Abstract
The mutation and reduction of mitochondrial DNA (mtDNA) have been suggested as factors in the carcinogenesis. However, whether the depletion of mtDNA induces multidrug resistance in cancer cells has not been fully investigated. To elucidate the association of cellular mtDNA content and drug resistance, we generated HCT-8 colon cancer cells which revealed a marked decrease in cellular mtDNA and ATP content, concomitant with a lack of mRNAs encoded by mtDNA. The mtDNA-depleted cells showed a decreased sensitivity and accumulation of anti-cancer drugs, suggesting that mtDNA depletion could develop multidrug resistance (MDR) phenotype in HCT-8 cells. We found that the expression level of MDR1 mRNA and its translated product P-glycoprotein was increased in the mtDNA-depleted cells, indicating that the decrease of sensitivity and accumulation of anti-cancer drug in the mtDNA-depleted cells might be due to a substantial increase in the expression of P-glycoprotein. Furthermore, increased expression of MDR1 mRNA and P-glycoprotein was due to an increase of mRNA stability rather than transcriptional activation. Taken together, these results indicate that mtDNA depletion can induce an increased P-glycoprotein expression via an increase of mRNA stability and suggest that the mtDNA depletion in cancer cells plays an important role in the induction of MDR phenotype.
Keywords: colonic neoplasms; DNA, mitochondrial; drug resistance; P-glycoprotein; RNA stability
Introduction
It has been reported that long-term treatment of cells with low doses (0.1-2 µg/ml) of ethidium bromide (EtBr), an inhibitor of DNA/RNA synthesis, specifically suppresses the replication and transcription of extrachromosomal genetic components such as mtDNA without affecting nuclear DNA replication and transcription (Zylber et al., 1969; Desjardins et al., 1985; Hayakawa et al., 1998). Therefore, mtDNA-depleted cells have been important tools in the investigation of the cellular components regulated by mtDNA (King and Attardi, 1989). The role of mitochondrial dysfunction in tumorigenesis has been investigated extensively using multiple approaches (Shay and Werbin, 1987; Cavalli and Liang, 1998). Previous studies have reported that a wide rage of tumors exhibit mtDNA abnormality such as point mutations and deletions (Horton et al., 1996; Polyak et al., 1998; Fliss et al., 2000; Yeh et al., 2000). Although it is not clear whether mtDNA abnormalities are direct contributing factor or indirect effect of cancer development because the genes involved in tumorigenicity are largerly nuclear encoded, recent studies showed that mitochondria-to-nucleus stress signaling induces tumor progression and cell invasion (Amuthan et al., 2001, 2002).
The development of multidrug resistance (MDR) phenotype is a major impediment to cancer chemotherapy for many malignancies (Gottesman et al., 2002). One of major mechanisms of drug resistance in cancer cells is overexpression of P-glycoprotein that is encoded by the MDR1 gene (Ivy et al., 1996; Borst, 1997). P-glycoprotein is a 170-kDa transmembrane protein that belongs to the ATP-binding cassette (ABC) transporter superfamily (Juliano and Ling, 1976). P-glycoprotein functions as an energy-dependent efflux pump that reduces the intracellular concentration of cytotoxic compounds and thus confers resistance to unrelated drugs that differ widely with respect to molecular structure and target specificity such as paclitaxel, vincristine, and doxorubicin (Gottesman et al., 2002). Recently, several lines of evidence obtained from mtDNA-depleted cells have revealed that mitochondrial stress resulting from the reduction of mtDNA causes substantial resistance of apoptosis (Park et al., 2004; Biswas et al., 2005), suggesting that the depletion of mtDNA could induce resistance for cancer chemotherapy. However, it is not clear whether the mtDNA-depletion is a contributing factor in the expression of molecules responsible for drug resistance.
In this study, we provide evidence that the depletion of mtDNA induces MDR phenotype in HCT-8 colon cancer cells. Furthermore, we show that this is due to an increase in mRNA stability.
Materials and Methods
Reagents and antibodies
Doxorubicin was purchased from Sigma (St. Louis, Mo). [3H]-paclitaxel was obtained from Moravek Biochemicals (Labrea, CA). Monoclonal antibody directed against P-glycoprotein was purchased from Calbiochem. Polyclonal antibody directed against actin was obtained from Sigma. HRP-conjugated anti-mouse IgG and anti-rabbit IgG were obtained from Santa Cruz (Santa Cruz, CA). Oligonucleotides primers were from Bionics (Seoul, Korea). All biochemicals were of analytical grade and were purchased from commercial suppliers.
Cells culture
HCT-8 human colon cancer cells were grown in RPMI-1640 medium supplemented with 10% (v/v) FBS (Invitrogen, Grand Island, NY), penicillin (100 IU/ml) and streptomycin (100 µg/ml). Depletion of mtDNA was induced by treatment with ethidium bromide (0.2 µg/ml) for 2-3 weeks. The cell lines exhibiting reduced mtDNA content were grown in the presence of 1 mM sodium pyruvate (Sigma) and 50 µg/ml uridine (Sigma). The control HCT-8 cells were maintained for the same time period under normal culture condition.
