Abstract
Expression of protein kinase C-δ (PKCδ) is up-regulated by apoptosis-inducing stimuli. However, very little is known about the signaling pathways that control PKCδ gene transcription. In the present study, we demonstrate that JNK stimulates PKCδ gene expression via c-Jun and ATF2 in response to the anticancer agent doxorubicin (DXR) in mouse lymphocytic leukemia L1210 cells. Luciferase reporter assays showed that DXR-induced activation of the PKCδ promoter was enhanced by ectopic expression of JNK1, c-Jun, or ATF2, whereas it was strongly reduced by expression of dominant negative JNK1 or by treatment with the JNK inhibitor SP600125. Furthermore, point mutations in the core sequence of the c-Jun/ATF2 binding site suppressed DXR-induced activation of the PKCδ promoter. Our results suggest an additional role for a JNK signaling cascade in DXR-induced PKCδ gene expression.
Keywords: activating transcription factor 2, apoptosis, doxorubicin, JNK mitogen-activated protein kinases, protein kinase C-δ, proto-oncogene proteins c-jun
Introduction
PKC family of serine-threonine kinases is a key regulator of cellular signaling pathways that are activated by diverse extracellular stimuli. These pathways include those regulating cell proliferation, differentiation, apoptosis, and tumor suppression (Blobe et al.,1994; Nishizuka, 1995; Newton, 1997). Based upon differences in their co-factor requirements and in the structures of their regulatory domains, the proteins of the PKC family are categorized into three different subgroups: classical PKCs (α, βI, βII, γ), novel PKCs (δ, ε, η, and θ), and atypical PKCs (ζ, λ/ι) ((Blobe et al.,1994; Nishizuka, 1995; Newton, 1997).
Upon activation by apoptotic stimuli, PKCδ promotes the progression of apoptosis (Ghayur et al., 1996; Denning et al., 1998; Li et al., 1999; Majumder et al., 2000; Brodie and Blumberg, 2003; Gavrielides et al., 2006; Liu et al., 2006; Reyland, 2007; Yoshida, 2007). Interestingly, during the apoptotic response, PKCδ is proteolytically cleaved by caspase-3 to release a catalytically active fragment (Ghayur et al., 1996; Denning et al., 1998). This cleavage is also associated with ubiquitin-dependent proteasomal degradation that terminates the PKCδ kinase activity (Lu et al., 1998), suggesting that expression of PKCδ is tightly regulated after irreversible proteolytic cleavage, to maintain its cellular level or to promote apoptosis progression. In deed, cellular levels of PKCδ have been reported to increase upon exposure to various apoptotic stimuli (Morrish and Rumsby, 2002; Suh et al., 2003; Shin et al., 2004; Gavrielides et al., 2006; Liu et al., 2006). However, very little is known about the signaling pathways that control the activity of the PKCδ gene promoter during the apoptotic response.
The goal of the present study was to identify the signaling pathway that stimulates PKCδ gene expression in mouse lymphocytic leukemia L1210 cells. Our data demonstrate that the PKCδ promoter is activated by JNK via c-Jun and ATF2 in response to the anticancer agent doxorubicin (DXR).
Materials and Methods
Plasmids
The plasmid pRL-null, which encodes Renilla luciferase, was purchased from Promega (Madison, WI). The plasmids expressing pcDNA/PKCδ, pcDNA3/JNK1 and dominant negative (dn)-JNK1 (pSRα/HA-JNK T183A/Y185F) were obtained from Dr. D. S. Min (Department of Molecular Biology, Pusan National University, Korea). The plasmid expressing ATF2 (pCMX/ATF2) was from Dr. Jae Hun Cheong (Department of Molecular Biology, Pusan National University, Korea), and the c-Jun pl(pcDNA3/c-Jun) plasmid was from Jae-Hong Kim (School of Life Sciences and Biotechnology, Korea University, Korea).
Northern blot analysis
Total RNA was prepared using RNA STAT-60 Reagent (Tel-Test, Friendswood, TX) according to the manufacturer's instructions. The total RNA (10 µg) was separated and transferred to a Hybond N+ nylon membrane (Amersham Pharmacia Biotech, Piscataway, NJ). Northern blotting was performed with a [γ-32P]-dCTP-labeled PKCδ cDNA probe, followed by hybridization with a GAPDH cDNA probe.
Western blot analysis
Western blots were performed as described previously (Shin et al., 2004). Anti-PKCδ antibody was purchased from Transduction Laboratories (Lexington, KY). Anti-PKCα, -PKCβI, -PKCβII, -PKCγ, and -PKCζ antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), and anti-JNK, -phospho-JNK (Thr183/Tyr185), -phosphoc-Jun (Ser73), and -phospho-ATF2 (Thr71) antibodies were from Cell Signaling Laboratories (Beverly, MA).
Generation of L1210 transfectants overexpressing PKCδ
In order to generate a stable cell line that overexpresses mouse PKCδ, the pcDNA3/PKCδ or empty pcDNA3 plasmid, respectively, was transfected into mouse lymphocytic leukemia L1210 cells using the LipofectAMINE Plus reagent (Invitrogen), as recommended by the manufacturer. The transfected cells were selected with 200 µg/ml G418 sulfate for about 3 weeks. All of the transfectants were screened by Western blot analysis using the anti-PKCδ antibody.
