Abstract
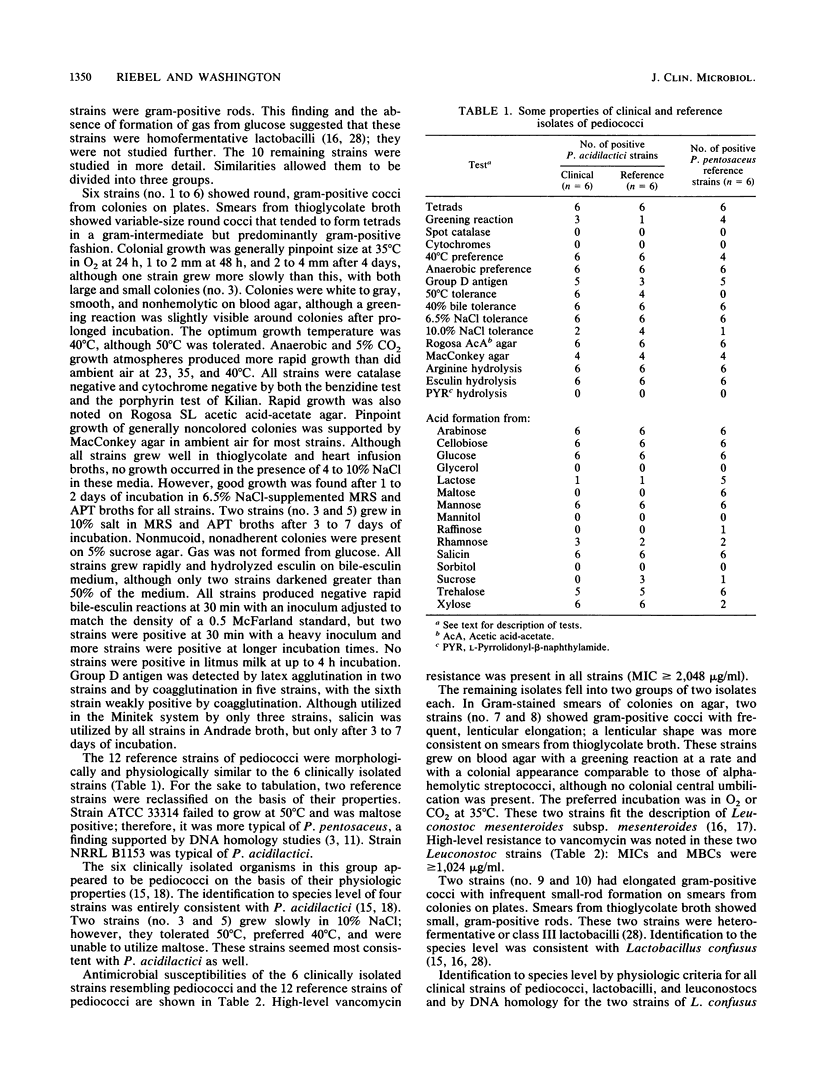
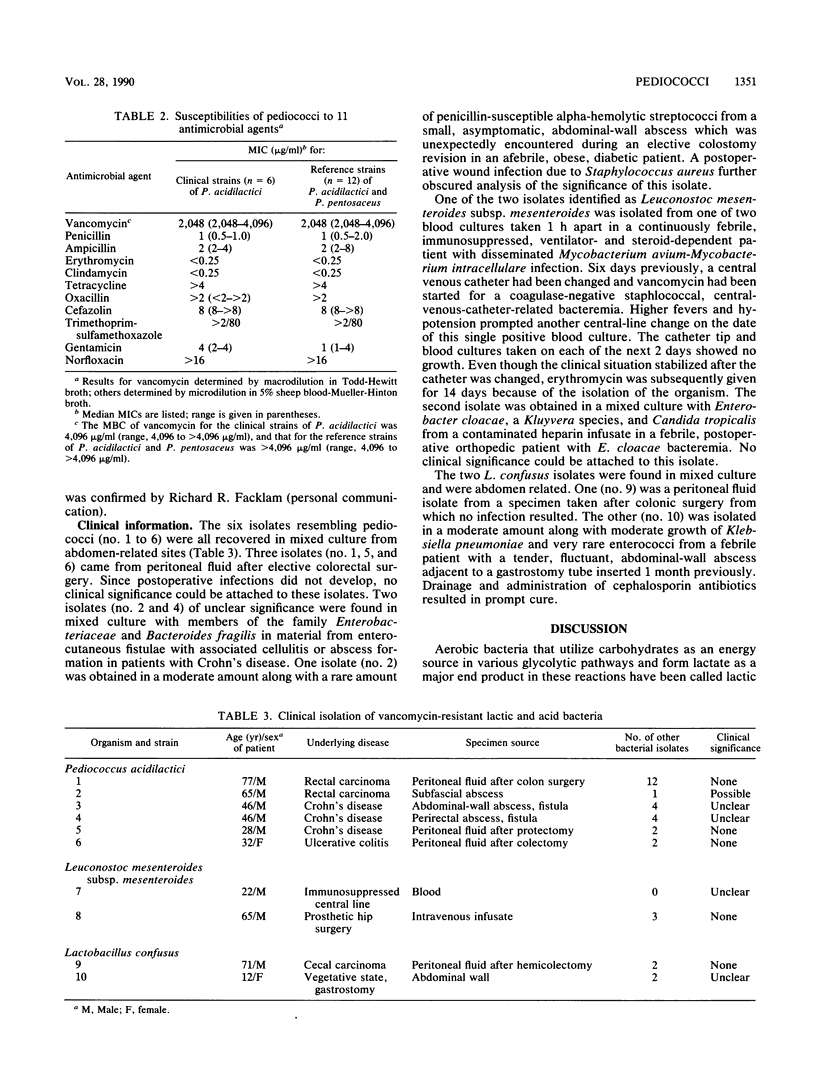
Over a 43-month period, 23 separate isolates of nonenterococcal alpha- and nonhemolytic streptococci were reported by our clinical microbiology laboratory to be resistant to vancomycin. This constituted 0.32% of nonenterococcal alpha- and nonhemolytic streptococci reported and 4.4% of such streptococci upon which susceptibility testing was performed. Of 13 isolates which were available for further study, all were highly resistant to vancomycin (MIC greater than or equal to 1,024 micrograms/ml), but none were actually streptococci. Three were clearly gram-positive rods by Gram stain and were found to be homofermentative lactobacilli. Two strains with elongated gram-positive cocci from colonies on agar showed small gram-positive rods when grown in thioglycolate broth and were physiologically identified as Lactobacillus confusus. Two isolates with lenticular gram-positive cocci appeared to be Leuconostoc mesenteroides subsp. mesenteroides. Six gram-positive isolates with round cells from growth on agar and from broth were arranged in tetrads in broth and closely resembled Pediococcus acidilactici. Twelve additional strains of pediococci that were not of human origin were also found to be highly resistant to vancomycin. These findings confirm published reports of clinical isolation of organisms resembling pediococci and suggest that clinically isolated, vancomycin-resistant bacteria which superficially resemble streptococci are probably other lactic acid bacteria.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourgault A. M., Wilson W. R., Washington J. A., 2nd Antimicrobial susceptibilities of species of viridans streptococci. J Infect Dis. 1979 Sep;140(3):316–321. doi: 10.1093/infdis/140.3.316. [DOI] [PubMed] [Google Scholar]
- Buu-Hoï A., Branger C., Acar J. F. Vancomycin-resistant streptococci or Leuconostoc sp. Antimicrob Agents Chemother. 1985 Sep;28(3):458–460. doi: 10.1128/aac.28.3.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman G., Efstratiou A. Vancomycin-resistant leuconostocs, lactobacilli and now pediococci. J Hosp Infect. 1987 Jul;10(1):1–3. doi: 10.1016/0195-6701(87)90025-9. [DOI] [PubMed] [Google Scholar]
- Coovadia Y. M., Solwa Z., van Den Ende J. Potential pathogenicity of Leuconostoc. Lancet. 1988 Feb 6;1(8580):306–306. doi: 10.1016/s0140-6736(88)90395-9. [DOI] [PubMed] [Google Scholar]