Measurement of cellular ATP levels
Cellular ATP levels were measured using a Somatic cell ATP assay kit (Sigma) as previously described (Biswas et al., 1999), which is based on the assay of ATP-driven luciferin luciferase activity. Briefly, cells were lysed with an ATP releasing reagent (Sigma), and the lysates were assayed for luciferase activity in accordance with the manufacturer's instructions. ATP-dependent formation of light was measured in a Luminometer (Lumat LB 9501) with appropriate ATP standards.
Total DNA extraction and PCR
Total cellular DNA was extracted using a DNeasy Tissue kit in accordance with the manufacturer's instructions (Qiagen, Hilden, Germany). The amplification of mtDNA was performed under the following conditions: 94℃ for 2 min (initial denaturation); 94℃ for 30 s, 60℃ for 30 s, 72℃ for 45 s (25 cycles); and 72℃ for 10 min (final extension). The primers used in this study are shown in Table 1.
Table 1.
DNA sequences of primers used for genomic DNA PCR and real-time PCR.
gDNA, genomic DNA; N/A, not applicable
RNA extraction and RT-PCR
Total RNA was extracted from cells using Trizol reagent in accordance with the manufacturer's instructions (Invitrogen), and the quantity and quality of the isolated RNA was then determined by measuring the absorbance at 260 and 280 nm. The reverse transcription reaction was performed in a reaction mixture comprised of 2 µg of total RNA, 200 ng of oligo (dT)15 primer, 1 × reverse transcription buffer, 0.5 mM deoxynucleotide triphosphate mixture, RNasin recombinant ribonuclease inhibitor (Promega, Madison, WI), and 200 U of M-MLV reverse transcriptase (Promega). After incubation for 50 min at 42℃, the reverse transcription reaction was terminated by heating it for 15 min at 70℃. The newly synthesized cDNA was then amplified by PCR in a reaction mixture comprised of 2 µl of cDNA template, 1.5 mM MgCl2, 1 U of Tag polymerase, and 0.3 µM of primers. The amplification was performed under the following conditions: 94℃ for 2 min (initial denaturation); 94℃ for 30 s, 58℃ for 30 s, 72℃ for 60 s (30 cycles); and 72℃ for 7 min (final extension). PCR products were electrophoresed on 2% agarose gels, and then visualized by ethidium bromide staining. Band intensities were quantitated by densitometric scanning using an analytical scanning system (AlphaImager 1220) and normalized to GAPDH expression levels.
Quantitative gene expression analysis
In order to confirm gene expression levels, quantitative real time RT-PCR (qRT-PCR) was carried out in Rotor Gene 2000 (Corbett Research, Mortlake, Australia) using a SYBR-Green PCR Master Mix in accordance with the manufacturer's instructions (Qiagen, Valencia, CA). All of the gene-specific primer sets used for qRT-PCR are listed in Table 1. After amplification, the authenticity of the PCR products was verified by melting curve analysis and agarose gel electrophoresis. Images from electrophoresed gels were captured by a camera in a computer-assisted imaging system (AlphaImager 1220). The qRT-PCR results were analyzed using Rotor-Gene analysis software version 6.0 (Corbett Research). The comparative cycle threshold (CT) method was used to analyze the data by generating relative values of the amount of target cDNA. Relative quantitation for any given gene, expressed as percentage variation over control, was calculated after determination of the difference between CT of the given gene A and that of the calibrator gene B (β-actin) in the depleted cells (ΔCT1 = ΔCT1A.ΔCTB) and control cells (ΔCT0 = ΔCT0A - ΔCTB) using the 2-ΔΔCT(1-0) formula. CT values are means of triplicate measurements.
Cell proliferation assay
Cell viability was assessed using a CellTiter 96 Aqueous One Solution cell proliferation assay kit (Promega), as previously described (Ahn et al., 2006). Briefly, 1.0 × 103 cells per well were seeded in 96-well plates to which were added a dilution series of doxorubicin and vincristine in triplicate and incubated under standard culture conditions. After 4 days, a volume of 20 µl of the detection solution were added to each well and incubated for 90 min at 37℃ prior to measuring absorbance at 490 nm using a microplate reader (Biorad).
Paclitaxel accumulation
Steady-state paclitaxel accumulation was conducted as previously described (Wu et al., 2003). In brief, parent HCT-15 cells and transfectants were seeded in 6-well plates and grown for 48 h. Then, the growth medium was aspirated and replaced with 1 ml of RPMI 1640 containing 50 nM of [3H]-paclitaxel (10.0 Ci/mmol; Moravek Biochemicals). After incubation for 2 h at 37℃, the cells were cooled on ice, washed three times with ice-cold PBS, and solubilized with 0.2 ml of 1% SDS. The radioactivity in each sample was determined by scintillation counting.
