Abstract
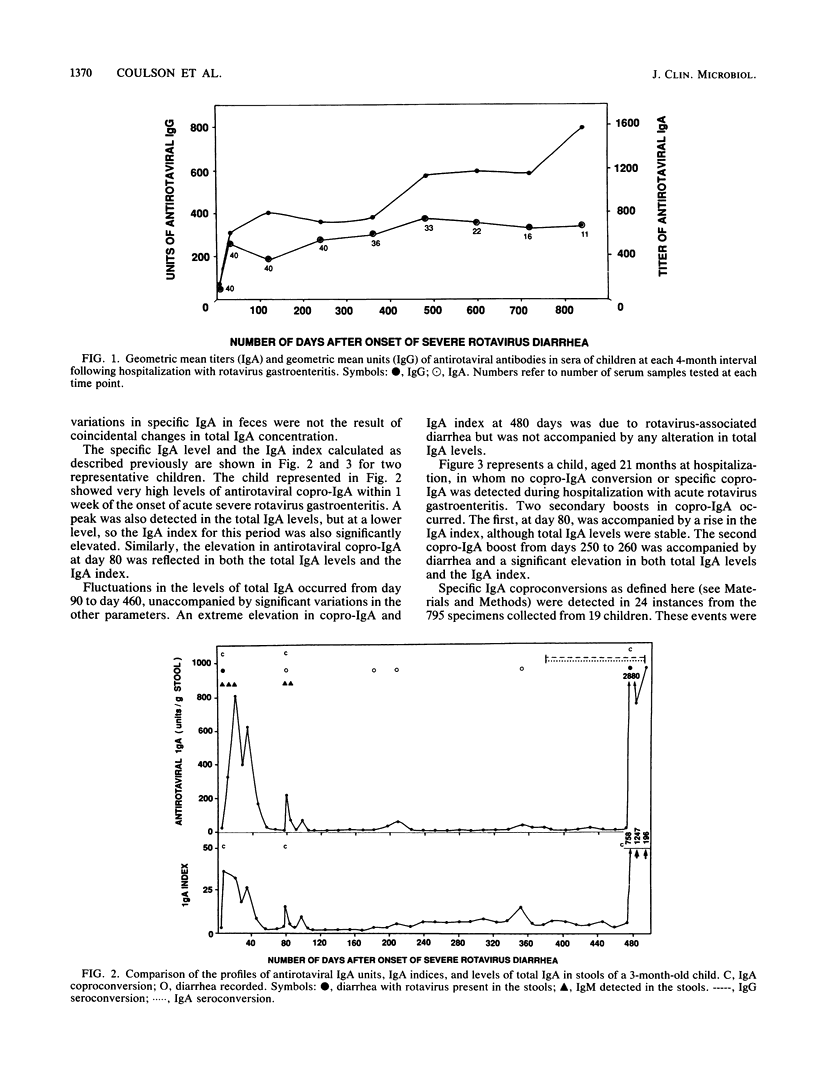
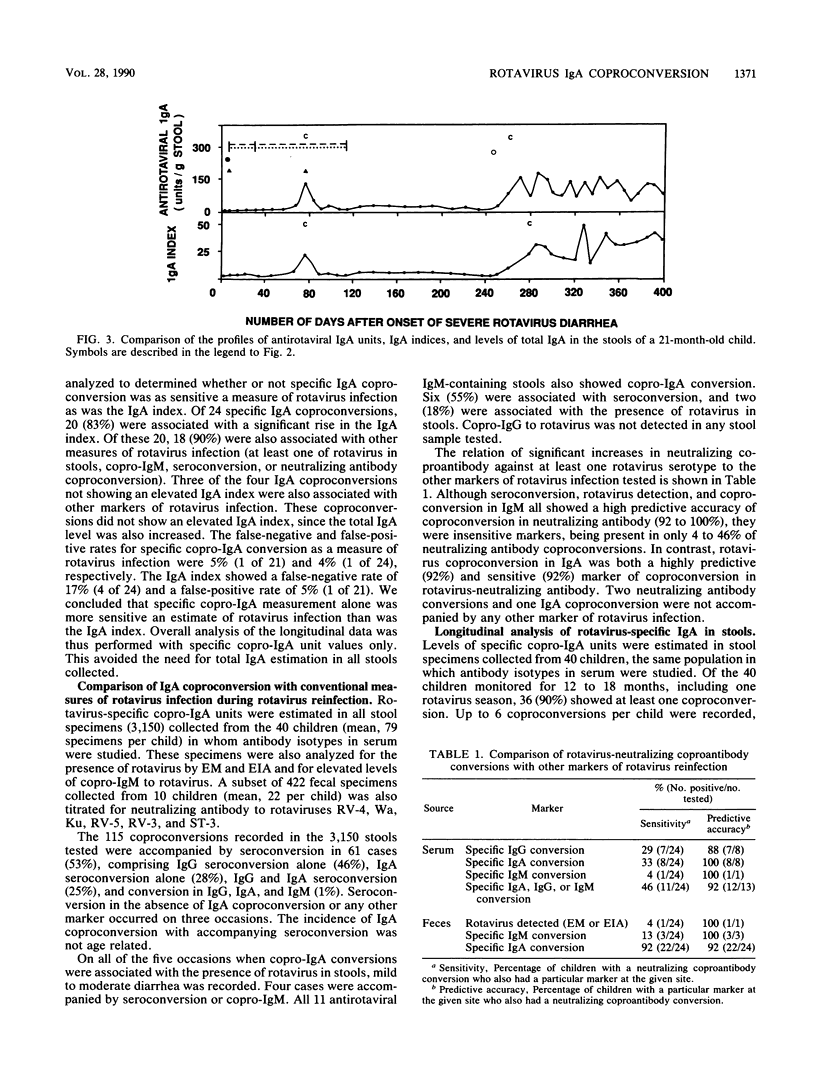
In order to determine the sensitivity and reliability of antirotaviral fecal immunoglobulin A (IgA) as an indicator of rotavirus reinfection, the antibody responses to rotavirus of 44 infants with severe rotavirus gastroenteritis recruited on admission to a hospital were studied. Feces were collected daily during hospitalization and weekly thereafter, and sera were obtained every 4 months, for 6 to 32 months (median, 17 months). Antirotaviral IgG, IgA, and IgM were measured by enzyme immunoassay in all samples. Rotavirus antigen, rotavirus-neutralizing antibody, and total IgA were measured in feces. The results showed that use of an IgA index (ratio of specific IgA to total IgA) was unnecessary to identify copro-IgA conversion to rotavirus. The other markers of rotavirus infection tested showed a high level of predictive accuracy of coproconversion in rotavirus-neutralizing antibody. Copro-IgM, serum IgM, and virus in feces were insensitive measures of neutralizing antibody coproconversion. Seroconversion in IgG or IgA was detected in 46% of neutralizing coproconversions. The most sensitive marker, present in 92% of neutralizing coproconversions, was antirotaviral fecal IgA conversion. This correlation of fecal IgA with fecal neutralizing antibody suggests that coproconversions in IgA represent true elevations in antirotaviral IgA with neutralizing capacity. A coproconversion in IgA appears to indicate genuine rotavirus infection. Copro-IgA conversions in feces collected weekly are likely to be more sensitive markers of rotavirus reinfection than are seroconversion and virus detection combined in epidemiological studies of acute diarrhea in children and in rotavirus vaccine trials.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert M. J., Bishop R. F. Cultivation of human rotaviruses in cell culture. J Med Virol. 1984;13(4):377–383. doi: 10.1002/jmv.1890130409. [DOI] [PubMed] [Google Scholar]
- Angeretti A., Magi M. T., Merlino C., Ferrara B., Negro Ponzi A. Specific serum IgA in rotavirus gastroenteritis. J Med Virol. 1987 Dec;23(4):345–349. doi: 10.1002/jmv.1890230406. [DOI] [PubMed] [Google Scholar]
- Bachmann P. A., Hess R. G. Local immunity in rotaviral infections. Ann Rech Vet. 1983;14(4):502–506. [PubMed] [Google Scholar]
- Bernstein D. I., Ziegler J. M., Ward R. L. Rotavirus fecal IgA antibody response in adults challenged with human rotavirus. J Med Virol. 1986 Dec;20(4):297–304. doi: 10.1002/jmv.1890200402. [DOI] [PubMed] [Google Scholar]
