Abstract
In order to develop an anti-human TNF-α mAb, mice were immunized with recombinant human TNF-α. A murine mAb, TSK114, which showed the highest binding activity for human TNF-α was selected and characterized. TSK114 specifically bound to human TNF-α without cross-reactivity with the homologous murine TNF-α and human TNF-β. TSK114 was found to be of IgG1 isotype with κ light chain. The nucleotide sequences of the variable regions of TSK114 heavy and light chains were determined and analyzed for the usage of gene families for the variable (V), diversity (D), and joining (J) segments. Kinetic analysis of TSK114 binding to human TNF-α by surface plasmon resonance technique revealed a binding affinity (KD) of ~5.3 pM, which is about 1,000- and 100-fold higher than those of clinically relevant infliximab (Remicade) and adalimumab (Humira) mAbs, espectively. TSK114 neutralized human TNF-α-mediated cytotoxicity in proportion to the concentration, exhibiting about 4-fold greater efficiency than those of infliximab and adalimumab in WEHI 164 cells used as an in vitro model system. These results suggest that TSK114 has the potential to be developed into a therapeutic TNF-α-neutralizing antibody with picomolar affinity.
Keywords: antibodies, monoclonal; antibody affinity; antibody specificity; tumor necrosis factor-α
Introduction
TNF-α is a pleiotropic cytokine that has been implicated as a crucial mediator in the pathogenesis of infectious, inflammatory and autoimmune diseases (Beutler and Cerami, 1988; Beutler, 1999; Smyth and Johnstone, 2000). TNF-α is produced primarily by macrophages and monocytes, but also by many other cell types including T cells, B cells and fibroblasts (Vassalli, 1992). TNF-α is expressed as a 26 kDa integral transmembrane precursor protein from which a 17 kDa subunit is released after proteolytic cleavage (Kriegler et al., 1988). TNF-α forms a homotrimer and activates signaling cascades via two receptors, TNFR1 (55 kDa) and TNFR2 (75 kDa) (Bazzoni and Beutler, 1996). The wide range of TNF-α activities is explained by the presence of TNF receptors on almost all nucleated cell types. TNFR1 is expressed on a wide range of cell types and mediates many of the proinflammatory actions of TNF-α. TNFR2 is expressed on a more limited range of cell types including leukocytes and epithelial cells, and its actions are less clear.
The natural functions of TNF-α are thought to include modulation of host immune and inflammatory responses to a variety of infectious, malignant and autoimmune conditions as part of a complex regulatory mechanism in which numerous other cytokines participate. While initial TNF-α production in response to infection or injury is beneficial, elevated serum levels of TNF-α, usually produced by activated monocytes and macrophages, can result in significant pathological changes in diseases such as sepsis, rheumatoid arthritis, inflammatory bowel disease, Crohn's disease, ankylosing spondylitis, etc. (Balkwill and Burke, 1989). Therapeutic strategies targeted towards reducing TNF-α have been developed using a variety of biotechnology methods. These include the development of anti-TNF-α chimeric or fully human mAbs, recombinant soluble TNF receptors, and small anti-TNF-α molecules that inhibit TNF-α mRNA synthesis, the signaling pathway leading to activation of TNF gene expression, or interaction of TNF-α with its receptors (Smolen and Steiner, 2003). The chimeric anti-TNF-α mAb, infliximab (Remicade), the fully human anti-TNF-α mAb, adalimumab (Humira), and the recombinant dimeric soluble TNF receptor, etanercept (Enbrel), have been used clinically for the treatment of rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and Crohn's disease (Maini et al., 1999; Feldmann and Maini, 2001; Benucci et al., 2005). These TNF-α antagonists are also being successfully explored for use in other diseases such as inflammatory bowel disease, sepsis, asthma and uveitis in Behcet's diseaseand, suggesting that the drug market for anti-TNF-α will expand in the future (Sfikakis et al., 2001, Su et al., 2002). However, many rheumatoid arthritis patients treated with such TNF-α blocking agents have been reported to produce antibodies against these agents, requiring to switch to another TNF-α blocking agents (Gatto, 2006). Recently, many efforts have been made to develop new anti-TNF-α mAbs with higher binding affinity and better neutralizing activity, which may allow lower dosage and thus could minimize the possibility of antibody production against anti-TNF-α mAbs, for the treatment of a more diverse range of patients with TNF-α related diseases.
