Abstract
Viral proteins of γ-2 herpesviruses, such as LMP2A of Epstein Barr virus (EBV) and Tip of herpesvirus saimiri (HVS) dysregulate lymphocyte signaling by interacting with Src family kinases. K15 open reading frame of Kaposi's sarcoma associated herpesvirus (KSHV), located at the right end of the viral genome, encodes several splicing variants differing in numbers of transmembrane domains. Previously, we demonstrated that the cytoplasmic tail of the K15 protein interfered with B cell receptor signal transduction to cellular tyrosine phosphorylation and calcium mobilization. However, the detailed mechanism underlying this phenomenon was not understood. In the C-terminal cytoplasmic region of K15, putative binding domains for Src-SH2 and -SH3 were identified. In this study, we attempted to characterize these modular elements and cellular binding protein(s) by GST pull down and co-immunoprecipitation assays. These studies revealed that K15 interacted with the major B cell tyrosine kinase Lyn. In vitro kinase and transient co-expression assays showed that the expression of K15 protein resulted in activation of Lyn kinase activity. In addition, GST pull down assay suggested that the SH2 domain of Lyn alone was necessary for interaction with the C-terminal SH2B (YEEV) of K15, but the addition of Lyn SH3 to the SH2 domain increases the binding affinity to K15 protein. The data from luciferase assays indicate that K15 expression in BJAB cells induced NFAT and AP1 activities. The tyrosine residue in the C-terminal end of K15 required for the Lyn interaction appeared to be essential for NFAT/AP1 activation, highlighting the significance of the C-terminal SH2B of K15 as a modular element in interfering with B lymphocyte signaling through interaction with Lyn kinase.
Keywords: herpesvirus 8, human; K15 protein, human herpesvirus 8; lyn protein-tyrosine kinase; membrane microdomains; virus latency
Introduction
Kaposi's Sarcoma associated Herpesvirus (KSHV) or HHV8 (Human Herpesvirus 8) is the most recently identified member of human herpesviruses (Chang et al., 1994; Moore and Chang, 1995) and is consistently identified in all forms of Kaposi's sarcoma (KS), primary effusion lymphoma (PEL) and multicentric Castleman's disease (MCD) (Cesarman et al., 1995; Soulier et al., 1995; Gessain et al., 1996). Like other herpesviral infections, most of the KSHV-associated infections are also latent. Only a minor fraction of latently infected cells are known to undergo spontaneous lytic reactivation, spreading the viruses to other susceptible cells. B or T lymphocytes are the common targets of infection by γ-herpesviruses (Damania et al., 2000a; Damania and Jung, 2001). Upon antigenic engagement, BCR and TCR are activated to initiate a cascade of cellular signaling such as tyrosine phosphorylation, calcium mobilization and transcriptional modulation (Tsubata and Wienands, 2001; Gauld et al., 2002; Mustelin et al., 2002). However, several latency associated-viral proteins such as herpesvirus saimiri (HVS) Tip and Epstein Barr Virus (EBV) LMP2A are able to interfere with the antigenic receptor signaling that potentially minimizes untimely viral replication and/or antiviral activity (Jung et al., 1995; Longnecker and Miller, 1996). Although Tip is known to interact with Lck kinase, two groups reported a conflicting result as for the Lyn activity; inhibition (Lund et al., 1999) or activation (Jung et al., 1995).
EBV LMP2A is well characterized for its interaction thorough the N-terminal part with non receptor family of Src kinases including Lyn, Fyn (Burkhardt et al., 1992; Busson et al., 1995), Csk (Scholle et al., 1999) and Syk (Merchant et al., 2000). The genetic mutation of the N-terminal tyrosine residues of LMP2A renders the mutant recombinant virus infected cells to be responsive to BCR stimulation and lytic reactivation (Fruehling et al., 1998; Merchant et al., 2000). Although Lyn activity has recently been shown to be down regulated by LMP2A and E3 ligase-mediated ubiquitination/degradation (Ikeda et al., 2001), it is still not clear about its biological outcome and how LMP2A affects the activities of several tyrosine kinases.
Lyn and other Src kinases participate in the initial signaling cascade of antigen receptors. However, Lyn appears to be unique among many Src kianses, due to its ability to induce negative feedback inhibition soon after the activation signaling emanating from the BCR (Chan et al., 1997; DeFranco et al., 1998). Thus, any viral proteins or cellular genetic alterations that can elicit an activation of the Lyn or Lck kinase are shown and/or presumed to be competent with creating a cellular environment either antagonistic to lymphocyte activation or setting up a higher threshold for activation of the respective antigen receptor. With these possibilities in mind, we investigated the potentialities of the putative multi-transmembrane protein-K15 to interfere with the proximal signaling of BCR signal transduction pathway.