Apoptosis analysis
Cellular apoptosis was assessed by flow cytometric analysis. After L1210 tranfectants were treated with 1 µg/ml DXR for 24 h, the cells were treated with RNase A (250 µg/ml) and propidium iodide (50 µg/ml) for 30 min as described previously (Jung et al., 2008). Analysis of cellular DNA contents of propidium iodide-stained nuclei was measured by fluorescence-activated cell sorter (FACS; Becton Dickinson). The cell populations with subdiploid DNA content (sub-G0/G1) were determined as apoptotic cells.
Construction and mutagenesis of a PKCδ promoter-reporter construct
A mouse PKCδ promoter fragment spanning nts -1192 to +10 was isolated from mouse genomic DNA (Promega) by PCR using the primers 5'-CATGGGGTTTCTCACAGCCA-3' (forward; PKCδ-F) and 5'-GATGGAGCCTGGAGTGAGATTG-3' (reverse; PKCδ-R). A deletion construct spanning nts -683 to +10 was synthesized using PCR with the forward primer 5'-TGGACAGCAACTGTGGTGTG-3' and the reverse primer PKCδ-R. The amplified PCR products were cloned into a pT&A vector (Real Biotech Corporation, Taipei, Taiwan) and then ligated into the KpnI and BglII sites of the pGL3-Basic luciferase-encoding reporter vector (Promega). The resultant plasmids were designated mPKCδ-Luc(-1192/+10) and mPKCδ-Luc (-683/+10), respectively.
Site-directed mutagenesis of the c-Jun/ATF2 heterodimer binding site was performed using a two-step PCR reaction. In the first step, two fragments with overlapping regions were generated using the primer pairs (i) PKCδ-F (forward) and 5'-GAAACACTGcCTTCcGCCTGC-3' (reverse), and (ii) 5'-GCAGGCgGAAGgCAGTGTTTC-3' (forward) and PKCδ-R (reverse). Mutated bases are shown in lower-case text. In the second step, a mixture of the two overlapping DNA fragments was amplified using the PKCδ-F and PKCδ-R primers. The resulting fragment containing a mutated c-Jun/ATF2 heterodimer element was ligated into pGL3basic-Luc to generate mPKCδ-Luc(-1192/ +10)mtATF2. All constructs were verified by DNA sequencing and by restriction enzyme digestion analysis.
Promoter reporter assay
NIH 3T3 cells were seeded into a 12-well plate and transfected with 0.5 µg of PKCδ promoter-reporter construct using Lipofectamine 2000 as described previously (Yun et al., 2008). To monitor the transfection efficiency, the pRL-null plasmid (5 ng) encoding Renilla luciferase was included in all samples. Where indicated, mammalian expression vector encoding c-Jun, JNK1, DN-JNK1, or ATF2 was also included. At 24-h post-transfection, the cells were treated with DXR. After 6 to 12 h, the firefly and Renilla luciferase activities in a single sample were measured sequentially using a Dual-Glo Luciferase Assay System (Promega; Madison, WI). Luminescence was measured with a luminometer (Centro LB960; Berthold Tech, Bad Wildbad, Germany). The firefly luciferase luminescence data were normalized by dividing by the Renilla luciferase luminescence data. Results are expressed relative to the activity in control cells.
Statistical evaluation
Each experiment was performed at least three times. The data are shown as the mean ± standard deviation (S.D.). Comparisons between control and treatment were performed using Student's paired t-test, and differences with P < 0.05 were considered statistically significant.
Results
DXR accumulates the PKCδ protein to accelerate the apoptosis in L1210 cells
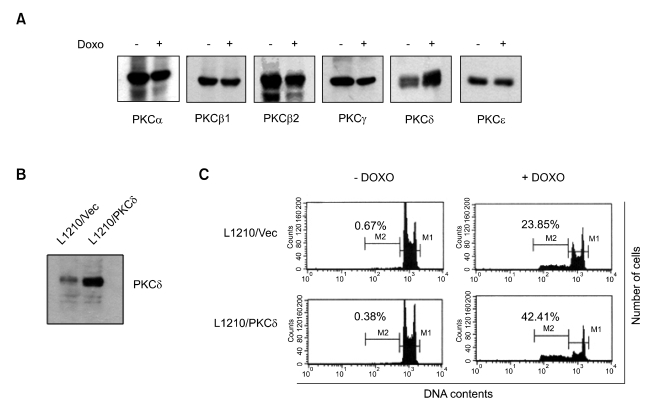
Doxorubicin (DXR) induced the accumulation of the PKCδ protein, but had no effect on the level of PKCα, PKCβ1, PKCβ2, PKCγ or PKCε, in L1210 cells (Figure 1A), suggesting that up-regulation of PKCδ isoform may be a specific event during DXR-induced cellular responses in L1210 cells. To address whether DXR-induced PKCδ up-regulation is functionally important for apoptosis progression, we established L1210 transfectants, PKCδ- transfected L1210/PKCδ cells and empty vector- transfected L1210/Vec cells (Figure 1B). When these cells were treated with 1 µg/ml doxorubicin for 24 h, apoptotic sub-G0/G1 population was 24.9% in L1210/Vec cells, while 42.4% in L1210/ PKCδ cells (Figure 1C). These data suggest that an increase in PKCδ expression induced by DXR may contribute to apoptotic responses in L1210 cells.
