Abstract
Tumor migration/invasion is the main cause of tumor progression and STAT3 is needed to enhance tumor migration/invasion by up-regulating MMP-9. Thus, agents that inhibit STAT3 activation may be used as an anticancer drug. We present herein that 6-methyl-2-propylimino-6, 7-dihydro-5H-benzo [1, 3]-oxathiol-4-one (LYR71) , a derivative of trimeric resveratrol, has an anticancer activity through inhibition of STAT3 activation. We found that LYR71 suppressed STAT3 activation and inhibited the expression and activity of MMP-9 in RANTES-stimulated breast cancer cells. In addition, LYR71 reduced RANTES-induced MMP-9 transcripts by blocking STAT3 recruitment, dissociating p300 and deacetylating histone H3 and H4 on the MMP-9 promoter. Furthermore, LYR71 inhibited tumor migration/invasion in RANTES-treated breast cancer cells and consequently blocked tumor progression in tumor-bearing mice. Taken together, the results of this study suggest that LYR71 can be therapeutically useful due to the inhibition effect of STAT3-mediated MMP-9 expression in breast cancer cells.
Keywords: chemokine CCL5, LYR71, matrix metalloproteinase 9, neoplasm metastasis, STAT3 transcription factor
Introduction
Previous reports have shown that tumor develops and progresses through tumor migration/invasion and that the ability to digest the extracellular matrix is a crucial step in tumor progression as a part of tumor invasion (Westermarck and Kahari, 1999; Freije et al., 2003). Matrix metalloproteinases (MMPs), a zinc-dependent endopeptidase family, are responsible for degradation of various components of the extracellular matrix. MMPs consist of at least 26 kinds of MMPs which are classified on the basis of their substrate specificity, and especially MMP-2 (gelatinase A) and MMP-9 (gelatinase B) prefer to degrade components of the basement membrane (Westermarck and Kahari, 1999; Freije et al., 2003; Cohen et al., 2006). MMP-9 is expressed at the transcriptional level via multiple signaling transduction pathways and is secreted as an inactive precursor form, proMMP-9. Activity of MMP-9 occurs through proteolytic processes by other proteases or autocatalysis (Dechow et al., 2004; Freitas et al., 2007).
STATs (signal transducers and activators of transcription) are cytoplasmic transcription factors which transmit signals to the nucleus and regulate gene expression by binding to the specific target sequence (Joung et al., 2005; Kim et al., 2006a; Yu et al., 2007). Many studies have demonstrated that STAT3 is aberrantly activated in a wide variety of cancers and contributes to cancer progression by upregulating oncogenes such as MMP-9 (Page et al., 2000; Wei et al., 2003). These facts imply that STAT3 inhibition leads to reduction of MMP-9 expression, and consequently suppresses tumor progression.
The present study was attempted to determine whether LYR71, a derivative of alpha-viniferin, which is a trimer of resveratrol, blocks tumor progression by inhibiting STAT3-mediated MMP-9 expression in vivo and in vitro.
Materials and Methods
Cell culture
The human breast cancer cell line, MDA-MB-231 cell, was purchased from the American Culture Collection and maintained in RPMI containing 10% FBS.
Western blot analysis
Cells were incubated with RANTES in the presence or absence of LYR71. After harvesting, the cells were washed with ice-cold PBS and lysed. Proteins were prepared and loaded onto SDS-PAGE gels, and the gel was then transferred onto a nitrocellulose membrane. The membranes were incubated with the primary antibody and washed. The membranes were then incubated with the peroxidase-conjugated secondary antibody (Santa Cruse Biotechnology, CA), washed and visualized using the ECL system (Amershem, Piscataway, NJ).
RT-PCR
RNA extraction and RT-PCR were performed as previously described (Kim et al., 2006b). The sequences of MMP-9 primers were 5'-GGCCCTTCTACGGCCACT-3' for forward and 5'-CAGAGAATCGCCAGTACTT-3' for reverse, and GADPH primers were 5'-CCATGGAGAAGGCTGGGG-3' for forward and 5'-CAAAGTTGTCATGGATGACC-3' for reverse.
Zymography
The conditioned media of MDA-MB-231 cells were collected and concentrated using a centricon Y-30 (Millipore, Bedford, MA). All conditioned media were mixed with the SDS sample buffer which did not contain β-mercaptorthanol and loaded onto SDS-PAGE gels containing 0.2% gelatin. After running, the gels were washed in 2.5% Triton X-100 twice, rinsed in 1× developing buffer (50 mM Tris-HCl (pH 7.6), 0.2 M NaCl, 5 mM CaCl2 and 0.02% Brij 35), and incubated in 1× developing buffer at 37℃ for 24 h. The gels were washed in double distilled water, stained with coomassie blue and destained with double distilled water. Areas of MMP activity were shown as clear bands on the dark blue background.