- Coovadia Y. M., Solwa Z., van den Ende J. Meningitis caused by vancomycin-resistant Leuconostoc sp. J Clin Microbiol. 1987 Sep;25(9):1784–1785. doi: 10.1128/jcm.25.9.1784-1785.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEIBEL R. H., EVANS J. B. Modified benzidine test for the detection of cytochrome-containing respiratory systems in microorganisms. J Bacteriol. 1960 Mar;79:356–360. doi: 10.1128/jb.79.3.356-360.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyas A., Chauhan N. Vancomycin-resistant Leuconostoc. Lancet. 1988 Feb 6;1(8580):306–306. doi: 10.1016/s0140-6736(88)90396-0. [DOI] [PubMed] [Google Scholar]
- Facklam R., Hollis D., Collins M. D. Identification of gram-positive coccal and coccobacillary vancomycin-resistant bacteria. J Clin Microbiol. 1989 Apr;27(4):724–730. doi: 10.1128/jcm.27.4.724-730.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland S. E., Speck M. L. Use of the Minitek system for characterizing lactobacilli. Appl Environ Microbiol. 1977 Jun;33(6):1289–1292. doi: 10.1128/aem.33.6.1289-1292.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golledge C. Vancomycin resistant lactobacilli. J Hosp Infect. 1988 Apr;11(3):292–292. doi: 10.1016/0195-6701(88)90109-0. [DOI] [PubMed] [Google Scholar]
- Gonzalez C. F., Kunka B. S. Evidence for Plasmid Linkage of Raffinose Utilization and Associated alpha-Galactosidase and Sucrose Hydrolase Activity in Pediococcus pentosaceus. Appl Environ Microbiol. 1986 Jan;51(1):105–109. doi: 10.1128/aem.51.1.105-109.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C. F., Kunka B. S. Plasmid transfer in Pediococcus spp.: intergeneric and intrageneric transfer of pIP501. Appl Environ Microbiol. 1983 Jul;46(1):81–89. doi: 10.1128/aem.46.1.81-89.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder E. J., Wilkowske C. J., Washington J. A., 2nd, Geraci J. E. Streptococcus mutans endocarditis. Ann Intern Med. 1974 Mar;80(3):364–368. doi: 10.7326/0003-4819-80-3-364. [DOI] [PubMed] [Google Scholar]
- Hardy S., Ruoff K. L., Catlin E. A., Ignacio Santos J. Catheter-associated infection with a vancomycin-resistant gram-positive coccus of the Leuconostoc sp. Pediatr Infect Dis J. 1988 Jul;7(7):519–520. doi: 10.1097/00006454-198807000-00018. [DOI] [PubMed] [Google Scholar]
- Holliman R. E., Bone G. P. Vancomycin resistance of clinical isolates of lactobacilli. J Infect. 1988 May;16(3):279–283. doi: 10.1016/s0163-4453(88)97676-1. [DOI] [PubMed] [Google Scholar]
