Abstract
Heat shock protein 70 (HSP70), which evidences important functions as a molecular chaperone and anti-apoptotic molecule, is substantially induced in cells exposed to a variety of stresses, including hypertonic stress, heavy metals, heat shock, and oxidative stress, and prevents cellular damage under these conditions. However, the molecular mechanism underlying the induction of HSP70 in response to hypertonicity has been characterized to a far lesser extent. In this study, we have investigated the cellular signaling pathway of HSP70 induction under hypertonic conditions. Initially, we applied a variety of kinase inhibitors to NIH3T3 cells that had been exposed to hypertonicity. The induction of HSP70 was suppressed specifically by treatment with protein kinase C (PKC) inhibitors (Gö6976 and GF109203X). As hypertonicity dramatically increased the phosphorylation of PKCµ, we then evaluated the role of PKCµ in hypertonicity-induced HSP70 expression and cell viability. The depletion of PKCµ with siRNA or the inhibition of PKCµ activity with inhibitors resulted in a reduction in HSP70 induction and cell viability. Tonicity-responsive enhancer binding protein (TonEBP), a transcription factor for hypertonicity-induced HSP70 expression, was translocated rapidly into the nucleus and was modified gradually in the nucleus under hypertonic conditions. When we administered treatment with PKC inhibitors, the mobility shift of TonEBP was affected in the nucleus. However, PKCµ evidenced no subcellular co-localization with TonEBP during hypertonic exposure. From our results, we have concluded that PKCµ performs a critical function in hypertonicity-induced HSP70 induction, and finally cellular protection, via the indirect regulation of TonEBP modification.
Keywords: HSP70 heat-shock proteins; NFAT5 protein, human; protein kinase C; protein kinase inhibitors
Introduction
Because all organisms face environmental and pathophysiological stresses, they need to develop appropriate protective mechanisms. One of the best-known protective mechanisms in this regard is the rescue of cells against a variety of insults via the induction of stress response proteins (Flanagan et al., 1995; Scliess et al., 1999). Inducible HSP70 has been implicated as a molecule which performs a pivotal role in the protection of cells against a variety of stresses. HSP70 synthesis has been identified in response to a broad range of chemicals and biological signals, including hypertonic stress, amino acid analogues, energy metabolism inhibitors, radiation, oxidative stress, and heavy metals (Williams and Morimoto, 1990; Hatayama et al., 1993; Abe et al., 1995; Wagner et al., 1999). This means that HSP70 is involved in a general cellular defense mechanism. Recently, the cytoprotective roles of HSP70 were clearly identified and the molecular mechanisms inherent to the anti-apoptotic function of HSP70 were clearly elucidated (Beere et al., 2000; Li et al., 2000; Ravagnan et al., 2001; Lee et al., 2005).
Inducible HSP70 is encoded from both hsp70.1 and hsp70.3 genes, which evidence a high degree of similarity in their coding sequences and a linked tandem array with the MHC region of the same chromosome. Two inducible hsp70 genes differ from each other in their 5'- and 3'-untranslated regions (Walter et al., 1994). We observed previously that hsp70.1 and hsp70.3 genes respond differentially to different types of stress (Lee and Seo, 2002). Hypertonicity induced only hsp70.1 expression, because only hsp70.1 harbors tonicity-responsive enhancer (TonE) sites, which have been shown to perform an important function in the response to hypertonicity (Heo et al., 2006).
HSP70 accumulation in cells exposed to stress is known to generally depend on the activation of heat-shock factor (HSF) (Abravaya et al., 1991; Morimoto et al., 1992). The stress-induced rapid activation of HSF is a very common phenomenon. However, it has been recently suggested that inducible HSP70 synthesis appears to be regulated via alternative transcription activators. As the hsp70.1 promoter harbors TonE sites, the expression of HSP70 may be regulated by the tonicity-responsive enhancer binding protein (TonEBP) under hypertonic conditions (Woo et al., 2002). The regulatory mechanism of hsp70 transcription during exposure to hypertonicity remains to be well-defined.
The principal objective of this study was to elucidate the manner in which HSP70 induction is regulated in response to hypertonic conditions. We determined that HSP70 expression is regulated by the transcription factor, TonEBP, via the activation of PKCµ, ultimately resulting in the protection of cells against hypertonic stress conditions.
Materials and Methods
Materials
PD98059, LY293002, H-89, and Gö6976 were purchased from Calbiochem (La Jolla, CA) and GF109203X was from LC Laboratories (Woburn, MA). Anti-HSP70, anti-actin, anti-TonEBP, anti-Lamin, and secondary antibodies were from Santa Cruz (Santa Cruz, CA). Anti-phospho-PKC and anti-PKCµ antibodies were from Cell Signaling Technology (Beverly, MA).
Cell culture
NIH3T3 mouse fibroblast and M-1 human renal epithelial cells were grown in DMEM with 10% FBS, penicillin (100 µg/ml), and streptomycin (100 µg/ml) in a 5% CO2 humidified atmosphere at 37℃. In order to generate hypertonic condition, NaCl solution was added to the medium to 130 mM final concentration of NaCl.
Preparation of whole cell lysate and Western blot analysis
Cells were washed with PBS, scraped and collected in extraction buffer (25 mM Tris-HCl, pH 8.0, 1 mM EDTA, 1% Triton X-100, 100 mM NaCl, 1 mM PMSF, 10 mM sodium azide, 1 mM orthovanidate, 20 µg/ml aprotinin, and 3 mM dithiothreitol). The collected cells were incubated on ice for 30 min. The lysate was centrifuged and quantitated with a Bradford Assay Reagents (Bio-Rad, Hercules, CA). Equal amount of proteins was loaded onto an 8 or 10% SDS-PAGE gel. After separation of proteins depending on their molecular weights, it was transferred to a nitrocellulose membrane. The membrane blot was incubated with antibody at 4℃ overnight, and washed three times in TBST. Protein bands were detected by sequential treatment with an HRP-conjugated secondary antibody (Santa Cruz, CA), and an enhanced chemiluminescence substrate kit (Amersham Bioscience, NJ).
MTT assay
Cells were applied to each well of 25-well culture plates and incubated overnight. One hundreds µl of 5 mg/ml MTT dissolved in PBS was added to each well and incubated for 4 h. The purple formazan formed by viable cells was dissolved by the addition of DMSO and absorbance was measured at 540 nm with VERSAmax (Molecular Device, Sunnyvale, CA).