KSHV K15 open reading frame exhibits a dramatic sequence variation among different isolates of PEL cells in addition to a complex splicing pattern (Glenn et al., 1999; Poole et al., 1999; Choi et al., 2000). We previously demonstrated that the cytoplasmic region of K15 inhibited the proximal signal transduction of BCR to cellular tyrosine phosphorylation and calcium mobilization. Based on these results, we hypothesized that K15 is not only a structural homolog but also a functional homolog to EBV LMP2A protein (Choi et al., 2000). However, detailed molecular mechanism remained unclear. Here we report that K15 targeted the major B cell Src kinase-Lyn and this interaction appeared to be important in transcriptional modulation of NFAT/AP1 activities.
Materials and Methods
Cell culture and transfection
BJAB and 293T cells were grown in RPMI and DMEM media containing 10% fetal calf serum respectively. A fugene (Roche) transfection was used according to the manufacture's instruction for transient expression of plasmid constructs in 293T cells. For electroporation, 5 millions of BJAB or 293T cells were washed once in the plain RPMI or DMEM and electroporated at 240 V and 9750 µF in 400 ul of serum-free medium. 24 h after, the cells were harvested for the luciferase and western blot analyses.
Plasmid construction
Full length K15 cDNA from BCBL1 was cloned into pCS4-3xflag (BamHI and NcoI sites) and a kozak sequence (GCCACC) was placed in front of the initiation methionine as described in Choi et al. (2000). The preparation of CD-delta ( = CD8-Δ) and CD8-K15 chimera constructs is described in Choi et al. (2000).
All mutations in K15 gene were generated by PCR using oligonucleotide-directed mutagenesis. The K15 PG mutant construct was obtained by changing the nucleotides for proline residues of 386th-391th to those of glycines and K15 YF mutant construct was generated by changing the nucleotide residues for 481th tyrosine residue to those of phenylalanine. K15 Dm mutant construct was made by introducing the YF mutation into PG mutant plasmid by PCR mutagenesis. The amplified DNA sequences containing mutations in K15 were cloned into the pCS4 vector. Each K15 mutant was completely sequenced to verify the presence of the mutation and the absence of any other changes.
Immunoprecipitation and immunoblot
Cells were harvested and lysed with lysis buffer (0.15M NaCl, 1% Nonidet P-40, 50mM HEPES buffer [pH 8.0]) or radio-immunoprecipitation assay buffer (0.15 M NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris [pH 7.5]) containing 0. 1mM Na2VO3, 1 mM NaF, and protease inhibitors (leupeptin, aprotinin, PMSF, and bestatin). Immunoprecipitated proteins from cleared cell lysates were separated by SDS-PAGE and reacted in immunoblot assays. For protein immunoblots, polypeptides in cell lysates corresponding to 105 cells were resolved by SDS-PAGE and transferred to a nylon membrane filter. Immunoblot detection was performed with a 1:1,000 dilution of primary antibody with an ECL kit (Amersham). The Fuji PhosphorImager was used for Western blot imaging and for quantification of protein bands where indicated.
In vitro kinase assay
For in vitro protein kinase assays, complexes prepared as described above were washed once more with kinase buffer (10 mM MgCl2, 1 mM DTT, 20 mM Tris, pH 7.0) and resuspended in 20 µl of the same buffer containing 5 µCi of [γ-32P]ATP (6,000 Ci/mmol; NEN) for 30 min at room temperature and separated by SDS-PAGE. The SDS-PAGE gel was treated briefly with 0.1N HCl for 10 min and rinsed in distilled water before gel dry and imaging with a Fuji PhosphorImager.
Sucrose gradient for lipid raft preparation
Bjab cells (2 × 107) were electroporated with twenty microgram of K15 cDNA in pCS4-3xflag or pCS4-3xflag respectively under the condition in the above and the cells were incubated for 2 days before washing with ice cold PBS and then subjected to lysis on ice in 1% Triton X-100 in TNEV (10 mM Tris-HCl [pH 7.5], 150 mM NaCl, 5 mM EDTA) containing phosphatase inhibitors and protease inhibitor cocktail (Roche, Mannheim, Germany). The lysates were completely homogenized with 10 strokes in a Wheaton loose-fitting Dounce homogenizer. Nuclei and cellular debris were pelleted by centrifugation at 900 × g for 10 min at 4℃. For discontinuous sucrose gradient, 1 ml of the cleared cell lysates was mixed with 1 ml of ice cold 85% sucrose in TNEV and transferred to the bottom of a Beckman centrifuge tube. The diluted lysates were carefully overlaid with 6 ml of ice cold 35% sucrose in TNEV and finally with ice cold 3.5 ml of 5% sucrose in TNEV. The samples were then centrifuged in an SW41 rotor at 200,000 × g for 16 h at 4℃, and 1-ml fraction was harvested from the top of the gradient with cut tip.
