Abstract
Lung cancer is one of the deadliest and commonly diagnosed neoplasms. Early diagnosis of this disease is critical for improving clinical outcome and prognosis. Because the early stages of lung cancer often produce no symptoms, it is necessary to identify biomarkers for early detection, prognostic evaluation, and recurrence monitoring of the cancer. To identify potential lung cancer biomarkers, we analyzed the differential protein secretion from transformed bronchial epithelial cells (1198 and 1170-I) as compared to immortalized normal bronchial epithelial cells (BEAS-2B) and non-transformed cells (1799) all of which are derived from BEAS-2B and represent multistage bronchial epithelial carcinogenesis. The proteins recovered from the conditioned media of the cells were separated on two-dimensional gels. There was little difference between the secretome of the BEAS-2B and 1799 cells, whereas the patterns between the transformed 1198 and 1170-I cells and non-transformed 1799 cells were significantly different. Using mass spectrometry and database search, we identified 20 proteins including protein gene product 9.5 (PGP9.5), translationally controlled tumor protein (TCTP), tissue inhibitors of metalloproteinases-2 (TIMP-2), and triosephosphate isomerase (TPI), that were either increased or decreased simultaneously in conditioned media of both 1198 and 1170-I cells. Furthermore, levels of PGP9.5, TCTP, TIMP-2, and TPI were significantly increased not only in the conditioned media of both transformed cell lines when compared to those of BEAS-2B and 1799 cells, but also in plasmas and tissues from lung cancer patients when compared to those in normal controls. We suggest the PGP9.5, TCTP, TIMP-2, and TPI as promising candidates for lung cancer serum biomarkers.
Keywords: biological markers, lung neoplasms, proteomics, serum
Introduction
According to global cancer statistics, lung cancer is the most commonly diagnosed cancer (1.35 million new cases in 2002) and the most common cause of death from cancer (1.18 million deaths in 2002) (Chen et al., 2003; Parkin et al., 2005). Despite improvements in diagnostic and therapeutic procedures, high case fatality persists (ratio of mortality to incidence, 0.87) (Chen et al., 2003; Parkin et al., 2005). In Korea, the mortality rates from lung cancer are the highest since 2000 (21.4% in 2006), and the 5-year overall survival rate for patients receiving treatment is just 13.7% for past 5 years since 1998 (Korea National Cancer Center). The best chance for successful treatment is offered by surgical resection in the early stages. However, the majority of lung cancers have been reported to reach stages III/IV (32%/41%) and Regional/Distant stages (35%/42%) at the time of diagnosis, and thereby show poor prognosis (Fry et al., 1999; Hoffman et al., 2000; Jemal et al., 2008). Detection of lung cancer at an early disease stage is therefore critical for successful clinical outcome such as improved prognosis and survival rate.
Serological markers can be exploited in cancer screening, monitoring of response to anticancer therapy and cancer progression, and surveillance of recurrence. Although several proteins such as carcinoembryonic antigen, neuron-specific enolase, tissue polypeptide antigen, chromogranin A, carbohydrate antigen 125, carbohydrate antigen 19-9, cytokeratin 19 fragment marker, and progastrin releasing peptide have been reported as putative serum biomarkers for lung cancer, they are not ideal for the detection of lung cancer due to their low specificity and/or sensitivity (Tarro et al., 2005; Schneider, 2006). Identification of biomarkers with high specificity and sensitivity is essential for more effective lung cancer diagnosis.
The emergence of cancer-specific autocrine and paracrine signals originating from tumor cells often includes the supraphysiological expression of secretory proteins or their receptors (Sporn and Roberts, 1985; Welsh et al., 2003; Park et al., 2005). Proteins that are secreted from neoplastic cells into the extracellular microenvironment are taken-up into the bloodstream. The secreted proteins, whose serum levels increase in the relatively early stages of cancer development and correlate with cancer cell proliferation and/or protein overexpression, can be considered as potential serum biomarkers of cancer and new molecular targets for therapeutic intervention (Welsh et al., 2003; Wu et al., 2005). In general, serum is the preferred specimen for the early diagnosis of cancer because it provides several key advantages, including low invasiveness, minimal cost, and easy sample collection and processing (Veenstra et al., 2005; Pan et al., 2008). However, a major limitation in the identification of candidate cancer biomarkers by serum proteome analysis is that high-abundant proteins such as albumin and globulin may obscure the detection of low-abundant proteins. Thus, much interest is currently being focused on the proteins secreted from isolated cancer cells in order to identify potential diagnostic and therapeutic markers.
Search for cancer biomarkers using cell lines established from different individuals is complicated since the genetic background of the cell lines are not identical. In that case, the possibility cannot be excluded that the difference in secreted proteins originates from the difference in genetic backgrounds rather than from the malignancy itself. It is well known that most lung cancers arise from the bronchial epithelium (Vogelstein and Kinzler, 2002). Therefore, in this study, we employed the following cell lines; non-transformed (1799), transformed but non-tumorigenic (1198), and tumorigenic (1170-I) cell lines. Each of cell lines is derived from a single immortalized human normal bronchial epithelial cell line, BEAS-2B. They not only possess the same genetic background but also represent multistep bronchial carcinogenesis (Reddel et al., 1988; Klein-Szanto et al., 1992).
The differentially secreted proteins from these cell lines are expected to be applicable as biomarkers for early detection of lung cancer. Although identification of differentially expressed genes in the human lung carcinogenesis model using cDNA microarray techniques has been previously reported (Feng et al., 2001; Lacroix et al., 2006), this is the first time, to our knowledge, that the differential secretion of proteins has been investigated in this model. We successfully demonstrated that PGP9.5, TCTP, TIMP-2, and TPI were differentially secreted from both transformed 1198 and 1170-I cells, and were present in significantly high concentrations in plasmas and tissue samples of lung cancer patients. These results suggest that these four proteins are strong candidates for diagnostic markers of lung cancer and that secretome analysis of cell lines representing multistage human lung carcinogenesis is a valuable approach to the identification of novel and specific tumor biomarkers.
Materials and Methods
Cells, cell culture, and conditioned medium collection
An in vitro lung carcinogenesis model comprised of a series of immortalized normal (BEAS-2B), non-transformed (1799), transformed but non-tumorigenic (1198), and tumorigenic (1170-I) human bronchial epithelial cells was used in this study. BEAS-2B is a normal human bronchial epithelial cell line derived by immortalization using a hybrid of adenovirus and SV40 (Ad12SV40) (Reddel et al., 1988). The 1198 and 1170-I cells were derived from BEAS-2B exposed in vivo to beeswax pellets containing cigarette smoke condensate (CSC), and 1799 cells were derived from BEAS-2B exposed in vivo to beeswax alone as control (Klein-Szanto et al., 1992). The CSC induced in vivo phenotypic changes in BEAS-2B cells similar to the progressive changes that occur during human lung carcinogenesis. The BEAS-2B, 1799, 1198, and 1170-I cells were obtained from Dr. Y. H. Kim (Korea Univ., Seoul).