Chromatin immunoprecipitation assay
Chromatin Immunoprecipitation (ChIP) assay was described in a previous report (Ye et al., 2001). Briefly, MDA-MB-231 cells were treated with RANTES in the presence or absence of LYR71, and the cells were then fixed with formaldehyde. Soluble chromatin samples were immunoprecipitated with antibodies against p-STAT3, p300, Ac-H3 or Ac-H4. Isolated DNA was amplified by PCR. The sequences of promoter-specific primer were 5'-TTGGGGAGGATATCTGACCT-3' for sense and 5'-GGTAGGGTTTTGCAAACTGC-3' for antisense.
Matrigel invasion assay
Matrigel invasion assay was conducted with an 8.0-µm porous polycarbonate filter (3422, Corning Costa, Cambridge, MA) and Matrigel Basement Membrane Matrix (354234, BD Biosciences, Bedford, MA). Matrigel (10 mg/ml) was coated on the upper side of the filter, and collagen was coated on the lower side of the filter. The upper chamber was filled with cells in serum-free medium, and the lower chamber was filled with 5% FBS-containing medium. After incubation with RANTES in the presence or absence of LYR71, the invaded cells were fixed and stained with hematoxylin and eosin. The filters were separated from the chambers, soaked into xylen and then mounted.
Immunohistochemistry
Immunohistochemistry was performed as previously described (Jung et al., 2007). Briefly, sections were deparaffinized and rehydrated, and antigens were retrieved by heating in sodium citrate buffer. The sections were blocked in blocking solution and incubated overnight with antibodies against p-STAT3 or MMP-9. The sections were then washed and incubated with biotinylated secondary antibodies, followed by incubation with the avidin-biotin-horseradish peroxidase complex. The sections were visualized with diaminobenzidine.
Xenograft
Four-week female nude mice (BALB/c-nu) were obtained (Central Lab, Animal Inc, Seoul, Korea) and housed under specific pathogen-free conditions. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the College of Medicine, Seoul National University, Korea. MDA-MB-231 cells (5 × 106 cells) were subcutaneously inoculated with cold liquid matrigel (10 mg/ml) in the flank of the nude mice. LYR71 was injected at a dose of 20 mg/kg or 40 mg/kg every 3 days for 30 days from the 13th day after inoculation. The tumor growth rate was measured using an electronic caliper every 6 days, and the tumor weight was calculated using the following equation: tumor weight (g) = {length (cm) × [width (cm)] 2} × 0.5 (Simpson-Herren and Lloyd, 1970).
MTT assay
MDA-MB-231 cells were incubated with LYR71 at concentrations of 5, 10, 20, 40, and 80 µM for various times then 20 µl of 5 mg/ml MTT (3-[4,5-dimethylthiazol-2-yl].-2,5-diphenylterazolium bromide) solution was added to each well (0.5 mg/well). After incubating for 4 h, the formazan crystals in each well were dissolved in 100 µl of DMSO. A540 was read on a scanning multi-well spectrophotometer (Molecular Device Co., Sunnyvale, CA).
Statistical analysis
The data were presented as mean±SEM. Statistical analyses were performed using Statview statistic software (SAS Institute, Cary, NC). Comparisons were made using the Duncan's post hoc analysis. Differences with a P value of < 0.05 were considered to be statistically significant.