Cellular fractionation
Cells were incubated with 400 µl of buffer A (10 mM HEPES, pH 7.9, 10 mM KCl, 0.1 mM EDTA, 1 mM PMSF, 10 mM sodium azide, 1 mM orthovanidate, 20 µg/ml aprotinin, and 1 mM DTT) on ice for 5 min. These samples were mixed with 25 µl of 10% Nonidet P-40 and vortexed for 10 s. After centrifugation at 14,000 rpm for 1 min, the supernatant was saved as cytoplasmic fraction. The pellets were washed once with the same buffer, resuspended in buffer B (20 mM HEPES, pH 7.9, 400 mM NaCl, 1 mM EDTA, 1 mM PMSF, 10 mM sodium azide, 1 mM orthovanidate, 20 µg/ml aprotinin, and 1 mM DTT), and vortexed on ice for 10 min. After centrifugation at 14,000 rpm for 10 min, the supernatant was waved as nuclear fraction.
Transient transfection
Recombinant plasmid 3-TonE-HSP70 promoter region up to -2 Kb from the transcription start point was obtained from PCR with mouse genomic DNA. PCR product (HSP70 promoter region) was ligated to promoterless EGFP vector (Clontech, Palo Alto, CA), and generated recombinant plasmid, pH70pro-EGFP. Plasmids, pcDNA-PKCα-KD-HA and pcDNA-PKCβI-KD-HA were thankfully obtained from Dr. YS Lee (Korea Institute of Radiological and Medical Sciences, Seoul, Korea). Recombinant plasmids were transfected to NIH-3T3 cells using Lipofectamine (Invitrogen, Carlsbad, CA) according to the instructions of the manufacturer. After transfection, the gene expression was monitored by Western blotting analysis.
Immunostaining and confocal microscopy
Cells were fixed in 4% paraformaldehyde in PBS for 30 min and permeabilized with 0.5% Triton X-100 for 10 min. The cells were then blocked with 2% BSA in PBS for 1 h, washed with PBS, and incubated with primary antibody diluted 1:200 in PBS containing 2% BSA. Then, cells were washed with PBS three times. Secondary FITC-conjugated antibody (1:200 dilutions in PBS containing 2% BSA) was applied to the coverslip. After washing three times with PBS, the cells were stained with DAPI for the detection of nuclei. After mounting, the cells were examined under a Zeiss confocal laser scanning microscope (Carl Zeiss, Jena, Germany). Images were captured and merged with LSM 5 PASCAL software (Carl Zeiss, Jena, Germany).
Treatment of siRNA
The stealth RNAi duplex targeting nucleotides of the PKCµ cDNA and negative control oligomer (BLOCK iT Oligo) were synthesized by Invitrogen (Carlsbad, CA). The stealth RNAi sequences of the PKCµ were as followed: sense 5'-UGCCAAAGCCAUCUCAGUAUCUUGG-3' and antisense 5'-CCAAGAUACUGAGAUGGCUUUGGCA-3'. Cells were transfected with 25 µM of either the PKCµ siRNA duplex or control dsRNA using the Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions.
Real time PCR
SYBR green-based real time PCR technique was used to detect the expression of HSP70 and PKCµ. Total RNA was prepared from NIH-3T3 cells, and 1 µg of total RNA was reverse-transcribed into cDNA in the presence of RT-premix (Bioneer, Daejeon, Korea) and 1 µg of random primers (Invitrogen, Carlsbad, CA) in a total volume of 20 µl at 42℃ for 1 h. The PCR mixture consisted of 1 µl cDNA, 10 µl of SYBR green-containing PCR master mixture (Qiagen, Valencia, CA), 0.75 µl of ROX (passive fluorescent dye) and 10 pmol of each primer in a total volume of 20 µl. The specific primers for real time PCR were designed by using the Primer3 software as follows: HSP70.1 (untranslated region in upstream of the start site) sense 5'-GAGACATGGACAAGCAAGCA-3' and antisense 5'-GGTGGTGAGAGTGTGGGACT-3'; PKCµ sense CGACGTCATCATCCATCAAC and antisense ACTGTGGTACCCTGCTCTGG; GAPDH sense 5'-GGAGCGAGACCCCACTAACA-3' and antisense 5'-ACATACTCAGCACCGGCCTC-3'. Real time PCR was performed using the ABI7000 thermocycler (Applied Biosciences, Foster City, CA), and the cycling conditions used were 95℃ for 15 s, 60℃ for 10 s and 72℃ for 30 s for 40 cycles, followed by a melting point determination that results in a single peak if the amplification is specific. The ROX-normalized fluorescence measurements were exported to Microsoft Excel. The results were normalized to the housekeeping gene GAPDH mRNA in the same sample. The relative expression of each gene in NIH-3T3 cells was calculated using a derivative of the comparative CT method.
Results
PKC signaling pathway involves hypertonicity-induced HSP70 expression
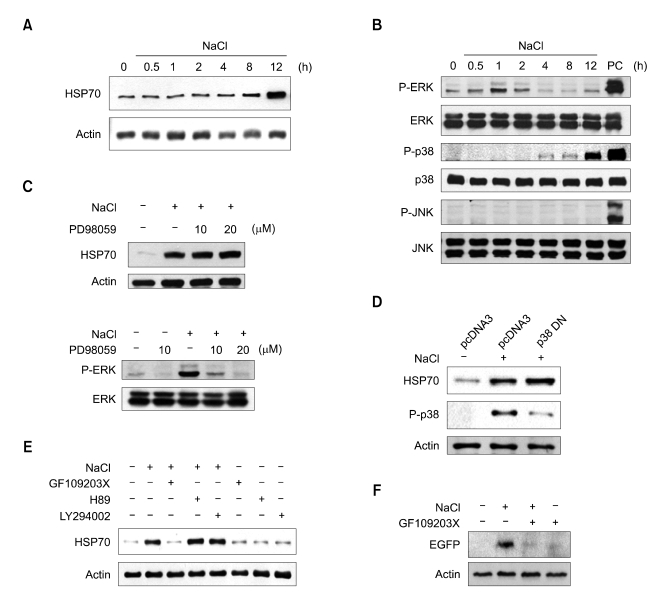
In order to determine the essential signaling pathway for the hypertonicity-induced HSP70 expression, we treated NIH3T3 cells with a variety of kinase inhibitors, and evaluated the induction of HSP70. When cells were exposed to hypertonic conditions (with the administration of an additional 130 mM NaCl to the medium), the levels of intracellular HSP70 were increased dramatically (Figure 1A). As ERK and p38 were activated in cells exposed to hypertonic conditions (Figure 1B), we attempted to determine whether ERK or p38 were involved in hypertonicity-induced HSP70 expression. Treatment with an ERK inhibitor (PD98059) exerted no effects on hypertonicity-induced HSP70 expresssion (Figure 1C). When we conducted transfection with a dominant negative form of p38 (p38 DN), the induction of HSP70 remained unaltered (Figure 1D). PKA inhibitor (H89) and PI-3K inhibitor (LY293002) also had no effects on the level of HSP70 induction (Figure 1E). However, when cells were treated with a broad range of PKC inhibitor (GF109203X), hypertonicity-induced HSP70 expression was inhibited completely (Figure 1E). In order to verify the inhibitory effects of GF109203X on the hypertonicity-induced expression of HSP70, we transfected the recombinant plasmid, pHSP70pro-EGFP (recombinant plasmid containing HSP70 promoter and EGFP coding regions), into NIH3T3 cells and detected EGFP expression under hypertonic conditions. Whereas the NIH3T3 cells evidenced EGFP protein expression as the result of NaCl treatment, GF109203X clearly inhibited the hypertonicity-induced expression of EGFP under the control of the HSP70 promoter (Figure 1F).