Gel electrophoresis and immunoblotting
Equal amount (20 µg) of total cell lysates were subjected to SDS-PAGE on 7 or 10% resolving gels as previously described (Park et al., 2005). Separated proteins were electrophoretically transferred to nitrocellulose membrane (Bio-Rad), blocked with 5% skim milk in Tris-buffered saline (TBS), and then incubated overnight at 4℃ with antibody directed against P-glycoprotein or actin in TBS-T (Tris-buffered saline plus 0.1% Tween 20) containing 1% skim milk. After washes with TBS-T, the membranes were incubated with HRP-conjugated anti-mouse or anti-rabbit IgG. Immunoreactive bands were visualized using an enhanced chemiluminescence (ECL) kit (Amersham, Little Chalfont, Buckinghamshire, UK).
Dual luciferase reporter assay
Control and mtDNA-depleted HCT-8 cells in 6-well plate were cotransfected with pGL3/basic or pMDR1-luc vector together with pRL-SV40 for constitutive expression of Renilla luciferase as an internal control. Luciferase activity was measured 48 h after transfection using a Dual Luciferase assay kit in accordance with the manufacturer's instructions (Promega). The ratio of firefly luciferase activity to Renilla luciferase activity was presented in arbitrary units as the relative luciferase activities.
mRNA stability assay
Control and mtDNA-depleted HCT-8 cells were incubated with 12.5 µg/ml actinomycin D to inhibit transcription. The cells were harvested at six different time points after actinomycin addition, and total RNA was isolated as described above. The level of MDR1 transcript was analyzed by RT-PCR and real-time PCR.
Statistical analysis
The statistical significance was assessed by the T-test. A P < 0.05 was considered to be statistically significant.
Results
Establishment of mtDNA-depleted HCT-8 cells
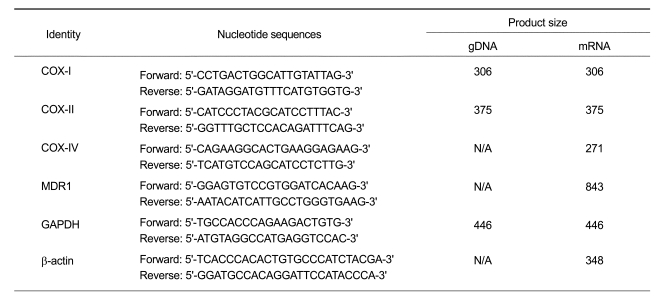
To develop partially mtDNA-depleted cell lines, we exposed HCT-8 cells to EtBr (0.2 µg/ml) in RPMI-1640 medium supplemented with pyruvate and uridine, which are shown to be essential for the growth of mtDNA-depleted cells (King and Attardi, 1989). The mtDNA content from HCT-8 cells cultured with or without EtBr was monitored routinely by amplifying total DNA. As shown in Figures 1A and B, cytochrome oxidase subunits I (COX I) and II (COX II) which are encoded only in mtDNA were hardly amplified from the total DNA of the cells treated with EtBr. In contrast, nuclear DNA-encoded GAPDH was equally detected in both control and EtBr-treated cells (Figure 1A), indicating that prolonged treatment with EtBr selectively depleted mtDNA without altering the nuclear DNA replication in HCT-8 cells. Under these experimental conditions, mtDNA was depleted to < 30% of normal cells after 3 week of growth in the presence of EtBr. We next analyzed the differential expressions of mitochondrial and nuclear genes such as COX I, II, and IV in the control and mtDNA-depleted cells. mRNA contents of COX I and COX II, which are mtDNA-encoded genes were significantly reduced in the depleted cells, whereas mRNA content of COX IV, which are encoded by nuclear DNA, were not changed in the depleted cells (Figure 1C), suggesting that mitochondrial mRNA levels correlates with the cellular mtDNA contents in these cells. In addition, the depleted cells showed a substantial decrease in total cellular ATP when compared with control cells (Figure 1D).
Figure 1.
Characterization of mtDNA-depleted HCT-8 cells. (A) Genomic DNA content. Genomic DNA was isolated from control and mtDNA-depleted HCT-8 cells, and mtDNA-encoded genes including cytochrome c oxidase subunit I (COX-I) and cytochrome c oxidase subunit II (COX-II) were amplified by PCR. GAPDH is used as a nuclear DNA-encoded control. The PCR products were electrophoresed on 2% agarose gel and then visualized by EtBr staining. A representative result of three independent experiments is shown. (B) The PCR products were quantified by densitometry, normalized to GAPDH expression, and expressed as a percentage relative to that obtained in control HCT-8 cells. The results are expressed as the means ± SD of three independent experiments. T-test: *P < 0.01. (C) RT-PCR analysis of mRNAs transcribed from mtDNA and nuclear DNA. Total RNA was extracted from control and mtDNA-depleted HCT-8 cells, and mtDNA-encoded transcripts such as COX-I and COX-II, and nuclear DNA-encoded transcripts such as COX-IV were analyzed by real-time PCR. The mRNA levels were expressed as a percentage relative to that obtained in control HCT-8 cells. The results are expressed as the means ± SD of three independent experiments. T-test: *P < 0.01. (D) Cellular ATP content. Total cellular ATP levels were measured using a somatic cell ATP assay kit as described in Materials and Methods. ATP content was expressed as a percentage relative to that observed in control HCT-8 cells. The results are expressed as the means ± SD of three independent experiments. T-test: *P < 0.05.