Results
LYR71 inhibits the STAT3 phosphorylation in MDA-MB-231 cells
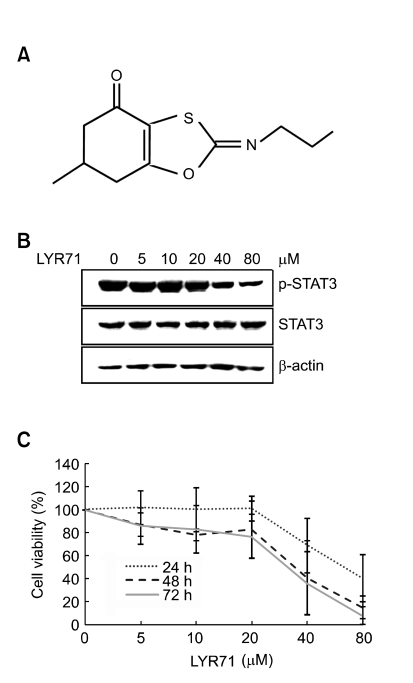
In our recent data, LYR71 inhibits inflammatory signal pathway in IFN-γ stimulated murine macrophages RAW 264.7 and human monocytes THP-1 cell lines. These effects were due to inhibition of NF-κB signaling but not PI3K-AKT pathway (unpublished data). LYR71 may has a wide spectrum of biological effects, e.g. antioxidant activity and anti-inflammatory properties (data not shown), which are similar to the effects of some anticancer agents. Although its mode of action was not elucidated, these facts led us to hypothesize that LYR71 has a potential role as an anti-cancer agent that act by inhibiting STAT3 signaling which is crucial for tumor-cell proliferation and survival, tumor angiogenesis and invasion/metastasis. To test this, we first evaluated whether LYR71 downregulates STAT3 phosphorylation as an anticancer effect (Figure 1B). MDA-MB-231 cells were exposed to LYR71 of various concentrations for 3 h and the phosphorylation state in tyrosin-705 of STAT3 was analyzed by western blot analysis using tyrosine phosphorylation antibody of STAT3. As shown in Figure 1B, LYR71 suppressed STAT3 tyrosine phosphorylation in MDA-MB-231 cells in a dose-dependent manner. LYR71 has STAT3 inhibiting effect with an IC50 of 20 µM. In addition, to rule out the possibility that cytotoxic effects of LYR71 has affected the STAT3 inactivation, we used the MTT assay to evaluate cell viabilities after treating LYR71 at 5-80 µM for 24, 48 and 72 h. As shown in Figure 1C, LYR71 did not have any specific toxicity effects at 20 µM LYR71 for 24 h incubation. Thus, 20 µM LYR71-treated MDA-MB-231 cells were prepared and examined. Also, after confirming the biological effects, a toxicological study of LYR71 should be performed.
Figure 1.
LYR71 inhibits the STAT3 phosphorylation in MDA-MB-231 cells. (A) Chemical structure of LYR71 (6-methyl-2-propylimino-6,7-dihydro-5H-benzo[1,3]-oxathiol-4-one, C11H15NO2S). (B) MDA-MB-231 cells were treated with LYR71 for 3 h. Total cellular protein extracts were subjected to western blot analysis using phospho-STAT3 (Y705) and anti-STAT3 antibodies. (C) LYR71 toxicity was measured using MTT assay. The MDA-MB-231 cells were incubated with LYR71 in various concentrations for 24, 48, 72 h. All experiments were performed in triplicate.
LYR71 has an anticancer effect on xenografted tumors
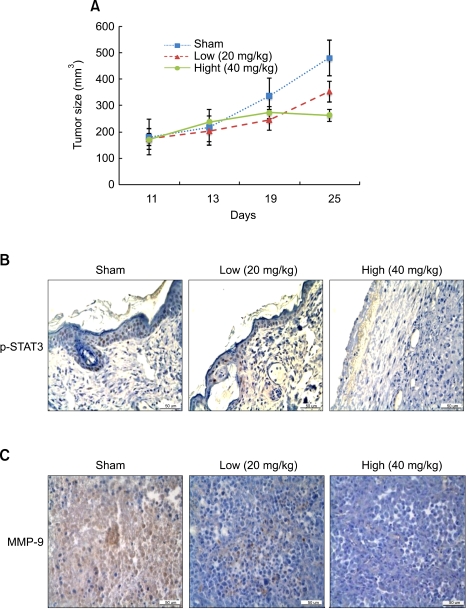
We investigated whether LYR71 inhibits tumor growth in vivo (Figure 1A). MDA-MB-231 cells and an equal volume of cold liquid matrigel (10 mg/ml) were subcutaneously inoculated in the flank of 4-week female nude mice (BALB/c-nu). Mice bearing MDA-MB-231 cells were randomly assigned to one of 3 groups. The first group (n = 3) received the vehicle (PBS) as the control group, the second group (n = 3) received 20 mg/kg of LYR71 and the third group (n = 3) received 40 mg/kg of LYR71 every 3 days for 2 weeks from the 11th day after inoculation. The tumor growth rate was measured using an electronic caliper and the tumor weight was calculated as previously described in Materials and Methods. As shown in Figure 2A, PBS treatment did not affect tumor growth, while LYR71 treatment significantly suppressed tumor growth in tumor-bearing mice. To determine how LYR71 suppresses tumor progression, we investigated STAT3 phosphorylation because STAT3 inhibitors are regarded as anticancer drugs on the basis of their oncogenic effects (Wei et al., 2003; Jing and Tweardy, 2005; Leeman et al., 2006; Jung et al., 2007). Consistent with the results of tumor growth, p-STAT3 immunoreactive cells were observed in the tumors of PBS-treated mice, but they were markedly reduced in the tumors of LYR71-treated mice, indicating that STAT3 is key modulator in tumorigenesis (Figure 2B). As tumor migration/invasion is a main cause for tumor progression (Freije et al., 2003), we hypothesized that LYR71 inhibits tumor migration/invasion. To answer this hypothesis, we investigated the expression of MMP-9, a key molecule of tumor invasion. As shown in Figure 2C, MMP-9-immunoreactive cells were significantly smaller in the second and third group than in the first group, suggesting that LYR71 suppresses tumor progression through inhibition of tumor migration/invasion.