Figure 1.
Involvement of PKC in hypertonicity-induced HSP70 expression. (A) HSP70 induction in NIH-3T3 cells exposed to hypertonic conditions. Cells were exposed to hypertonic conditions for the indicated time periods and analyzed via immunoblot assay. (B) Phosphorylation status of ERK, p38, and JNK in hypertonicity. Phosphorylated proteins were detected with phospho-specific ERK, p38, and JNK antibodies. PC designates the positive control sample, which was NIH3T3 cells treated for 30 min at 43℃. (C) The effects of ERK activity on hypertonicity-induced HSP70 expression. ERK inhibitor (10 µM and 20 µM PD98059) was added 1 h prior to exposure to hypertonicity. The cell lysates were prepared, and ERK phosphorylation (lower panel) and HSP70 expression (upper panel) were detected. (D) The effects of dominant negative p38 (p38 DN) on hypertonicity-induced HSP70 expression. The cells were transiently transfected with p38 DN and exposed to hypertonicity. P38 phosphorylation and HSP70 induction were detected at 4 h and 12 h after exposure to hypertonicity, respectively. (E) The effects of PKC inhibitor on hypertonicity-induced HSP70 expression. NIH-3T3 cells were treated with inhibitors (25 µM GF109203X, 2 µM H89, or 10 µM LY294002) for 1 h prior to exposure to hypertonicity for 12 h. Cell lysates were resolved via SDS-PAGE and blotted with anti-HSP70 antibody. (F) Effects of PKC inhibitor on HSP70 promoter activity. Cells were transiently transfected with recombinant plasmid, pHSP70promoter-EGFP (see Materials and Methods) and treated with 25 µM GF109203X prior to hypertonicity treatment. Cell lysates were resolved by SDS-PAGE and blotted with anti-EGFP antibody.
PKCµ is essential for hypertonicity-induced HSP70 expression and cytoprotection against lethal stress
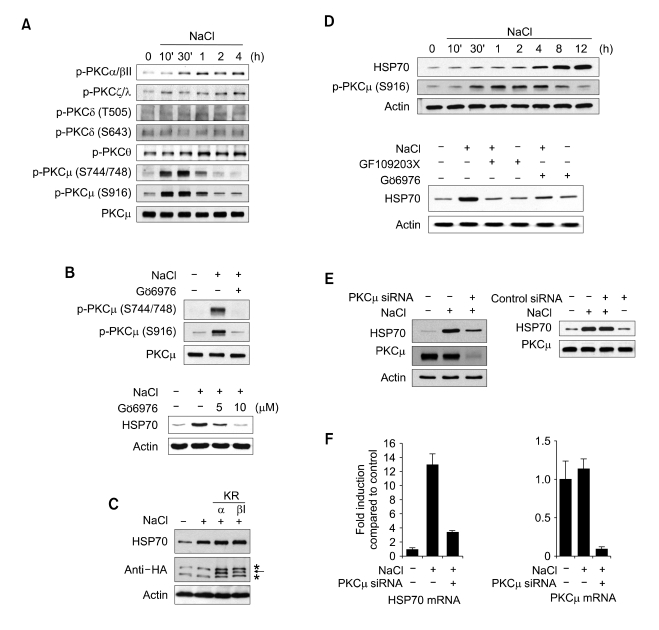
Because we observed the involvement of PKC in hyperonicity-induced HSP70 expression, we attempted to determine which PKC isoforms could be activated under hypertonic conditions. The phosphorylation of PKCµ was most evident under hypertonic stress condition among the various isoforms of PKC (Figure 2A). We then treated the cells with Gö6976, a PKCµ inhibitor, in order to evaluate the involvement of PKCµ in the pathway of hypertonicity-induced HSP70 expression. Treatment with Gö6976 completely inhibited hypertonicity-induced PKCµ phosphorylation. Hypertonicity-induced HSP70 expression was also inhibited completely by treatment with Gö6976, and this effect occurred in a dose-dependent manner (Figure 2B). It has been reported that Gö6976 also inhibits PKC isoforms, including PKCα and PKCβI (Martiny-Baron et al., 1993). In order to assess the possibility that PKCα and βI phosphorylation were involved in the pathway inherent to hypertonicity-induced HSP70 expression, we transfected kinase-inactive mutants of PKCα or PKCβI (PKCα KR and PKCβI KR). Both kinase-inactive PKC mutants did not alter the expression of HSP70 under hypertonic conditions (Figure 2C). We conducted the same experiments in human M-1 kidney cells. Under hypertonic stress conditions, PKCµ was phosphorylated and HSP70 was induced in M-1 cells. When GF109203X and Gö6976 were applied to the M-1 cells, the induction of HSP70 was also inhibited completely (Figure 2D).
Figure 2.
