Abstract
Many in vivo and in vitro studies have demonstrated the targeted migration of neural stem cells (NSC) to infiltrating brain tumors, including malignant glioma, highlighting a potential therapeutic approach. However, there is not enough information to apply this approach to clinical therapy. The most important things in stem cell therapy for brain tumors involve selecting the appropriate neural progenitor type and optimizing the efficiency of the cell engraftment. By histological analysis using two different live-dyes, human NSCs were shown to migrate away from the transplanted site in the direction of the expanding C6 glioma and to intermix with the tumor bed, especially with the tumor core. This intermixing occurred within 7 days when NSCs were implanted into glioma model. The time course of migratory HB1.F5 with the greatest mobility of three NSC lines was as follows. As early as 3 days after transplantation, several NSCs were found leaving the implant site, primarily approaching microsatellites and frontier cells located near the site of NSC implantation. Through 7 days post-transplantation, massive numbers of NSCs continued to be attracted to and interspersed with C6 glioma, and were finally distributed extensively throughout the whole tumor bed, including the core and penumbra of the tumor mass. However, NSCs appeared to penetrate into the tumor mass very well, whereas normal fibroblast cells could not migrate. These findings strengthen the potential for human NSCs as attractive vehicles to improve therapeutic gene delivery to cancer or glioma if they are optimized to selectively kill neoplastic cells.
Keywords: glioma, stem cells, tissue therapy, tropism
Introduction
Some malignant brain tumors, such as glioma and glioblastoma, are virtually resistant to current treatments and frequently recur, with a mean survival period of less than a year despite extensive surgical excision, radiation and chemo-therapy (Cho and Klemke, 2000; Mariani et al., 2001). One impediment to eliminating glioma cells by conventional therapy is their exceptional infiltration into surrounding neural tissues, often distinct from primary tumor localization. These microsatellites act as seeds for recurrent tumor growth (Giese et al., 2003). Therefore, new therapeutic strategies are needed to selectively target tumor cells, even going as far as to seek out cells that have escaped the main tumor mass.
Gene therapy appears to be an ideal candidate for treating brain tumors because of its selective toxicity to tumor cells (Du et al., 2006). However, the advancements in gene therapy for brain tumors have not succeeded in overcoming the major limiting factors of the delivery system. Because commonly used vectors have little or no migratory potential or specific tropism, gene therapy is quite limited in reaching glioma cells that have infiltrated the normal brain parenchyma (Rainov and Kramm, 2001). Therefore, development of an effective vehicle to deliver therapeutic molecules to areas of glioma cell invasion has been a long-standing issue. Many in vivo and in vitro studies have demonstrated that neural stem cells (NSCs) have the unique capacity to migrate throughout the brain and to aggressively target invading tumor cells, such as glioma, thus highlighting their therapeutic potential (Zhenggang et al., 2004; Khalid et al., 2005). The ability of NSC to target glioma cells for delivery of desired product(s) makes NSC a very promising delivery system in gene therapy for brain tumors. NSC-based gene therapy may be stably engrafted in the brain and chase tumor cells that are expanding out while expressing (a) therapeutic transgene product(s) (Kim et al., 2005, 2006).
The aim of this study was to investigate the migratory behavior of human NSCs in relation to aggressively invasive experimental intracranial glioma in adult rodent brain. To better distinguish NSC and glioma cells, we used DyeCycle green and Dil red to raise the color of the glioma cells and NSCs, respectively. Our findings lend further support to the amelioration of safer, more effective NSC-based gene therapy for malignant glioma.
Materials and Methods
Cell culture and establishment of stable cell lines
Rat glioma cells, C6, and human neural stem cells were grown in DMEM with 10% FBS, 10 µg/ml penicillin-streptomycin (Gibco, Grand island, NY) and incubated at 37℃ in an incubator of 5% CO2/95% air. To generate lac+ hNSC, pLHC-lacZ plasmids were transfected into hNSCs with lipofectamine (Invitrogen, Carlsbad, CA). Cells stably expressing lacZ were then selected with 200 µg/ml hygromycin (Invitrogen). C6 and human neural stem cells were labeled with Vybrant® DyeCycle™ Green stain (Molecular Probes, Eugene, Oregon) and Vybrant™DiI solution (Molecular Probes), respectively, according to the manufacturer's instruction for visualization after transplantation into rat brain.