Figure 1.
Role of PKCδ expression in DXR-induced apoptosis. (A) L1210 cells were treated with 1 µg/ml DXR for 12 h. Total cell lysates were prepared and subjected to Western blotting with indicated PKC isoform-specific antibodies. The blots are representatives of results obtained from two independent experiments. (B) Generation of L1210 transfectants. Whole cell lysates from empty vector-transfected (L1210/Vec) or PKCδ-overexpressed (L1210/PKCδ) cells were prepared and subjected to Western blotting with anti-PKCδ antibody. (C) L1210 transfectants, L1210/Vec and L1210/PKCδ, were treated with 1 µg/ml DXR for 24 h and cellular apoptosis was identified by FACS analysis using propidium iodide. The percentage of cells with subdiploid DNA content, representing apoptotoc cells (M2 gate), is shown in panels.
DXR up-regulates the PKCd gene expression at the transcriptional level
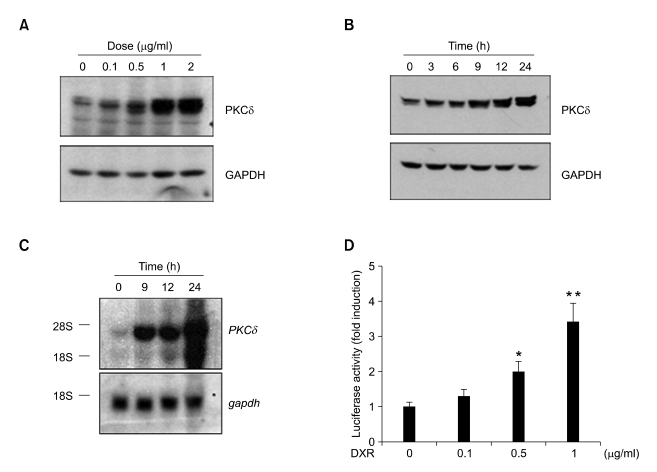
To further investigate the effects of DXR on PKCδ gene expression, we exposed mouse L1210 leukemia cells to various concentrations of DXR (0-2 µg/ml) for 12 h and then analyzed the amount of PKCδ protein by Western blotting. DXR increased the amount of PKCδ protein in a dose-dependent manner, with a near-maximum effect at 1 µg/ml DXR (Figure 2A). In a time-course study, PKCδ levels began to rise within 3 h of DXR treatment, and they gradually increased over 24 h (Figure 2B).
Figure 2.
Up-regulation of PKCδ expression by doxorubicin (DXR). (A, B) L1210 cells were treated with the indicated concentrations of DXR for 12 h (A) or with 1 µg/ml DXR for the indicated lengths of time (B). Total cell lysates were prepared and subjected to Western blotting with anti-PKCδ antibody (upper panels). The same blot was re-probed with anti-gapdh antibody as an internal control (lower panels). The blots shown are representative of results obtained in three independent experiments. (C) Total RNA was isolated from cells treated as in (B) and subjected to Northern blotting. The blot was hybridized with a 32P-labeled PKCδ cDNA probe (upper panel), stripped, and re-probed with a 32P-labeled GAPDH probe (lower panel). The blots shown are representative of results obtained from two independent experiments. (D) Sub-confluent NIH 3T3 cells grown in 12-well plates were transfected with 0.5 µg of mPKCδ-Luc(-1192/+10) along with 5 ng of pRL-null vector. Twenty-four hours after transfection, the cells were treated with DXR (0.1, 0.5, or 1 µg/ml) for 8-12 h. Firefly luciferase activity was normalized to Renilla luciferase activity. The data shown represent the mean ± S.D. (error bars) of three independent experiments performed in triplicate. The statistical significance of the assay was evaluated using Student's t-test (*, P < 0.05; **, P < 0.01 compared with untreated control).
To determine whether DXR up-regulates PKCδ expression at the transcriptional level, we used Northern blotting to measure PKCδ mRNA levels. In L1210 cells treated with DXR at 1 µg/ml, a marked, time-dependent increase in PKCδ mRNA levels was observed, whereas levels of control (gapdh) mRNA remained constant over the entire time period (Figure 2C).
We next isolated the mouse PKCδ promoter region located within 1.2 kb upstream of the transcriptional start site and subcloned it into the pGL3-Luc luciferase reporter vector to generate mPKCδ-Luc(-1192/+10). This construct was transfected into NIH 3T3 fibroblasts rather than L1210 cells because the latter displayed poor transfection efficiency. Twenty-four hours after transfection, the NIH 3T3 cells were treated with various concentrations of DXR (0-1 µg/ml), and reporter gene activity was measured. As shown in Figure 2D, DXR caused a dose-dependent elevation in luciferase reporter activity. A 3.4-fold increase in reporter activity was observed at 1 µg/ml DXR (P < 0.01, compared to mock-treated control). These results indicate that DXR activates transcription of the PKCδ gene.