Essential role of PKCµ in hypertonicity-induced HSP70 expression. (A) Phosphorylation status of various PKC isoforms under hypertonic conditions. Cells were maintained in media containing an additional 130 mM NaCl for the indicated time periods. Cell lysates were blotted with anti-p-PKCα/βII, anti-p-PKCζ/λ, anti-p-PKCθ, anti-p-PKCδ, and anti-p-PKCµ antibodies. (B) Effect of PKCµ inhibitor, Gö6976 on hypertonicity-induced HSP70 expression. Cells were pretreated with Gö6976 (5 µM or 10 µM) for 1 h, and then kept in hypertonic media for either 30 min for the detection of p-PKCµ or 12 h for the detection of HSP70. Cell lysates were resolved via SDS-PAGE and blotted with anti-p-PKCµ and anti-PKCµ antibodies (upper panel) or anti-HSP70 antibody (low panel). (C) Effect of kinase-inactive mutants (KR) of PKCα and PKCβI on hypertoniciy-induced HSP70 expression. Cells were transiently transfected with HA-PKCα KR or HA-PKCβI KR constructs. After maintaining transfectant cells in hypertonic media for 12 h, the cell lysates were prepared. Exogenous PKCα and PKCβI (indicated by arrow) and HSP70 were detected with anti-HA antibody. The asterisk designates non-specific signals. (D) Effect of PKCµ inhibitor on HSP70 expression in hypertonically stressed M-1 cells. Human renal epithelial M-1 cells were pretreated with inhibitors (25 µM GF109203X or 10µmM Gö6976) for 1 h and exposed to hypertonicity for the indicated time periods (upper panel) or 12 h (lower panel). Cell lysates were resolved on SDS-PAGE and blotted with anti-HSP70 and anti-p-PKCµ antibodies. (E) Effect of PKCµ knock-down on hypertonicity-induced HSP70 expression. NIH-3T3 cells were transiently transfected with RNAi duplexes (25 µM negative control or 25 µM PKCµ RNAi duplexes). After 48 h of transfection, the cells were exposed to hypertonic conditions for 12 h. The cell lysates were resolved via SDS-PAGE and blotted with anti-HSP70 and anti-PKCµ antibodies. (F) Effect of PKCµ knock-down on hypertonicity-induced HSP70 mRNA transcription. Cells were transiently transfected with PKCµ RNAi duplexes. After 48 h of transfection, the cells were exposed to hypertonic conditions for 4 h. Cells were harvested and real time PCR was conducted as described in Materials and Methods.
In order to verify whether PKCµ was involved in hypertonicity-induced HSP70 expression per se, we conducted an RNA interference assay. In NIH-3T3 cells transfected with PKCµ siRNA, no PKCµ expression was detected, and hypertonicity-induced HSP70 expression was reduced at both the mRNA and protein levels, as compared to what was observed in cells treated with a negative control siRNA duplex (Figure 2E and F).
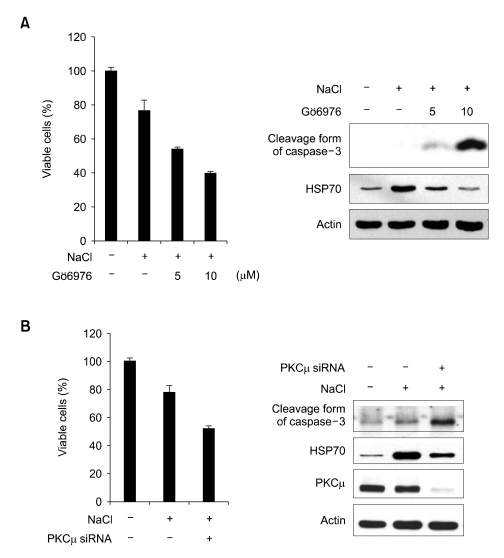
Induced HSP70 performs an anti-apoptotic role via the inhibition of caspase-3 activation under lethal conditions (Li et al., 2000). We evaluated the effects of PKCµ inhibition or depletion on cell viability under hypertonic conditions. Either the inhibition of PKCµ activity as the result of Gö6976 treatment or PKCµ depletion with PKCµ siRNA resulted in reduced cell viabilities due to the activation of caspase-3 (Figure 3A and B). Our data indicate that PKCµ is crucial for cytoprotection via the mechanism of HSP70 induction and the prevention of caspase-3 activation under hypertonic stress conditions.
Figure 3.
The critical role of PKCµ on cell viability under hypertonic condition. Increase of hypertonicity-induced cell death due to the treatment of PKCµ inhibitor (A) or PKCµ Si duplex (B) NIH-3T3 cells were treated with Gö6976 or transiently transfected with PKCµ RNAi duplexes prior to exposure to hypertonic conditions for 12 h. Cell viability was assessed via MTT assay. Values are expressed as the means ± standard deviation of triplicate determinations. Cell lysates were also resolved via SDS-PAGE and blotted with anti-HSP70 and anti-caspase-3 antibodies.
PKCµ partially regulates TonEBP modification in the nucleus under hypertonic conditions
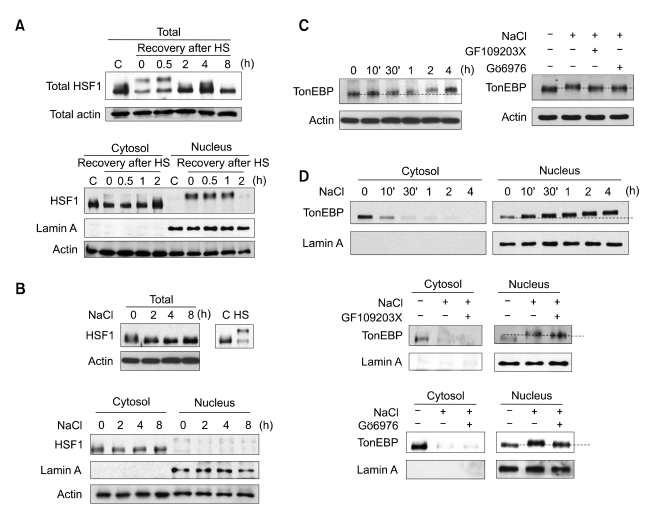
HSF1 is a well-known general transcription factor for the induction of HSP70 under a variety of stresses (Morimoto et al., 1992). However, it has been previously reported that hypertonicity-induced HSP70 expression was mediated by another transcription factor, TonEBP (Woo et al., 2002). First, we attempted to determine whether or not HSF1 was involved in hypertonicity-induced HSP70 expression. HSF1 was immediately modified and translocated into the nucleus during heat shock (HS) treatment, and was relocated to the cytosol and dephosphorylated during the recovery period (Figure 4A). However, HSF1 was neither modified nor translocated into the nucleus under hypertonic conditions (Figure 4B). TonEBP was translocated immediately into the nucleus, then gradually modified in the nucleus under hypertonic conditions (Figure 4C and D). Lamin A was utilized as a marker protein for nuclear fractionation.