In this study, we report an anti-human TNF-α murine mAb, TSK114, raised by immunization of mice with recombinant human TNF-α. The specificity, binding affinity, and neutralization activity of TSK114 to human TNF-α was analyzed.
Materials and Methods
Cell lines and mice
Sp2/o cells were purchased from ATCC (Manasa, VA) and maintained in RPMI-1640 with 10% heat inactivated fetal calf serum (Sigma, St. Louis, MO). The cell lines were maintained in a humidified chamber with 5% CO2 at 37℃. The mouse fibrosarcoma cell line, WEHI 164 clone 13, was purchased from ATCC (Manasa, VA). BALB/c mice used for immunization were purchased from Oriental Co. (Osan, Korea).
Generation of hybridoma cells
Male BALB/c mice (6-weeks old) were injected intraperitoneally with 30 µg of purified recombinant human TNF-α (Biosource, Camarillo, CA) emulsified in complete Freund's adjuvant. Three additional injections (30 µg) of recombinant human TNF-α emulsified in incomplete Freund's adjuvant followed at weekly intervals starting one week after the first immunization. Four weeks later, the mice were given a final booster injection with 30 µg of human TNF-α. Three days after the last injection, spleen cells from the immunized mice were fused with the myeloma Sp2/o cells according to the procedure of Rathjen et al. (1986). A solid-phase radioimmunoassay was employed for screening monoclonal antibodies specific for human TNF-α. The hybridoma line secreting a mAb, TSK114, was chosen and the antibody from the cell supernatant was purified by protein A-Sepharose 4 fast flow (Pharmacia, Uppsala, Sweden).
Isotying and isoelectric focusing
The isotype of TSK114 was determined by adding 25 µl of the cell culture supernatant containing TSK114 with 200 µl assay buffer to wells coated with each of the rabbit anti-mouse antibodies from mouse MonoAb ID kit (Zymed, San Francisco, CA) against IgG1, IgG2a, IgG2b, IgG3, IgA, IgM, kappa, and lambda. Detection of bound mAb was by goat anti-rabbit IgG-HRP conjugated antibody (Zymed, San Francisco, CA). IEF was performed in IEF gel pH 3-7 (Invitrogen, Carlsbad, CA). Electrophoresis was run following the manufacturer's protocol.
ELISA
Ninety-six-well flat-bottom plates (NUNC, Roskilde, Denmark) were coated with serially diluted human TNF-α, murine TNF-α and human TNF-β. Plates were then washed three times with PBS containing 0.05% Tween 20. Non-specific sites were blocked with 1% BSA at 37℃ for 2 h. TSK114 was added (100 ng/well) and incubated at 37℃ for 2 h. After washing with Tween/PBS, an HRP-conjugated goat anti-mouse IgG (Sigma, St. Louis, MO) was used for detection.
Western blot analysis
Recombinant human TNF-α, murine TNF-α and human TNF-β (Biosource, Camarillo, CA) were subjected to SDS-PAGE according to the standard method described by Laemmli (1970) and transferred to 0.45 µm nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA) for 1 h. The blots were then blocked with Tris-buffered saline (TBS) containing 0.1% (w/v) casein for 2 h at room temperature. After washing three times with TBS, the blots were incubated for 1 h at 37℃ with TBS buffer containing 1 µg/ml of TSK114. The membranes were washed three times with TBS and incubated with goat anti-mouse IgG conjugated to HRP (Bio-Rad Laboratories, Hercules, CA) for 1 h at 37℃. Following the washes with TBS, the plates were incubated with the enzyme substrate solution containing 0.5 mg/ml 4-chloro-1-naphthol (Sigma, St. Louis, MO), 0.15% (v/v) hydrogen peroxide and 25% (v/v) methanol.