MDR1 mRNA and P-glycoprotein are up-regulated in mtDNA-depleted cells
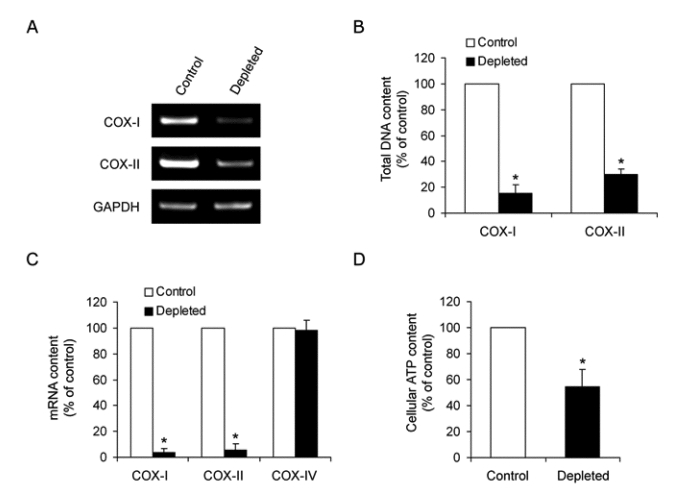
To demonstrate whether the depletion of mtDNA content in HCT-8 cells induces drug resistance, we measured the sensitivity of anti-cancer drug in the control and mtDNA-depleted cells using a cell proliferation assay. The sensitivity to doxorubicin and vincristine, P-glycoprotein-transportable compounds, is reduced in the mtDNA-depleted cells when compared with that observed in control cells (Figure 2A). We next investigated intracellular drug accumulation in mtDNA-depleted cells. As shown in Figure 2B, the uptake of paclitaxel is decreased by approximately 70% in the mtDNA-depleted cells when compared with control cells. Therefore, these results indicate that mtDNA depletion provokes impediment to chemotherapy in HCT-8 cells and suggest that the reduction of mtDNA is associated with MDR phenotype.
Figure 2.
The sensitivity to doxorubicin and the accumulation of paclitaxel. (A) 1.0 × 103 cells per well were seeded in 96-well plate to which were added a dilution series of doxorubicin (left panel) and vincristine (right panel) in triplicate and incubated for 4 days under standard culture conditions. Cell viability was assessed using a CellTiter 96 Aqueous One Solution cell proliferation assay kit (Promega). Results are expressed as means ± SD of three independent experiments. T-test: *P < 0.05. (B) Control and mtDNA-depleted HCT-8 cells were seeded in 6-well plates and grown for 48 h. The cells were then incubated with 50 nM [3H]-paclitaxel for 2 h at 37℃. At the end of incubation, the cells were cooled on ice, washed three times with ice-cold PBS, and solubilized with 0.2 ml of 1% SDS. The radioactivity in each sample was determined by scintillation counting. The Results are expressed as the means ± SD of three independent experiments. T-test: *P < 0.05.
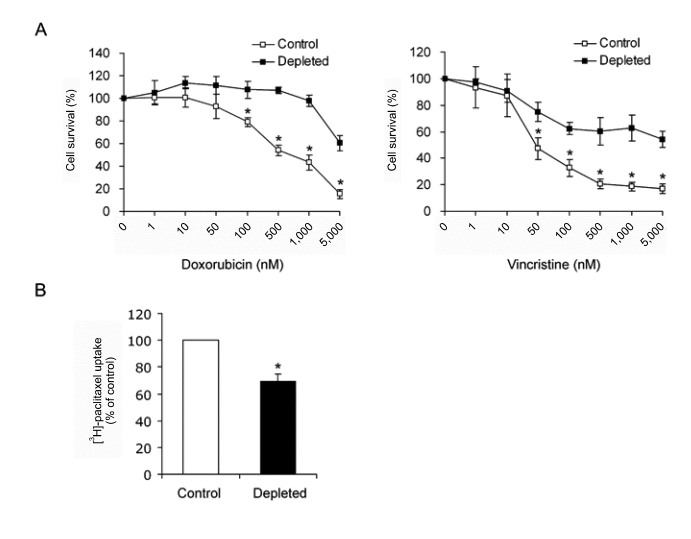
To investigate the cellular mechanism involved in the induction of MDR phenotype by mtDNA reduction, we measured the expression level of P-glycoprotein, which is encoded by the MDR1 gene and known to play a key role in drug resistance of cancer cells. Immunoblot analysis showed an increased P-glycoprotein expression in the mtDNA-depleted cells when compared with control cells (Figure 3A). The level of MDR1 mRNA is also significantly increased in mtDNA-depleted HCT-8 cells (Figure 3B). These results suggest that the decrease of sensitivity and accumulation of anti-cancer drug in the mtDNA-depleted cells might be due to a substantial increase in stead-state MDR1 mRNA levels.