DXR activates the JNK pathway in L1210 cells
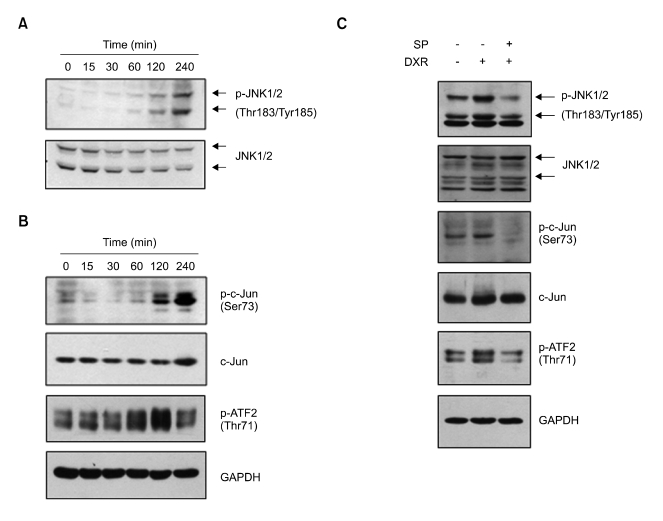
The JNK pathway has long been associated with the apoptotic response induced by DXR and other DNA-damaging agents (Verheij et al., 1996; Davis, 2000; Chang and Karin, 2001; Lin, 2003; Panaretakis et al., 2005). To determine whether DXR can activate JNK signaling in L1210 cells, serum-starved cells were treated with DXR (1 µg/ml) for various lengths of time (0-4 h), and the JNK activation status was assessed by immunoblotting with anti-phospho-JNK (Thr183/Tyr185) antibody. After 1 h of treatment, DXR had a detectable effect on JNK phosphorylation; this effect gradually increased as the treatment duration increased for up to 4 h (Figure 3A).
Figure 3.
DXR activation of the JNK pathway in L1210 cells. (A) L1210 cells were serum-starved (grown in 0.5% serum) for 24 h and then exposed to 1 µg/ml DXR for the indicated lengths of time up to 240 min. Total cell lysates were prepared and subjected to Western blotting with anti-phospho-JNK1/2 (Thr183/Tyr185) antibody. The blot was stripped and re-probed with anti-total JNK antibody as an internal control. (B) L1210 cells were treated as in (A). Total cell lysates were subjected to Western blotting with anti-phospho-c-Jun (Ser73) and anti-phospho-ATF2 (Thr71) antibodies. The blots were re-probed with antibodies against total c-Jun and GAPDH as internal controls. (C) L1210 cells were treated with 0 or 40 µM SP600125 (SP) for 30 min and then treated with 0 or 1 µg/ml DXR for 2 h as indicated. Total cell lysates were prepared and subjected to Western blotting with antibodies specific for phospho-JNK1/2 (Thr183/Tyr185), -phospho-c-Jun (Ser73), or phospho-ATF2 (Thr71). The blots were stripped and re-probed with anti-JNK, -c-Jun, or -GAPDH antibody. Blots shown are representative of at least three separate experiments.
DXR induction of JNK phosphorylation coincided with phosphorylation of c-Jun (Ser73) and ATF2 (Thr71), which are known JNK targets (Figure 3B). To confirm the dependence of JNK on the phosphorylation of c-Jun and ATF2, cells were pretreated with SP600125 before addition of DXR, and c-Jun and ATF2 phosphorylation were measured. Treatment with SP600125 strongly inhibited DXR-induced phosphorylation of c-Jun and ATF2, as well as JNK (Figure 3C), suggesting that both c-Jun and ATF2 are downstream mediators of DXR-stimulated JNK. Thus, JNK signaling is activated by DXR in L1210 cells.
JNK activates the PKCδ promoter activity
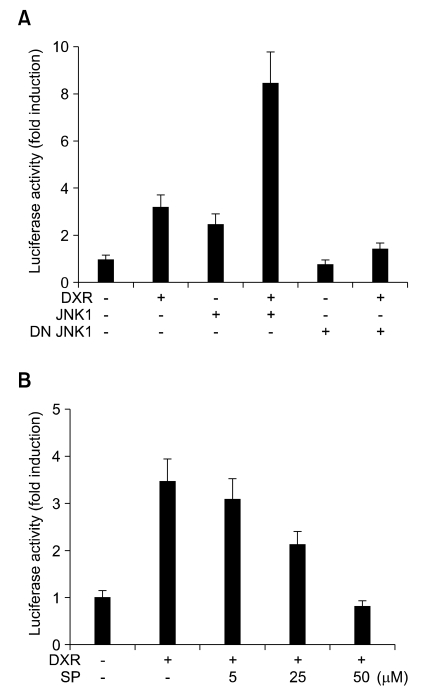
To determine whether JNK signaling pathway is involved in DXR-induced PKCδ expression, we first examined the effect of forced expression of JNK in PKCδ promoter activation. NIH 3T3 cells were co-transfected with the mPKCδ-Luc(-1192/+10) promoter-reporter construct and expression plasmid for JNK1. Figure 4A shows that the reporter-inducing activity of DXR was synergized by transient expression of JNK1, while reduced by expression of dominant negative (DN)-JNK1. When NIH 3T3 cells transfected with the mPKCδ-Luc(-1192/+10) construct were pretreated with SP600125, a selective inhibitor of JNK, DXR-induced reporter activity was attenuated dose-dependently by SP600125 (Figure 4B). These results suggest that the JNK signaling pathway may play a role in mediating DXR-induced PKCδ expression.