Animal studies in vivo
To establish intracranial tumors, the C6 rat glioma line (Kim et al., 2003) was implanted into the brains of adult female Sprague Dawley (SD) rats as previously described (Tamiya et al., 1995). Briefly, animals received stereotactically guided injections (KORF Instrum 900) into the right hemisphere (Coordinates: AP +0.4; ML -2.3; DV -4.5) of 1 × 106 Vybrant® DyeCycle™ Green source stained or non-stained C6 cells suspended in 3 µl of HBSS. Five days later, animals received a second implant of NSCs through stereotactical injection of LacZ+ or DiI-labeled human NSCs into the frontal cortex (Coordinates: AP +0.4; ML -2.3; DV -2.0). On the specified days, the brains were processed as described below (e.g., for X-gal staining or fluorescence imaging of live dyes).
Tissue processing, immunohistochemistry and X-gal staining
Immunohistochemistry was performed at 3, 5 and 7 days after transplantation of the human neural stem cells. Rats were deeply anesthetized with chloral hydrate and transcardially perfused with isotonic saline and ice-cold 4% paraformaldehyde. The brains were removed, immersed in 30% sucrose in 0.1 M phosphate buffer at 4℃, and then frozen and sectioned coronally at 30 µm on a cryostat through the striatum. A series of consecutive sections/brain were prepared for further processing (Jung et al., 2007). Beta-galactosidase was detected by incubating brain sections for 3 h at 37℃ in reaction solution consisting of PBS containing 4 mM potassium ferricyanide (Sigma, St. Louis, MO), 4 mM potassium ferrocyanide (Sigma), 4 mM magnesium chloride (Sigma), and 0.4 mg/ml 5-bromo-4-chloro-3-indolyl β-D-galactoside (X-gal, Sigma). After rinsing in PBS, the sections were counterstained with hematoxylin and eosin, and mounted for microscopy. DyeCycle™-labeled C6 rat glioma cells or DiI-labeled hNSCs were mounted on slices with Vectashield® Hard Set™ mounting medium (Vector, CA), and were observed with an Olympus IX71 confocal laser scanning microscope (Olympus, Tokyo, Japan).
Results
Infiltrating nature of C6 glioma cells
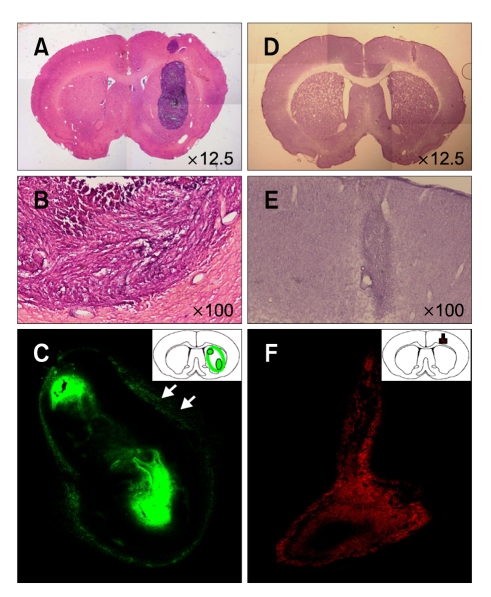
To visualize the growth of the tumor mass, SD rat was stereotactically injected with Vybrant® DyeCycle™ Green-stained C6 rat glioma cells in right striatum. Five days later, the animal received another injection of HBSS in the right cortex to emulate minor trauma in the CNS. After another five or seven days, the animal was sacrificed to observe the brain sections (Figure 1A-C). C6 rat glioma cells developed into a huge mass, occupying almost the entire striatum (dark hematoxylin-stained mass in Figure 1A and B). The live-dye, Vybrant® DyeCycle™ Green, provided contrastive images: the fluorescence was detected much more clearly in the quiescent cells than in the proliferating cells of the glioma mass since the cellular dye was diluted by repeated mitosis. Therefore, as DyeCycle-labeled C6 cells were transplanted and established a glioma mass, DyeCycle fluorescence was selectively detected only in the non-mitotic zone of the tumor mass, including the tumor core and invading edge (Figure 1C). Interestingly, the DyeCycle image of the glioma mass clearly displayed not only the frontier cells of the tumor mass infiltrating into normal tissue, but also some solitary tumor cells escaping far from the tumor mass (Figure 1C, white arrow). As a whole, C6 glioma cells were found to expand expeditiously around the implanted site regardless of the presence (Figure 6A) or absence (Figure 1A-C) of NSCs, implicating their extremely infiltrating nature.