The BEAS-2B and 1799 cells were grown in Keratinocyte serum free media (Gibco BRL, Eggstein, Germany) supplemented with EGF (Gibco BRL), and pituitary extract (P.E., Gibco BRL). The 1198 and 1170-I cells were cultured in the medium described above, except that 3% FBS (Gibco BRL) was added to the medium. Cells were cultivated at 37℃ in a humidified atmosphere of 5% CO2. The medium was changed every 2 or 3 days. To prepare conditioned media, cells were cultured until reaching subconfluency and then washed 3 times with PBS. The cells were maintained for 24 h in fresh medium for medium conditioning. To remove cells and cell debris, the collected media were centrifuged for 10 min at 14,000 rpm and 4℃, and supernatants were used as conditioned media in the following study.
Lactate dehydrogenase (LDH) activity assay
To evaluate the extent of cell death that may occur during medium conditioning, LDH activities were analyzed in conditioned media. The assay was performed with LDH assay kit (Sigma Chemical Co., St. Louis, MO) according to the manufacture's protocol. The enzyme reaction was performed under dark conditions at room temperature and terminated by adding 1N HCl. The absorbance at wavelengths of 490 and 690 nm was measured using a Gemini microplate reader (Molecular Devices Co., Sunnyvale, CA). The LDH activities were determined by calculating absorbance difference and normalized by cell numbers.
Sample preparation for two-dimensional electrophoresis (2-DE)
Samples for proteomic analysis were prepared from the equivalent volume of conditioned media (normalized by cell number). Protein in the medium was concentrated using TCA precipitation. Briefly, the protein was precipitated by adding 100% TCA in an amount equivalent to 1/10 volume of conditioned medium. The mixture was incubated for 2 h at 4℃ and thereafter centrifuged for 20 min at 14,000 rpm and 4℃. The protein pellet was resuspended in ice-cold acetone for 1 h and centrifuged under the same conditions as described above. The pellet was immediately resuspended and dissolved in lysis buffer [2 M thiourea, 7 M urea, 4% w/v CHAPS, protease inhibitors cocktail (Complete, EDTA-free, Roche Applied Science, Indianapolis, IN), 1% w/v DTT, and 2% v/v Immobilized pH gradient (IPG) buffer] for isoelectric focusing of 2-DE analysis.
Preparation of clinical specimens
This study was approved by the Institutional Review Board of Korea University College of Medicine (Approval No. 2005-1114-1).
Matched normal and tumor tissues from 11 lung cancer patients [6 squamous cell carcinomas (SCCs) and 5 adenocarcinomas] were obtained from Korea Lung Tissue Bank (Seoul, Korea) assigned and supported by Korea Science and Engineering Foundation in the Ministry of Science and Technology. Frozen tissues were homogenized in lysis buffer at 30,000 rpm on ice for 15 cycles of 5 s using a Tissue Tearor (BioSpec Products Inc., Bartlesville, OK). The tissue homogenates were next sonicated with Vibra-Cell Ultrasonic Processors (Sonics & Materials Inc., Newtown, CT) on ice for 6 cycles of 3 s each with 10 s-intervals and centrifuged at 14,000 rpm at 4℃ for 10 min. The tissue lysates were then aliquoted and stored at -70℃ until use.
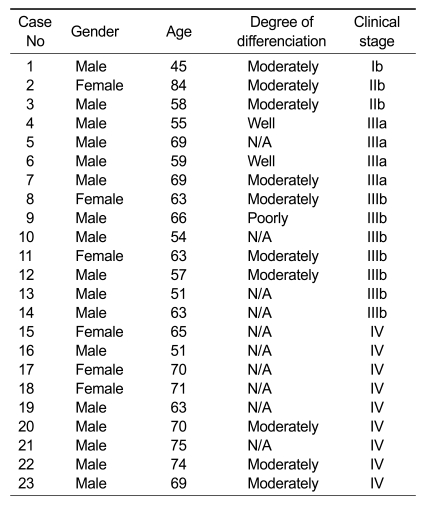
Human plasma samples (treated with EDTA), which were collected from lung cancer patients and normal controls, were obtained from Korea University Anam Hospital (Seoul, Korea) and BioInfra (Seoul, Korea) and used for Western blot analysis and ELISA. Clinical information on the patients is summarized in Table 1. There were 23 SCCs (ages 51-84 years; 6 females and 17 males; clinical stage I-IV). The control group consisted of 17 healthy donors (ages 55-72 years; 5 females and 12 males). The EDTA-treated blood was centrifuged at 2,000 rpm for 10 min. The supernatants were recovered as plasma and stored at -70℃ until use. Protein concentration was determined using the Bradford Method.
Table 1.
Clinical features of lung cancer patients who donated plasmas.
N/A, data not available
2-DE and protein identification
Isoelectric focusing, the first dimension of 2-DE was performed using a Multiphor II Electrophoresis System (Amersham Pharmacia, Uppsala, Sweden) as described in previous reports (Rabilloud, 1998; Choe and Lee, 2000; Gorg et al., 2000; Zuo and Speicher, 2000; Lim et al., 2006). IPG gel strips (Linear pH 4-7, 18 cm long) were rehydrated overnight (~16 h) at room temperature in rehydration buffer (2 M thiourea, 7 M urea, 2% w/v CHAPS, 0.3% w/v DTT, 2% v/v IPG buffer and bromophenol blue). Each sample protein dissolved in lysis buffer was applied to the IPG strip using cup-loading. The first dimension was performed at 53 kVhr using the following conditions at 20℃: 300 V for 5 h, 3,500 V for 5 h, and 3,500 V for 12 h. SDS-PAGE, the second dimension of 2-DE was performed using 10% gel (18 cm × 1.5 mm) at 15 mA/gel for 15 min and then 30 mA/gel until the front of the bromophenol blue dye reached the bottom of the gels as previously described (Rabilloud, 1998; Gorg et al., 2000; Zuo and Speicher, 2000). Following electrophoresis, gels were visualized by silver staining (Gharahdaghi et al., 1999; Yan et al., 2000) and scanned in a flatbed densitometer (ImageScanner, Amersham Pharmacia). Spot detection and image analysis were performed by Progenesis (Nonlinear Dynamics Group, Newcastle upon Tyne, UK).
For peptide mass fingerprinting, protein spots of interest were excised from gels, destained and treated with 20 μl of trypsin (10-15 μg/ml) at 37℃ for 16-24 h, and the proteins were extracted. Matrix-assisted Laser Desorption/Ionization Mass Spectrometry (MALDI-MS) was performed at Yonsei Proteome Research Center (Seoul, Korea) and Genomine, Inc. (Pohang, Korea). The peptide masses were measured as mono-isotopic masses. To identify proteins, the measured mono-isotopic masses of peptides were analyzed using multiple Web-based search programs such as Mascot (http://www.matrixscience.com), MS-Fit (http://prospector.ucsf.edu), and ProFound (http://prowl. rockefeller.edu/prowl-cgi/profound.exe) and public protein databases such as the National Center for Biotechnology Information (NCBI, http://www.ncbi.nlm.nih.gov) and Swiss-Prot/TrEMBL (http://www.expasy.org/sprot).