Figure 4.
Involvement of JNK in DXR-induced PKCδ promoter activation. (A) NIH 3T3 cells grown in 12-well plates were co-transfected with 0.5 µg of mPKCδ-Luc(-1192/+10) and 5 ng of pRL-null vector, along with or without 0.2 µg expression plasmid for JNK1 or DN-JNK1, as indicated. At 24 h post-transfection, the cells were treated with 1 µg/ml DXR for 8 h, as indicated. (B) NIH 3T3 cells grown in 12-well plates were co-transfected with 0.5 µg of PKCδ-Luc(-1192/+10) and 5 ng of pRL-null vector. After 24 h, the cells were pretreated with the indicated concentrations of SP600125 for 30 min, followed by addition of 1 µg/ml DXR for 8 h. Firefly luciferase activity was normalized to Renilla luciferase activity. The data shown represent the mean ± S.D. (error bars) of three independent experiments performed in triplicate.
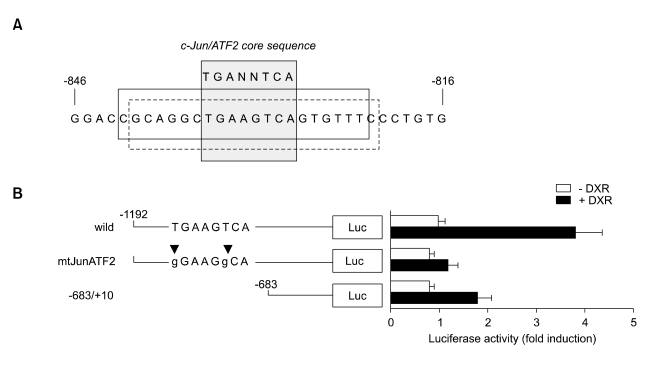
c-Jun/ATF2 binding site in the PKCδ gene is responsive to DXR
Activation of JNK stimulates gene expression by phosphorylating ATF2, which may form a heterodimer with phosphorylated c-Jun. Given that the phosphorylation of both c-Jun and ATF2 is increased by DXR (Figure 3), we speculated that c-Jun and ATF2 might be involved in DXR-induced PKCδ expression as JNK targets. To test this hypothesis, we used a web-based database (Genomatix) to search the promoter region of the PKCδ gene for the known binding site consensus sequence motif of ATF2/c-Jun. Analysis of the 1.2-kb region upstream of the transcription start site revealed a putative c-Jun/ATF2 heterodimer binding sequence located at nucleotides -841 to -821. This sequence is 5'-cgcaggcTGAAGTCAgtgtttc-3', which contains the core sequence 5'-TGANNTCA-3' (Figure 5A). To assess the relevance of the putative c-Jun/ATF2 heterodimer binding motif, we performed a site-directed mutagenesis. As shown in Figure 5B, point mutations in the core sequence of the c-Jun/ATF2 binding site (TGAAGTCA → gGAAGgCA) severely attenuated the PKCδ promoter activity. Furthermore, deletion of the distal region from nucleotides -1192 to -684, yielding mPKCδ-Luc(-683/+10), which lacks the c-Jun/ATF2 binding motif, similarly reduced DXR-induced PKCδ promoter activity. These data suggest that the c-Jun/ATF2 binding site at -841 to -821 in the enhancer region of the PKCδ gene is responsible for DXR-induced PKCδ promoter activation.
Figure 5.
Role of c-Jun/ATF2 binding motif in DXR-induced PKCδ promoter activation. (A) Nucleotide sequence of the 30 bp (-846 to -816) in the 5'-flanking region of the mouse PKCδ gene. The predicted binding site for c-Jun or ATF2 is marked as a solid or dotted box, respectively. Consensus c-Jun/ATF2 heterodimer binding site is marked as a grey box. (B) NIH 3T3 cells were transfected with wild-type mPKCδ-Luc(-1192/+10), mPKCδ-Luc(-1192/+10)mtJunATF2, or mPKCδ-Luc(-683/+10), along with 5 ng of pRL-null vector. The putative c-Jun/ATF2 binding motif is shown schematically. Arrowheads indicate the sites of point mutations. After 24 h post-transfection, the cells were either untreated or treated with 1 µg/ml DXR for 8 h. Firefly luciferase activity was normalized to the Renilla luciferase activity. The data shown represent the mean ± S.D. (error bars) of three independent experiments performed in triplicate.
