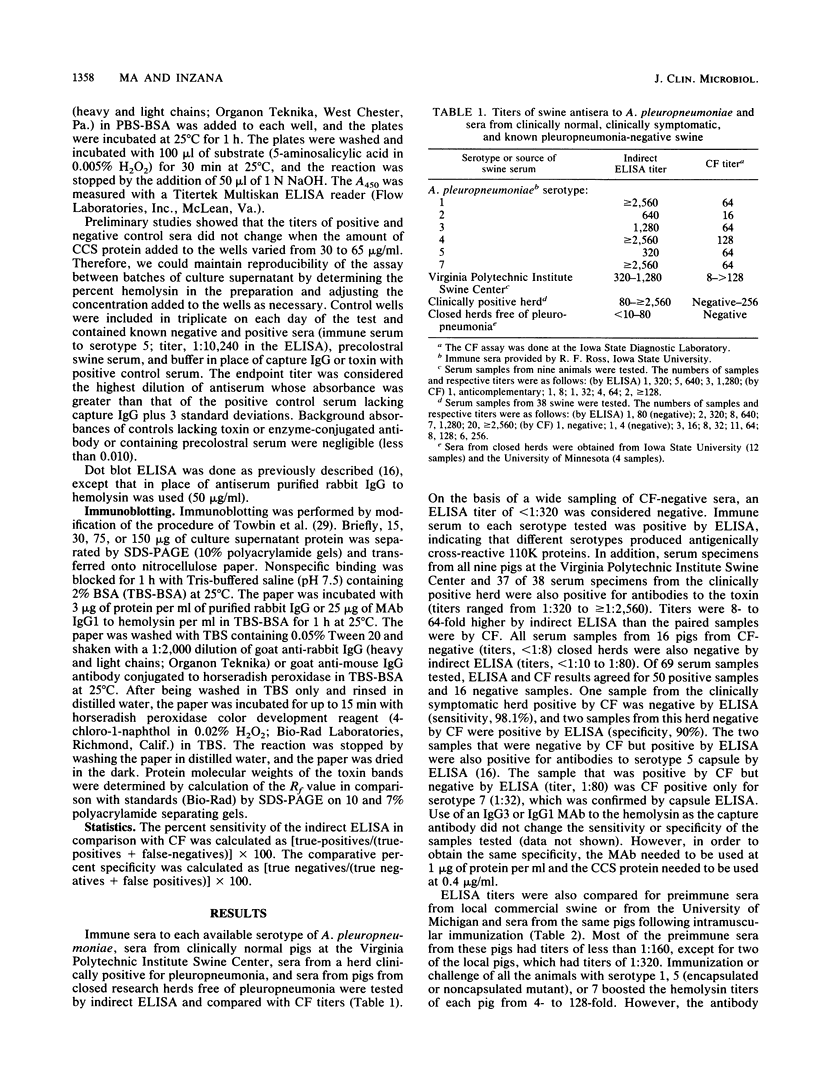
Abstract
An indirect enzyme-linked immunosorbent assay (ELISA) was developed to detect swine antibody to a 110,000-molecular-weight hemolysin (110K hemolysin) of Actinobacillus pleuropneumoniae. Affinity-purified rabbit polyclonal or mouse monoclonal immunoglobulin G to the hemolysin of A. pleuropneumoniae serotype 5 strain J45, followed by hemolysin-rich concentrated culture supernatant, was used to bind swine antibody to hemolysin to microdilution plates. Sixty-nine serum samples from swine that were clinically normal, presented with clinical evidence of pleuropneumonia, were experimentally immunized or challenged, or were free of pleuropneumonia were tested, and their ELISA titers were compared with complement fixation (CF) titers. On the basis of serum samples from swine that were clinically normal and negative by CF, an ELISA titer of 1:320 or greater was considered positive. In comparison with CF, the sensitivity of the ELISA was 98.1% and the specificity was 90%. The two samples negative by CF and positive by indirect ELISA were, however, also positive for antibody to serotype 5 capsule by ELISA. Immunization of normal pigs with whole cells or purified hemolysin boosted titers 4- to 128-fold within 4 weeks. Immunoblotting demonstrated that the affinity-purified immunoglobulin G to hemolysin used for capture in the assay recognized only a 110K protein of A. pleuropneumoniae serotypes 1 to 7, although the reactivity was quantitatively variable between serotypes. Therefore, the indirect ELISA is capable of identifying animals infected with or exposed to most, if not all, serotypes of A. pleuropneumoniae. If an indirect ELISA titer of 1:320 or greater is considered positive, the assay can be a valuable diagnostic tool in both clinical and research laboratories.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendixen P. H., Shewen P. E., Rosendal S., Wilkie B. N. Toxicity of Haemophilus pleuropneumoniae for porcine lung macrophages, peripheral blood monocytes, and testicular cells. Infect Immun. 1981 Sep;33(3):673–676. doi: 10.1128/iai.33.3.673-676.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. F., Young R., Post D., Struck D. K. Identification and characterization of the Pasteurella haemolytica leukotoxin. Infect Immun. 1987 Oct;55(10):2348–2354. doi: 10.1128/iai.55.10.2348-2354.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. F., Young R., Struck D. K. Cloning and characterization of a hemolysin gene from Actinobacillus (Haemophilus) pleuropneumoniae. DNA. 1989 Nov;8(9):635–647. doi: 10.1089/dna.1.1989.8.635. [DOI] [PubMed] [Google Scholar]
- Devenish J., Rosendal S. Identification of the heat-labile hemolysin of Actinobacillus pleuropneumoniae serotype 1. Can J Vet Res. 1989 Apr;53(2):251–254. [PMC free article] [PubMed] [Google Scholar]
- Devenish J., Rosendal S., Johnson R., Hubler S. Immunoserological comparison of 104-kilodalton proteins associated with hemolysis and cytolysis in Actinobacillus pleuropneumoniae, Actinobacillus suis, Pasteurella haemolytica, and Escherichia coli. Infect Immun. 1989 Oct;57(10):3210–3213. doi: 10.1128/iai.57.10.3210-3213.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Fedorka-Cray P. J., Huether M. J., Stine D. L., Anderson G. A. Efficacy of a cell extract from Actinobacillus (Haemophilus) pleuropneumoniae serotype 1 against disease in swine. Infect Immun. 1990 Feb;58(2):358–365. doi: 10.1128/iai.58.2.358-365.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey J., Nicolet J. Hemolysin patterns of Actinobacillus pleuropneumoniae. J Clin Microbiol. 1990 Feb;28(2):232–236. doi: 10.1128/jcm.28.2.232-236.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey J., Nicolet J. Regulation of hemolysin expression in Actinobacillus pleuropneumoniae serotype 1 by Ca2+. Infect Immun. 