Figure 1.
Infiltrating nature of C6 glioma and NSC HB1.F5 in normal brain. A-C: SD rat was stereotactically injected with Vybrant® DyeCycle™ Green-stained C6 rat glioma cells in the right striatum. Five days later, the right cortex of the animal received another injection with HBSS to emulate minor trauma in CNS. Seven days later, the brain sections of the recipient were observed. C6 rat glioma cells were developed to form a huge mass, occupying almost the entire striatum. (A and B) Hematoxylin and eosin-stained and (C) DyeCycle-labeled C6 glioma. D-F: SD rat received the 1st injection of C6 cell-free HBSS in the right striatum; five days later, the right cortex of the animal received a 2nd stereotaxic injection of Dil-labeled HB1.F5 cells. The brain sections of the recipient were then assessed. Whole NSCs remained at the original injection site as long as a week. (D) Hematoxylin and eosin-stained, and (E and F) Dil-labeled HB1.F5 cells.
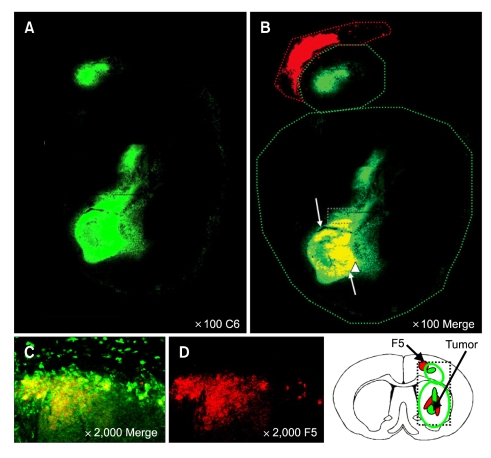
Figure 6.
NSCs were distributed throughout the tumor bed. Dil-labeled HB1.F5 cells were infused into rodent brain previously implanted with DyeCycle™-labeled C6 glioma cells. Seven days later, recipients were sacrificed and their brain sections were processed to detect fluorescing cells. NSCs invaded the tumor bed until they were mixed throughout the tumor bed that occupied the whole striatum and cortex, especially in the tumor core as noted by the white arrow in B. (A and B) Low-power (100 ×) and (C and D) High-power (2,000 ×) confocal images. (B and C) Merged images of DyeCycle-labeled C6 and Dil-labeled NSCs. Green: DyeCycle-labeled C6, Red: Dil-labeled NSC.
NSC in normal brain conditions
To determine the behavior of NSCs in normal brain conditions, SD rat received an injection of C6 cell-free HBSS in the right striatum. Five days later, the animal received an injection of human NSCs stereotactically in the right cortex. The brain sections of the recipient demonstrated that the vast majority of NSCs remained at the original injection site for as long as a week, forming a small mass of NSC without any sign of expansion. This finding indicated that NSCs themselves were not tumorigenic, which was consistent with previous reports (Aboody et al., 2000). Meanwhile, a few NSCs moved slightly to the needle track of the 2nd injection (Figure 1D-F), implying that NSCs could not be driven to move far away by simple trauma such as an injection wound.
Tumor tropism of human NSCs : HB1.F5 can migrate powerfully
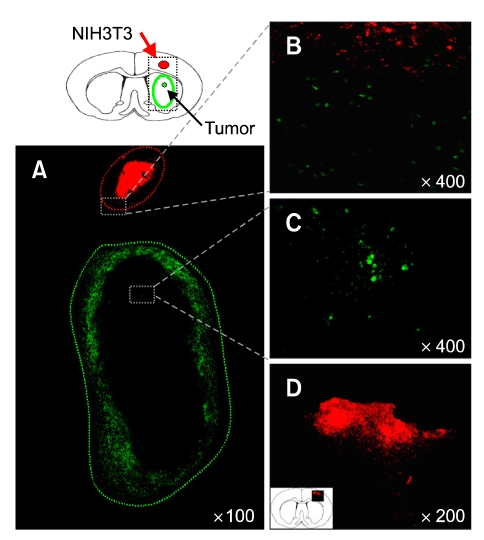
To confirm that the migration of NSCs to brain tumors is NSC-specific, mouse fibroblast cells NIH3T3 were, at first, transplanted to the cortex of rat tumor model. Majority of NIH3T3 cells did not migrate and remained at the original injection site in brain tumor model as well as in normal brain condition (Figure 2). In addition, there was no intermixing of tumor cells and NIH3T3 cells in the boundary of tumor mass even 2 weeks after transplantation. This result indicated that fibroblast cells do not have the ability of tropism for tumor.