Recombinant GST-Lyn protein
For purification of recombinant Lyn protein from Escherichia coli, the Lyn DNA fragment corresponding to the SH3, SH2 and SH3-SH2 of Lyn was cloned into pGEX4T-1 in frame to express GST-Lyn chimera proteins. Clones were sequenced to ensure the presence of the exact desired sequence. When E. coli-BL21 containing plasmid pGEX4T-Lyn reached an optical density at 600 nm of approximately 0.6, 2 mM IPTG (isopropyl-[β]-D-thiogalactopyranoside) was added, and the cells were harvested 5 h after induction. Cells were solubilized with NP40 containing lysis buffer followed by a brief centrifugation to remove cellular debris. The precleared cell lysates were subjected to GST purification as described elsewhere (Kim et al., 2007).
Reporter assays
All transfections and electroporations included 3 µg of pGKβ-gal, which expresses β-galactosidase from a phosphoglucokinase promoter, and 5 µg of 3X-NFAT-luc, which has three copies of the NFAT binding site from the murine MHC class I promoter upstream of a minimal fos promoter and a luciferase gene. The AP-1 luciferase reporter plasmid (pGL3-2 × AP-1) was provided by Jae U. Jung (NERPRC, Harvard Medical School). 24 h after transfections or electroporations, cells were washed once in PBS and lysed in 200 µl of reporter lysis buffer (Promega). Assays for luciferase activity were performed with a Luminometer. Values were normalized for β-galactosidase activity as described elsewhere (Kim et al., 2003).
Results
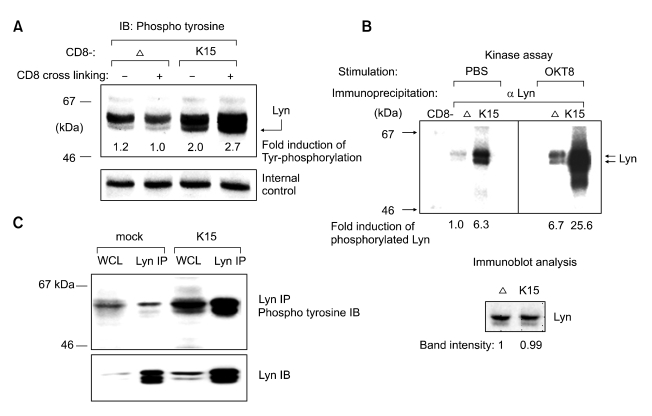
Expression of K15 protein as a CD8 chimera induces tyrosine phosphorylation of cellular proteins (Figure 1)
Figure 1.
Increased tyrosine phosphorylation of 53 kDa by K15 expression. A total of 2 × 106 Bjab cells were stimulated with either PBS or OKT8 (anti-CD8) antibody for 5 min and the cell extracts were subjected to a western blot with antiphospho tyrosine immunoblot. At the bottom, the cell surface expression of CD8 chimera proteins were measured by anti CD8-PE antibody.
Previously, we showed that the K15 protein when expressed as a CD8 chimera was tyrosine phosphorylated and able to block the BCR signal transduction. To better understand the molecular mechanisms, we searched for cellular targets of the K15 protein in a B lymphoma cell line-BJAB. As in Figure 1A, upon CD8 cross linking by α CD8 dyna beads (M-450) and α phospho tyrosine immuno blotting, the cells expressing CD8-K15 chimera exhibited phospho tyrosine band at about 53 kDa. We reported previously that the CD8-K15 chimera protein itself co-migrates at about 51kDa. The fact that the amount of phospho tyrosine band at 55 kDa increased in extracts of CD8-K15 compared to that of CD8-Δ led us to test whether it can be of a major B cell Src kinase-Lyn. Thus, we did immuno-precipitation with anti-Lyn antibody followed by kinase assay using radioactive 32P-γ-ATP. As in panel A of Figure 1B, Lyn protein migrated as a doublet band at 55 kDa in both CD8-Δ and CD8-K15 lanes. Without stimulation, Lyn activity was evidently higher in CD8-K15 cells than in CD8-Δ cells, but following CD8 cross-linking the total Lyn activity increased significantly in CD8-K15 compared to that of CD8-Δ cells. This result therefore suggests that the 55 kDa phospho tyrosine bands in Figure 1A is likely the Lyn kinase. Subsequently, we checked whether full length K15 can modulate Lyn activity. To this end, Lyn protein was immnoprecipitated by monoclonal antibody against Lyn followed by immunoblotting with anti-phosphotyrosine antibody. As shown in Figure 1C, the whole cell lysate and the immunoprecipitate sample from K15 expressing cells showed clearly increased phosphotyrosine signals compared to those of vector transfected cells. Thus, these data in Figure 1 strongly support our interpretation that K15 increase Lyn kinase activity in BJAB cells.