Western blot analysis and quantification
Protein samples were prepared as described in "Sample Preparation for 2-DE" and "Preparation of Clinical Specimens", separated on 10% or 12.5% SDS-polyacrylamide gel, and then transferred to nitrocellulose membranes using semidry blotting (Bio-Rad Laboratories, Inc., Hercules, CA). Blots were blocked overnight in TBS-T (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.05% Tween 20) containing 5% skim milk and then incubated for 2 h with primary antibodies (500 ×) against β-actin, GAPDH, Lamin B, PGP9.5, peroxiredoxin 6 (PRX6), secreted protein, acidic, cysteine-rich (SPARC), TCTP, TIMP-2, and TPI (Abcam, Cambridge, MA). Immunodetection was carried out with an enhanced chemiluminescent (ECL) kit (Amersham Pharmacia) according to the manufacturer's protocol. To determine protein abundance, band intensities were quantified using a flatbed densitometer (ImageScanner, Amersham Pharmacia) and image analysis software (LabWorks 4.6, UVP, Upland, CA). Quantitative analysis of TIMP-2 protein in human plasma was carried out using human TIMP-2 Quantikine ELISA Kit (R&D Systems, Minneapolis, MN) according to the manufacturer's protocol.
Statistical analysis
Statistical analyses were performed by the unpaired t-test or the Mann-Whitney U test, depending on the outcome of the normality test using SigmaStat 2.0 (Systat Software, Inc., San Jose, CA).
Results
Optimization of medium conditioning
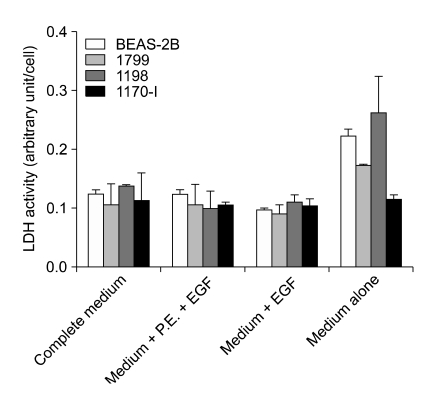
Because P.E. and FBS contain complex mixtures of proteins, their addition to the medium for conditioning may complicate the discovery of low-abundant secreted proteins. When the protein profiles of unconditioned media were examined, media supplemented with P.E. and/or FBS showed a large number of protein spots, in contrast to medium alone or medium supplemented only with EGF, as expected (data not shown). Although some components necessary for preparing complete medium, such as EGF, P.E., and FBS, can be omitted to increase the probability of identification of differentially secreted proteins, it may also result in increased cell death, and consequent contamination of the conditioned medium with intracellular proteins released from the dead cells. To determine the difference in death rate between cells cultured in media from which some supplements were omitted, LDH activities were measured in the conditioned media (Figure 1). Medium supplemented only with EGF showed almost no difference in LDH activities of the four cell lines when compared to complete medium or medium containing P.E. and EGF. In contrast, death rates were high and also there was a significant difference in the rates of the four cell lines when cultured in the medium lacking all of P.E., FBS, and EGF. Taken together, these results indicate that medium containing only EGF was sufficient to maintain the viability of the cells in culture and was therefore used as the conditioning medium for the identification of differentially secreted proteins.
Figure 1.
Optimization of medium conditioning. BEAS-2B, 1799, 1198, and 1170-I cells were cultured for 24 h in fresh media as indicated. Cells and cell debris were removed by centrifugation from the collected media, and supernatants were used as conditioned media. LDH activities were measured in the conditioned media and normalized by cell number. All of the data represent the means ± standard deviations obtained from four independent experiments. EGF and P.E. indicate epidermal growth factor and pituitary extract, respectively.
Identification of differentially secreted proteins in conditioned medium
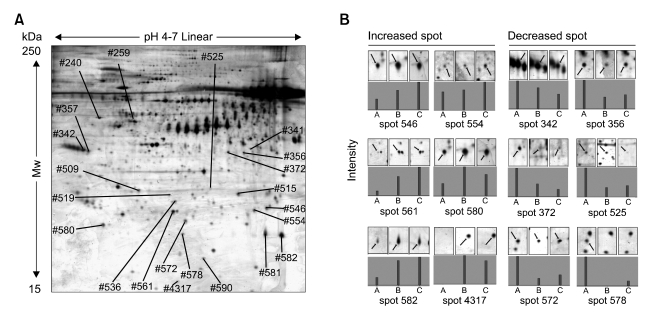
Approximately 600 spots in the pH range 4-7 and molecular mass ranges of 15-120 kDa were detected in the conditioned media from BEAS-2B, 1799, 1198, and 1170-I cells (Figure 2). Proteomic patterns were similar between the conditioned media of BEAS-2B and 1799 (non-transformed) cells, and between those of 1198 (transformed, non-tumorigenic) and 1170-I (transformed, tumorigenic) cells. In contrast, the patterns obtained from the conditioned media from 1198 and 1170-I were markedly different from the patterns of BEAS-2B and 1799 cells (data not shown).
Figure 2.
Representative 2-dimensional reference map of 1170-I-conditioned medium (A) and change in secretion pattern across 1799, 1198 and 1170-I cells (B). Proteins in the equivalent volume of conditioned media (normalized by cell number) were concentrated using TCA precipitation and separated on pH 4 to 7 linear IPG strips and 10% SDS-PAGE gels. Protein spots were visualized by silver staining, and spot intensities were measured by densitometer. Twenty proteins whose levels were changed by more than 1.5-fold were identified using MALDI-MS and annotated by spot numbers. Corresponding protein names were listed in Table 2. The map, spot images, and graphs represent one of five independent experiments with similar results. A, B, and C indicate 1799, 1198 and 1170-I cells, respectively.
Out of the secreted proteins whose levels were changed by more than 1.5-fold, 33 were increased and 38 were decreased in 1198-conditioned medium, and 38 were increased and 61 were decreased in 1170-I-conditioned medium compared with 1799-conditioned medium. Of those proteins that were identified as differentially secreted in the transformed 1198 and 1170-I cells, 16/32 were increased or decreased in both transformed cells.