Surface plasmon resonance (SPR)
Kinetic interactions of TSK114, infliximab, and adalimumab with TNF-α were measured at 25℃ using a Biacore 2000 SPR biosensor (Biacore AB, Uppsala, Sewden) as described previously (Kim et al., 2003, 2006). After immobilization of the antibodies onto the carboxymethylated dextran surface of a CM5 sensor chip at a level of about 2,500-3,000 response units, 60 µl of serially diluted human TNF-α in a buffer containing 10 mM HEPES (pH 7.4), 150 mM NaCl, 3 mM EDTA, and 0.005% polysorbate was injected into the flow cell at a rate of 30 µl/min (Gil et al, 2002). As a control, BSA (0.04 mg/ml) was simultaneously immobilized onto the reference surface under the same conditions to correct for instrument and buffer artifacts. Dissociation (koff) and association (kon) rate constants were obtained by nonlinear regression analysis of the primary sensorgram data according to a 1 : 1 binding model using the BIAevaluation version 3.2 software provided by the manufacturer. The dissociation constant KD was calculated using the formula KD = koff / kon (Kim et al., 2006).
Cloning of cDNAs for the variable regions of TSK114 and sequence analysis
Total cellular RNA was isolated from the cell line using RNeasy Mini Spin kit according to the manufacturer's instructions (QIAgen, Hilden, Germany). cDNA was produced via reverse transcription using 10 pg to 10 µg of RNA template, oligodT primer and ThermoScript reverse transcriptase (Invitrogen, Carlsbad, CA). Reactions were incubated at 50℃ for 1 h. The resulting cDNA was used as a template for PCR amplification using Amplitaq polymerase (Perkin-Elmer, MA) and 5' and 3' primers of Ig-prime kit (Novagen, Darmstadt, Germany) specific for mouse Vh and Vl genes. PCR reactions were incubated at 95℃ for 5 min, followed by 35 cycles of 94℃ for 1 min, 56℃ for 1 min, and 72℃ for 2 min. A final extension at 72℃ for 10 min completed the reactions. PCR products were inserted into the pCR2.1-TOPO TA vector (Invitrogen, Carlsbad, CA) and sequenced in both strands. The sequence analysis of TSK114 heavy and light chains were done in the web site of http://www.ncbi.nlm.nih.gov/igblast/.
Bioassays for neutralization of TNF-α-mediated cytotoxicity
Neutralizing activities of TSK114 and infliximab against human TNF-α were measured on the mouse WEHI 164 cell line treated with actinomycin D according to the method described previously (Austgulen et al., 1986; Khabar et al., 1995). Briefly, WHEI 164 cells were seeded in triplicate at 1 × 104 cells/well into a 96-well plate and cultured in RPMI 1640 medium supplemented with 10% (v/v) FBS for 20 h. Then, serially diluted antibodies (final concentration: 0.5-50 ng/ml) in the medium containing 2 µg/ml actinomycin D were added to the cell culture together with 0.1 ng/ml of human TNF-α. The cells were incubated for an additional 20 h at 37℃ and cell viability was analyzed using a colorimetric MTT-based Cell Growth Determination kit (Sigma, St. Louis, MO). The ED50 value was calculated by complex sigmoid non-linear regression analysis using Sigma plot software (Systat software, Inc. Richmond, CA).