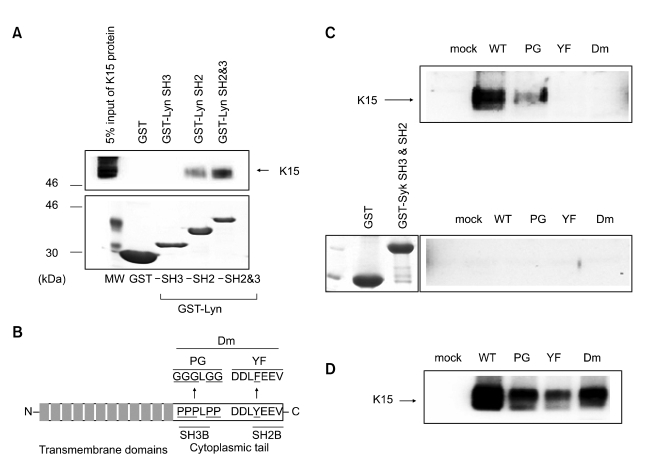
The tyrosine residue in the C-terminal end of K15 is important for an interaction with the SH2 and SH3 domains of Lyn (Figure 2)
Figure 2.
The SH2 domain of Lyn interacts with the C-terminal and tyrosine (490th) of K15. (A) Identification of Lyn domain(s) necessary for interaction with K15. (B) Schematic diagram of the putative modular domains (SH3B and SH2B) in K15. (C) The cell lysates of K15 WT or each K15 mutant were prepared from 293T cells and subjected to pull down with GST-Lyn SH2 and 3 or GST-Syk SH3 and 3. In panel (A), the presence of K15 protein was checked by α flag K15 antibody. The cell lysates were checked for the expression of K15 proteins by α flag K15 antibody in panel (C).
To identify K15 domain(s) necessary for Lyn binding, two motifs from K15 protein sequence that are highly conserved among different primary effusion lymphoma isolates were mutated for the binding study: Five prolines of PPPLPP (386th-391th) and a tyrosine residue of YEEV (481th) were changed to five glycines (PG) and a phenylalanine (YF) respectively or both domains were mutated to generate Dm mutant construct. K15 wild type (wt) or individual K15-mutant was co-expressed with Lyn in 293T cells and cleared cell lysates were subjected to anti-Lyn immuno-precipitation and immuno-blotted with anti-K15 antibody. As shown in Figure 2C, Wt K15 and PG mutant were able to bind with Lyn, whereas YF and Dm K15 mutants could not bind with Lyn, suggesting that the tyrosine of the C-terminal end is essential for this interaction. Even though the proline rich region-SH3B was not required for interaction with Lyn but clearly it increased the binding affinity toward Lyn. The way that K15 interacted with Src family kinases were similar to that of another src kinase-Fyn interaction with K15. Conversely, the Lyn domains required for interaction with K15 protein were investigated using GST Lyn-SH2-SH3 chimeric protein. Wt K15 protein prepared from 293T cells were incubated with the purified GST-Lyn chimera proteins and subjected to pulldown assays. In a western blot using anti-K15 antibody, GST Lyn-SH2-SH3 immnuoprecipitated Wt K15 and PG mutant, while it failed to pulldown K15-YF and -Dm mutants. Thus, the C-terminal SH2B domain seemed to play a more important role in Lyn interaction while the SH3B domain contribute to the enhancement of the interaction with Lyn. On the other hand, there was no appreciable difference in Syk kinase activity between CD8-Δ and CD8-K15 cell extracts (data not shown) and in addition, GST-Syk pull down experiment did not reveal any interaction with K15 protein as shown in Figure 2C, suggesting that unlike LMP2A, K15 protein does not contain ITAM motif and is incapable of interacting with Syk kinase (Merchant et al., 2000).
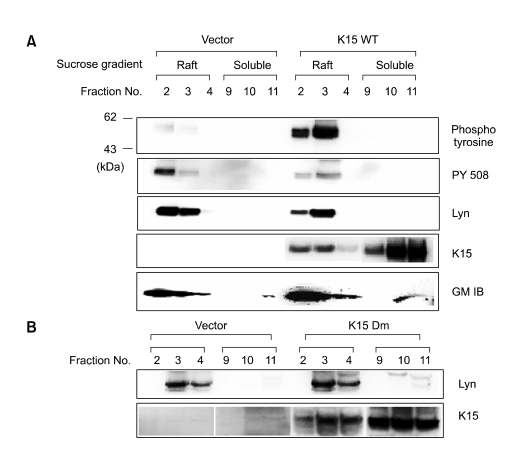
K15 increases the level of tyrosine phosphorylation and Lyn activity in the membrane raft fraction (Figure 3)
Figure 3.
Modulation of cellular signaling by K15 in BJAB Frt-to.