- Bishop R. F., Cipriani E., Lund J. S., Barnes G. L., Hosking C. S. Estimation of rotavirus immunoglobulin G antibodies in human serum samples by enzyme-linked immunosorbent assay: expression of results as units derived from a standard curve. J Clin Microbiol. 1984 Apr;19(4):447–452. doi: 10.1128/jcm.19.4.447-452.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black R. E., Greenberg H. B., Kapikian A. Z., Brown K. H., Becker S. Acquisition of serum antibody to Norwalk Virus and rotavirus and relation to diarrhea in a longitudinal study of young children in rural Bangladesh. J Infect Dis. 1982 Apr;145(4):483–489. doi: 10.1093/infdis/145.4.483. [DOI] [PubMed] [Google Scholar]
- Brüssow H., Werchau H., Lerner L., Mietens C., Liedtke W., Sidoti J., Sotek J. Seroconversion patterns to four human rotavirus serotypes in hospitalized infants with acute rotavirus gastroenteritis. J Infect Dis. 1988 Sep;158(3):588–595. doi: 10.1093/infdis/158.3.588. [DOI] [PubMed] [Google Scholar]
- Cameron D. J., Bishop R. F., Veenstra A. A., Barnes G. L. Noncultivable viruses and neonatal diarrhea: fifteen-month survey in a newborn special care nursery. J Clin Microbiol. 1978 Jul;8(1):93–98. doi: 10.1128/jcm.8.1.93-98.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy C., Madore H. P., Pichichero M. E., Gala C., Pincus P., Vosefski D., Hoshino Y., Kapikian A., Dolin R. Field trial of rhesus rotavirus vaccine in infants. Pediatr Infect Dis J. 1988 Sep;7(9):645–650. doi: 10.1097/00006454-198809000-00009. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Dolan K. T., Horton-Slight P., Palmer J., Plotkin S. A. Diverse serologic response to rotavirus infection of infants in a single epidemic. Pediatr Infect Dis. 1985 Nov-Dec;4(6):626–631. doi: 10.1097/00006454-198511000-00006. [DOI] [PubMed] [Google Scholar]
- Coulson B. S., Fowler K. J., Bishop R. F., Cotton R. G. Neutralizing monoclonal antibodies to human rotavirus and indications of antigenic drift among strains from neonates. J Virol. 1985 Apr;54(1):14–20. doi: 10.1128/jvi.54.1.14-20.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson B. S., Grimwood K., Bishop R. F., Barnes G. L. Evaluation of end-point titration, single dilution and capture enzyme immunoassays for measurement of antirotaviral IgA and IgM in infantile secretions and serum. J Virol Methods. 1989 Oct;26(1):53–65. doi: 10.1016/0166-0934(89)90074-8. [DOI] [PubMed] [Google Scholar]
- Coulson B. S., Unicomb L. E., Pitson G. A., Bishop R. F. Simple and specific enzyme immunoassay using monoclonal antibodies for serotyping human rotaviruses. J Clin Microbiol. 1987 Mar;25(3):509–515. doi: 10.1128/jcm.25.3.509-515.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson G. P., Barnes G. L. Structural and functional abnormalities of the small intestine in infants and young children with rotavirus enteritis. Acta Paediatr Scand. 1979 Mar;68(2):181–186. doi: 10.1111/j.1651-2227.1979.tb04986.x. [DOI] [PubMed] [Google Scholar]