Results
Characterization of a mAb, TSK114
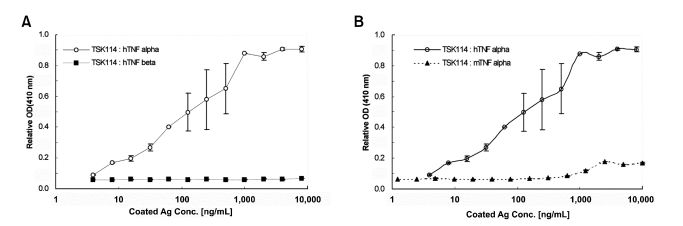
For the development of an anti-human TNF-α mAb, eleven BALB/c mice were immunized with purified recombinant human TNF-α. ELISA was employed to screen hybridoma culture supernatants with binding activity for human TNF-α. Among the positive hybridoma lines a mAb, TSK114, which showed the highest binding activity for human TNF-α, was selected and further characterized. Isotyping of TSK114 by sandwich ELISA using class and sub-class specific anti-mouse antibodies revealed that TSK114 belongs to IgG1 isotype with κ light chain. IEF of TSK114 showed that pI value of TSK114 is in a range of 7.0-7.5. The purified TSK114 was also evaluated by ELISA for its ability to bind to human TNF-α and its homologous proteins, murine TNF-α and human TNF-β. As shown in Figure 1, TSK114 binds to human TNF-α in a concentration dependent manner, but not to murine TNF-α and human TNF-β, even at very high concentrations of proteins. Specific binding of TSK114 to human TNF-α was also confirmed by Western blot analysis (Figure 2). TSK114 bound to the 17 kDa and 34 kDa protein bands corresponding to the monomeric and dimeric forms of human TNF-α , respectively, but not to murine TNF-α and human TNF-β. These results suggest that TSK114 is a murine mAb highly specific to human TNF-α without crossreactivity to the homologous murine TNF-α and human TNF-β.
Figure 1.
Specific binding analysis of TSK114 for human TNF-α by ELISA. Serially diluted antigens (human TNF-α, murine TNF-α, or human TNF-β) were coated onto microtiter plates and TSK114 was added as described in Materials and methods. The binding of TSK114 to antigens was detected with HRP-conjugated goat anti-mouse IgG. (A) human TNF-α (○) and human TNF-β (■). (B) human TNF-α (○) and murine TNF-α (▲).
Figure 2.
Specific binding analysis of TSK114 for human TNF-α by Western blot. Antigens were resolved under non-reducing conditions with 10% SDS-PAGE. Protein bands were visualized by either Commassie blue staining (A) or Western blot (B). For Western blotting, the resolved gel was transferred to a blotting membrane and incubated with TSK114. Bound antibody was detected with an HRPconjugated goat anti-mouse IgG. Protein bands of human TNF-α of about 17 kDa and 34 kDa were recognized by TSK114 and are marked by arrows. M, Protein size marker; Lane 1, human TNF-α; Lane 2, murine TNF-α; Lane 3, human TNF-β.
Kinetic analysis of TSK114 binding to human TNF-α
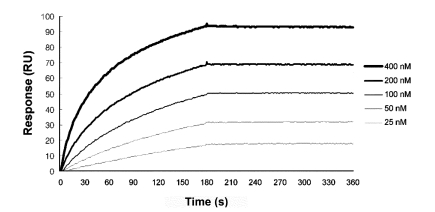
Binding kinetics of TSK114 for human TNF-α was determined at 25℃ by SPR technique by flowing human TNF-α over TSK114-immobilized surface (Figure 3). For comparisons, the binding interactions of infliximab and adalimumab with human TNF-α were also measured under the same conditions as TSK114. A representative sensogram for TSK114 and kinetic binding parameters for each antibody are shown in Figure 3 and Table 1, respectively. The dissociation constant (KD) of TSK114, infliximab and adalimumab for human TNF-α were approximately 5.3 pM, 9.1 nM, and 0.6 nM, respectively (Table 1). The KD values of infliximab and adalimumab determined in this study were higher than the values previously reported, ~0.6 nM of infliximab measured by solid-phase radioimmuno assay (Knight et al., 1993) and ~0.1 nM of adalimumab determined by SPR (Santora et al., 2001). The affinity of TSK114 with human TNF-α was about 1,000- and 100-fold greater than those of infliximab and adalimumab, respectively, mainly as a result of the almost same magnitude reduction in the dissociation constant (koff) (Table 1).
Figure 3.