Figure 2.
NIH3T3 cells did not migrate to developing tumor bed. (A) NIH3T3 cells were transplanted to the same hemisphere in which tumor cells was received. Almost all NIH3T3 cells did not migrate to tumor mass (B and C). The high power images of (A) Green: dyecycle green labeled C6, Red: Dil labeled NIT3T3. (D) In addition, NIH3T3 cells did not move to anywhere in normal brain.
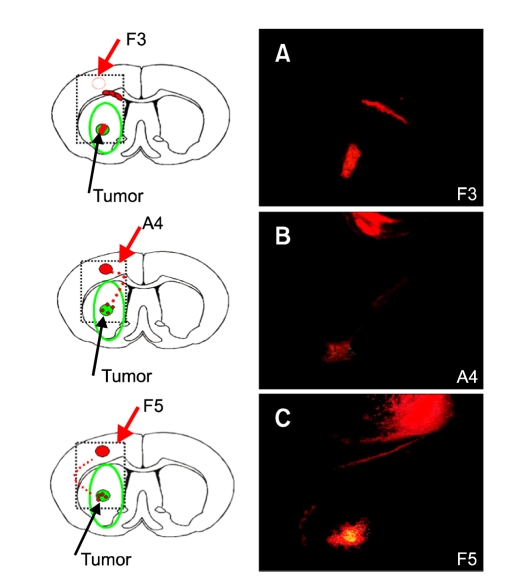
To compare the tumor tropism of various lineages of human NSCs (HB1.F3, A4 or F5), Dil-labeled HB1.F3, A4 or F5 cells were infused into rodent brain that was previously implanted with DyeCycle™-labeled C6 glioma cells. Five days later, the rat was sacrificed to assess the migratory capacity and the routes of NSC to target the glioma mass, compared with the responses to the cell-free HBSS-injected control. HB1.F3, A4 and F5 migrated toward the tumor mass and seemed to seek out the infiltrating microsatellites, far from their initial seeding site, through non-stereotypical routes (Figure 3). Some HB1.F3 cells migrated and populated the tumor core while others remained near the corpus callosum (Figure 3A). HB1.A4 and HB1.F5 left behind a few cells along the tracking path, but HB1.F3 cells did not. HB1.A4 cells migrated through the corpus callosum beside the subventricular zone to reach the tumor core (Figure 3B). HB1.F5 cells migrated along the corpus callosum rather than through it (Figure 3C). The motility of the three NSC lineages, HB1.F3, A4 and F5, seemed to vary. Actually, HB1.F5 cells had the strongest motility compared with the migration of HB1.A4 and HB1.F3 cells.
Figure 3.
Tumor tropism of HB1.F3, A4 or F5 cells: F5 migrate powerfully. Dil-labeled HB1.F3, A4 or F5 cells were infused into rodent brain previously implanted with DyeCycle™-labeled C6 glioma cells. Five days later, the rat was sacrificed to compare the migratory capacity and routes to target the glioma mass. The migration of HB1.F3, A4 and F5 was directed toward the tumor mass and seemed to seek out the infiltrating microsatellites, far from their initial seeding site, along non-stereotypical routes. (A) HB1.F3, (B) HB1.A4, (C) HB1.F5 cells.