Figure 4.
Effect of PKC inhibitors on TonEBP mobility shift under hypertonic conditions. (A) HSF1 mobility shift and translocation to the nucleus by the treatment of heat shock (HS). NIH-3T3 cells were treated with 42℃ heat shock for 30 min and recovered at 37℃ for the indicated time periods. Then, the whole cell lysates, cytosolic fraction, and nuclear fraction were prepared as described in the Materials and Methods section. Proteins were resolved via SDS-PAGE on 8% low-bias gels and blotted with anti-HSF1 and anti-lamin A antibodies. (B) HSF1 status under hypertonic conditions. NIH-3T3 cells were exposed to hypertonic conditions for the indicated time periods. Prepared whole cell lysates, cytosolic fraction, and nucleus fractions were analyzed via immunoblot assay. (C) Effect of PKC inhibitors on the mobility shift of TonEBP. Cells were exposed to hypertonic conditions either for the indicated time periods without any pretreatment (left panel) or for 2 h with pretreatment of 25 µM GF109203X or 10 µM Gö6976 (right panel). Cell lysates were prepared and analyzed via immunoblot assay. (D) Effect of PKC inhibitors on TonEBP translocation. Cells were maintained in hypertonic media for either the indicated time periods without inhibitor treatment (upper panel) or for 2 h with pretreatment of 25 µM GF109203X (middle panel) and 10 µM Gö6976 (lower panel). Cell lysates were prepared and analyzed via immunoblot assay for the dectection of TonEBP translocation.
As we determined that the phosphorylation of PKCµ was critical for hypertonicity-induced HSP70 expression, we then assessed the effects of PKC inhibitors (GF109203X and Gö6976) on the translocation and modification of TonEBP under hypertonic conditions. Neither GF109203X nor Gö6976 treatment prevented the translocation of TonEBP (Figure 4D). Although GF109203X and Gö6976 did not prevent the translocation of TonEBP from the cytoplasm to the nucleus, TonEBP evidenced a slightly lesser mobility shift in the nuclear fraction, due to the inhibitor treatment (Figure 4C and D). Hypertonicity induced a gradual mobility shift of TonEBP in a time dependent-manner. The inhibition of PKCµ activity induced an incomplete mobility shift of TonEBP, as was shown in Figure 4D.
PKCµ indirectly regulates TonEBP modification under hypertonic condition
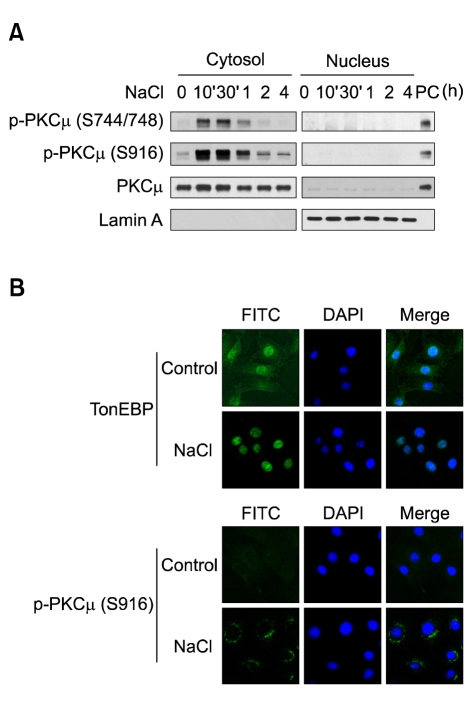
We determined that PKCµ mediated a complete mobility shift of TonEBP, thereby inducing HSP70 expression under hypertonic conditions. We attempted to determine whether hypertonicity-activated PKCµ could directly regulate TonEBP modification. TonEBP was translocated rapidly into the nucleus under hypertonic conditions, as is shown in Figure 4C. However, phosphorylated PKCµ was not detected in the nuclei (Figure 5A). Phosphorylated PKCµ and TonEBP evidenced different subcellular localization characteristics (Figure 5B). Whereas TonEBP was translocated into the nucleus, phosphorylated PKCµ accumulated in the peri-nuclear region. We observed no physical interaction occurring between PKCµ and TonEBP (data not shown). These results show that PKCµ indirectly regulates the activation of TonEBP, thereby inducing the expression of HSP70.
Figure 5.
Subcellular localization of PKCµ and TonEBP under hypertonic conditions. (A) Subcellular localization of PKCµ and phosphorylated PKCµ. NIH-3T3 cells were exposed to hypertonic conditions for the indicated time periods. Cytosolic and nuclear fractions were prepared as described in the Materials and Methods section. The cellular fractions were resolved via SDS-PAGE and blotted with anti-PKCµ and anti-phospho PKCµ antibodies. PC designates the positive control sample (cytosol fraction of cells treated with 130 mM NaCl for 30 min). B. Immunofluorescence detection of PKCµ localization. Immunofluorescence stainings were conducted using anti-TonEBP and anti-phospho PKCµ primary antibodies and FITC-conjugated secondary antibodies in cells exposed for 30 min to hypertonicity.
Discussion
HSP70 is one of the most conserved proteins from bacteria to humans. Whereas HSC70 is expressed constitutively, HSP70 can be induced by a variety of stresses (Morimoto et al., 1992; Wagner et al., 1999). Induced HSP70 protects cells against stresses by virtue of its ability to chaperone denatured proteins and to prevent the activation of the apoptotic pathway (Lee et al., 2005). We reported previously that hyperosmolarity induced only hsp70.1 expression (Lee and Seo, 2002). It has been reported that the hsp70.1 gene harbors TonE cis-acting elements within its promoter region (Heo et al., 2006). As the trans-acting factor for the TonE site, TonEBP, was recently identified (Miyakawa et al., 1998), it could be surmised that TonEBP is activated in the induction of hsp70.1 gene expression under hypertonic conditions. Several papers have reported that TonEBP stimulates the transcription of HSP70 in response to hypertonicity (Woo et al., 2002; Na et al., 2003; Wang, 2006; Neuhofer et al., 2007). However, until now, there have been no reports concerning the precise signaling pathway of TonEBP activation and HSP70 induction under hypertonic conditions.