Representative sensorgrams for the kinetic binding interactions between human TNF-α and TSK114. SPR sensograms were obtained from injections of human TNF-α at 25, 50, 100, 200 and 400 nM over a TSK114-immobilized surface.
Table 1.
Kinetic binding parameters for the interactions of TSK114, infliximab, and adalimumab with human TNF-α.
All parameters were determined by Biacore analysis and represent the mean ± SD from four separate determinations.
Sequence analysis of the variable regions of TSK114
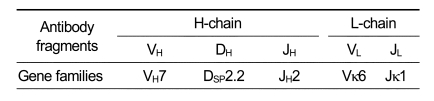
To determine the nucleotide sequences of the variable regions of TSK114 heavy (H) and light (L) chains, the cDNAs for the variable regions of TSK114 were amplified from the total RNA of the hybridoma line secreting TSK114 by PCR and sequenced after cloning. The nucleotide sequences and deduced amino acid sequences for the variable regions of TSK114 are shown in Figure 4. From the sequence analysis, the usage of gene families for the variable (V), diversity (D), and joining (J) segments of H- and L-chains could be identified (Table 2). TSK114 was found to utilize VH7, DSP2.2, and JH2 gene segments for the variable region of H-chain, and Vκ6 and Jκ1 for the variable region of L-chain. Comparison of the amino acid sequences of TSK114 with those of mouse immunoglobulin-encoding genes listed in the GenBank database showed that the amino acid sequence of TSK114 has not been previously reported.
Figure 4.
Nucleotide and deduced amino acid sequences of the cDNA encoding the variable regions of the heavy (A) and light chain (B) of TSK114 (Patent number registered in Korea: 10-0772800). CDRs are underlined.
Table 2.
Family usage of gene segments of the variable regions of TSK114.
Gene family usage was defined according to the determination in the web site of http://www.ncbi.nlm.nih.gov/igblast/.
Neutralization of TNF-α-mediated cytotoxicity by TSK114
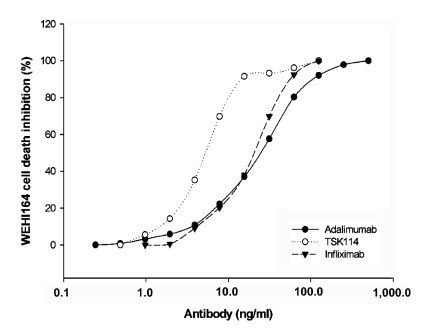
To examine the neutralization activities of TSK114, the ability of TSK114 to block the cytotoxic effect of human TNF-α on the TNF-α sensitive WEHI 164 clone 13 cells was analyzed and also compared to those of infliximab and adalimumab. As shown in Figure 5, TNF-α-mediated toxicity in the WEHI 164 cells treated with 0.1 ng/ml of human TNF-α was effectively neutralized by TSK114 in a dose-dependent fashion with an ED50 of ~5.3 ng/ml. Infliximab and adalimumab also showed neutralization activities with ED50 of ~24.3 ng/ml and ~24.4 ng/ml, respectively. Thus TSK114 exhibited about 4-fold more effective neutralizing activity than infliximab and adalimumab.
Figure 5.
Neutralization of TNF-α-mediated cytotoxicity in WEHI 164 cells by antibodies. Various concentrations of TSK114 (●), nfliximab (○), and adalimumab (▼) were added to WEHI 164 cells cultured with 0.1ng/ml of human TNF-α. The cells were incubated for 20 h at 37℃ and cell viability was analyzed using a colorimetric MTT assay.
Discussion
In the present study, a novel anti-human TNF-α murine mAb, TSK114, was raised and characterized. ELISA and Western blot analysis showed that TSK114 was highly specific to human TNF-α and did not cross-react with either murine TNF-α or human TNF-β, both of which are closely related to human TNF-α, being about 80% and 35% homologous to human TNF-α, respectively. The binding affinity of TSK114 to human TNF-α was approximately 1,000- and 100-fold higher than those of infliximab and adalimumab, respectively, largely due to its extremely slow dissociation rate (Table 1).