Figure 2.
LYR71 has an anticancer effect on xenografted tumors. (A) MDA-MB-231 cells were injected subcutaneously into the flank of female nude mice. After 11 days, mice were treated with LYR71 (20 or 40 mg/kg) or PBS (vehicle) every 3 days for 2 weeks. Tumor growth was measured every 6 days using a digital caliper, and tumor weight was calculated using an equation: tumor weight (g) = {length (cm) × [width (cm)]2} × 0.5. (B and C) Mice were euthanized, tumors were removed, fixed and embedded in paraffin. Tumor sections were prepared for immunohistochemical staining with antibodies against p-STAT3 (B) or MMP-9 (C). The dark brown staining indicated cells that were positive for p-STAT3 or MMP-9, while the dark blue color was the counterstain (400×).
LYR71 inhibits migration and invasion of breast cancer cells
Since MMP-9 is a main molecule for tumor migration/invasion and highly expressed in response to RANTES (Azenshtein et al., 2002; Van Kempen and Coussens, 2002; Freije et al., 2003), we investigated whether LYR71 would inhibit RANTES-induced tumor migration/invasion. Cells were prepared in serum-free medium in the upper chamber and treated with RANTES in the presence or absence of LYR71. RANTES enhanced cell migration but LYR71 inhibited RANTES-induced cell migration (Figure 3A). In concert with cell migration assay, we tested cell invasion assay. Because cell invasion assay uses chemoattractant property, the cells were prepared with serum-free medium in the upper chamber, and medium containing 5% FBS were provided in the lower chamber. As shown in Figure 3B, LYR71 reduced RANTES-induced cell invasion.
Figure 3.
LYR71 suppresses tumor migration and invasion in RANTES-induced MDA-MB-231 cells. (A and B) The migration/invasion assay was performed using a 24-well transwell chamber with an 8.0-µm porous polycarbonate filter. Cells were seeded onto the upper chamber and incubated with RANTES (25 ng/ml) for 24 h in the presence or absence of LYR71 (20 µM). (A) For migration assay, both the upper and lower chambers were filled with serum-free medium without matrigel coating. (B) For invasion assay, the upper chamber was coated with matrigel and 5% FBS was supplied for the lower chamber as a chemoattractant. The number of migrated/invaded cells was counted and presented (*P < 0.0001 Con vs. RANTES; #P < 0.0001 RANTES vs. RANTES with LYR71 using post hoc analysis with the Duncan's test). (C) Conditioned medium was obtained under the same condition as tumor migration/invasion assay. Medium was concentrated, and Western blotting (upper panel) and zymography (lower panel) were performed to measure expression and activity of MMP-9.
LYR71 suppresses MMP-9 expression and activity in RANTES-induced breast cancer cells
As LYR71 inhibits MMP-9 expression in tumor bearing mice and blocks RANTES-induced tumor migration/invasion, we examined expression and activity of MMP-9 in RANTES-induced MDA-MB-231 cells. MDA-MB-231 cells were treated with RANTES for 24 h in the presence or absence of LYR71, and the cells and conditioned media were separately harvested. Conditioned media were used to measure expression and activity of MMP-9. Western blotting showed that LYR71 inhibited RANTES-induced MMP-9 expression (Figure 3C, upper panel). Since MMP-9 is secreted in its inactive precursor form and then activated by proteolytic processing (Freije et al., 2003), we performed zymography using gelatin. MMP-9 activity was coincident with MMP-9 expression. Zymography showed that RANTES induced MMP-9 activity, but LYR71 significantly inhibited MMP-9 activity (Figure 3C, middle panel).
