] [Google Scholar]
- 18.Mayhew TM, Myklebust R, Whybrow A, Jenkins R. Epithelial integrity, cell death and cell loss in mammalian small intestine. Histol Histopathol. 1999;14:257–267. doi: 10.14670/HH-14.257. [DOI] [PubMed] [Google Scholar]
- 19.Miyano K, Ueno N, Takeya R, Sumimoto H. Direct involvementof the small GTPase Rac in activation of the superoxide-producing NADPH oxidase Nox1. J Biol Chem. 2006;281:21857–21868. doi: 10.1074/jbc.M513665200. [DOI] [PubMed] [Google Scholar]
- 20.Murdoch WJ, Wilken C, Young DA. Sequence of apoptosis and inflammatory necrosis within the formative ovulatory site of sheep follicles. J Reprod Fertil. 1999;117:325–329. doi: 10.1530/jrf.0.1170325. [DOI] [PubMed] [Google Scholar]
- 21.Ohba M, Ishino K, Kashiwagi M, Kawabe S, Chida K, Huh NH, Kuroki Y. Induction of differentiation in normal human keratinocytes by adenovirus-mediated introduction of the eta and delta isoforms of protein kinase C. Mol Cell Biol. 1998;18:5199–5207. doi: 10.1128/mcb.18.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olive PL, Vikse CM, Vanderbyl S. Increase in the fraction of necrotic, not apoptotic, cells in SiHa xenograft tumours shortly after irradiation. Radiother Oncol. 1999;50:113–119. doi: 10.1016/s0167-8140(98)00104-2. [DOI] [PubMed] [Google Scholar]
- 23.Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM, Murphy MP, Beckman JS. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci USA. 2006;103:15038–15043. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sata N, Klonowski-Stumpe H, Han B, Haussinger D, Niederau C. Menadione induces both necrosis and apoptosis in rat pancreatic acinar AR4-2J cells. Free Radic Biol Med. 1997;23:844–850. doi: 10.1016/s0891-5849(97)00064-6. [DOI] [PubMed] [Google Scholar]
- 25.Soltoff SP. Rottlerin is a mitochondrial uncoupler that decreases cellular ATP levels and indirectly blocks protein kinase C delta tyrosine phosphorylation. J Biol Chem. 2001;276:37986–37992. doi: 10.1074/jbc.M105073200. [DOI] [PubMed] [Google Scholar]
- 26.Takeya R, Ueno N, Kami K, Taura M, Kohjima M, Izaki T, Nunoi H, Sumimoto H. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J Biol Chem. 2003;278:25234–25246. doi: 10.1074/jbc.M212856200. [DOI] [PubMed] [Google Scholar]
- 27.Tillman DM, Izeradjene K, Szucs KS, Douglas L, Houghton JA. Rottlerin sensitizes colon carcinoma cells to tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis via uncoupling of the mitochondria independent of protein kinase C. Cancer Res. 2003;63:5118–5125. [PubMed] [Google Scholar]
- 28.Troyano A, Sancho P, Fernandez C, de Blas E, Bernardi P, Aller P. The selection between apoptosis and necrosis is differentially regulated in hydrogen peroxide-treated and glutathione-depleted human promonocytic cells. Cell Death Differ. 2003;10:889–898. doi: 10.1038/sj.cdd.4401249. [DOI] [PubMed] [Google Scholar]
- 29.Ueyama T, Geiszt M, Leto TL. Involvement of Rac1 in activation of multicomponent Nox1- and Nox3-based NADPH oxidases. Mol Cell Biol. 2006;26:2160–2174. doi: 10.1128/MCB.26.6.2160-2174.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vanden Berghe T, Kalai M, Van Loo G, Declercq W, Vandenabeele P. Disruption of HSP90 function reverts tumor necrosis factor-induced necrosis to apoptosis. J Biol Chem. 2003;278:5622–5629. doi: 10.1074/jbc.M208925200. [DOI] [PubMed] [Google Scholar]
- 31.Vercammen D, Beyaert R, Denecker G, Goossens V, Van Loo G, Declercq W, Grooten J, Fiers W, Vandenabeele P. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998;187:1477–1485. doi: 10.1084/jem.187.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]