From the cloning of the cDNA encoding the variable regions of TSK114 and nucleotide sequence analysis, the usage of gene families for the V, D, and J segments of H- and L-chains was determined, and CDR1, 2, and 3 regions of TSK114 could be identified (Figure 4). Interestingly, the CDR3 region (Tyr-Asp-Ser-Arg-Gly-Phe-Asp-Cys) of TSK114 H-chain contains a cysteine residue, which is very unusual (Figure 4). It was reported that cysteine residue is least likely to be found in mouse mature B-cell CDRs as an overall frequency of 0.05% (Raaphorst et al., 1997). However, most of antibodies produced in shark and camel contain 1 or 2 cysteine residues in the CDR3 region of H-chain (Raaphorst et al., 1997), which is critical for the stability of antibody by inhibiting the degradation in vivo. It will be interesting to see if the cysteine residue in the CDR3 of TSK114 could be also involved in the stability of antibody.
Using human TNF-α sensitive WEHI 164 cells, TSK114 was shown to have an excellent neutralizing activity against human TNF-α. The neutralizing activity of TSK114 was about 4-fold higher than infliximab and adalimumab. One of the critical factors for the neutralizing activity of anti-TNF-α antibodies might be the high affinity binding between antagonists and TNF-α to maintain the stable neutralizing complexes (Feldmann and Maini, 2001). The much higher binding affinity of TSK114 for TNF-α with the lower dissociation rate constants than those of infliximab and adalimumab indicates that TSK114-TNF-α complex is much more stable once it is formed, compared with infliximab-TNF-α and adalimumab-TNF-α complexes. In other words, dissociation of TNF-α from the TSK114-TNF-α complex would be much slower than those of infliximab-TNF-α or adalimumab-TNF-α complexes. Thus, the much higher affinity of TSK114 for TNF-α to form stable neutralizing complexes might explain its much more efficient neutralizing activity against TNF-α-induced cytotoxicity in vitro, as compared those of infliximab and adalimumab (Figure 5). The preliminary in vivo efficacy test using the human TNF-α transgenic (TTg) mice (Le Buanec et al., 2006) suggests that TSK114 can suppress arthritic symptoms in the animal model of polyarthritis with a comparable efficiency to infliximab (data not shown). In addition, interestingly enough, the human TNF-α concentration in the sera of the human TNF-α transgenic mice (TTg mice) treated with infliximab for 12 weeks was higher (50-60 pg/ml) than PBS-treated TTg mice (5-10 pg/ml) (data not shown), which was consistent with previously reported observations (Charles et al., 1999, Zwerina et al., 2004). However, almost no human TNF-α was detected in the sera of TSK114-treated TTg mice. At this point, it is not clear why the infliximab treatment caused the mice to accumulate higher serum concentrations of TNF-α than those of PBS-treated mice. A previous study showed that TNF-α dissociated from the etanercept-TNF-α complex was bioactive enough to exert cytotoxicity (Scallon et al., 2002). In this context, TSK114 has the potential to be developed into a more effective TNF-α-neutralizing antibody than infliximab and adalimumab. However, further in vivo studies are required to titrate and compare the effective concentration of TSK114 with those of infliximab and adalimumab, and also to make clear any correlationships among their affinities, serum concentrations of TNF-α, and their in vivo efficacies for suppressing arthritis.
In conclusion, we characterized TSK114, a murine mAb against human TNF-α with a picomolar binding affinity and high neutralizing activity. Future studies, including in vivo studies and humanization of TSK114, may provide us with a powerful TNF-α-neutralizing antibody for the treatment of rheumatoid arthritis.