Previous studies suggested that EBV-LMP2A, a positional and structural homolog of K15, is known to localize in the membrane raft fraction, where it interacts with Lyn (Dykstra et al., 2001). In this study, the location on the plasma membrane of K15 in BJAB cells was investigated by sucrose gradient ultracentrifugation. Along this line, Doxycycline inducible BJAB-Frt-To cells expressing K15 Wt and Dm were established and the expressions of two kinds of K15 were analyzed by western blotting. This indicated that like EBV LMP2A, K15 Wt and Dm seemed to be localized in both membrane raft fraction and soluble fractions. Analysis of cellular tyrosine phosphorylation by 4G10 antibody reveals that there was an increased amount of phosphor-tyrosine band (first row) migrating at the same position with Lyn (second row) in K15 expressing cells. K15 seemed to increase the level of tyrosine phosphorylation at 53 kDa and in this regard, K15 appeared to be distinct from EBV LMP2A which increased the Lyn protein in the raft fraction. The analysis of inhibitory tyrosine phosphorylation of Lyn by anti-PY 508 antibody indicated when compared to vector control cells the K15 expression in Bjab cells correlated with decreased level of the inhibitory phosphorylation. In addition, K15 was found to be co-localizing with a known marker for membrane raft-ganglioside GM in a confocal study (data not shown). Thus, K15 is similar with EBV-LMP2A with respect to structurally, positionally on the virus genome and functionally by membrane raft localization and Lyn kinase interaction.
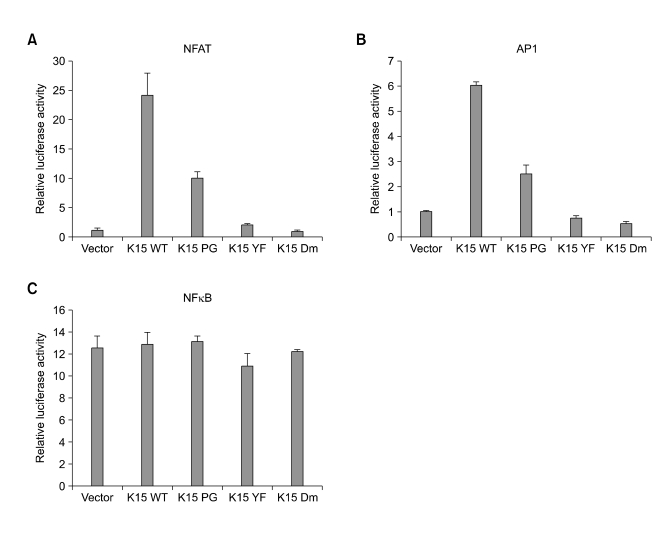
Induction of NFAT and AP1 activities by K15 (Figure 4)
Figure 4.
Modulation of NFAT activity by K15. (A) Each of K15 expression plasmids was co-expressed in 293T cells with a luciferase-NFAT and β-gal reporter plasmids. 24 h later, the cell lysates were prepared for luciferase assays and the readouts were compensated by the activity of β-gal. (B) The K15 expression was confirmed with anti-flag K15 antibody. The NFAT assays were repeated 4 times. The readouts on the Y axis is shown as relative luciferase unit.
A number of lymphotropic viral proteins are known to modulate the NFAT activities to control the transcription of cellular genes (Kennedy et al., 1998; Lagunoff et al., 1999; Damania et al., 2000b; Manninen et al., 2001; Ding et al., 2002). In addition, BCR signaling is known to modulate NFAT and NFκB activities, which ultimately contribute to the immune responses. We tested whether K15 can modulate NFAT/NFκB/AP1 activities in transient luciferase assays. BJAB cells were electroporated with a plasmid vector, K15-WT, -PG, -YF and -Dm mutant together with β-gal gene as an internal control and harvested for analysis 24 h after the electroporation. As shown in Figure 4A, NFAT activity is up-regulated by wt K15 for about 25 folds compared to the vector control. On the other hand, PG mutant could activate NFAT to a lesser extent by 2-3 folds, but the YF and the Dm mutants failed to activate the NFAT. In a separate assay, Wt K15 and PG mutant induced about 6 and 2.5 folds of AP1 activity respectively, while YF and Dm mutants failed to do. However, K15 was unable to activate NFκB activity (Figure 4C). This is an interesting observation since not only BCR signaling but also several viral oncoproteins such as EBV LMP1 and KSHV K1 are found to increase NFκB activity. In this regard, the K15 may generate a distinct cellular signaling to contribute to the latency of the herpesvirus infected cells.
Discussion
γ herpesviruses, which include EBV, HVS, MHV68, RRV and KSHV, are well known for their prominent tropism to B or T lymphocytes. Antigenic receptors and their downstream responses, such as production of cytokines and antibodies, constitute the major line of adaptive immunity against pathogens like γ-herpesviruses. Not surprisingly, this group of viruses has outsmarted the host defensive tactics by interfering with the antigenic receptor signaling and antiviral activities. EBV LMP2A and HVS Tip are reported to alter Lyn or Lck activity, a key factor in relaying antigenic receptor activation to calcium mobilization and Ras activation. In the virus infected lymphocytes, calcium mobilization in vitro by chemicals such as ionomycin and thapsigargin is known to initiate lytic viral replication, to produce virus particle (Zoeteweij et al., 2001), necessitating the viral inhibition of the cellular signaling to calcium mobilization.