NSCs approached glioma cells in the vicinity of the NSC mass
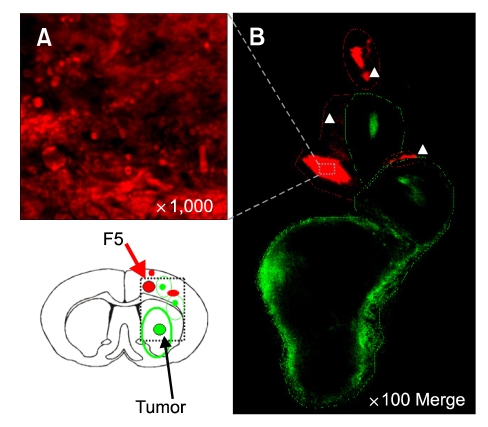
To investigate the tumor tropism of NSCs in a time-dependent manner, we transplanted Dil-labeled HB1.F5, having the high motility activity, into the rat glioma model. Recipients were sacrificed at 2-day intervals after NSC transplantation and brain sections were processed to detect NSC in relation to glioma cells in hematoxylin and eosin stained images and in fluorescent images with green glioma and red NSCs. As a whole, extensive migration of Dil-labeled red HB1.F5 cells was noted in the vicinity of the green-stained infiltrating glioma (Figure 4, 5 and 6). As early as three days after HB1.F5 transplantation, some NSCs had migrated away from the transplantation site toward the developing C6 tumor bed, directed toward two satellite tumor masses in preference to the main tumor mass. However, most NSCs still remained around the seeding site, without being co-localized with the glioma mass (Figure 4). We also observed that a few NSCs appeared to track microsatellites, single tumor cells escaping from the tumor mass, as demarcated by white arrowheads in Figure 4B. In the meantime, glioma cells expanded to distribute themselves through the right-most hemisphere, passing over the corpus callosum as they migrated as far as the cortex to build two satellite tumor masses, each with a core as outlined by the green line in Figure 4B.
Figure 4.
NSCs approached glioma cells in the vicinity of NSC mass. Dil-labeled HB1.F5 cells were infused into the rodent brain previously implanted with DyeCycle™-labeled C6 glioma cells. Three days later, recipients were sacrificed and their brain sections processed to detect fluorescing cells. Most NSC localized near the transplanted site (red arrows in left lower panel). Extensive migration of Dil-labeled red HB1.F5 was noted in the vicinity of the green-stained infiltrating glioma (as demarcated by the white arrow in B). Green: DyeCycle green-labeled C6, Red: Dil-labeled NSCs (A) High resolution (1,000 ×) of transplanted NSC. (B) 100 × merged image.
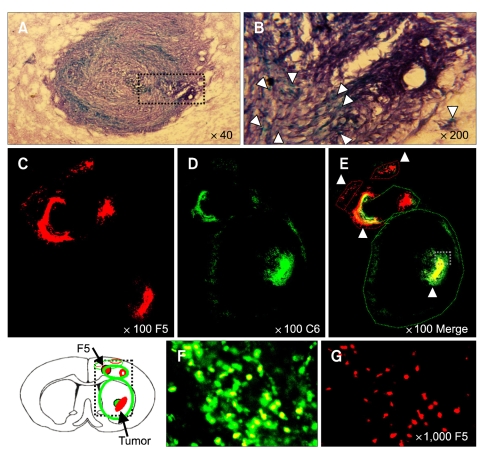
Figure 5.
NSCs progressively invade the tumor core, surrounding the glioma mass. Dil-labeled HB1.F5 cells were infused into the rodent brain previously implanted with DyeCycle™-labeled C6 glioma cells. Five days later, recipients were sacrificed and their brain sections processed for observation. C6 rat glioma cells continued to disseminate themselves through the entire striatum of the recipient's brain and their mass enlarged to reach almost to the brain surface. X-gal-stained blue NSCs were located in virtual juxtaposition along the border of the C6 tumor mass stained by hematoxylin where the tumor interfaced with normal tissue that was being aggressively infiltrated (as indicated by white arrowheads in B). Several NSCs were also found invading deeply into the tumor mass and up to the core and intermixing with tumor cells as shown in the merged images (E and F). In the cortex, NSCs appeared to surround the tumor mass and to prevent the tumor cells from moving into the normal brain tissue. A few NSCs pursued the solitary tumor cells that escaped from the cerebral cortex (B and E). It was particularly noteworthy that numerous NSCs reached the tumor core, far from the original injection site and intermixed with the tumor cells as shown in the merged images and noted by the white arrowhead in B, E and F. (A) Low-resolution (40 ×) and (B) High-resolution images of Hematoxylin-stained C6 and X-gal stained NSC. (C-E) Low-power and (F and G) High-power confocal images. (E and F) merged images. Green: DyeCycle-labeled C6, Red: Dil-labeled NSC.