Figure 3.
Expression of MDR1 mRNA and P-glycoprotein in control and mtDNA-depleted HCT-8 cells. (A) Cell lysates were prepared from control and mtDNA-depleted HCT-8 cells. Equal amounts (20 µg proteins) of cell lysates were separated via 7% SDS-PAGE and subjected to immunoblotting with anti-P-glycoprotein or anti-actin antibody. A representative result of three independent experiments is shown. (B) Total RNA was isolated from control and mtDNA-depleted HCT-8 cells, and the level of MDR1 transcript was analyzed by real-time PCR. The relative values were expressed as fold increase relative to that observed in control HCT-8 cells. The Results are expressed as the means ± SD of three independent experiments. T-test: *P < 0.01.
Up-regulation of MDR1 is mediated by the increase of mRNA stability
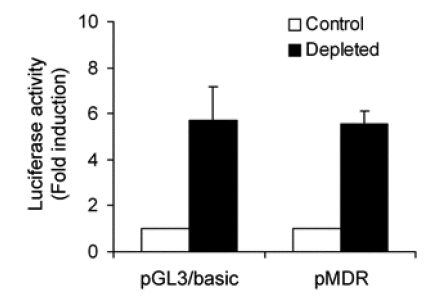
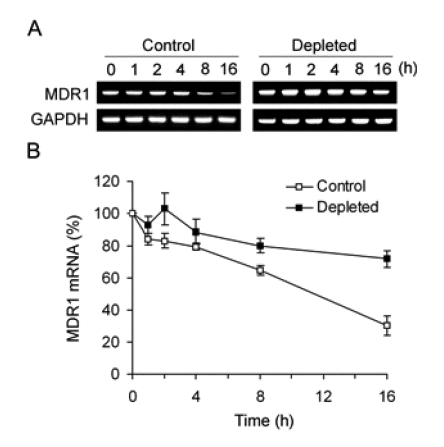
To determine whether MDR1 mRNA up-regulation was due to transcriptional activation, we performed a gene reporter assay in which an MDR1 promoter fragment was placed upstream of the luciferase reporter gene. However, promoter activity was equivalent in both control and mtDNA-depleted cells (Figure 4), indicating that the depletion of mtDNA did not upregulate the activity of MDR1 promoter. Since the increase of steady-state MDR1 mRNA was not due to transcriptional activation, we investigated whether change in the rates of mRNA decay might be involved. To determine the half-lives of MDR1 mRNA in control and mtDNA-depleted HCT-8 cells, we treated cells with actinomycin D, which is a transcriptional inhibitor that blocks further incorporation of uridine into RNA and thereby prevents increase of mRNA, and monitored the disappearance of MDR1 mRNA in the presence of actinomycin D. The results showed that the half-life of MDR1 mRNA was prolonged from approximately 10 h in normal HCT-8 cells to greater than 16 h in the mtDNA-depleted cells (Figure 5). In contrast, the half-life of a long-lived message (GAPDH) was unchanged in both cells. Thus, up-regulation of MDR1 mRNA levels following mtDNA depletion is due to an increase in mRNA stability.
Figure 4.
Analysis of MDR1 promoter activity in control and mtDNA-depleted HCT-8 cells. Promoter activity of a luciferase reporter gene driven by the MDR1 promoter was measured after transfection of control and mtDNA-depleted cells with pMDR1. Luciferase activity was normalized to Renilla luciferase activity, and shown as fold increase of luciferase activity over the value obtained from the empty pGL3 reporter vector alone. The Results are expressed as the means ± SD of three independent experiments.
Figure 5.
Stability of MDR1 mRNA in control and mtDNA-depleted HCT-8 cells. (A) Control and mtDNA-depleted HCT-8 cells were treated with 12.5 µg/ml actinomycin D for various times, followed by extraction and analysis of RNA via RT-PCR. GAPDH is used as an internal control. A representative result of three independent experiments is shown. (B) The level of MDR1 transcript was analyzed by real-time PCR. The level of MDR1 mRNA was expressed as a percentage relative to that observed at time 0. The Results are expressed as the means ± SD of three independent experiments.