LYR71 suppresses RANTES-induced STAT3 phosphorylation, which directly regulates MMP-9 expression
We next examined whether LYR71 inhibits RANTES-induced STAT3 activation in order to further investigate the underlying mechanisms of LYR71 action. Cells were incubated with RANTES for 3 h in the presence or absence of LYR71 and the STAT3-phosphorylated level was measured. As shown in Figure 4A, LYR71 reduced STAT3 activation in the RANTES-induced MDA-MB-231 cells. Since MMP-9 has STAT3-binding sites on its promoter, we determined whether STAT3 directly regulates MMP-9 production. We prepared constitutively active form of STAT3 (CA STAT3)-expressing cells and dominant negative form of STAT3 (DN STAT3)-expressing cells by transient transfection. As shown in Figure 4B (upper panel), activated-STAT3 strongly upregulated MMP-9 expression and inactivated-STAT3 suppressed MMP-9 expression. MMP-9 expression by activated-STAT3 led to enhanced MMP-9 activity (Figure 4B, zymography, middle panel). Furthermore, we examined MMP-9 transcription by RT-PCR and found that CA STAT3 enhanced MMP-9 transcription, while DN STAT3 suppressed it (Figure 4B, lower panel), indicating that MMP-9 is required for STAT3-mediated transcription.
Figure 4.
LYR71 inhibits RANTES-induced STAT3 phosphorylation which directly regulates MMP-9 expression and activity. (A) MDA-MB-231 cells were pretreated with LYR71 (20 µM) for 15 min and incubated with RANTES (25 ng/ml) for 3 h. Total protein was extracted and the STAT3 phosphorylation level was analyzed using western blotting. β-actin was used as a loading control. (B) To examine the relation between STAT3 and MMP-9, CA STAT3 and DN STAT3 were transiently transfected into MDA-MB-231 cells. Western blotting was performed to measure the expression level of MMP-9 (upper panel) and zymography was performed to determine the activity of MMP-9 (middle panel) with a conditioned medium. Transcription level of MMP-9 was determined using RT-PCR (lower panel). (C) Total RNA was extracted and cDNA was prepared. To examine the inhibitory effect of LYR71 on MMP-9 transcripts, RT-PCR was performed. GAPDH was indicated as a control (upper panel). Changes in the MMP-9 promoter structure were determined using ChIP assay. Cells were incubated with RANTES (25 ng/ml) or LYR71 (20 µM). The cells were then fixed and chromatin samples were prepared and immunoprecipitated with antibodies against p-STAT3, p300, Ac-H3 or Ac-H4. Purified DNA was amplified by PCR using MMP-9 promoter-specific primers (lower panel). (D) The diagram of LYR action. LYR inhibits RANTES-induced phosphorylation of STAT3, and in turn suppresses expression of MMP-9.
To affirm transcriptional regulation of MMP-9 by LYR71, RT-PCR was performed. As expected, LYR71 suppressed RANTES-induced MMP-9 transcription in MDA-MB-231 cells. As STAT3 regulates gene expression by translocating to the nucleus and binding to the specific sequence on the promoter of target genes (Jung et al., 2005; Yu et al., 2007), we tested whether RANTES induces STAT3 recruitment into MMP-9 promoter and LYR71 does inhibit it. Besides we examined structural change of MMP-9 promoter by RANTES or LYR71, because chromatin has open structure to increase transcriptional activity. As shown in Figure 4C, RANTES induced recruitment of STAT3 and p300 into the MMP-9 promoter region and acetylation of histone H3 and H4, while LYR71 strongly suppressed the recruitment of STAT3 and p300 and deacetylated histone H3 and H4 (Figure 4C).
Discussion
In this study, we demonstrated that LYR71 is a novel anticancer agent acting through inhibition of STAT3 activation. LYR71 suppressed RANTES-induced STAT3 activation and in turn inhibited expression and activity of MMP-9 in breast cancer cells. Also, LYR71 inhibited the recruitment of STAT3 and p300 into the MMP-9 promoter region and led to structural changes in histone through deactylation of histone H3 and H4 in RANTES-induced MDA-MB-231 cells. Consequently, LYR71 attenuated RANTES-induced tumor migration/invasion and suppressed tumor progression in tumor bearing mice. However, this report on the mechanism underlying the anti-tumor effects of LYR71 is limited to studies on the inhibition of STAT3. Because our studies have not showed that STAT3 disruption by LYR71 is accomplished by inhibiting upstream molecules, such as JAK or Src tyrosine kinase. Our unpublished data showed that JAK2 kinase may be a potential target in the anti-inflammatory activity of LYR71 (data not shown). Although it is thus likely that LYR71 could exert MMP-9 inhibition effects, we can speculate that this inhibitory effect on JAK2-STAT3 signal pathway was significant when cells were treated with LYR71.