NSCs progressively invade the tumor core, surrounding glioma mass
C6 rat glioma cells continued to disseminate throughout the recipient's entire striatum and their mass enlarged, reaching the brain surface after 5 days. At the same time, X-gal-stained blue NSCs were located in virtual juxtaposition along the border of the C6 tumor mass stained by hematoxylin where the tumor interfaced with normal tissue that was being aggressively infiltrated (Figure 5A and B, white arrowheads). Several NSCs also invaded deeply into the tumor mass and up to the core, and intermixed with tumor cells as shown in the merged images (Figure 5C-F). In the cortex, NSCs appeared to surround the tumor mass and to shield the tumor cells so that they could not move into the normal brain tissue. A few NSCs pursued the solitary tumor cells escaping from the cerebral cortex, analogous to the observation on the 3rd day of NSC transplantation (Figure 5B and E). It was particularly noteworthy that numerous NSCs reached the tumor core, far from the original injection site, and intermixed with the tumor cells, as shown in the merged images and noted by the white arrowheads (Figure 5B, E and F).
NSCs were mixed throughout the tumor bed
Invasion of NSCs into the tumor bed progressed until they were intermixed throughout the tumor bed that occupied the whole striatum and cortex, especially in the tumor core as shown by the merged image (white arrows in Figure 6B). Although transplanted NSCs appeared to prevent the expansion of the tumor mass in cortex, the tumor mass was not regressed by transplanted HB1.F5 compared with the control group (Figure 1E). Consistent with previous reports that showed tumor tropism of murine or human NSCs (Aboody et al., 2000; Kim et al., 2005), these findings suggest that soluble chemotactic factors produced by glioma cells, particularly from the tumor core and from solitary tumor cells infiltrating normal brain tissues, are in part responsible for inducing and guiding NSC migration.
Discussion
The benefit derived from traditional cancer therapies is low for malignant brain tumors including glioma because of defects in apoptotic machinery, which account for their resistance to radiation- and chemo-therapy, and their infiltrating nature, which provokes frequent recurrence despite extensive excision (Giese et al., 2003). Although recent studies suggest several promising therapies, such as oncolytic virus, gene therapy, etc., aggressive brain tumors remain largely incurable. The major reasons for this failure are defective vector, limited survival of viral vector due to immune rejection and inability of therapeutic vectors to access tumor cells because of their infiltrating nature. Therefore, new vehicles are required to effectively deliver therapeutic molecules to specific areas of rapidly invading tumors, e.g., glioma as shown in Figure 1. The extensive directed-migration of NSCs has made them useful in therapeutic paradigms. NSCs actually seek out tumor foci that have infiltrated far from the main tumor mass, a common occurrence with glioma, suggesting that NSCs armed with oncolytic molecules are capable of effectively and specifically delivering these molecules to areas of glioma cell invasion to increase survival time, (Kim et al., 2005, 2006). The motility pattern and velocity of three different NSC lineages, HB1.F3, A4 and F5, varied. HB1.F5 had the greatest motility (Figure 3). Initially NSCs targeted the glioma cells in their own vicinity, e.g., the penumbra and border of the tumor mass. Then, they progressively invaded the main tumor mass, especially up to the core region, where they interspersed with glioma cells (Figure 4, 5 and 6). Nevertheless, the tumor mass did not regress with NSC invasion compared with the control group (Figure 1E). These relationships between NSC and glioma were elucidated using live dyes, DyeCycle green and Dil red. Interestingly, the DyeCycle image of established glioma clearly displayed that the frontier cells of tumor mass were infiltrating normal tissue and single cells from the penumbra were escaping from the tumor mass (Figure 1C) according to contrastive staining of Dil-labeled NSC.