Discussion
The cellular treatment with low dose EtBr specifically inhibits transcription and replication of circular DNA by deleting RNA primers required for the initiation of replication (Zylber et al., 1969; Desjardins et al., 1985; Hayakawa et al., 1998 ), and it has been a useful tool for the study of various cellular responses affected by changes in mtDNA contents (Biswas et al., 1999; Amuthan et al., 2001, 2002). Studies using mtDNA-depleted cells to evaluate the role of mtDNA in the expression of MDR1 and P-glycoprotein have yielded mixed results. Pillay and colleagues found that P-glycoprotein is up-regulated in rat hepatoma ρ0 cells (Pillay et al., 1998), whereas Liang and Ullyatt found that mRNA expression of MDR1 and multidrug resistance-associated protein 1 (MRP1/ ABCC1), putatively associated drug resistance was unchanged in mtDNA-depleted U937 cells (Liang and Ullyatt, 1998). Although Moye-Rowley and colleagues also reported that multiple signals from dysfunctional mitochondria activate the pleiotropic drug resistance pathway in S. cerevisiae (Hallstrom and Moye-Rowley, 2000; Moye-Rowley, 2005), it is not clear whether the mtDNA-depletion is a contributing factor in the expression of molecules responsible for drug resistance. In this study, we generated HCT-8 cells containing partially depleted mtDNA (<80% of control level) by EtBr treatment. The results of our study showed that the depletion of mtDNA led to an increased expression of MDR1 mRNA and P-glycoprotein in HCT-8 colon cancer cells.
Transcriptional activation is generally accepted to be the principle mechanism for up-regulating MDR1 gene expression (Labialle et al., 2002). Several regulatory elements such as Sp1, AP-1, NF-Y, and C/EBPβ, have been characterized. It is reported that MDR1 expression in cancer cells is up-regulated by activation of NF-κB (Kuo et al., 2002; Bentires-Alj et al., 2003). However, recent studies reported that the expression of MDR1 is also regulated by post-transcriptional levels (Yague et al., 2003; Gomez-Martinez et al., 2007). In this study, we found that up-regulation of MDR1 gene in the mtDNA-depleted cells results from an increase in mRNA stability. The cis-determinants involved in mRNA stability is located in the 3'-untranslated region of the transcripts (Wilusz et al., 2001). The MDR1 3' UTR contains several potential AU-binding protein recognition sites that could regulate rapid mRNA decay (Marino et al., 1990). The half-life of MDR1 mRNA in HCT-8 cells is similar to that observed in the human hepatoma cell line HepG2 (Prokipcak et al., 1999), and significantly increased by the depletion of mtDNA. Although our results demonstrated that the depletion of mtDNA induces the up-regulation of MDR1 gene via an increase of mRNA stability, a recent study provided evidence that MDR1 expression is also regulated at translational initiation (Yague et al., 2003). Thus, it remains the possibility that translational regulation is also involved in up-regulation of P-glycoprotein in the mtDNA-depleted cells. Further studies are needed for elucidating whether MDR1 expression is regulated at the translational level under mtDNA-depleted conditions.
In this study, we showed that mtDNA-depleted HCT-8 cells are resistant to doxorubicin and vincristine, P-glycoprotein-transportable compounds. Our results indicate that drug resistance is mediated by a decreased accumulation of drug via up-regulation of P-glycoprotein. However, a previous study provided evidence that the depletion of mtDNA induces a resistance on cell death via an adaptive increase in the expression of anti-oxidant enzymes (Park et al., 2004). We found that the expression of catalase is up-regulated in the mtDNA-depleted HCT-8 cells (unpublished data). Furthermore, several investigators have demonstrated that alteration of mtDNA quantity and quality induces mitochondria-to-nucleus stress signaling which changes the expression level of various nuclear-encoded genes, including anti-apoptotic proteins, Bax and bcl-2 (Amuthan et al., 2001, 2002). Therefore, it appears more likely that drug resistance by the depletion of mtDNA may be mediated by a more complicated mechanism that remains to be established.
In summary, our results showed that the depletion of mtDNA increases MDR1 gene expression in HCT-8 colon cancer cells. Furthermore, we demonstrated that up-regulation of MDR1 expression is due to mRNA stabilization. Considering that MDR1 has an important role in drug resistance of cancer cells, our identification would help to elucidate the molecular mechanism by which drug resistance is induced in the mtDNA-depleted cells.
Acknowledgments
This work was supported by the grant No. RTI04-01-01 from the Regional Technology Innovation Program of the MOCIE, Advanced Medical Technology Cluster for Diagnosis and Prediction at Kyungpook National University from MOCIE and by a grant from Korea Health 21 R&D Project, Ministry of Health and Welfare, Korea (00-PJ3-PG6-GN07-0001).