In this study, we attempted to address the question as to the manner in which TonEBP is involved in the expression of hsp70.1 under hypertonic conditions. First, in order to determine which kinases mediate hypertonicity-induced HSP70 expression, we administered several kinase inhibitors during the process of hypertonicity-induced HSP70 expression. MAPKs perform critical functions in a variety of signaling pathways in mammalian cells. It has been reported that p38 kinase is an essential kinase for the hypertonic induction of HSP70, and the dual control of p38 and Fyn tyrosine kinase regulates the functioning of TonEBP via the transactivation domain (Sheikh-Hamad et al., 1998; Ko et al., 2002). ERK has been studied as a signaling molecule for the induction of the de novo expression of proteins including HSP70, BGT-1 (sodium/chloride/betain cotransporter 1), SMIT (sodium/ myoinosito cotransporter), and TauT (sodium/chloride/taurine cotransporter) under hypertonic conditions (Ho, 2003; Uhlik et al., 2003; Tsai et al., 2007). We determined that hypertonicity activated ERK and p38, but not JNK, during hypertonicity treatment. However, we found no evidence to suggest that MAPKs are involved in the hypertonicity-induced expression of HSP70 (Figure 1B-D). GF109203X (an inhibitor of novel and conventional PKC isoforms) and Gö6976 (an inhibitor of PKCµ, PKCα, and PKCβI isoforms) caused a reduction in TonEBP-dependent HSP70 expression (Figure 1E). More specifically, when cells were transfected with PKCµ siRNA, the induction of HSP70 was inhibited (Figure 2E and 3B). The effects of PKC inhibition on TonEBP activation were also observed. The mobility shift of TonEBP located in the nucleus was affected by treatment with PKC inhibitors (Figure 4C and D). Since it has been established that the PLC/DAG/PKC signaling cascade performs a crucial function in the activation of PKCµ (Rozengurt et al., 2005; Wang, 2006), we surmised that the activation of PKCµ by hypertonicity might be mediated by the upstream kinase PKC. To the best of our knowledge, this study is the first report to demonstrate that PKCµ plays an essential role in hypertonicity-induced HSP70 expression.
Even though HSF1 is a general transcription activator for the induction of HSP70 under a variety of stressful conditions (Morimoto et al., 1996), we demonstrated that HSF1 was neither activated nor translocated to the nucleus under hypertonic conditions, by way of contrast with heat shock treatment (Figure 4A and B). Instead of HSF1, TonEBP was translocated into the nucleus and then post-translationally modified to respond to hypertonicity (Figure 4 C and D). TonEBP is a member of the Rel family of transcriptional activators, which includes NF-κB and NFAT (nuclear factor of activated T-cells) (Woo et al., 2002). TonEBP stimulates the transcription of several genes, including BGT1, SMIT, TauT, and AT (aldorase reductase), to protect cells against the deleterious effects of hypertonicity, which principally occurs via the attenuation of cellular ionic strength (Jeon et al., 2006). TonEBP also regulates the induction of HSP70. However, the action mechanism of HSP70, which is induced by TonEBP in hypertonic situations, operates differently. Hypertonicity causes double-stranded DNA breaks and increases mitochondrial ROS generation, finally resulting in apoptosis (Zhou et al., 2006). We demonstrated that HSP70 protects against hyperosmolarity-induced apoptosis and cellular damage via the prevention of caspase-3 activation (Lee et al., 2005). HSP70 induced via the mechanism of PKCµ and TonEBP activation also prevents the activation of caspase-3, the executioner of the hypertonicity-induced apoptosis pathway, ultimately protecting against apoptotic cell death (Figure 3).
TonEBP is activated via subsequent events, including phosphorylation, dimerization, and nuclear translocation under hypertonic conditions (Dahl et al., 2001; Lopez-Rodriguez et al., 2001; Lee et al., 2002). We observed an upward shift in TonEBP which appeared to be the result of phosphorylation, and this event occurred exclusively in the nucleus (Figure 4C and D). TonEBP is gradually modified in a time-dependent manner under hypertonic conditions. Previous research has shown that TonEBP activation is regulated by several kinases, including p38 and Fyn, ATM, and PKA (Ferraris et al., 2002; Ko et al., 2002; Irarrazabel et al., 2006). However, the kinases that directly phosphorylate TonEBP have yet to be clearly identified (Jeon et al., 2006). In addition, we determined that, although the PKC and PKCµ inhibitors inhibited hypertonicity-induced HSP70 expression almost completely, hypertonicity-induced TonEBP modification was partially affected. Therefore, upstream kinases and the molecular mechanisms inherent to the modification of TonEBP under hypertonic conditions remain to be clearly elucidated.
Finally, to assess whether PKCµ is colocalized with TonEBP in the nucleus, we evaluated the distribution of PKCµ under hypertonic conditions. The activated PKCµ remained in the perinuclear region, which appeared to have accumulated in the Golgi apparatus (Figure 5). It has been reported that PKCµ is localized in several intracellular compartments, including the plasma membrane, Golgi apparatus, mitochondria, and nucleus (Rey and Rozengurt, 2001; Rey et al., 2004; Waldron et al., 2004; Storz et al., 2005). In particular, the precise functions of PKCµ in the Golgi apparatus in the context of hypertonicity-induced HSP70 expression via the activation of TonEBP will require further study.
PKCµ activates NF-κB to protect the cells from oxidative stress-related cell death. Src-Abl activated by oxidative stress induces phosphorylation of PKCµ and leads activation of downstream IKKNF-κB signaling (Storz and Toker 2003). Additionally, mitochondrial ROS also activate PKCµ and induce the expression of manganese-dependent superoxide dismutase (MnSOD) through activating NF-κB (Storz et al., 2005). Reduced ROS production by antioxidant suppresses NaCl-induced TonEBP activation and BGT1 expression (Zhou et al., 2006). Therefore, we suggest that PKCµ is a mediator between ROS and TonEBP activation in hypertonic condition. Since, when we inhibited PKCµ using specific inhibitor or siRNA knock-down method, TonEBP phosphorylation and HSP70 induction were evidently decreased, we could conclude that increased ROS by hypertonicity might be the main cause of PKCµ activation and TonEBP-mediated HSP70 gene expression.