- Grimwood K., Lund J. C., Coulson B. S., Hudson I. L., Bishop R. F., Barnes G. L. Comparison of serum and mucosal antibody responses following severe acute rotavirus gastroenteritis in young children. J Clin Microbiol. 1988 Apr;26(4):732–738. doi: 10.1128/jcm.26.4.732-738.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwith M., Wenman W., Hinde D., Feltham S., Greenberg H. A prospective study of rotavirus infection in infants and young children. J Infect Dis. 1981 Sep;144(3):218–224. doi: 10.1093/infdis/144.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelt K., Grauballe P. C., Schiøtz P. O., Andersen L., Krasilnikoff P. A. Intestinal and serum immune response to a naturally acquired rotavirus gastroenteritis in children. J Pediatr Gastroenterol Nutr. 1985 Feb;4(1):60–66. doi: 10.1097/00005176-198502000-00012. [DOI] [PubMed] [Google Scholar]
- Lanata C. F., Black R. E., del Aguila R., Gil A., Verastegui H., Gerna G., Flores J., Kapikian A. Z., Andre F. E. Protection of Peruvian children against rotavirus diarrhea of specific serotypes by one, two, or three doses of the RIT 4237 attenuated bovine rotavirus vaccine. J Infect Dis. 1989 Mar;159(3):452–459. doi: 10.1093/infdis/159.3.452. [DOI] [PubMed] [Google Scholar]
- Losonsky G. A., Rennels M. B., Lim Y., Krall G., Kapikian A. Z., Levine M. M. Systemic and mucosal immune responses to rhesus rotavirus vaccine MMU 18006. Pediatr Infect Dis J. 1988 Jun;7(6):388–393. doi: 10.1097/00006454-198806000-00004. [DOI] [PubMed] [Google Scholar]
- Mata L., Simhon A., Urrutia J. J., Kronmal R. A., Fernández R., García B. Epidemiology of rotaviruses in a cohort of 45 Guatamalan Mayan Indian children observed from birth to the age of three years. J Infect Dis. 1983 Sep;148(3):452–461. doi: 10.1093/infdis/148.3.452. [DOI] [PubMed] [Google Scholar]
- McLean B., Sonza S., Holmes I. H. Measurement of immunoglobulin A, G, and M class rotavirus antibodies in serum and mucosal secretions. J Clin Microbiol. 1980 Sep;12(3):314–319. doi: 10.1128/jcm.12.3.314-319.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio O., Ishihara Y., Isomura S., Inoue H., Inouye S. Long-term follow-up of infants from birth for rotavirus antigen and antibody in the feces. Acta Paediatr Jpn. 1988 Aug;30(4):497–504. doi: 10.1111/j.1442-200x.1988.tb02542.x. [DOI] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F. Protection against rotavirus-induced gastroenteritis in a murine model by passively acquired gastrointestinal but not circulating antibodies. J Virol. 1985 Apr;54(1):58–64. doi: 10.1128/jvi.54.1.58-64.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberton D. M., Forrest P. J., Frangoulis E., Jones C. L., Mermelstein N. Early induction of secretory immunity in infancy: specific antibody in neonatal breast milk. Arch Dis Child. 