K15 gene is a positional and structural homolog to EBV LMP2 gene being located on the right most end of the viral genome possessing multiple membrane spanning domains and the putative SH2B/SH3B motifs. The similarity between two genes was further substantiated by our previous data in which K15 cytoplasmic region in CD8 chimera efficiently inhibited calcium mobilization and cellular tyrosine phosphorylation (Choi et al., 2000).
In this study, we identified Lyn src kinase as a target of K15. Lyn is the major src kinase that can be either activated by BCR or inactivated by Csk (Ingley et al., 2006). Expression of full length K15 or K15 cytoplasmic region as a CD8 chimera in BJAB was enough to activate the Lyn kinase dramatically, as evidenced by increased tyrosine phosphorylation of 53kDa in K15 cell extracts compared to the vector control. The fact that Lyn kinase can be activated not only by K15 but also by BCR, which raises an interesting question regarding a nature of cellular signaling downstream of the Lyn kinase. However, binding partners, the cross talks and the duration of each of the signaling may be different between K15 and BCR, through which K15 may avoid a catastrophic antiviral response against viruses and the infected cells.
While the K15-YF mutant protein lost the interaction with Lyn, the PG mutation diminished the binding toward the GST-Lyn-SH2-SH3 to a lesser extent. This finding points to a possibility for sequential interaction between K15 and Lyn to achieve a long lasting interaction: a highly specific but transient binding conferred by C-terminal tyrosine residue of K15 versus a stable interaction conferred by the proline rich region. The fact that the SH2B and SH3B of K15 interacts with Lyn and Fyn in a similar fashion suggests that other Src family kinases including Yes, Lck and Hck can also interact with the SH2B and SH3B domains resulting in activation of the kinase activity. It is an interesting finding that a single tyrosine residue in the C-terminal of K15 is critical in the interaction with Lyn and also in modulating the kinase activity. The increased activity of Lyn upon K15 expression appeared to be counter-balanced by concomitant increase of a negative feedback signaling including Csk and Cbp (unpublished data). Like EBV LMP2A, K15 is found to be localized in the membrane raft and cytosol fractions. Unlike LMP2A that increased the amount of Lyn protein in the raft, K15 increased the tyrosine phosphorylation of 53kDa protein while reducing the level of inhibitory tyrosine phosphorylation at 508th residue of Lyn after Doxycylin induction for K15 expression in BJAB cells.
The lucierase reporter assays showed that K15 expression significantly increased the transcription activity of NFAT and AP1. Since the YF and Dm mutants failed to induce NFAT and AP1 activities, the C-terminal tyrosine of K15 appears to be required for both Lyn interaction and induction of NFAT/AP1 activities. Along this line, mT of polyoma virus interacts with Src kinase resulting in induction of NFAT and mT mutant incapable of binding Src can not induce NFAT activity. In contrast, NFκB activity was not altered by K15 and this suggests that K15 mediated signaling is different from those displayed by BCR activation and herpesviral oncogenes such as EBV LMP1 and KSHV K1. While we are preparing this manuscript, Brinkmann et al. also reported the significance of the C-terminal tyrosine residue of K15 in modulating the transcriptional activity of cellular genes such as IL8, NFAT and Cox-2 (Brinkmann et al., 2007). Taken together, it remains to be further studied as for the outcome of increased Lyn kinase activity by K15 in lymphocytes latently infected with KSHV.
Acknowledgements
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MOST) (R13-2007-001-01001-0 (2007)) to JK Choi and also was supported by the "GRRC project of Gyeonggi Provincial Government, Republic of Korea to JK Choi. NH Cho was supported by a Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund; KRF-2005-003-E00052). We would like to appreciate Dr. Jae U. Jung at Harvard Medical School for his guide and support throughout this study. Also, we thank Hye-ryun Kim and Arunkumar for help in preparation of this manuscript.