In summary, PKCµ is a novel mediator of hypertonicity-induced HSP70 expression. We have demonstrated herein that the PKC/PKCµ/TonEBP signaling cascade performs a function in inhibiting the hypertonicity-induced apoptotic pathway via the induction of the HSP70 protein.
Acknowledgements
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-2006-351-E00005).
Abbreviations
- HSF1
heat shock transcription factor 1
- HSP70
heat shock protein 70
- TonEBP
the tonicity-responsive enhancer binding protein
References
- 1.Abe K, Kogure K, Itoyama Y. Rapid and semiquantitative analysis of HSP72 and HSC73 heat shock mRNAs by mimic RT-PCR. Brain Res. 1995;683:251–253. doi: 10.1016/0006-8993(95)00272-r. [DOI] [PubMed] [Google Scholar]
- 2.Abravaya K, Phillips B, Morimoto RI. Attenuation of the heat shock response in HeLa cells is mediated by the release of bound heat shock transcription factor and is modulated by changes in growth and in heat shock temperatures. Genes Dev. 1991;5:2117–2127. doi: 10.1101/gad.5.11.2117. [DOI] [PubMed] [Google Scholar]
- 3.Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000;2:469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- 4.Dahl SC, Handler JS, Kwon HM. Hypertonicity-induced phosphorylation and nuclear localization of the transcription factor TonEBP. Am J Physiol Cell Physiol. 2001;280:C248–C253. doi: 10.1152/ajpcell.2001.280.2.C248. [DOI] [PubMed] [Google Scholar]
- 5.Ferraris JD, Persaud P, Williams CK, Chen Y, Burg MB. cAMP-independent role of PKA in tonicity-induced transactivation of tonicity-responsive enhancer/ osmotic response element-binding protein. Proc Natl Acad Sci. 2002;99:16800–16805. doi: 10.1073/pnas.222659799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flanagan SW, Ryan AJ, Gisolfi CV, Moseley PL. Tissue-specific HSP70 response in animals undergoing heat stress. Am J Physiol. 1995;268:R28–R32. doi: 10.1152/ajpregu.1995.268.1.R28. [DOI] [PubMed] [Google Scholar]
- 7.Hatayama T, Asai Y, Wakatsuki T, Kitamura T, Imahara H. Regulation of hsp70 synthesis induced by cupric sulfate and zinc sulfate in thermotolerant HeLa cells. J Biochem (Tokyo) 1993;114:592–597. doi: 10.1093/oxfordjournals.jbchem.a124222. [DOI] [PubMed] [Google Scholar]
- 8.Heo JI, Lee MS, Kim JH, Lee JS, Kim J, Park JB, Lee JY, Han JA, Kim JI. The role of tonicity responsive enhancer sites in the transcriptional regulation of human hsp70-2 in response to hypertonic stress. Exp Mol Med. 2006;38:295–301. doi: 10.1038/emm.2006.35. [DOI] [PubMed] [Google Scholar]
- 9.Ho SN. The role of NFAT5/TonEBP in establishing an optimal intracellular environment. Arch Biochem Biophys. 2003;413:151–157. doi: 10.1016/s0003-9861(03)00130-9. [DOI] [PubMed] [Google Scholar]
- 10.Irarrazabal CE, Burg MB, Ward SG, Ferraris JD. Phosphatidylinositol 3-kinase mediates activation of ATM by high NaCl and by ionizing radiation: Role in osmoprotective transcriptional regulation. Proc Natl Acad Sci. 2006;103:8882–8887. doi: 10.1073/pnas.0602911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeon US, Kim JA, Sheen MR, Kwon HM. How tonicity regulates genes: story of TonEBP transcriptional activator. Acta Physiol (Oxf) 2006;187:241–247. doi: 10.1111/j.1748-1716.2006.01551.x. [DOI] [PubMed] [Google Scholar]
- 12.Ko BC, Lam AK, Kapus A, Fan L, Chung SK, Chung SS. Fyn and p38 signaling are both required for maximal hypertonic activation of the osmotic response element-binding protein/tonicity-responsive enhancer-binding protein (OREBP/TonEBP) J Biol Chem. 2002;277:46085–46092. doi: 10.1074/jbc.M208138200. [DOI] [PubMed] [Google Scholar]
- 13.Lee JS, Lee JJ, Seo JS. HSP70 deficiency results in activation of c-Jun N-terminal Kinase, extracellular signal-regulated kinase, and caspase-3 in hyperosmolarity-induced apoptosis. J Biol Chem. 2005;280:6634–6641. doi: 10.1074/jbc.M412393200. [DOI] [PubMed] [Google Scholar]
- 14.Lee JS, Seo JS. Differential expression of two stress-inducible hsp70 genes by various stressors. Exp Mol Med. 2002;34:131–136. doi: 10.1038/emm.2002.19. [DOI] [PubMed] [Google Scholar]
- 15.Lee SD, Woo SK, Kwon HM. Dimerization is required for phosphorylation and DNA binding of TonEBP/NFAT5. Biochem Biophys Res Commun. 2002;294:968–975. doi: 10.1016/S0006-291X(02)00572-7. [DOI] [PubMed] [Google Scholar]
- 16.Li CY, Lee JS, Ko YG, Kim JI, Seo JS. Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase-3 activation. J Biol Chem. 2000;275:25665–25671. doi: 10.1074/jbc.M906383199. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Rodriguez C, Aramburu J, Jin L, Rakeman AS, Michino M, Rao A. Bridging the NFAT and NF-kappaB families: NFAT5 dimerization regulates cytokine gene transcription in response to osmotic stress. Immunity. 2001;15:47–58. doi: 10.1016/s1074-7613(01)00165-0. [DOI] [PubMed] [Google Scholar]
- 18.Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Gö6976. J Biol Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- 19.Miyakawa H, Woo SK, Chen CP, Dahl SC, Handler JS, Kwon HM. Cis- and trans-acting factors regulating transcription of the BGT1 gene in response to hypertonicity. Am J Physiol. 1998;274:F753–F761. doi: 10.1152/ajprenal.1998.274.4.F753. [DOI] [PubMed] [Google Scholar]