Abbreviations
- D fragment
diversity fragment
- H-chain
heavy chain
- J fragment
joining fragment
- L-chain
light chain
- SPR
surface plasmon resonance
- TBS
tris-buffered saline
- TNFR
TNF-α receptor
- TTg mice
human TNF-α transgenic mice
- V fragment
variable fragment
References
- 1.Austgulen R, Hammerstrom J, Espevik T, Nissen-Meyer J. Human monocyte cytotoxic factor mediates cytolysis of WEHI 164 cells. Cell Immunol. 1986;98:211–220. doi: 10.1016/0008-8749(86)90281-9. [DOI] [PubMed] [Google Scholar]
- 2.Balkwill FR, Burke F. The cytokine network. Immunol Today. 1989;10:299–304. doi: 10.1016/0167-5699(89)90085-6. [DOI] [PubMed] [Google Scholar]
- 3.Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med. 1996;334:1717–1725. doi: 10.1056/NEJM199606273342607. [DOI] [PubMed] [Google Scholar]
- 4.Benucci M, Li GF, Del Rosso A, Manfredi M. Adalimumab (anti-TNF-alpha) therapy to improve the clinical course of adult-onset Still's disease: the first case report. Clin Exp Rheumatol. 2005;23:733. [PubMed] [Google Scholar]
- 5.Beutler B, Cerami A. Tumor necrosis, cachexia, shock and inflammation: a common mediator. Ann Rev Biochem. 1988;57:505–518. doi: 10.1146/annurev.bi.57.070188.002445. [DOI] [PubMed] [Google Scholar]
- 6.Beutler BA. The role of tumor necrosis factor in health and disease. J Rheumatol Suppl. 1999;26:16–21. [PubMed] [Google Scholar]
- 7.Charles P, Elliott MJ, Davis D, Potter A, Kalden JR, Antoni C, Breedveld FC, Smolen JS, Eberl G, deWoody K, Feldmann M, Maini RN. Regulation of cytokines, cytokine inhibitors, and acute-phase proteins following anti-TNF-alpha therapy in rheumatoid arthritis. J Immunol. 1999;1631:1521–1528. [PubMed] [Google Scholar]
- 8.Feldmann M, Maini RN. Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol. 2001;19:163–196. doi: 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]
- 9.Gatto B. Biologics targeted at TNF: design, production and challenges. Reumatismo. 2006;58:94–103. doi: 10.4081/reumatismo.2006.94. [DOI] [PubMed] [Google Scholar]
- 10.Gil MC, Lee MH, Seo JI, Choi YL, Kim MK, Jung KC, Park SH, Kim TJ. Characterization and epitope mapping of two monoclonalantibodies against human CD99. Exp Mol Med. 2002;34:411–418. doi: 10.1038/emm.2002.58. [DOI] [PubMed] [Google Scholar]
- 11.Khabar KS, Siddiqui S, Armstrong JA. WEHI-13VAR: a stable and sensitive variant of WEHI 164 clone 13 fibrosarcoma for tumor necrosis factor bioassay. Immunol Lett. 1995;46:107–110. doi: 10.1016/0165-2478(95)00026-2. [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Lee MH, Jung KI, Na HY, Cha HS, Koh EM, Kim TJ. Detection of antibodies against glucose 6-phosphate isomerase in synovial fluid of rheumatoid arthritis using surface plasmon resonance (BIAcore) Exp Mol Med. 2003;35:310–316. doi: 10.1038/emm.2003.42. [DOI] [PubMed] [Google Scholar]
- 13.Kim YR, Kim JS, Lee SH, Lee WR, Sohn JN, Chung YC, Shim HK, Lee SC, Kwon MH, Kim YS. Heavy and light chain variable single domains of an anti-DNA binding antibody hydrolyze both double- and single-stranded DNAs without sequence specificity. J Biol Chem. 2006;281:15287–15295. doi: 10.1074/jbc.M600937200. [DOI] [PubMed] [Google Scholar]
- 14.Knight DM, Trinh H, Le J, Siegel S, Shealy D, McDonough M, Scallon B, Moore MA, Vilcek J, Daddona P, Ghrayeb J. Construction and initial chracterization of a house-human chimeric antibody. Mol Immunol. 1993;30:1443–1453. doi: 10.1016/0161-5890(93)90106-l. [DOI] [PubMed] [Google Scholar]