The mechanism of tumor tropism by NSC has been proposed as follows: 1) the tumor itself elaborates (a) tropic signal(s) to drive NSC migration, by both contact- and non-contact-mediated factors; or 2) this motility is partly due to tropic cytokines released by damaged tissue and NSCs are presumably drawn by the degenerative or inflammatory reaction in the close vicinity of the tumor to surround the invading tumor border or the wound due to the tissue damage of establishing an experimental tumor bed (Snyder et al., 1997; Aboody et al., 2000; Sun et al., 2004). We could not replicate the powerful tropism of NSCs in animals in which a needle was inserted to emulate the tissue damage of establishing an experimental tumor bed but without the actual implantation of glioma cells (Figure 1B); thus, we surmise that minor CNS destruction alone could not prompt the dramatic migration seen in the glioma model. In addition, NIH3T3 cells did not migrate to brain tumor of animal model, suggesting that cell migration to brain tumor is NSC-specific. These findings agreed with a previous in vitro study in which NSCs migrated rapidly and interspersed throughout the glioma monolayer, far from their initial seeding site, in contrast to fibroblasts which remained localized to the initial seeding area (Aboody et al., 2000). In comparison, Figure 1D-F displays that trauma due to NSC-injection rendered NSCs to move slightly, suggesting that the presence of tropic agent(s) in conjunction with minor trauma was not sufficient for migration. We also observed that the NSCs migrated freely through the tumor, but slowed down without direction in normal adult brain parenchyma where the environment seemed to be less permissive for their migration. Moreover, NSCs appeared to preferentially track microsatellites, single tumor cells that leave the tumor mass, as demarcated by the white arrows in Figures 4B and 5E, raising the possibility that powerful tropic signal(s) could be instilled from the tumor mass, especially from the frontier cells and microsatellites. Recently, NSCs is reported to localized to neuroblastoma metastases in bone marrow besides brain tumor (Aboody et al., 2006). All these features of NSC migration lead to the conclusion that the migratory behavior of NSCs is mediated by various signals derived from multiple sources, including attractants, adhesion and substrate molecules, chemokines, etc. For instance, as gliomas progress and invade, extensive modulation of the extracellular matrix occurs (Ziu et al., 2006). In addition, the brain tumor-targeting behavior of NSCs was recently suggested to be mediated by chemoattractant molecules and their respective receptors, including stem cell factor (SCF)/c-Kit (Sun et al., 2004), stromal cell-derived factor 1 (SDF-1)/CXC chemokine receptor 4 (CXCR4; Ehtesham et al., 2004; Ratajczak et al., 2006) and VEGF/VEGF receptor (VEGFR)-1 and VEGFR2 (Schmidt et al., 2005).
Our findings fortify the potential of NSC-based gene therapy aimed at malignancies. In the near future, neural stem cells might be used in parallel with standard therapies to reduce the incidence of recurrence and to improve patient survival.
Acknowledgments
This research was supported by grants from the BK21 Program of the Ministry of Education and Human Resource Development, from a Neurobiology Research Program grant from the Korea Ministry of Science and Technology, and a grant (SC3090) from Stem Cell Research Center of the 21st Century Frontier Research Program, funded by the Ministry of Science and Technology, Republic of Korea. This work was also supported by a Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-2006-532-C00010).
Abbreviations
- AP
anterior posterior
- DV
depth
- ML
mid line
- NSC
neural stem cell
References
- 1.Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, Small JE, Herrlinger U, Ourednik V, Black PM, Breakefield XO, Snyder EY. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aboody KS, Bush RA, Garcia E, Metz MZ, Najbauer J, Justus KA, Phelps DA, Remack JS, Yoon KJ, Gillespie S, Kim SU, Glackin CA, Potter PM, Danks MK. Development of a tumor-selective approach to treat metastatic cancer. PLoS ONE. 2006;20(1):e23. doi: 10.1371/journal.pone.0000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho SY, Klemke RL. Extracellular-regulated kinase activation and CAS/Crk coupling regulate cell migration and suppress apoptosis during invasion of the extracellular matrix. J Cell Biol. 2000;149:223–226. doi: 10.1083/jcb.149.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du ZX, Zhang HY, Gao da X, Wang HQ, Li YJ, Liu GL. Antisurvivin oligonucleotides inhibit growth and induce apoptosis in human medullary thyroid carcinoma cells. Exp Mol Med. 2006;38:230–240. doi: 10.1038/emm.2006.28. [DOI] [PubMed] [Google Scholar]