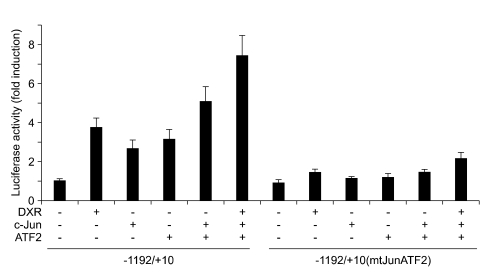
c-Jun and ATF2 transactivate the PKCδ gene through the putative c-Jun/ATF2 binding motif
To further assess whether PKCδ is a possible target gene of the c-Jun/ATF2, the wild-type mPKCδ-Luc(-1192/+10) or mutant construct of c-Jun/ATF2 binding site (mPKCδ-Luc(-1192/+10) mtJunATF2) was co-transfected into NIH 3T3 cells together with c-Jun and ATF2 expression vectors. Forced expression of c-Jun or ATF2 elevated basal reporter activity in the absence of DXR treatment, and combined transfection of both plasmids further enhanced DXR-induced reporter activity (Figure 6). In contrast, c-Jun and ATF2 had no appreciable effect on the mPKCδ-Luc(-1192/+10)mtJunATF2 construct. These results suggest that the mutation in the c-Jun/ATF2 binding site blocks the binding of c-Jun/ATF2 to the PKCδ promoter, thus abolishing the transacting activity of c-Jun/ATF2.
Figure 6.
Role of c-Jun and ATF2 in DXR-induced PKCδ promoter activation. NIH 3T3 cells were transfected with either wild-type mPKCδ-Luc (-1192/+10) or c-Jun/ATF2 binding mutant mPKCδ-Luc(-1192/+10) mtJunATF2 construct in the absence or presence of expression plasmids for c-Jun and ATF2, as indicated. 5 ng of pRL-null vector was included in all samples. After 24 h, the cells were either untreated or treated with 1 µg/ml DXR for 8 h. Firefly luciferase activity was normalized to the Renilla luciferase activity. The data shown represent the mean ± S.D. (error bars) of three independent experiments performed in triplicate.
Taken together, our results demonstrate that PKCδ is transcriptionally regulated by DXR and that JNK is involved in DXR-induced PKCδ gene transcription through the putative c-Jun/ATF2 binding motif in the distal promoter region.
Discussion
PKCδ plays important roles in various cellular functions, including cell survival and cell death (Blobe et al.,1994; Nishizuka, 1995; Newton, 1997; Reyland, 2007; Yoshida, 2007). Cellular levels of PKCδ have been reported to increase upon exposure to various apoptotic stimuli (Morrish and Rumsby, 2002; Suh et al., 2003; Shin et al., 2004; Gavrielides et al., 2006; Liu et al., 2006), indicating that PKCδ expression is tightly regulated. Previously, we have found that treatment with some genotoxic agents, including doxorubicin (DXR), etoposide, and camptothecin, resulted in an increase in the amount of the full-length PKCδ protein, whereas cisplatin, ultraviolet C irradiation or taxol had little effect in L1210 mouse leukemia cells (Shin et al., 2004). However, since most PKCδ studies have focused on its biological functions, the signaling pathways that control the transcription of the PKCδ gene are poorly understood. The present study shows the molecular mechanism in which JNK contributes to DXR-induced PKCδ expression by activating c-Jun/ATF2 transcription factors. While PKCδ lies upstream of JNK in response to DNA damage (Yoshida et al., 2002; Shin et al., 2004; Panaretakis et al., 2005; Liu et al., 2006), our findings provide further support for cross-talk between PKCδ and JNK during apoptosis progression.
Our results show that DXR stimulates the phosphorylation of JNK and its downstream mediators, c-Jun and ATF2, and that pretreatment of cells with SP600125, a selective JNK inhibitor, inhibits DXR-stimulated phosphorylation of c-Jun and ATF2. Based on these findings, we speculate that DXR-induced PKCδ gene expression is controlled by c-Jun/ATF2 as a downstream target of JNK. To test this possibility, we analyzed a 1.2-kb region upstream of the PKCδ transcription start site to search the promoter region of the PKCδ gene for the known binding site consensus sequence motif of ATF2/c-Jun. We found a putative c-Jun/ATF2 heterodimer binding site (TGAAGTCA) located at nts -841 to -821. The c-Jun/c-Fos heterodimer, the most prominent AP1 dimer, is involved in the activation of a number of target genes (Karin et al., 1997). The AP1 dimer can also be composed of c-Jun and ATF2 (Ivashkiv et al., 1990). However, the c-Jun/c-Fos heterodimer binds to the TGA-NTCA consensus sequence, whereas the c-Jun/ATF2 heterodimer binds preferentially to the TGANNTCA consensus sequence (Ivashkiv et al., 1990; Hai and Curran, 1991). In our luciferase reporter assays, combined transfection of c-Jun and ATF2 further enhanced DXR-induced PKCδ promoter activation. To corroborate the functional role of the c-Jun/ATF2 heterodimer binding site in mediating DXR-induced PKCδ promoter activity, we made two point mutations in the core sequence of the c-Jun/ATF2 binding site (TGAAGTCA → gGAAGgCA) to create the mutant promoter-reporter construct, mPKCδ-Luc(-1192/+10)mtJun-ATF2. These mutations significantly suppressed DXR-induced activity of the reporter gene. Thus, DXR appears to regulate the PKCδ gene at the transcriptional level via the c-Jun/ATF2 heterodimer-binding element. This conclusion is further supported that DXR activation of the PKCδ promoter was significantly reduced for another reporter construct, mPKCδ-Luc(-683/+10), which lacks the c-Jun/ATF2 binding site.