- 15.Kriegler M, Perez C, DeFay K, Albert I, Lu SD. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988;53:45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Le Buanec H, Delavallée L, Bessis N, Paturance S, Bizzini B, Gallo R, Zagury D, Boissier MC. TNFα kinoid vaccinationinduced neutralizing antibodies to TNFα protect mice from autologous TNFα-driven chronic and acute inflammation. Proc Natl Acad Sci USA. 2006;103:19442–19447. doi: 10.1073/pnas.0604827103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, Smolen J, Emery P, Harriman G, Feldmann M, Lipsky P. Infliximab (chimeric anti-tumour necrosis factoralpha mAb) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999;354:1932–1939. doi: 10.1016/s0140-6736(99)05246-0. [DOI] [PubMed] [Google Scholar]
- 19.Raaphorst FM, Raman CS, Nall BT, Teale JM. Molecular mechanisms governing reading frame choice of immunoglobulin diversity genes. Immunol Today. 1997;18:37–43. doi: 10.1016/s0167-5699(97)80013-8. [DOI] [PubMed] [Google Scholar]
- 20.Rathjen DA, Underwood PA, Whalley JM. An evaluation of some in vivo immunization strategies for the production of monoclonal antibodies to insulin and ACTH. J Biol Stand. 1986;14:1–10. doi: 10.1016/s0092-1157(86)80003-8. [DOI] [PubMed] [Google Scholar]
- 21.Santora LC, Kaymakcalan Z, Sakorafas P, Krull IS, Grant K. Characterization of noncovalent complexes of recombinant human monoclonal antibody and antigen using cation exchange, size exclusion chromatography, and BIAcore. Anal Biochem. 2001;299:119–129. doi: 10.1006/abio.2001.5380. [DOI] [PubMed] [Google Scholar]
- 22.Scallon B, Cai A, Solowski N, Rosenberg A, Song XY, Shealy D, Wagner C. Binding and functional comparisons of two types of tumor necrosis factor antagonists. J Pharmacol Exp Ther. 2002;301:418–426. doi: 10.1124/jpet.301.2.418. [DOI] [PubMed] [Google Scholar]
- 23.Sfikakis PP, Theodossiadis PG, Katsiari CG, Kaklamanis P, Markomichelakis NN. Effect of infliximab on sight-threatening panuveitis in Behcet's disease. Lancet. 2001;358:295–296. doi: 10.1016/s0140-6736(01)05497-6. [DOI] [PubMed] [Google Scholar]
- 24.Smolen JS, Steiner G. Therapeutic strategies for rheumatoid arthritis. Nat Rev Drug Discov. 2003;2:473–488. doi: 10.1038/nrd1109. [DOI] [PubMed] [Google Scholar]
- 25.Smyth MJ, Johnstone RW. Role of TNF in lymphocytemediated cytotoxity. Microsc Res Tech. 2000;50:196–208. doi: 10.1002/1097-0029(20000801)50:3<196::AID-JEMT3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 26.Su C, Salzberg BA, Lewis JD, Deren JJ, Kornbluth A, Katzka DA, Stein RB, Adler DR, Lichtenstein GR. Efficacy of anti-tumor necrosis factor therapy in patients with ulcerative colitis. Am J Gastroenterol. 2002;97:2577–2584. doi: 10.1111/j.1572-0241.2002.06026.x. [DOI] [PubMed] [Google Scholar]
- 27.Vassalli P. The pathphysiology of tumor necrosis factor. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 28.Zwerina J, Hayer S, Tohidast-Akrad M, Bergmeister H, Redlich K, Feige U, Dunstan C, Kollias G, Steiner G, Smolen J, Schett G. Single and combined inhibition of tumor necrosis factor, interleukin-1, and RANKL pathways in tumor necrosis factor-induced arthritis: effects on synovial inflammation, bone erosion, and cartilage destruction. Arthritis Rheum. 2004;50:277–290. doi: 10.1002/art.11487. [DOI] [PubMed] [Google Scholar]