- Horowitz H. W., Handwerger S., van Horn K. G., Wormser G. P. Leuconostoc, an emerging vancomycin-resistant pathogen. Lancet. 1987 Dec 5;2(8571):1329–1330. doi: 10.1016/s0140-6736(87)91217-7. [DOI] [PubMed] [Google Scholar]
- Isenberg H. D., Vellozzi E. M., Shapiro J., Rubin L. G. Clinical laboratory challenges in the recognition of Leuconostoc spp. J Clin Microbiol. 1988 Mar;26(3):479–483. doi: 10.1128/jcm.26.3.479-483.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. H., Gilligan P. H., Facklam R. R. Recovery of resistant enterococci during vancomycin prophylaxis. J Clin Microbiol. 1988 Jun;26(6):1216–1218. doi: 10.1128/jcm.26.6.1216-1218.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M. A rapid method for the differentiation of Haemophilus strains. The porphyrin test;. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Dec;82(6):835–842. doi: 10.1111/j.1699-0463.1974.tb02381.x. [DOI] [PubMed] [Google Scholar]
- Leclercq R., Derlot E., Duval J., Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med. 1988 Jul 21;319(3):157–161. doi: 10.1056/NEJM198807213190307. [DOI] [PubMed] [Google Scholar]
- London J., Chace N. M. Aldolases of the lactic acid bacteria. Demonstration of immunological relationships among eight genera of Gram positive bacteria using an anti-pediococcal aldolase serum. Arch Microbiol. 1976 Oct 11;110(1):121–128. doi: 10.1007/BF00416976. [DOI] [PubMed] [Google Scholar]
- Lütticken R., Kunstmann G. Vancomycin-resistant Streptococcaceae from clinical material. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Jan;267(3):379–382. doi: 10.1016/s0176-6724(88)80054-3. [DOI] [PubMed] [Google Scholar]
- Orberg P. K., Sandine W. E. Common occurrence of plasmid DNA and vancomycin resistance in Leuconostoc spp. Appl Environ Microbiol. 1984 Dec;48(6):1129–1133. doi: 10.1128/aem.48.6.1129-1133.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orberg P. K., Sandine W. E. Survey of antimicrobial resistance in lactic streptococci. Appl Environ Microbiol. 1985 Mar;49(3):538–542. doi: 10.1128/aem.49.3.538-542.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucci M. J., Monteschio M. E., Kemker C. L. Intergeneric and intrageneric conjugal transfer of plasmid-encoded antibiotic resistance determinants in Leuconostoc spp. Appl Environ Microbiol. 1988 Feb;54(2):281–287. doi: 10.1128/aem.54.2.281-287.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGOSA M., SHARPE M. E. Species differentiation of human vaginal lactobacilli. J Gen Microbiol. 1960 Aug;23:197–201. doi: 10.1099/00221287-23-1-197. [DOI] [PubMed] [Google Scholar]