To date, many studies including our previous reports, have demonstrated the role of STAT3 in the development and progression of tumors (Wei et al., 2003; Jung et al., 2005). STAT3 is highly activated in most tumors (Page et al., 2000; Wei et al., 2003), and acts as an oncogene through transcriptional regulation of STAT3-responsive target genes (Wei et al., 2003; Pedranzini et al., 2004). These results imply that development of a new drug which inhibits STAT3 activation is valuable in treating cancers. In addition, many studies have focused on anticancer therapy through STAT3 inhibition (Jing and Tweardy, 2005; Leeman et al., 2006). In this study, we also found that LYR71 suppressed tumor growth by inhibiting STAT3 activation and consequently suppressed MMP-9 production (Figure 2 and 4). MMP-9 is overexpressed in invasive tumors and thus it may play an important role in cancer invasion through its enzymatic degradation of the extracellular matrix (Westermarck and Kahari 1999; Freije et al., 2003). Tumor develops and progresses through tumor migration/invasion not only in its original site but also in other sites (Chen and Yates 2006; Sleeman and Cremers 2007). Thus, drugs to block tumor migration/invasion can have a potent anticancer activity.
LYR71 is a derivative of alpha-viniferin, which is a trimer of resveratrol possessing anti-inflammation, anti-oxidant and anti-cancer activities (Aziz et al., 2005; Garvin et al., 2006; Busquets et al., 2007; Hwang et al., 2007). Previous reports have shown that resveratrol suppresses proinflammatory mediators, such as TNF-α, IL-1β, COX-2 or iNOS, by inhibiting NF-κB or AP-1 signaling (Manna et al., 2000; Birrell et al., 2005; Kowalski et al., 2005) and that it prevents tumor progression through the inhibition of MMP-9 expression (Yu et al., 2007; Tang et al., 2008). Alpha-viniferin also has an inhibitory effect similar to that of resveratrol in inflammation and cancer (Birrell et al., 2005; Kowalski et al., 2005), indicating that alpha-viniferin can be used as a therapeutic agent in inflammation and cancer. However, it is not easy and simple to synthesize alpha-viniferin. Thus we have synthesized over 100 kinds of derivatives of alpha-viniferin and have screened their activity. Among them, derivatives possessing the benzoxathiolone structure show a strongly inhibitory effect in inflammation and cancer. This effect might be attributed to their benzoxthiol structure because the benzoxathiol structure has an antioxidant effect (Povalishev et al., 2006). We also found that LYR71 had an anti-inflammatory effect as well as an anticancer effect and that its anti-inflammatory effect was produced through the inhibition of NF-κB, a modulator of inflammation (data not shown).
Taken together, the results of this study may provide the possibility of LYR71 as an anticancer agent and may be worth for further studies with respect to clinical applications.
Acknowledgements
This work was supported by a grant from the 2007 National R&D Program for Cancer Control, the Ministry of Health and Welfare (Project No.: 800-20070230), and the Korean Science and Engineering Foundation (R01-2006-000-10977-0).