1986 May;61(5):489–494. doi: 10.1136/adc.61.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez W. J., Kim H. W., Brandt C. D., Schwartz R. H., Gardner M. K., Jeffries B., Parrott R. H., Kaslow R. A., Smith J. I., Kapikian A. Z. Longitudinal study of rotavirus infection and gastroenteritis in families served by a pediatric medical practice: clinical and epidemiologic observations. Pediatr Infect Dis J. 1987 Feb;6(2):170–176. doi: 10.1097/00006454-198702000-00006. [DOI] [PubMed] [Google Scholar]
- Ryder R. W., Singh N., Reeves W. C., Kapikian A. Z., Greenberg H. B., Sack R. B. Evidence of immunity induced by naturally acquired rotavirus and Norwalk virus infection on two remote Panamanian islands. J Infect Dis. 1985 Jan;151(1):99–105. doi: 10.1093/infdis/151.1.99. [DOI] [PubMed] [Google Scholar]
- Shinozaki T., Araki K., Ushijima H., Kim B., Fujii R. Frequency of successive rotavirus infections among infants in a nursery home measured by coproantibody conversion. Eur J Pediatr. 1986 Oct;145(5):450–451. doi: 10.1007/BF00439262. [DOI] [PubMed] [Google Scholar]
- Simhon A., Mata L., Vives M., Rivera L., Vargas S., Ramírez G., Lizano L., Catarinella G., Azofeifa J. Low endemicity and low pathogenicity of rotaviruses among rural children in Costa Rica. J Infect Dis. 1985 Dec;152(6):1134–1142. doi: 10.1093/infdis/152.6.1134. [DOI] [PubMed] [Google Scholar]
- Snodgrass D. R., Wells P. W. Passive immunity in rotaviral infections. J Am Vet Med Assoc. 1978 Sep 1;173(5 Pt 2):565–568. [PubMed] [Google Scholar]
- Stuker G., Oshiro L. S., Schmidt N. J. Antigenic comparisons of two new rotaviruses from rhesus monkeys. J Clin Microbiol. 1980 Feb;11(2):202–203. doi: 10.1128/jcm.11.2.202-203.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesikari T., Kapikian A. Z., Delem A., Zissis G. A comparative trial of rhesus monkey (RRV-1) and bovine (RIT 4237) oral rotavirus vaccines in young children. J Infect Dis. 1986 May;153(5):832–839. doi: 10.1093/infdis/153.5.832. [DOI] [PubMed] [Google Scholar]
- Wright P. F., Tajima T., Thompson J., Kokubun K., Kapikian A., Karzon D. T. Candidate rotavirus vaccine (rhesus rotavirus strain) in children: an evaluation. Pediatrics. 1987 Oct;80(4):473–480. [PubMed] [Google Scholar]
- Wyatt R. G., Kapikian A. Z., Mebus C. A. Induction of cross-reactive serum neutralizing antibody to human rotavirus in calves after in utero administration of bovine rotavirus. J Clin Microbiol. 1983 Sep;18(3):505–508. doi: 10.1128/jcm.18.3.505-508.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H., Inouye S., Yamauchi M., Morishima T., Matsuno S., Isomura S., Suzuki S. Anamnestic response in fecal IgA antibody production after rotaviral infection of infants. J Infect Dis. 1985 Aug;152(2):398–400. doi: 10.1093/infdis/152.2.398. [DOI] [PubMed] [Google Scholar]