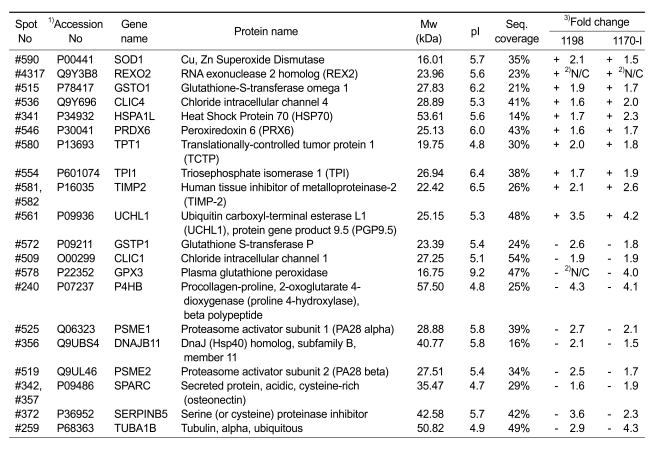
To identify the proteins that were differentially secreted in the conditioned media of both 1198 and 1170-I cells (16 increased and 32 decreased), the protein spots of interest were excised from the silver-stained 2-DE gels and subjected to in-gel digestion. The peptide mass fingerprints (PMFs) were obtained by MALDI-MS and were then used to query the NCBI and Swiss-Prot/TrEMBL protein databases with Mascot, MS-Fit, and ProFound softwares. Ten increased and ten decreased proteins were successfully identified (annotated in Figure 2A). The data for all identified protein spots are shown in Table 2, together with their corresponding spot numbers, Swiss-Prot/TrEMBL accession numbers, gene and protein names, molecular weights and pI values, peptide matched sequence coverage, and fold changes as compared to the levels of each protein in the conditioned medium of 1799 cells. Proteins whose secretion was increased in both 1198 and 1170-I cells include glutathione-S-transferase omega 1, PGP9.5, PRX6, TCTP, TIMP-2, and TPI, and those whose secretion was decreased in the cells include plasma glutathione peroxidase, protein disulfide isomerase, proteasome activator subunit 1, proteasome activator subunit 2, and SPARC.
Table 2.
Protein identification of 20 differentially secreted proteins with recurring ≥ 4 times out of the 5 pairs of samples.
1) Accession number from Swiss-Prot/TrEMBL
2) N/C (Not calculatable): the spots on one of the paired gels were too weak or non-detectable.
3) Fold change in secretion compared with 1799
Validation of differentially secreted proteins in conditioned medium
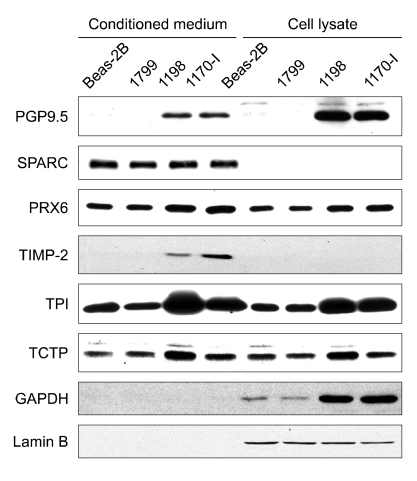
To verify the reliability of proteomic analysis, the levels of PGP9.5, PRX6, SPARC, TCTP, TIMP-2, and TPI were examined in conditioned media by Western blot analysis (Figure 3). It was observed that the levels of PGP9.5, PRX6, TCTP, TIMP-2, and TPI were significantly higher in the conditioned media from both transformed 1198 and 1170-I cells than from BEAS-2B and 1799 cells. This finding is in agreement with the proteomic analysis results and was reproduced in whole cell lysates, with the exception that TIMP-2 was not detectable. In contrast, there was no difference in the levels of SPARC between the conditioned media of four cell lines, and SPARC was undetectable in the whole cell lysates. It is probable that the differential secretion of proteins is a secondary event resulting from a generalized increase in protein expression. To address this point, GAPDH levels were evaluated in the conditioned media and whole cell lysates of four cell lines, because overexpression of many glycolytic enzymes including GAPDH is observed in a variety of tumors including lung cancer and accompanied during malignant transformation (Tokunaga et al., 1987; Pelicano et al., 2006). Although GAPDH levels were markedly increased in the lysates of both transformed 1198 and 1170-I cells contrasted with BEAS-2B and 1799 cells, the protein was not detected in the conditioned media of four cell lines, indicating that the differential secretion of proteins observed in the present study is a tumor-specific phenomenon.
Figure 3.
Immunoblot analysis of differentially secreted proteins in conditioned media and whole cell lysates. The conditioned media and whole cell lysates were prepared from BEAS-2B, 1799, 1198 and 1170-I cells. Proteins were separated on 10% or 12.5% SDS-PAGE gels, and visualized using ECL method after incubation with the primary antibodies against GAPDH, PGP9.5, PRX6, SPARC, TCTP, TIMP-2, and TPI. Lamin B proteins were used as an internal control to correct protein loading errors. Data are a representative of three or four independent experiments with similar results.
Validation in lung cancer patients' plasmas and tissues
Next, a comparative analysis of the levels of PGP9.5, PRX6, TCTP, TIMP-2, and TPI was performed in tissues (matched normal and tumor tissues of 11 patients) and plasmas of 23 lung cancer patients and 17 normal controls using immunoblot analysis and ELISA.
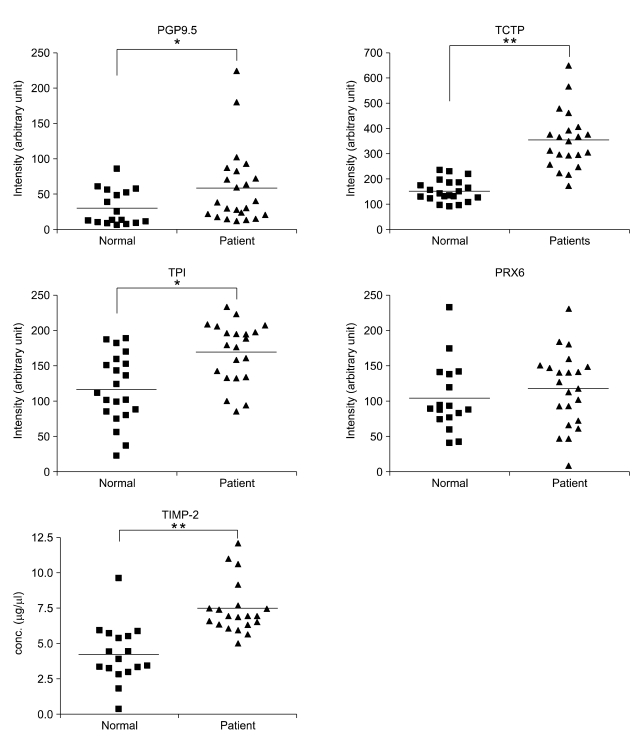
In the tissues, levels of PGP9.5, PRX6, TCTP, and TPI that were detected by immunoblot analysis were significantly increased, and TIMP-2 was once again not detected (data not shown). These findings are consistent with the results observed in whole cell lysates as described above (Figure 4). Plasma levels of the proteins were quantified using immunoblot densitometry or ELISA (Figure 5). The levels of PGP9.5, TCTP, TIMP-2, and TPI were statistically significantly increased in the plasmas of lung cancer patients compared to normal controls. However, the level of PRX6 in the plasmas of lung cancer patients was not different from normal control plasmas. Altogether, PGP9.5, TCTP, TIMP-2, and TPI are identified as potential serological markers for lung cancer to be used in the diagnosis and surveillance of lung cancer.