Our finding showed that point mutation and deletion of the c-Jun/ATF2 binding site did not completely abolish the DXR response (Figure 5B). These data suggest that a c-Jun/ATF2 heterodimer response element is necessary but not sufficient for the full activation of the PKCδ gene by DXR, and that an additional pathway may be required for full activation. Given that DXR activates NF-κB in L1210 cells (Shin et al., 2006), NFκB might be involved in this additional pathway. Indeed, a recent study showed that NF-κB binding sites located at nucleotides -83/-74 and -52/-43 in the proximal region of the PKCδ gene contribute to PKCδ expression and promote UV-induced apoptosis (Liu et al., 2006). NFκB is also involved in insulin-induced PKCδ mRNA expression in L6 skeletal muscle cells (Horovitz-Fried and Sampson, 2007). Further studies will be required to fully elucidate the regulatory signal pathway responsible for PKCδ transcriptional activation in response to DXR treatment.
In a previous report, we showed that the chemotherapy drug etoposide induces PKCδ gene expression in L1210 cells, and that this induction is impaired by inhibition of PKCδ activity, but not of JNK activity, implicating a JNK-independent PKCδ autoregulatory loop in etoposide-induced PKCδ gene expression (Shin et al., 2004). In the present study, we confirmed that a PKCδ autoregulatory mechanism is also involved in DXR-induced PKCδ expression; we observed that rottlerin, a selective inhibitor of PKCδ, dose-dependently blocked DXR-induced PKCδ accumulation in L1210 cells (data not shown). However, DXR-induced PKCδ expression was attenuated by inhibition of JNK activity. Thus, it is conceivable that PKCδ selects JNK-dependent or -independent downstream effectors to up-regulate its own expression depending on the nature of the stimuli.
In summary, the results of this present investigation suggest a role for a JNK signaling cascade in DXR-induced expression of the PKCδ gene. Stimulation of JNK by DXR leads to the activation of the c-Jun and ATF2 transcription factors in the activation of the PKCδ gene promoter.
Acknowledgments
This research was supported by a grant from the Disease Network Research Program (M10751050003-07N5105-00310) of the Korea Science and Engineering Foundation (KOSEF), and a grant from Basic Research Promotion Fund (KRF-2005-041-E00065).
Abbreviations
- DXR
doxorubicin
- PKCδ
porein kinase Cδ
References
- 1.Blobe GC, Obeid LM, Hannun YA. Regulation of protein kinase C and role in cancer biology. Cancer Metastasis Rev. 1994;13:411–431. doi: 10.1007/BF00666107. [DOI] [PubMed] [Google Scholar]
- 2.Brodie C, Blumberg PM. Regulation of cell apoptosis by protein kinase c δ. Apoptosis. 2003;8:19–27. doi: 10.1023/a:1021640817208. [DOI] [PubMed] [Google Scholar]
- 3.Chang L, Karin M. Mammalian MAP kinase signaling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 4.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;1003:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 5.Denning MF, Wang Y, Nickoloff BJ, Smith-Wrone T. Protein kinase C δ is activated by caspase-dependent proteolysis during ultraviolet radiation-induced apoptosis of human keratinocytes. J Biol Chem. 1998;273:29995–30002. doi: 10.1074/jbc.273.45.29995. [DOI] [PubMed] [Google Scholar]
- 6.Gavrielides MV, Gonzalez-Guerrico AM, Riobo NA, Kazanietz MG. Androgens regulate protein kinase C δ transcription and modulate its apoptotic function in prostate cancer cells. Cancer Res. 2006;66:11792–11801. doi: 10.1158/0008-5472.CAN-06-1139. [DOI] [PubMed] [Google Scholar]
- 7.Ghayur T, Hugunin M, Talanian RV, Ratnofsky S, Quinlan C, Emoto Y, Pandey P, Datta R, Huang Y, Kharbanda S, Allen H, Kamen R, Wong W, Kufe D. Proteolytic activation of protein kinase C δ by an ICE/CED 3-like protease induces characteristics of apoptosis. J Exp Med. 1996;184:2399–2404. doi: 10.1084/jem.184.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hai T, Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci USA. 1991;88:3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horovitz-Fried M, Sampson SR. Involvement of PKCα in insulin-induced PKCδ expression: Importance of SP-1 and NFκB transcription factors. Biochem Biophys Res Commun. 2007;352:78–83. doi: 10.1016/j.bbrc.2006.10.149. [DOI] [PubMed] [Google Scholar]