- Raccach M. Pediococci and biotechnology. Crit Rev Microbiol. 1987;14(4):291–309. doi: 10.3109/10408418709104442. [DOI] [PubMed] [Google Scholar]
- Rubin L. G., Vellozzi E., Shapiro J., Isenberg H. D. Infection with vancomycin-resistant "streptococci" due to Leuconostoc species. J Infect Dis. 1988 Jan;157(1):216–216. doi: 10.1093/infdis/157.1.216. [DOI] [PubMed] [Google Scholar]
- Ruoff K. L., Kuritzkes D. R., Wolfson J. S., Ferraro M. J. Vancomycin-resistant gram-positive bacteria isolated from human sources. J Clin Microbiol. 1988 Oct;26(10):2064–2068. doi: 10.1128/jcm.26.10.2064-2068.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwalbe R. S., Stapleton J. T., Gilligan P. H. Emergence of vancomycin resistance in coagulase-negative staphylococci. N Engl J Med. 1987 Apr 9;316(15):927–931. doi: 10.1056/NEJM198704093161507. [DOI] [PubMed] [Google Scholar]
- Setterstrom J. A., Gross A., Stanko R. S. Comparison of Minitek and conventional methods for the biochemical characterization of oral streptococci. J Clin Microbiol. 1979 Oct;10(4):409–414. doi: 10.1128/jcm.10.4.409-414.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlaes D. M., Marino J., Jacobs M. R. Infection caused by vancomycin-resistant Streptococcus sanguis II. Antimicrob Agents Chemother. 1984 Apr;25(4):527–528. doi: 10.1128/aac.25.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims W. The isolation of pediococci from human saliva. Arch Oral Biol. 1966 Oct;11(10):967–972. doi: 10.1016/0003-9969(66)90198-1. [DOI] [PubMed] [Google Scholar]
- Swenson J. M., Facklam R. R., Thornsberry C. Antimicrobial susceptibility of vancomycin-resistant Leuconostoc, Pediococcus, and Lactobacillus species. Antimicrob Agents Chemother. 1990 Apr;34(4):543–549. doi: 10.1128/aac.34.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttley A. H., Collins C. H., Naidoo J., George R. C. Vancomycin-resistant enterococci. Lancet. 1988 Jan 2;1(8575-6):57–58. doi: 10.1016/s0140-6736(88)91037-9. [DOI] [PubMed] [Google Scholar]
- Wenocur H. S., Smith M. A., Vellozzi E. M., Shapiro J., Isenberg H. D. Odontogenic infection secondary to Leuconostoc species. J Clin Microbiol. 1988 Sep;26(9):1893–1894. doi: 10.1128/jcm.26.9.1893-1894.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J. D. Porphyrin test as an alternative to benzidine test for detecting cytochromes in catalase-negative gram-positive cocci. J Clin Microbiol. 1987 Oct;25(10):2006–2007. doi: 10.1128/jcm.25.10.2006-2007.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