Figure 4.
Immunoblot analysis of differentially secreted proteins in lung cancer tissues and adjacent normal lung tissues. Matched normal and tumor tissues obtained from 11 lung cancer patients [6 SCCs and 5 adenocarcinomas] were used. Proteins (20 to 50 μg) were separated on 10% or 12.5% SDS-PAGE gels and then transferred to nitrocellulose membranes. The membranes were incubated with the primary antibodies against β-actin, PGP9.5, PRX6, TCTP, and TPI. Proteins were visualized by ECL method. β-actin proteins were used as an internal control to correct protein loading errors. Data represent one of three independent experiments with similar results.
Figure 5.
Plasma levels of PGP9.5, PRX6, TCTP, TIMP-2, and TPI in lung cancer patients and healthy controls. The plasmas of lung cancer patients (n = 23) with SCCs and healthy donors (n = 17) were used. Plasma levels of PGP9.5, PRX6, TCTP, and TPI were quantified using immunoblot densitometry, and those of TIMP-2 were measured by ELISA. Proteins in 7 μl plasma were separated on 10% or 12.5% SDS-PAGE gels and blotted to nitrocellulose membranes. The blot membranes were incubated with the primary antibodies against PGP9.5, PRX6, TCTP, and TPI, and were visualized by ECL method. Intensities of protein bands were quantified using densitometry and image analysis software. All data points are shown and each point is mean of four or five independent experiments. The mean values are denoted by horizontal bars. The asterisks designate significant differences (**P < 0.001 and *P < 0.05) in the mean values compared to each control group.
Discussion
To identify potential early biomarkers for lung cancer, we performed comparative secretome analysis in a series of non-transformed (BEAS-2B and 1799) and transformed (1198 and 1170-I) cell lines all derived from a single human bronchial epithelial cell line, BEAS-2B. Among the 48 protein spots (16 increased and 32 decreased spots) that were differentially secreted in the conditioned media of both transformed cell lines, 20 proteins (10 increased and 10 decreased proteins) were identified by PMF. Interestingly, these proteins can be classified into two groups based on their change in secretion pattern across 1799, 1198 and 1170-I cells: one group showing a gradual increase or decrease across all three cell lines, and another group showing a marked change in 1198 cells compared to 1799 cells but not much difference between 1198 and 1170-I cells (Figure 2B). It is probable that proteins belonging to the latter group rather than the former are more promising biomarkers for early cancer detection, because secretion of the proteins is dramatically and similarly changed in both transformed cell lines irrespective of their tumorigenicity. Immunoblot analysis and ELISA in samples from tissues and plasmas of lung cancer patients as well as conditioned media demonstrated that four of these identified proteins, PGP9.5, TPI, TCTP, and TIMP-2, were specifically secreted at a high level from 1198 and 1170-I cells, and their levels in clinical specimens of lung cancer patients were significantly higher than those in normal controls, suggesting that the four proteins can serve as biomarkers for early detection of lung cancer. These results also demonstrate that our proteomic results are reliable.
PGP9.5 (ubiquitin COOH-terminal esterase L1, or UCHL1) is a ubiquitin hydrolase that is widely expressed in neuronal tissues at all stages of neuronal differentiation (Brichory et al., 2001). Ubiquitination of cellular proteins for ubiquitin-mediated proteolysis is an important mechanism for cell cycle control. Increasing clinical evidence shows that alterations in the ubiquitination of cell-cycle regulators play a significant role in the etiology of many human malignancies (Reed, 2003; Nakayama and Nakayama, 2006), although whether PGP9.5 plays a pivotal role in the oncogenic transformation of human lung epithelial cells remains unclear. PGP9.5 protein and/or transcript were observed in both small cell lung carcinoma (SCLC) and non SCLC (NSCLC) cell lines (Abbona et al., 1998; Hibi et al., 1998, 1999; Chen et al., 2002), and PGP9.5 expression was strongly associated with the pathological stage of the cancer in primary NSCLCs (Hibi et al., 1999). Moreover, it has been reported that PGP9.5 autoantibody (9 cases) and/or antigen (2 cases) was detected in the sera of 64 lung cancer patients regardless of the histological types. PGP9.5 protein was also detected in the conditioned medium of A549 human lung adenocarcinoma cells (Brichory et al., 2001; Huang et al., 2006). These reports suggest that PGP9.5 may serve as a biomarker for lung cancer.
TCTP, a highly conserved protein in various species such as Drosophila, S. cerevisiae, C. elegans, zebrafish, mouse, and human (Hsu et al., 2007) has been reported to have IgE-dependent histamine releasing activity (MacDonald et al., 1995, 1996). It has been reported to function as a guanine nucleotide dissociation inhibitor on the translation elongation factor, eEF1A and act as a guanine nucleotide exchange factor for Ras homologue enriched in brain (Rheb), which is involved in mammalian target of rapamycin (mTOR) signaling (Cans et al., 2003; Hsu et al., 2007). It has been suggested that TCTP can be a target of tumor reversion, a process by which some cancer cells lose their malignant phenotypic characteristics such as rapid cell growth and proliferation, activation of anti-apoptotic pathways, malignant transformation and tumorigenicity (Tuynder et al., 2002, 2004; Chen et al., 2007). The level of TCTP protein is increased in various human tumor tissues including breast, larynx, liver, lung, ovary, prostate, rectum, skin, thyroid, and uterus (Tuynder et al., 2002; Kuramitsu and Nakamura, 2006) and in the human SCLC cell line (Ziv et al., 2006). Furthermore, TCTP protein levels were up-regulated in chemo-resistant sublines of melanoma cells (Sinha et al., 2000). To our knowledge, detection of TCTP in the conditioned medium of human lung cancer cell lines and the serum of patients having solid tumors has never been reported, although TCTP was initially isolated as a secreted factor from U937-derived cultured supernatants (MacDonald et al., 1995) and confirmed by subsequent studies.