Abbreviations
- BCR
B cell receptor
- EBV
Epstein Barr virus
- HHV8
Human Herpesvirus 8
- HVS
Herpesvirus saimiri
- KSHV
Kaposi's sarcoma associated herpesvirus
- KS
Kaposi's sarcoma
- LMP2A
Latent membrane protein 2A
- MCD
multicentric Castleman's disease
- PEL
Primary effusion lymphoma
- TCR
T cell receptor
References
- 1.Brinkmann MM, Pietrek M, ttrich-Breiholz O, Kracht M, Schulz TF. Modulation of host gene expression by the K15 protein of Kaposi's sarcoma-associated herpesvirus. J Virol. 2007;81:42–58. doi: 10.1128/JVI.00648-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burkhardt AL, Bolen JB, Kieff E, Longnecker R. An Epstein-Barr virus transformation-associated membrane protein interacts with src family tyrosine kinases. J Virol. 1992;66:5161–5167. doi: 10.1128/jvi.66.8.5161-5167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busson P, Edwards RH, Tursz T, Raab-Traub N. Sequence polymorphism in the Epstein-Barr virus latent membrane protein (LMP)-2 gene. J Gen Virol. 1995;76:139–145. doi: 10.1099/0022-1317-76-1-139. [DOI] [PubMed] [Google Scholar]
- 4.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 5.Chan VW, Meng F, Soriano P, DeFranco AL, Lowell CA. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity. 1997;7:69–81. doi: 10.1016/s1074-7613(00)80511-7. [DOI] [PubMed] [Google Scholar]
- 6.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 7.Choi JK, Lee BS, Shim SN, Li M, Jung JU. Identification of the novel K15 gene at the rightmost end of the Kaposi's sarcoma-associated herpesvirus genome. J Virol. 2000;74:436–446. [PMC free article] [PubMed] [Google Scholar]
- 8.Damania B, Choi JK, Jung JU. Signaling activities of gammaherpesvirus membrane proteins. J Virol. 2000a;74:1593–1601. doi: 10.1128/jvi.74.4.1593-1601.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damania B, DeMaria M, Jung JU, Desrosiers RC. Activation of lymphocyte signaling by the R1 protein of rhesus monkey rhadinovirus. J Virol. 2000b;74:2721–2730. doi: 10.1128/jvi.74.6.2721-2730.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damania B, Jung JU. Comparative analysis of the transforming mechanisms of Epstein-Barr virus, Kaposi's sarcoma-associated herpesvirus, and Herpesvirus saimiri. Adv Cancer Res. 2001;80:51–82. doi: 10.1016/s0065-230x(01)80012-9. [DOI] [PubMed] [Google Scholar]
- 11.DeFranco AL, Chan VW, Lowell CA. Positive and negative roles of the tyrosine kinase Lyn in B cell function. Semin Immunol. 1998;10:299–307. doi: 10.1006/smim.1998.0122. [DOI] [PubMed] [Google Scholar]
- 12.Ding W, Albrecht B, Kelley RE, Muthusamy N, Kim SJ, Altschuld RA, Lairmore MD. Human T-cell lymphotropic virus type 1 p12(I) expression increases cytoplasmic calcium to enhance the activation of nuclear factor of activated T cells. J Virol. 2002;76:10374–10382. doi: 10.1128/JVI.76.20.10374-10382.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dykstra ML, Longnecker R, Pierce SK. Epstein-Barr virus coopts lipid rafts to block the signaling and antigen transport functions of the BCR. Immunity. 2001;14:57–67. doi: 10.1016/s1074-7613(01)00089-9. [DOI] [PubMed] [Google Scholar]
- 14.Fruehling S, Swart R, Dolwick KM, Kremmer E, Longnecker R. Tyrosine 112 of latent membrane protein 2A is essential for protein tyrosine kinase loading and regulation of Epstein-Barr virus latency. J Virol. 1998;72:7796–7806. doi: 10.1128/jvi.72.10.7796-7806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gauld SB, Dal Porto JM, Cambier JC. B cell antigen receptor signaling: roles in cell development and disease. Science. 2002;296:1641–1642. doi: 10.1126/science.1071546. [DOI] [PubMed] [Google Scholar]
- 16.Gessain A, Sudaka A, Briere J, Fouchard N, Nicola MA, Rio B, Arborio M, Troussard X, Audouin J, Diebold J, de TG. Kaposi sarcoma-associated herpes-like virus (human herpesvirus type 8) DNA sequences in multicentric Castleman's disease: is there any relevant association in non-human immunodeficiency virus-infected patients? Blood. 1996;87:414–416. [PubMed] [Google Scholar]
- 17.Glenn M, Rainbow L, Aurade F, Davison A, Schulz TF. Identification of a spliced gene from Kaposi's sarcoma-associated herpesvirus encoding a protein with similarities to latent membrane proteins 1 and 2A of Epstein-Barr virus. J Virol. 1999;73:6953–6963. doi: 10.1128/jvi.73.8.6953-6963.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikeda M, Ikeda A, Longnecker R. PY motifs of Epstein-Barr virus LMP2A regulate protein stability and phosphorylation of LMP2A-associated proteins. J Virol. 2001;75:5711–5718. doi: 10.1128/JVI.75.12.5711-5718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ingley E, Schneider JR, Payne CJ, McCarthy DJ, Harder KW, Hibbs ML, Klinken SP. Csk-binding protein mediates sequential enzymatic down-regulation and degradation of Lyn in erythropoietin-stimulated cells. J Biol Chem. 2006;281:31920–31929. doi: 10.1074/jbc.M602637200. [DOI] [PubMed] [Google Scholar]