- 20.Morimoto RI, Kroeger PE, Cotto JJ. The transcriptional regulation of heat shock genes: a plethora of heat shock factors and regulatory conditions. EXS. 1996;77:139–163. doi: 10.1007/978-3-0348-9088-5_10. [DOI] [PubMed] [Google Scholar]
- 21.Morimoto RI, Sarge KD, Abravaya K. Transcriptional regulation of heat shock genes. A paradigm for inducible genomic responses. J Biol Chem. 1992;267:21987–21990. [PubMed] [Google Scholar]
- 22.Na KY, Woo SK, Lee SD, Kwon HM. Silencing of TonEBP/NFAT5 transcriptional activator by RNA interference. J Am Soc Nephrol. 2003;14:283–288. doi: 10.1097/01.asn.0000045050.19544.b2. [DOI] [PubMed] [Google Scholar]
- 23.Neuhofer W, Steinert D, Fraek ML, Beck FX. Prostaglandin E2 stimulates expression of osmoprotective genes in MDCK cells and promotes survival under hypertonic conditions. J Physiol. 2007;583:287–297. doi: 10.1113/jphysiol.2007.135178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, Zamzami N, Mak T, Jaattela M, Penninger JM, Garrido C, Kroemer G. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol. 2001;3:839–843. doi: 10.1038/ncb0901-839. [DOI] [PubMed] [Google Scholar]
- 25.Rey O, Reeve JR, Zhukova E, Sinnett-Smith J, Rozengurt E. G protein-coupled receptor-mediated phosphorylation of the activation loop of protein kinase D: dependence on plasma membrane translocation and protein kinase Cepsilon. J Biol Chem. 2004;279:34361–34372. doi: 10.1074/jbc.M403265200. [DOI] [PubMed] [Google Scholar]
- 26.Rey O, Rozengurt E. Protein kinase D interacts with Golgi via its cysteine-rich domain. Biochem Biophys Res Commun. 2001;287:21–26. doi: 10.1006/bbrc.2001.5530. [DOI] [PubMed] [Google Scholar]
- 27.Rozengurt E, Rey O, Waldron RT. Protein kinase D signaling. J Biol Chem. 2005;280:13205–13208. doi: 10.1074/jbc.R500002200. [DOI] [PubMed] [Google Scholar]
- 28.Schliess F, Wiese S, Haussinger D. Osmotic regulation of the heat shock response in H4IIE rat hepatoma cells. FASEB J. 1999;13:1557–1564. doi: 10.1096/fasebj.13.12.1557. [DOI] [PubMed] [Google Scholar]
- 29.Sheikh-Hamad D, Di Mari J, Suki WN, Safirstein R, Watts BA, 3rd, Rouse D. p38 kinase activity is essential for osmotic induction of mRNAs for HSP70 and transporter for organic solute betaine in Madin-Darby canine kidney cells. J Biol Chem. 1998;273:1832–1837. doi: 10.1074/jbc.273.3.1832. [DOI] [PubMed] [Google Scholar]
- 30.Storz P, Doppler H, Toker A. Protein kinase D mediates mitochondrion-to-nucleus signaling and detoxification from mitochondrial reactive oxygen species. Mol Cell Biol. 2005;25:8520–8530. doi: 10.1128/MCB.25.19.8520-8530.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Storz P, Toker A. Protein kinase D mediates a stress induced NF-κB activation and survival pathway. EMBO J. 2003;22:109–120. doi: 10.1093/emboj/cdg009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai TT, Guttapalli A, Agrawal A, Albert TJ, Shapiro IM, Risbud MV. MEK/ERK signaling controls osmoregulation of nucleus pulposus cells of the intervertebral disc by transactivation of TonEBP/OREBP. J Bone Miner Res. 2007;22:965–974. doi: 10.1359/jbmr.070322. [DOI] [PubMed] [Google Scholar]
- 33.Uhlik MT, Abell AN, Johnson NL, Sun W, Cuevas BD, Lobel-Rice KE, Horne EA, Dell'Acqua ML, Johnson GL. Rac-MEKK3-MKK3 scaffolding for p38 MAPK activation during hyperosmotic shock. Nat Cell Biol. 2003;5:1104–1110. doi: 10.1038/ncb1071. [DOI] [PubMed] [Google Scholar]
- 34.Wagner M, Hermanns I, Bittinger F, Kirkpatrick CJ. Induction of stress proteins in human endothelial cells by heavy metal ions and heat shock. Am J Physiol. 1999;277:L1026–L1033. doi: 10.1152/ajplung.1999.277.5.L1026. [DOI] [PubMed] [Google Scholar]
- 35.Waldron RT, Rey O, Zhukova E, Rozengurt E. Oxidative stress induces protein kinase C-mediated activation loop phosphorylation and nuclear redistribution of protein kinase D. J Biol Chem. 2004;279:27482–27493. doi: 10.1074/jbc.M402875200. [DOI] [PubMed] [Google Scholar]
- 36.Walter L, Rauh F, Gunther E. Comparative analysis of the three major histocompatibility complex-linked heat shock protein 70 (Hsp70) genes of the rat. Immunogenetics. 1994;40:325–330. doi: 10.1007/BF01246673. [DOI] [PubMed] [Google Scholar]
- 37.Wang QJ. PKD at the crossroads of DAG and PKC signaling. Trends Pharmacol Sci. 2006;27:317–323. doi: 10.1016/j.tips.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Williams GT, Morimoto RI. Maximal stress-induced transcription from the human HSP70 promoter requires interactions with the basal promoter elements independent of rotational alignment. Mol Cell Biol. 1990;10:3125–3136. doi: 10.1128/mcb.10.6.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woo SK, Lee SD, Kwon HM. TonEBP transcriptional activator in the cellular response to increased osmolality. Pflugers Arch. 2002;444:579–585. doi: 10.1007/s00424-002-0849-2. [DOI] [PubMed] [Google Scholar]
- 40.Woo SK, Lee SD, Na KY, Park WK, Kwon HM. TonEBP/NFAT5 stimulates transcription of HSP70 in response to hypertonicity. Mol Cell Biol. 2002;22:5753–5760. doi: 10.1128/MCB.22.16.5753-5760.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou X, Ferraris JD, Burg MB. Mitochondrial reactive oxygen species contribute to high NaCl-induced activation of the transcription factor TonEBP/OREBP. Am J Physiol Renal Physiol. 2006;290:F1169–F1176. doi: 10.1152/ajprenal.00378.2005. [DOI] [PubMed] [Google Scholar]