1988 Oct;56(10):2570–2575. doi: 10.1128/iai.56.10.2570-2575.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey J., Perrin J., Nicolet J. Cloning and expression of a cohemolysin, the CAMP factor of Actinobacillus pleuropneumoniae. Infect Immun. 1989 Jul;57(7):2050–2056. doi: 10.1128/iai.57.7.2050-2056.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-González M., Bettinger S., Ott S., Olivier P., Kadouche J., Pouletty P. Purification of murine IgG3 and IgM monoclonal antibodies by euglobulin precipitation. J Immunol Methods. 1988 Jun 28;111(1):17–23. doi: 10.1016/0022-1759(88)90054-3. [DOI] [PubMed] [Google Scholar]
- Gunnarsson A. Evaluation of different antigens in the complement-fixation test for diagnosis of Haemophilus pleuropneumoniae (parahaemolyticus) infections in swine. Am J Vet Res. 1979 Nov;40(11):1564–1567. [PubMed] [Google Scholar]
- Hull S. I., Hull R. A., Minshew B. H., Falkow S. Genetics of hemolysin of Escherichia coli. J Bacteriol. 1982 Aug;151(2):1006–1012. doi: 10.1128/jb.151.2.1006-1012.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J., Clark G. F., Todd J. Detection of serotype-specific antibodies or capsular antigen of Actinobacillus pleuropneumoniae by a double-label radioimmunoassay. J Clin Microbiol. 1990 Feb;28(2):312–318. doi: 10.1128/jcm.28.2.312-318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J., Mathison B. Serotype specificity and immunogenicity of the capsular polymer of Haemophilus pleuropneumoniae serotype 5. Infect Immun. 1987 Jul;55(7):1580–1587. doi: 10.1128/iai.55.7.1580-1587.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J. Purification and partial characterization of the capsular polymer of Haemophilus pleuropneumoniae serotype 5. Infect Immun. 1987 Jul;55(7):1573–1579. doi: 10.1128/iai.55.7.1573-1579.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp E. M., van Leengoed L. A. Serotype-related differences in production and type of heat-labile hemolysin and heat-labile cytotoxin of Actinobacillus (Haemophilus) pleuropneumoniae. J Clin Microbiol. 1989 Jun;27(6):1187–1191. doi: 10.1128/jcm.27.6.1187-1191.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodrubetz D., Dailey T., Ebersole J., Kraig E. Cloning and expression of the leukotoxin gene from Actinobacillus actinomycetemcomitans. Infect Immun. 1989 May;57(5):1465–1469. doi: 10.1128/iai.57.5.1465-1469.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maudsley J. R., Kadis S. Growth and hemolysin production by Haemophilus pleuropneumoniae cultivated in a chemically defined medium. Can J Microbiol. 1986 Oct;32(10):801–805. doi: 10.1139/m86-147. [DOI] [PubMed] [Google Scholar]
- Rapp V. J., Ross R. F., Young T. F. Characterization of Haemophilus spp. isolated from healthy swine and evaluation of cross-reactivity of complement-fixing antibodies to Haemophilus pleuropneumoniae and Haemophilus taxon "minor group". J Clin Microbiol. 1985 Dec;22(6):945–950. doi: 10.1128/jcm.22.6.945-950.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendal S., Devenish J., MacInnes J. I., Lumsden J. H., Watson S., Xun H. Evaluation of heat-sensitive, neutrophil-toxic, and hemolytic activity of Haemophilus (Actinobacillus) pleuropneumoniae. Am J Vet Res. 1988 Jul;49(7):1053–1058. [PubMed] [Google Scholar]
- Sebunya T. N., Saunders J. R. Haemophilus pleuropneumoniae infection in swine: a review. J Am Vet Med Assoc. 1983 Jun 15;182(12):1331–1337. [PubMed] [Google Scholar]
- Strathdee C. A., Lo R. Y. Extensive homology between the leukotoxin of Pasteurella haemolytica A1 and the alpha-hemolysin of Escherichia coli. Infect Immun. 1987 Dec;55(12):3233–3236. doi: 10.1128/iai.55.12.3233-3236.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]