Abbreviations
- COX
cytochrome c oxidase
- EtBr
ethidium bromide
- MDR
multidrug resistance
- mtDNA
mitochondrial DNA
References
- 1.Ahn BH, Min G, Bae YS, Min DS. Phospholipase D is activated and phosphorylated by casein kinase-II in human U87 astroglioma cells. Exp Mol Med. 2006;38:55–62. doi: 10.1038/emm.2006.7. [DOI] [PubMed] [Google Scholar]
- 2.Amuthan G, Biswas G, Zhang SY, Klein-Szanto A, Vijayasarathy C, Avadhani NG. Mitochondria-to-nucleus stress signaling induces phenotypic changes, tumor progression and cell invasion. Embo J. 2001;20:1910–1920. doi: 10.1093/emboj/20.8.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amuthan G, Biswas G, Ananadatheerthavarada HK, Vijayasarathy C, Shephard HM, Avadhani NG. Mitochondrial stress-induced calcium signaling, phenotypic changes and invasive behavior in human lung carcinoma A549 cells. Oncogene. 2002;21:7839–7849. doi: 10.1038/sj.onc.1205983. [DOI] [PubMed] [Google Scholar]
- 4.Bentires-Alj M, Barbu V, Fillet M, Chariot A, Relic B, Jacobs N, Gielen J, Merville MP, Bours V. NF-kappaB transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene. 2003;22:90–97. doi: 10.1038/sj.onc.1206056. [DOI] [PubMed] [Google Scholar]
- 5.Biswas G, Adebanjo OA, Freedman BD, Anandatheerthavarada HK, Vijayasarathy C, Zaidi M, Kotlikoff M, Avadhani NG. Retrograde Ca2+ signaling in C2C12 skeletal myocytes in response to mitochondrial genetic and metabolic stress: a novel mode of inter-organelle crosstalk. Embo J. 1999;18:522–533. doi: 10.1093/emboj/18.3.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biswas G, Anandatheerthavarada HK, Avadhani NG. Mechanism of mitochondrial stress-induced resistance to apoptosis in mitochondrial DNA-depleted C2C12 myocytes. Cell Death Differ. 2005;12:266–278. doi: 10.1038/sj.cdd.4401553. [DOI] [PubMed] [Google Scholar]
- 7.Borst P. Multidrug resistant proteins. Semin Cancer Biol. 1997;8:131–134. doi: 10.1006/scbi.1997.0072. [DOI] [PubMed] [Google Scholar]
- 8.Cavalli LR, Liang BC. Mutagenesis, tumorigenicity, and apoptosis: are the mitochondria involved? Mutat Res. 1998;398:19–26. doi: 10.1016/s0027-5107(97)00223-6. [DOI] [PubMed] [Google Scholar]
- 9.Desjardins P, Frost E, Morais R. Ethidium bromide-induced loss of mitochondrial DNA from primary chicken embryo fibroblasts. Mol Cell Biol. 1985;5:1163–1169. doi: 10.1128/mcb.5.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fliss MS, Usadel H, Caballero OL, Wu L, Buta MR, Eleff SM, Jen J, Sidransky D. Facile detection of mitochondrial DNA mutations in tumors and bodily fluids. Science. 2000;287:2017–2019. doi: 10.1126/science.287.5460.2017. [DOI] [PubMed] [Google Scholar]
- 11.Gomez-Martinez A, Garcia-Morales P, Carrato A, Castro-Galache MD, Soto JL, Carrasco-Garcia E, Garcia-Bautista M, Guaraz P, Ferragut JA, Saceda M. Post-transcriptional regulation of P-glycoprotein expression in cancer cell lines. Mol Cancer Res. 2007;5:641–653. doi: 10.1158/1541-7786.MCR-06-0177. [DOI] [PubMed] [Google Scholar]
- 12.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 13.Hallstrom TC, Moye-Rowley WS. Multiple signals from dysfunctional mitochondria activate the pleiotropic drug resistance pathway in Saccharomyces cerevisiae. J Biol Chem. 2000;275:37347–37356. doi: 10.1074/jbc.M007338200. [DOI] [PubMed] [Google Scholar]
- 14.Hayakawa T, Noda M, Yasuda K, Yorifuji H, Taniguchi S, Miwa I, Sakura H, Terauchi Y, Hayashi J, Sharp GW, Kanazawa Y, Akanuma Y, Yazaki Y, Kadowaki T. Ethidium bromide-induced inhibition of mitochondrial gene transcription suppresses glucose-stimulated insulin release in the mouse pancreatic beta-cell line betaHC9. J Biol Chem. 1998;273:20300–20307. doi: 10.1074/jbc.273.32.20300. [DOI] [PubMed] [Google Scholar]
- 15.Horton TM, Petros JA, Heddi A, Shoffner J, Kaufman AE, Graham SD, Jr, Gramlich T, Wallace DC. Novel mitochondrial DNA deletion found in a renal cell carcinoma. Genes Chromosomes Cancer. 1996;15:95–101. doi: 10.1002/(SICI)1098-2264(199602)15:2<95::AID-GCC3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 16.Ivy SP, Olshefski RS, Taylor BJ, Patel KM, Reaman GH. Correlation of P-glycoprotein expression and function in childhood acute leukemia: a children's cancer group study. Blood. 1996;88:309–318. [PubMed] [Google Scholar]