- 20.Jung JU, Lang SM, Jun T, Roberts TM, Veillette A, Desrosiers RC. Downregulation of Lck-mediated signal transduction by tip of herpesvirus saimiri. J Virol. 1995;69:7814–7822. doi: 10.1128/jvi.69.12.7814-7822.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy AP, Sekulic A, Irvin BJ, Nilson AE, Dilworth SM, Abraham RT. Polyomavirus middle T antigen as a probe for T cell antigen receptor-coupled signaling pathways. J Biol Chem. 1998;273:11505–11513. doi: 10.1074/jbc.273.19.11505. [DOI] [PubMed] [Google Scholar]
- 22.Kim MA, Kim HJ, Brown AL, Lee MY, Bae YS, Park JI, Kwak JY, Chung JH, Yun J. Identification of novel substrates for human checkpoint kinase Chk1 and Chk2 through genome-wide screening using a consensus Chk phosphorylation motif. Exp Mol Med. 2007;39:205–212. doi: 10.1038/emm.2007.23. [DOI] [PubMed] [Google Scholar]
- 23.Kim JY, Lee JJ, Kim KS. Acetyl-CoA carboxylase β expression mediated by MyoD and muscle regulatory factor4 is differentially affected by retinoic acid receptor and retinoid X receptor. Exp Mol Med. 2003;35:23–29. doi: 10.1038/emm.2003.4. [DOI] [PubMed] [Google Scholar]
- 24.Lagunoff M, Majeti R, Weiss A, Ganem D. Deregulated signal transduction by the K1 gene product of Kaposi's sarcoma-associated herpesvirus. Proc Natl Acad Sci USA. 1999;96:5704–5709. doi: 10.1073/pnas.96.10.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Longnecker R, Miller CL. Regulation of Epstein-Barr virus latency by latent membrane protein 2. Trends Microbiol. 1996;4:38–42. doi: 10.1016/0966-842x(96)81504-6. [DOI] [PubMed] [Google Scholar]
- 26.Lund TC, Prator PC, Medveczky MM, Medveczky PG. The Lck binding domain of herpesvirus saimiri tip-484 constitutively activates Lck and STAT3 in T cells. J Virol. 1999;73:1689–1694. doi: 10.1128/jvi.73.2.1689-1694.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manninen A, Huotari P, Hiipakka M, Renkema GH, Saksela K. Activation of NFAT-dependent gene expression by Nef: conservation among divergent Nef alleles, dependence on SH3 binding and membrane association, and cooperation with protein kinase C-theta. J Virol. 2001;75:3034–3037. doi: 10.1128/JVI.75.6.3034-3037.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merchant M, Caldwell RG, Longnecker R. The LMP2A ITAM is essential for providing B cells with development and survival signals in vivo. J Virol. 2000;74:9115–9124. doi: 10.1128/jvi.74.19.9115-9124.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore PS, Chang Y. Detection of herpesvirus-like DNA sequences in Kaposi's sarcoma in patients with and without HIV infection. N Engl J Med. 1995;332:1181–1185. doi: 10.1056/NEJM199505043321801. [DOI] [PubMed] [Google Scholar]
- 30.Mustelin T, Abraham RT, Rudd CE, Alonso A, Merlo JJ. Protein tyrosine phosphorylation in T cell signaling. Front Biosci. 2002;7:d918–d969. doi: 10.2741/A821. [DOI] [PubMed] [Google Scholar]
- 31.Poole LJ, Zong JC, Ciufo DM, Alcendor DJ, Cannon JS, Ambinder R, Orenstein JM, Reitz MS, Hayward GS. Comparison of genetic variability at multiple loci across the genomes of the major subtypes of Kaposi's sarcoma-associated herpesvirus reveals evidence for recombination and for two distinct types of open reading frame K15 alleles at the right-hand end. J Virol. 1999;73:6646–6660. doi: 10.1128/jvi.73.8.6646-6660.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scholle F, Longnecker R, Raab-Traub N. Epithelial cell adhesion to extracellular matrix proteins induces tyrosine phosphorylation of the Epstein-Barr virus latent membrane protein 2: a role for C-terminal Src kinase. J Virol. 1999;73:4767–4775. doi: 10.1128/jvi.73.6.4767-4775.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d'Agay MF, Clauvel JP, Raphael M, Degos L. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood. 1995;86:1276–1280. [PubMed] [Google Scholar]
- 34.Tsubata T, Wienands J. B cell signaling. Introduction. Int Rev Immunol. 2001;20:675–678. doi: 10.3109/08830180109045584. [DOI] [PubMed] [Google Scholar]
- 35.Zoeteweij JP, Moses AV, Rinderknecht AS, Davis DA, Overwijk WW, Yarchoan R, Orenstein JM, Blauvelt A. Targeted inhibition of calcineurin signaling blocks calcium-dependent reactivation of Kaposi sarcoma-associated herpesvirus. Blood. 2001;97:2374–2380. doi: 10.1182/blood.v97.8.2374. [DOI] [PubMed] [Google Scholar]