Tissue inhibitors of metalloproteinases (TIMP-1, -2, -3 and -4) are endogenous inhibitors of metalloproteinase (MMP) activities. There is currently a great deal of interest in the MMP-independent, cell surface receptor-mediated, and cellular context-dependent functions of TIMPs. Of special interest are both pro- and anti-tumorigenic activities of TIMPs, that have an effect on a number of cellular processes, including cell growth and apoptosis, in a wide range of cells including cancer cells (Stetler-Stevenson, 2008). In contrast to our results on TIMP-2, it has been reported that TIMP-2 protein levels were significantly lower in the sera of patients with NSCLC than in normal control sera (Ylisirnio et al., 2000; Suemitsu et al., 2004), and that TIMP-2 abundance was decreased in human cancers both through genetic polymorphisms and epigenetic mechanisms involving hypermethylation of the TIMP-2 promoter (Stetler-Stevenson, 2008). The levels of TIMP-2 protein were significantly higher in the sera of patients with SCC than in those with adenocarcinoma (Suemitsu et al., 2004), while its level was more frequently increased in adenocarcinoma tissues than SCC tissues (Thomas et al., 2000), indicating the possibility that serum levels of TIMP-2 do not correlate to its levels in the corresponding tumor tissues. This may explain why TIMP-2 protein is detected in the conditioned medium of lung cancer cells and serum of patients having lung cancers whereas it is undetectable in the corresponding cell lysates and cancer tissues. To date, there are only few reports addressing the level of TIMP-2 in the sera and/or plasmas of lung cancer patients and the potential value of TIMP-2 as a serological marker of lung cancer. Therefore, whether TIMP-2 level is increased in lung cancer patients' sera/plasmas needs to be further investigated in large sample-size study.
TPI, is a highly conserved glycolytic enzyme that catalyze the interconversion of dihydroxyacetone phosphate and glyceraldehyde-3-phosphate. An increase in TPI expression was observed in a spontaneous transformant of an immortalized but non-tumorigenic cell line derived from alveolar type II pneumocytes as well as in lung tumor tissues versus normal lung tissues from a chemical carcinogenesis mouse model (Peebles et al., 2003; Kassie et al., 2008). TPI was identified by comparative proteomic analysis as being significantly overexpressed in lung cancer tissues compared with adjacent normal lung tissues (Chen et al., 2002; Kuramitsu and Nakamura, 2006). In the conditioned medium of A549 cells treated with Gefitinib (Iressa), an inhibitor of the EGF-R Tyrosine Kinase, decreased levels of TPI were observed when compared to vehicle-treated control cells (McClelland and Gullick, 2007). In addition, it has recently been reported that TPI autoantibody and/or antigen was detected in the sera of lung cancer patients, and the frequency of the autoantibody detection was significantly high in SCC lung cancer when compared to other types of lung cancer. (Li et al., 2006; Nakanishi et al., 2006; Okano et al., 2006; Yang et al., 2007).
In summary, this study produced results that are in accord with previous reports on PGP9.5 and TPI but in opposition to previous reports on TIMP-2. In the present study, we first demonstrated that TCTP is detected in the plasmas of lung cancer patients as well as in the conditioned medium of human lung cancer cells. Our results suggest that PGP9.5, TCTP, TIMP-2, and TPI are potential lung cancer biomarkers. Our finding also demonstrated that our approach, utilizing secretome analysis in multistep human lung carcinogenesis model, provides a reliable way to identify serological markers, even though large-sample investigation with quantitative analysis is necessary to validate whether the identified proteins are reliable and specific markers for early detection and prognostic evaluation of lung cancer.
Acknowledgements
This work was supported by the grants from the Korea Health 21 R&D Project (Ministry of Health, Welfare and Family Affairs, Republic of Korea, A010250 and A030003) and the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MOST) [No. R13-2003-016-04001-0 (2008)].
Abbreviations
- CSC
cigarette smoke condensate
- IPG
immobilized pH gradient
- LDH
lactate dehydrogenase
- MALDI-MS
matrix-assisted laser desorption/ionization mass spectrometry
- MMP
matrix metalloproteinase
- NSCLC
non-small cell lung carcinoma
- P.E.
pituitary extract
- PGP9.5
protein gene product 9.5
- PRX6
peroxiredoxin 6
- SCC
squamous cell carcinoma
- SCLC
small cell lung carcinoma
- SPARC
secreted protein, acidic, cysteine-rich
- TCTP
translationally controlled tumor protein
- TIMP-2
tissue inhibitors of metalloproteinases-2
- TPI
triosephosphate isomerase
- 2-DE
two-dimensional electrophoresis
References
- 1.Abbona G, Papotti M, Viberti L, Macri L, Stella A, Bussolati G. Chromogranin A gene expression in non-small cell lung carcinomas. J Pathol. 1998;186:151–156. doi: 10.1002/(SICI)1096-9896(1998100)186:2<151::AID-PATH154>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 2.Brichory F, Beer D, Le Naour F, Giordano T, Hanash S. Proteomics-based identification of protein gene product 9.5 as a tumor antigen that induces a humoral immune response in lung cancer. Cancer Res. 2001;61:7908–7912. [PubMed] [Google Scholar]
- 3.Cans C, Passer BJ, Shalak V, Nancy-Portebois V, Crible V, Amzallag N, Allanic D, Tufino R, Argentini M, Moras D, Fiucci G, Goud B, Mirande M, Amson R, Telerman A. Translationally controlled tumor protein acts as a guanine nucleotide dissociation inhibitor on the translation elongation factor eEF1A. Proc Natl Acad Sci U S A. 2003;100:13892–13897. doi: 10.1073/pnas.2335950100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen G, Gharib TG, Huang CC, Thomas DG, Shedden KA, Taylor JM, Kardia SL, Misek DE, Giordano TJ, Iannettoni MD, Orringer MB, Hanash SM, Beer DG. Proteomic analysis of lung adenocarcinoma: identification of a highly expressed set of proteins in tumors. Clin Cancer Res. 2002;8:2298–2305. [PubMed] [Google Scholar]