- 10.Ivashkiv LB, Liou HC, Kara CJ, Lamph WW, Verma IM, Glimcher LH. mXBP/CRE-BP2 and c-Jun form a complexwhich binds to the cyclic AMP, but not to the 12-O-tetradecanoylphorbol-13-acetate, response element. Mol Cell Biol. 1990;10:1609–1621. doi: 10.1128/mcb.10.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung EJ, Kim CW, Kim DR. Cytoplasmic accumulation of γH2AX is associated with tropomyosin-related kinase A-induced cell death in U2OS cells. Exp Mol Med. 2008;40:276–285. doi: 10.3858/emm.2008.40.3.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Lorenzo PS, Bogi K, Blumberg PM, Yuspa SH. Protein kinase Cδ targets mitochondria, and induces apoptosis in normal and neoplastic keratinocytes when overexpressed by an adenoviral vector. Mol Cell Biol. 1999;19:8547–8558. doi: 10.1128/mcb.19.12.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin A. Activation of the JNK signaling pathway: breaking the brake on apoptosis. Bioessays. 2003;25:17–24. doi: 10.1002/bies.10204. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Yang D, Minemoto Y, Leitges M, Rosner MR, Lin A. NF-κB is required for UV-induced JNK activation via induction of PKCδ. Mol Cell. 2006;21:467–480. doi: 10.1016/j.molcel.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 16.Lu Z, Liu D, Hornia A, Devonish W, Pagano M, Foster DA. Activation of protein kinase C triggers its ubiquitination and degradation. Mol Cell Biol. 1998;8:839–845. doi: 10.1128/mcb.18.2.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majumder PK, Pandey P, Sun X, Cheng K, Datta R, Saxena S, Kharbanda S, Kufe D. Mitochondrial translocation of protein kinase C δ in phorbol ester-induced cytochrome c release and apoptosis. J Biol Chem. 2000;275:21793–21796. doi: 10.1074/jbc.C000048200. [DOI] [PubMed] [Google Scholar]
- 18.Morrish BC, Rumsby MG. The 5' untranslated region of protein kinase Cδ directs translation by an internal ribosome entry segment that is most active in densely growing cells and during apoptosis. Mol Cell Biol. 2002;22:6089–6099. doi: 10.1128/MCB.22.17.6089-6099.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newton AC. Regulation of protein kinase C. Curr Opin Cell Biol. 1997;9:161–167. doi: 10.1016/s0955-0674(97)80058-0. [DOI] [PubMed] [Google Scholar]
- 20.Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- 21.Panaretakis T, Laane E, pokrovskaja K, Bjöorklund A-C, Moustakas A, Zhivotovsky B, Heyman M, Shoshan MC, Grandér D. Doxorubicin requires the sequential activation of capsase-2, protein kinase C δ, and c-Jun NH2-terminal kinase to induce apoptosis. Mol Biol Cell. 2005;16:3821–3831. doi: 10.1091/mbc.E04-10-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reyland ME. Protein kinase Cδ and apoptosis. Biochem Soc Trans. 2007;35:1001–1004. doi: 10.1042/BST0351001. [DOI] [PubMed] [Google Scholar]
- 23.Shin SY, Kim CG, Ko J, Min DS, Chang JS, Ohba M, Kuroki T, Choi YB, Kim YH, Na DS, Kim JW, Lee YH. Transcriptional and post-transcriptional regulation of the PKCδ gene by etoposide in L1210 murine leukemia cells: implication of PKCδ autoregulation. J Mol Biol. 2004;340:681–693. doi: 10.1016/j.jmb.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Shin SY, Choi BH, Kim JR, Kim JH, Lee YH. Suppression of P-glycoprotein expression by antipsychotics trifluoperazine in adriamycin-resistant L1210 mouse leukemia cells. Eur J Pharm Sci. 2006;28:300–306. doi: 10.1016/j.ejps.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Suh KS, Tatunchak TT, Crutchley JM, Edwards LE, Marin KG, Yuspa SH. Genomic structure and promoter analysis of PKC-δ. Genomics. 2003;82:57–67. doi: 10.1016/s0888-7543(03)00072-7. [DOI] [PubMed] [Google Scholar]
- 26.Verheij M, Bose R, Lin XH, Yao B, Jarvis WD, Grant S, Birrer MJ, Szabo E, Zon LI, Kyriakis JM, Haimovitz-Friedman A, Fuks Z, Kolesnick RN. Requirement for ceramide-initiated SAPK/JNK signaling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida K, Miki Y, Kufe D. Activation of SAPK/JNK signaling by protein kinase C δ in response to DNA damage. J Biol Chem. 2002;277:48372–48378. doi: 10.1074/jbc.M205485200. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida K. PKCδ signaling: Mechanisms of DNA damage response and apoptosis. Cell Signal. 2007;19:892–901. doi: 10.1016/j.cellsig.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 29.Yun HJ, Cho YH, Moon Y, Park YW, Yoon HK, Kim YJ, Cho SH, Lee YI, Kang BS, Kim WJ, Park K, Seol W. Tranascriptional targeting of gene expression in breast cancer by the promoters of protein regulator of cytokinesis 1 and ribonuclease reductase 2. Exp Mol Med. 2008;40:345–353. doi: 10.3858/emm.2008.40.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]