Abbreviations
- ChIP
chromatin immunoprecipitation
- LYR71
6-methyl-2-propylimino-6, 7-dihydro-5H-benzo [1, 3]-oxathiol-4-one
- MMP
matrix metalloproteinase
- RANTES
regulated on activation normal T cell expressed and secreted
- STAT
signal transducer and activator of transcription
References
- 1.Azenshtein E, Luboshits G, Shina S, Neumark E, Shahbazian D, Weil M, Wigler N, Keydar I, Ben-Baruch A. The CC chemokine RANTES in breast carcinoma progression: regulation of expression and potential mechanisms of promalignant activity. Cancer Res. 2002;62:1093–1102. [PubMed] [Google Scholar]
- 2.Aziz MH, Reagan-Shaw S, Wu J, Longley BJ, Ahmad N. Chemoprevention of skin cancer by grape constituent resveratrol: relevance to human disease? FASEB J. 2005;19:1193–1195. doi: 10.1096/fj.04-3582fje. [DOI] [PubMed] [Google Scholar]
- 3.Birrell MA, McCluskie K, Wong S, Donnelly LE, Barnes PJ, Belvisi MG. Resveratrol, an extract of red wine, inhibits lipopolysaccharide induced airway neutrophilia and inflammatory mediators through an NF-kappaB-independent mechanism. FASEB J. 2005;19:840–841. doi: 10.1096/fj.04-2691fje. [DOI] [PubMed] [Google Scholar]
- 4.Busquets S, Ametller E, Fuster G, Olivan M, Raab V, Argiles JM, Lopez-Soriano FJ. Resveratrol, a natural diphenol, reduces metastatic growth in an experimental cancer model. Cancer Lett. 2007;245:144–148. doi: 10.1016/j.canlet.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 5.Chen EI, Yates JR. Maspin and tumor metastasis. IUBMB Life. 2006;58:25–29. doi: 10.1080/15216540500531721. [DOI] [PubMed] [Google Scholar]
- 6.Cohen M, Meisser A, Bischof P. Metalloproteinases and human placental invasiveness. Placenta. 2006;27:783–793. doi: 10.1016/j.placenta.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Dechow TN, Pedranzini L, Leitch A, Leslie K, Gerald WL, Linkov I, Bromberg JF. Requirement of matrix metalloproteinase-9 for the transformation of human mammary epithelial cells by Stat3-C. Proc Natl Acad Sci U S A. 2004;101:10602–10607. doi: 10.1073/pnas.0404100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freije JM, Balbin M, Pendas AM, Sanchez LM, Puente XS, Lopez-Otin C. Matrix metalloproteinases and tumor progression. Adv Exp Med Biol. 2003;532:91–107. doi: 10.1007/978-1-4615-0081-0_9. [DOI] [PubMed] [Google Scholar]
- 9.Freitas VM, Vilas-Boas VF, Pimenta DC, Loureiro V, Juliano MA, Carvalho MR, Pinheiro JJ, Camargo AC, Moriscot AS, Hoffman MP, Jaeger RG. SIKVAV, a Laminin {alpha}1-Derived Peptide, Interacts with Integrins and Increases Protease Activity of a Human Salivary Gland Adenoid Cystic Carcinoma Cell Line through the ERK 1/2 Signaling Pathway. Am J Pathol. 2007;171:124–138. doi: 10.2353/ajpath.2007.051264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garvin S, Ollinger K, Dabrosin C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett. 2006;231:113–122. doi: 10.1016/j.canlet.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 11.Hwang JT, Kwak DW, Lin SK, Kim HM, Kim YM, Park OJ. Resveratrol induces apoptosis in chemoresistant cancer cells via modulation of AMPK signaling pathway. Ann N Y Acad Sci. 2007;1095:441–448. doi: 10.1196/annals.1397.047. [DOI] [PubMed] [Google Scholar]
- 12.Jing N, Tweardy DJ. Targeting Stat3 in cancer therapy. Anticancer Drugs. 2005;16:601–607. doi: 10.1097/00001813-200507000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Jung JE, Kim HS, Lee CS, Park DH, Kim YN, Lee MJ, Lee JW, Park JW, Kim MS, Ye SK, Chung MH. Caffeic acid and its synthetic derivative CADPE suppress tumor angiogenesis by blocking STAT3-mediated VEGF expression in human renal carcinoma cells. Carcinogenesis. 2007;28:1780–1787. doi: 10.1093/carcin/bgm130. [DOI] [PubMed] [Google Scholar]
- 14.Joung YH, Lim EJ, Lee MY, Park JH, Ye SK, Park EU, Kim SY, Zhang Z, Lee KJ, Park DK, Park TK, Moon WK, Yang YM. Hypoxia activates the cyclin D1 promoter via the Jak2/STAT5b pathway in breast cancer cells. Exp Mol Med. 2005;37:353–364. doi: 10.1038/emm.2005.45. [DOI] [PubMed] [Google Scholar]