- 5.Chen G, Gharib TG, Wang H, Huang CC, Kuick R, Thomas DG, Shedden KA, Misek DE, Taylor JM, Giordano TJ, Kardia SL, Iannettoni MD, Yee J, Hogg PJ, Orringer MB, Hanash SM, Beer DG. Protein profiles associated with survival in lung adenocarcinoma. Proc Natl Acad Sci U S A. 2003;100:13537–13542. doi: 10.1073/pnas.2233850100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen SH, Wu PS, Chou CH, Yan YT, Liu H, Weng SY, Yang-Yen HF. A knockout mouse approach reveals that TCTP functions as an essential factor for cell proliferation and survival in a tissue- or cell type-specific manner. Mol Biol Cell. 2007;18:2525–2532. doi: 10.1091/mbc.E07-02-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choe LH, Lee KH. A comparison of three commercially available isoelectric focusing units for proteome analysis: the multiphor, the IPGphor and the protean IEF cell. Electrophoresis. 2000;21:993–1000. doi: 10.1002/(SICI)1522-2683(20000301)21:5<993::AID-ELPS993>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Feng G, Xu X, Youssef EM, Lotan R. Diminished expression of S100A2, a putative tumor suppressor, at early stage of human lung carcinogenesis. Cancer Res. 2001;61:7999–8004. [PubMed] [Google Scholar]
- 9.Fry WA, Phillips JL, Menck HR. Ten-year survey of lung cancer treatment and survival in hospitals in the United States: a national cancer data base report. Cancer. 1999;86:1867–1876. doi: 10.1002/(sici)1097-0142(19991101)86:9<1867::aid-cncr31>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Gharahdaghi F, Weinberg CR, Meagher DA, Imai BS, Mische SM. Mass spectrometric identification of proteins from silver-stained polyacrylamide gel: a method for the removal of silver ions to enhance sensitivity. Electrophoresis. 1999;20:601–605. doi: 10.1002/(SICI)1522-2683(19990301)20:3<601::AID-ELPS601>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Gorg A, Obermaier C, Boguth G, Harder A, Scheibe B, Wildgruber R, Weiss W. The current state of two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis. 2000;21:1037–1053. doi: 10.1002/(SICI)1522-2683(20000401)21:6<1037::AID-ELPS1037>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 12.Hibi K, Liu Q, Beaudry GA, Madden SL, Westra WH, Wehage SL, Yang SC, Heitmiller RF, Bertelsen AH, Sidransky D, Jen J. Serial analysis of gene expression in non-small cell lung cancer. Cancer Res. 1998;58:5690–5694. [PubMed] [Google Scholar]
- 13.Hibi K, Westra WH, Borges M, Goodman S, Sidransky D, Jen J. PGP9.5 as a candidate tumor marker for non-small-cell lung cancer. Am J Pathol. 1999;155:711–715. doi: 10.1016/S0002-9440(10)65169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffman PC, Mauer AM, Vokes EE. Lung cancer. Lancet. 2000;355:479–485. doi: 10.1016/S0140-6736(00)82038-3. [DOI] [PubMed] [Google Scholar]
- 15.Hsu YC, Chern JJ, Cai Y, Liu M, Choi KW. Drosophila TCTP is essential for growth and proliferation through regulation of dRheb GTPase. Nature. 2007;445:785–788. doi: 10.1038/nature05528. [DOI] [PubMed] [Google Scholar]
- 16.Huang LJ, Chen SX, Huang Y, Luo WJ, Jiang HH, Hu QH, Zhang PF, Yi H. Proteomics-based identification of secreted protein dihydrodiol dehydrogenase as a novel serum markers of non-small cell lung cancer. Lung Cancer. 2006;54:87–94. doi: 10.1016/j.lungcan.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 18.Kassie F, Anderson LB, Higgins L, Pan Y, Matise I, Negia M, Upadhyaya P, Wang M, Hecht SS. Chemopreventive agents modulate the protein expression profile of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone plus benzo[a]pyrene-induced lung tumors in A/J mice. Carcinogenesis. 2008;29:610–619. doi: 10.1093/carcin/bgn014. [DOI] [PubMed] [Google Scholar]
- 19.Klein-Szanto AJ, Iizasa T, Momiki S, Garcia-Palazzo I, Caamano J, Metcalf R, Welsh J, Harris CC. A tobacco-specific N-nitrosamine or cigarette smoke condensate causes neoplastic transformation of xenotransplanted human bronchial epithelial cells. Proc Natl Acad Sci U S A. 1992;89:6693–6697. doi: 10.1073/pnas.89.15.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korea Cancer Registry. Korea National Cancer Center. 2006 http://wwwcancergokr/cms/statics/mortality/indexhtml.
- 21.Kuramitsu Y, Nakamura K. Proteomic analysis of cancer tissues: shedding light on carcinogenesis and possible biomarkers. Proteomics. 2006;6:5650–5661. doi: 10.1002/pmic.200600218. [DOI] [PubMed] [Google Scholar]
- 22.Lacroix L, Feng G, Lotan R. Identification of genes expressed differentially in an in vitro human lung carcinogenesis model. Cancer Biol Ther. 2006;5:665–673. doi: 10.4161/cbt.5.6.2870. [DOI] [PubMed] [Google Scholar]
- 23.Li C, Xiao Z, Chen Z, Zhang X, Li J, Wu X, Li X, Yi H, Li M, Zhu G, Liang S. Proteome analysis of human lung squamous carcinoma. Proteomics. 2006;6:547–558. doi: 10.1002/pmic.200500256. [DOI] [PubMed] [Google Scholar]
- 24.Lim BH, Cho BI, Kim YN, Kim JW, Park ST, Lee CW. Overexpression of nicotinamide N-methyltransferase in gastric cancer tissues and its potential post-translational modification. Exp Mol Med. 2006;38:455–465. doi: 10.1038/emm.2006.54. [DOI] [PubMed] [Google Scholar]
- 25.MacDonald SM, Rafnar T, Langdon J, Lichtenstein LM. Molecular identification of an IgE-dependent histamine-releasing factor. Science. 1995;269:688–690. doi: 10.1126/science.7542803. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald SM. Histamine-releasing factors. Curr Opin Immunol. 1996;8:778–783. doi: 10.1016/s0952-7915(96)80004-5. [DOI] [PubMed] [Google Scholar]
- 27.McClelland CM, Gullick WJ. Proteomic identification of secreted proteins as surrogate markers for signal transduction inhibitor activity. Br J Cancer. 2007;96:284–289. doi: 10.1038/sj.bjc.6603544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakanishi T, Takeuchi T, Ueda K, Murao H, Shimizu A. Detection of eight antibodies in cancer patients' sera against proteins derived from the adenocarcinoma A549 cell line using proteomics-based analysis. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;838:15–20. doi: 10.1016/j.jchromb.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Nakayama KI, Nakayama K. Ubiquitin ligases: cell-cycle control and cancer. Nat Rev Cancer. 2006;6:369–381. doi: 10.1038/nrc1881. [DOI] [PubMed] [Google Scholar]
- 30.Okano T, Kondo T, Kakisaka T, Fujii K, Yamada M, Kato H, Nishimura T, Gemma A, Kudoh S, Hirohashi S. Plasma proteomics of lung cancer by a linkage of multi-dimensional liquid chromatography and two-dimensional difference gel electrophoresis. Proteomics. 2006;6:3938–3948. doi: 10.1002/pmic.200500883. [DOI] [PubMed] [Google Scholar]
- 31.Pan J, Chen HQ, Sun YH, Zhang JH, Luo XY. Comparative Proteomic Analysis of Non-small-cell Lung Cancer and Normal Controls Using Serum Label-Free Quantitative Shotgun Technology. Lung. 2008;186:255–261. doi: 10.1007/s00408-008-9093-7. [DOI] [PubMed] [Google Scholar]