- 5.Ehtesham M, Yuan X, Kabos P, Chung NH, Liu G, Akasaki Y, Black KL, Yu JS. Glioma tropic neural stem cells consist of astrocytic precursors and their migratory capacity is mediated by CXCR4. Neoplasia. 2004;6:287–293. doi: 10.1593/neo.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21:1624–1636. doi: 10.1200/JCO.2003.05.063. [DOI] [PubMed] [Google Scholar]
- 7.Jung DS, Baek SY, Park KH, Chung YI, Kim HJ, Kim CD, Cho MK, Han ME, Park KP, Kim BS, Kim JB, Oh SO. Effects of retinoic acid on ischemic brain injury-induced neurogenesis. Exp Mol Med. 2007;39:304–315. doi: 10.1038/emm.2007.34. [DOI] [PubMed] [Google Scholar]
- 8.Khalid S, Emilie B, Kim DE, Atherine Y, Yi T, Ralph W, Xandra OB. Glioma therapy and real-time imaging of neural precursor cell migration and tumor regression. Ann Neurol. 2005;57:34–41. doi: 10.1002/ana.20306. [DOI] [PubMed] [Google Scholar]
- 9.Kim SK, Cargioli TG, Machluf M, Yang W, Sun Y, Al-Hashem R, Kim SU, Black PM, Carroll RS. PEX producing human neural stem cell inhibit tumor growth in a mouse glioma model. Clin Cancer Res. 2005;11:5965–5970. doi: 10.1158/1078-0432.CCR-05-0371. [DOI] [PubMed] [Google Scholar]
- 10.Kim SK, Kim SU, Park IH, Bang JH, Aboody KS, Wang KC, Cho BK, Kim M, Menon LG, Black PM, Carr RS. Human neural stem cells target experimental intracranial medulloblastoma and deliver a therapeutic gene leading to tumor regression. Clin Cancer Res. 2006;12:5550–5556. doi: 10.1158/1078-0432.CCR-05-2508. [DOI] [PubMed] [Google Scholar]
- 11.Kim TE, Lee HS, Lee YB, Hong SH, Lee YS, Ichimose H, Kim SU, Lee MA. Sonic hedgehog and FGF8 collaborate to induce dopaminergic phenotypes in the Nurr1overexpressing neural stem cell. Biochem Biophys Res Commun. 2003;305:1040–1048. doi: 10.1016/s0006-291x(03)00879-9. [DOI] [PubMed] [Google Scholar]
- 12.Mariani L, Beaudry C, McDonough WS. Glioma cell motility is associated with reduced transcription of proapoptotic and proliferation genes: a cDNA microarray analysis. J Neurooncol. 2001;53:161–176. doi: 10.1023/a:1012253317934. [DOI] [PubMed] [Google Scholar]
- 13.Rainov NG, Kramm CM. Vector delivery methods and targeting strategies for gene therapy of brain tumors. Current Gene Therapy. 2001;1:367–383. doi: 10.2174/1566523013348445. [DOI] [PubMed] [Google Scholar]
- 14.Ratajczak MZ, Zuba-Surma E, Kucia M, Reca R, Wojakowski W, Ratajczak J. The pleiotropic effects of the SDF-1.CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia. 2006;20:1915–1924. doi: 10.1038/sj.leu.2404357. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt NO, Przylecki W, Yang W, Ziu M, Teng Y, Kim SU, Black PM, Aboody KS, Carroll RS. Brain tumor tropism of transplanted human neural stem cells is induced by vascular endothelial growth factor. Neoplasia. 2005;7:623–629. doi: 10.1593/neo.04781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snyder EY, Yoon CH, Flax JD, Macklis JD. Targeted neocortical cell death in adult mice guides migration and differentiation of transplanted embryonic neurons. Proc Natl Acad Sci USA. 1997;94:11663–11668. [Google Scholar]
- 17.Sun L, Lee J, Fine HA. Neuronally expressed stem cell factor induces neural stem cell migration to areas of brain injury. J Clin Invest. 2004;113:1364–1374. doi: 10.1172/JCI20001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamiya T, Wei MX, Chase M, Ono Y, Lee F, Breakefield XO, Chiocca EA. Transgene inheritance and retroviral infection contribute to the efficiency of gene expression in solid tumors inoculated with retroviral vector producer cells. Gene Ther. 1995;2:531–538. [PubMed] [Google Scholar]
- 19.Zhenggang Z, Quan J, Feng J, Gaungliang D, Ruilan Z, Lei W, Li Z, Adam MR, Mark K, Michael C. In vivo magnetic resonance imaging tracks adult neural progenitor cell targeting of brain tumor. NeuroImage. 2004;23:281–287. doi: 10.1016/j.neuroimage.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 20.Ziu M, Schmidt NO, Cargioli TG, Aboody KS, Black PM, Carroll RS. Glioma-produced extracellular matrix influences brain tumor tropism of human neural stem cells. J Neurooncol. 2006;79:125–133. doi: 10.1007/s11060-006-9121-5. [DOI] [PubMed] [Google Scholar]