- 15.Jung JE, Lee HG, Cho IH, Chung DH, Yoon SH, Yang YM, Lee JW, Choi S, Park JW, Ye SK, Chung MH. STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. FASEB J. 2005;19:1296–1298. doi: 10.1096/fj.04-3099fje. [DOI] [PubMed] [Google Scholar]
- 16.Kim DH, Cho IH, Kim HS, Jung JE, Kim JE, Lee KH, Park T, yang YM, Seong SY, Ye SK, Chung MH. Anti- inflammatory effects of 8-hydroydeoxyguanosine in LPS- induced microglia activation: suppression of STAT3- mediated intercellular adhesion molecular-1 expression. Exp Mol Med. 2006a;38:417–427. doi: 10.1038/emm.2006.49. [DOI] [PubMed] [Google Scholar]
- 17.Kim HS, Ye SK, Cho IH, Jung JE, Kim DH, Choi S, Kim YS, Park CG, Kim TY, Lee JW, Chung MH. 8-hydroxydeoxyguanosine suppresses NO production and COX-2 activity via Rac1/STATs signaling in LPS-induced brain microglia. Free Radic Biol Med. 2006b;41:1392–1403. doi: 10.1016/j.freeradbiomed.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 18.Kowalski J, Samojedny A, Paul M, Pietsz G, Wilczok T. Effect of apigenin, kaempferol and resveratrol on the expression of interleukin-1beta and tumor necrosis factor- alpha genes in J774.2 macrophages. Pharmacol Rep. 2005;57:390–394. [PubMed] [Google Scholar]
- 19.Leeman RJ, Lui VW, Grandis JR. STAT3 as a therapeutic target in head and neck cancer. Expert Opin Biol Ther. 2006;6:231–241. doi: 10.1517/14712598.6.3.231. [DOI] [PubMed] [Google Scholar]
- 20.Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000;164:6509–6519. doi: 10.4049/jimmunol.164.12.6509. [DOI] [PubMed] [Google Scholar]
- 21.Page C, Huang M, Jin X, Cho K, Lilja J, Reynolds RK, Lin J. Elevated phosphorylation of AKT and Stat3 in prostate, breast, and cervical cancer cells. Int J Oncol. 2000;17:23–28. doi: 10.3892/ijo.17.1.23. [DOI] [PubMed] [Google Scholar]
- 22.Pedranzini L, Leitch A, Bromberg J. Stat3 is required for the development of skin cancer. J Clin Invest. 2004;114:619–622. doi: 10.1172/JCI22800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Povalishev VN, Polozov GI, Shadyro OI. Effects of alpha-tocopherol and related compounds on reactions involving various organic radicals. Bioorg Med Chem Lett. 2006;16:1236–1239. doi: 10.1016/j.bmcl.2005.11.078. [DOI] [PubMed] [Google Scholar]
- 24.Simpson-Herren L, Lloyd HH. Kinetic parameters and growth curves for experimental tumor systems. Cancer Chemother Rep. 1970;54:143–174. [PubMed] [Google Scholar]
- 25.Sleeman JP, Cremers N. New concepts in breast cancer metastasis: tumor initiating cells and the microenvironment. Clin Exp Metastasis. 2007;24:707–715. doi: 10.1007/s10585-007-9122-6. [DOI] [PubMed] [Google Scholar]
- 26.Tang FY, Chiang EP, Sun YC. Resveratrol inhibits heregulin-beta1-mediated matrix metalloproteinase-9 expression and cell invasion in human breast cancer cells. J Nutr Biochem. 2008;19:287–294. doi: 10.1016/j.jnutbio.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Van Kempen LC, Coussens LM. MMP-9 potentiates pulmonary metastasis formation. Cancer Cell. 2002;2:251–252. doi: 10.1016/s1535-6108(02)00157-5. [DOI] [PubMed] [Google Scholar]
- 28.Wei D, Le X, Zheng L, Wang L, Frey JA, Gao AC, Peng Z, Huang S, Xiong HQ, Abbruzzese JL, Xie K. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene. 2003;22:319–329. doi: 10.1038/sj.onc.1206122. [DOI] [PubMed] [Google Scholar]
- 29.Westermarck J, Kahari VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781–792. [PubMed] [Google Scholar]
- 30.Ye SK, Agata Y, Lee HC, Kurooka H, Kitamura T, Shimizu A, Honjo T, Ikuta K. The IL-7 receptor controls the accessibility of the TCRgamma locus by Stat5 and histone acetylation. Immunity. 2001;15:813–823. doi: 10.1016/s1074-7613(01)00230-8. [DOI] [PubMed] [Google Scholar]
- 31.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 32.Yu H, Pan C, Zhao S, Wang Z, Zhang H, Wu W. Resveratrol inhibits tumor necrosis factor-alpha-mediated matrix metalloproteinase-9 expression and invasion of human hepatocellular carcinoma cells. Biomed Pharmacother. 2008;62:366–372. doi: 10.1016/j.biopha.2007.09.006. [DOI] [PubMed] [Google Scholar]