- 32.Park M, Park H, Kim WH, Cho H, Lee JH. Presence of autocrine hepatocyte growth factor-Met signaling and its role in proliferation and migration of SNU-484 gastric cancer cell line. Exp Mol Med. 2005;37:213–219. doi: 10.1038/emm.2005.29. [DOI] [PubMed] [Google Scholar]
- 33.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 34.Peebles KA, Duncan MW, Ruch RJ, Malkinson AM. Proteomic analysis of a neoplastic mouse lung epithelial cell line whose tumorigenicity has been abrogated by transfection with the gap junction structural gene for connexin 43, Gja1. Carcinogenesis. 2003;24:651–657. doi: 10.1093/carcin/bgg008. [DOI] [PubMed] [Google Scholar]
- 35.Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–4646. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 36.Rabilloud T. Use of thiourea to increase the solubility of membrane proteins in two-dimensional electrophoresis. Electrophoresis. 1998;19:758–760. doi: 10.1002/elps.1150190526. [DOI] [PubMed] [Google Scholar]
- 37.Reddel RR, Ke Y, Gerwin BI, McMenamin MG, Lechner JF, Su RT, Brash DE, Park JB, Rhim JS, Harris CC. Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12 SV40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region genes. Cancer Res. 1988;48:1904–1909. [PubMed] [Google Scholar]
- 38.Reed SI. Ratchets and clocks: the cell cycle, ubiquitylation and protein turnover. Nat Rev Mol Cell Biol. 2003;4:855–864. doi: 10.1038/nrm1246. [DOI] [PubMed] [Google Scholar]
- 39.Schneider J. Tumor markers in detection of lung cancer. Adv Clin Chem. 2006;42:1–41. doi: 10.1016/s0065-2423(06)42001-1. [DOI] [PubMed] [Google Scholar]
- 40.Sinha P, Kohl S, Fischer J, Hutter G, Kern M, Kottgen E, Dietel M, Lage H, Schnolzer M, Schadendorf D. Identification of novel proteins associated with the development of chemoresistance in malignant melanoma using two-dimensional electrophoresis. Electrophoresis. 2000;21:3048–3057. doi: 10.1002/1522-2683(20000801)21:14<3048::AID-ELPS3048>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 41.Sporn MB, Roberts AB. Autocrine growth factors and cancer. Nature. 1985;313:745–747. doi: 10.1038/313745a0. [DOI] [PubMed] [Google Scholar]
- 42.Stetler-Stevenson WG. Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci Signal. 2008;1:re6. doi: 10.1126/scisignal.127re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suemitsu R, Yoshino I, Tomiyasu M, Fukuyama S, Okamoto T, Maehara Y. Serum tissue inhibitors of metalloproteinase-1 and -2 in patients with non-small cell lung cancer. Surg Today. 2004;34:896–901. doi: 10.1007/s00595-004-2853-y. [DOI] [PubMed] [Google Scholar]
- 44.Tarro G, Perna A, Esposito C. Early diagnosis of lung cancer by detection of tumor liberated protein. J Cell Physiol. 2005;203:1–5. doi: 10.1002/jcp.20195. [DOI] [PubMed] [Google Scholar]
- 45.Thomas P, Khokha R, Shepherd FA, Feld R, Tsao MS. Differential expression of matrix metalloproteinases and their inhibitors in non-small cell lung cancer. J Pathol. 2000;190:150–156. doi: 10.1002/(SICI)1096-9896(200002)190:2<150::AID-PATH510>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 46.Tokunaga K, Nakamura Y, Sakata K, Fujimori K, Ohkubo M, Sawada K, Sakiyama S. Enhanced expression of a glyceraldehyde-3-phosphate dehydrogenase gene in human lung cancers. Cancer Res. 1987;47:5616–5619. [PubMed] [Google Scholar]
- 47.Tuynder M, Susini L, Prieur S, Besse S, Fiucci G, Amson R, Telerman A. Biological models and genes of tumor reversion: cellular reprogramming through tpt1/TCTP and SIAH-1. Proc Natl Acad Sci U S A. 2002;99:14976–14981. doi: 10.1073/pnas.222470799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tuynder M, Fiucci G, Prieur S, Lespagnol A, Geant A, Beaucourt S, Duflaut D, Besse S, Susini L, Cavarelli J, Moras D, Amson R, Telerman A. Translationally controlled tumor protein is a target of tumor reversion. Proc Natl Acad Sci U S A. 2004;101:15364–15369. doi: 10.1073/pnas.0406776101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Veenstra TD, Conrads TP, Hood BL, Avellino AM, Ellenbogen RG, Morrison RS. Biomarkers: mining the biofluid proteome. Mol Cell Proteomics. 2005;4:409–418. doi: 10.1074/mcp.M500006-MCP200. [DOI] [PubMed] [Google Scholar]
- 50.Vogelstein B, Kinzler KW. The Genetic Basis of Human Cancer. 2nd Ed. New York: McGraw-Hill, Medical Pub. Division; 2002. [Google Scholar]
- 51.Welsh JB, Sapinoso LM, Kern SG, Brown DA, Liu T, Bauskin AR, Ward RL, Hawkins NJ, Quinn DI, Russell PJ, Sutherland RL, Breit SN, Moskaluk CA, Frierson HF, Jr, Hampton GM. Large-scale delineation of secreted protein biomarkers overexpressed in cancer tissue and serum. Proc Natl Acad Sci U S A. 2003;100:3410–3415. doi: 10.1073/pnas.0530278100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu CC, Chien KY, Tsang NM, Chang KP, Hao SP, Tsao CH, Chang YS, Yu JS. Cancer cell-secreted proteomes as a basis for searching potential tumor markers: nasopharyngeal carcinoma as a model. Proteomics. 2005;5:3173–3182. doi: 10.1002/pmic.200401133. [DOI] [PubMed] [Google Scholar]
- 53.Yan JX, Wait R, Berkelman T, Harry RA, Westbrook JA, Wheeler CH, Dunn MJ. A modified silver staining protocol for visualization of proteins compatible with matrix-assisted laser desorption/ionization and electrospray ionizationmass spectrometry. Electrophoresis. 2000;21:3666–3672. doi: 10.1002/1522-2683(200011)21:17<3666::AID-ELPS3666>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 54.Yang F, Xiao ZQ, Zhang XZ, Li C, Zhang PF, Li MY, Chen Y, Zhu GQ, Sun Y, Liu YF, Chen ZC. Identification of tumor antigens in human lung squamous carcinoma by serological proteome analysis. J Proteome Res. 2007;6:751–758. doi: 10.1021/pr0602287. [DOI] [PubMed] [Google Scholar]
- 55.Ylisirnio S, Hoyhtya M, Turpeenniemi-Hujanen T. Serum matrix metalloproteinases -2, -9 and tissue inhibitors of metalloproteinases -1, -2 in lung cancer--TIMP-1 as a prognostic marker. Anticancer Res. 2000;20:1311–1316. [PubMed] [Google Scholar]
- 56.Ziv T, Barnea E, Segal H, Sharon R, Beer I, Admon A. Comparative proteomics of small cell lung carcinoma. Cancer Biomark. 2006;2:219–234. doi: 10.3233/cbm-2006-2601. [DOI] [PubMed] [Google Scholar]
- 57.Zuo X, Speicher DW. Quantitative evaluation of protein recoveries in two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis. 2000;21:3035–3047. doi: 10.1002/1522-2683(20000801)21:14<3035::AID-ELPS3035>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]