- 17.Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455:152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- 18.King MP, Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science. 1989;246:500–503. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- 19.Kuo MT, Liu Z, Wei Y, Lin-Lee YC, Tatebe S, Mills GB, Unate H. Induction of human MDR1 gene expression by 2-acetylaminofluorene is mediated by effectors of the phosphoinositide 3-kinase pathway that activate NF-kappaB signaling. Oncogene. 2002;21:1945–1954. doi: 10.1038/sj.onc.1205117. [DOI] [PubMed] [Google Scholar]
- 20.Labialle S, Gayet L, Marthinet E, Rigal D, Baggetto LG. Transcriptional regulators of the human multidrug resistance 1 gene: recent views. Biochem Pharmacol. 2002;64:943–948. doi: 10.1016/s0006-2952(02)01156-5. [DOI] [PubMed] [Google Scholar]
- 21.Liang BC, Ullyatt E. Increased sensitivity to cis-diamminedichloroplatinum induced apoptosis with mitochondrial DNA depletion. Cell Death Differ. 1998;5:694–701. doi: 10.1038/sj.cdd.4400401. [DOI] [PubMed] [Google Scholar]
- 22.Marino PA, Gottesman MM, Pastan I. Regulation of the multidrug resistance gene in regenerating rat liver. Cell Growth Differ. 1990;1:57–62. [PubMed] [Google Scholar]
- 23.Moye-Rowley WS. Retrograde regulation of multidrug resistance in Saccharomyces cerevisiae. Gene. 2005;354:15–21. doi: 10.1016/j.gene.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 24.Park SY, Chang I, Kim JY, Kang SW, Park SH, Singh K, Lee MS. Resistance of mitochondrial DNA-depleted cells against cell death: role of mitochondrial superoxide dismutase. J Biol Chem. 2004;279:7512–7520. doi: 10.1074/jbc.M307677200. [DOI] [PubMed] [Google Scholar]
- 25.Park SY, Ryu J, Lee W. O-GlcNAc modification on IRS-1 and Akt2 by PUGNAc inhibits their phosphorylation and induces insulin resistance in rat primary adipocytes. Exp Mol Med. 2005;37:220–229. doi: 10.1038/emm.2005.30. [DOI] [PubMed] [Google Scholar]
- 26.Pillay V, Martinus RD, Hill JS, Phillips DR. Upregulation of P-glycoprotein in rat hepatoma rho (o) cells: implications for drug-DNA interactions. J Cell Biochem. 1998;69:463–469. [PubMed] [Google Scholar]
- 27.Polyak K, Li Y, Zhu H, Lengauer C, Willson JK, Markowitz SD, Trush MA, Kinzler KW, Vogelstein B. Somatic mutations of the mitochondrial genome in human colorectal tumours. Nat Genet. 1998;20:291–293. doi: 10.1038/3108. [DOI] [PubMed] [Google Scholar]
- 28.Prokipcak RD, Raouf A, Lee C. The AU-rich 3' untranslated region of human MDR1 mRNA is an inefficient mRNA destabilizer. Biochem Biophys Res Commun. 1999;261:627–634. doi: 10.1006/bbrc.1999.1101. [DOI] [PubMed] [Google Scholar]
- 29.Shay JW, Werbin H. Are mitochondrial DNA mutations involved in the carcinogenic process? Mutat Res. 1987;186:149–160. doi: 10.1016/0165-1110(87)90028-5. [DOI] [PubMed] [Google Scholar]
- 30.Wilusz CJ, Wormington M, Peltz SW. The cap-to-tail guide to mRNA turnover. Nat Rev Mol Cell Biol. 2001;2:237–246. doi: 10.1038/35067025. [DOI] [PubMed] [Google Scholar]
- 31.Wu H, Hait WN, Yang JM. Small interfering RNA-induced suppression of MDR1 (P-glycoprotein) restores sensitivity to multidrug-resistant cancer cells. Cancer Res. 2003;63:1515–1519. [PubMed] [Google Scholar]
- 32.Yague E, Armesilla AL, Harrison G, Elliott J, Sardini A, Higgins CF, Raguz S. P-glycoprotein (MDR1) expression in leukemic cells is regulated at two distinct steps, mRNA stabilization and translational initiation. J Biol Chem. 2003;278:10344–10352. doi: 10.1074/jbc.M211093200. [DOI] [PubMed] [Google Scholar]
- 33.Yeh JJ, Lunetta KL, van Orsouw NJ, Moore FD, Jr, Mutter GL, Vijg J, Dahia PL, Eng C. Somatic mitochondrial DNA (mtDNA) mutations in papillary thyroid carcinomas and differential mtDNA sequence variants in cases with thyroid tumours. Oncogene. 2000;19:2060–2066. doi: 10.1038/sj.onc.1203537. [DOI] [PubMed] [Google Scholar]
- 34.Zylber E, Vesco C, Penman S. Selective inhibition of the synthesis of mitochondria-associated RNA by ethidium bromide. J Mol Biol. 1969;44:195–204. doi: 10.1016/0022-2836(69)90414-8. [DOI] [PubMed] [Google Scholar]