Abstract
Ovarian carcinomas are a heterogeneous group of neoplasms traditionally sub-classified based on type and degree of differentiation. Although current clinical management of ovarian carcinoma largely fails to take this heterogeneity into account, it is becoming evident that each major histological type has characteristic genetic defects that deregulate specific signaling pathways in the tumor cells. Moreover, within the most common histological types, the molecular pathogenesis of low-grade versus high-grade tumors appears to be largely distinct. Mouse models of ovarian carcinoma have been developed that recapitulate many of the morphological features, biological behavior, and gene expression patterns of selected subtypes of ovarian cancer. Such models will likely prove useful for studying ovarian cancer biology and for pre-clinical testing of molecularly targeted therapeutics, which may ultimately lead to better clinical outcomes for women with ovarian cancer.
Keywords: endometrioid, serous, genetics, mouse models, classification
INTRODUCTION
Ovarian cancer causes more deaths in the United States than any other type of female reproductive tract cancer, with an estimated 22,430 new cases and 15,280 deaths in 2007 (1). Approximately 70% of ovarian cancers are diagnosed at advanced stage and only 30% of women with such cancers can expect to survive 5 years. While fewer than 20% of ovarian cancers are confined to the ovaries at diagnosis, the five-year survival of women with localized tumors exceeds 90%. Analysis of trends in overall five-year survival rates for women with ovarian cancer indicates some recent improvement for those diagnosed between 1996 and 2002, compared to the 1970’s and 1980’s (1). Nonetheless, these gains are rather modest and there clearly remains a need to better understand the molecular pathogenesis of ovarian cancer so new drug targets and biomarkers that facilitate early detection can be identified.
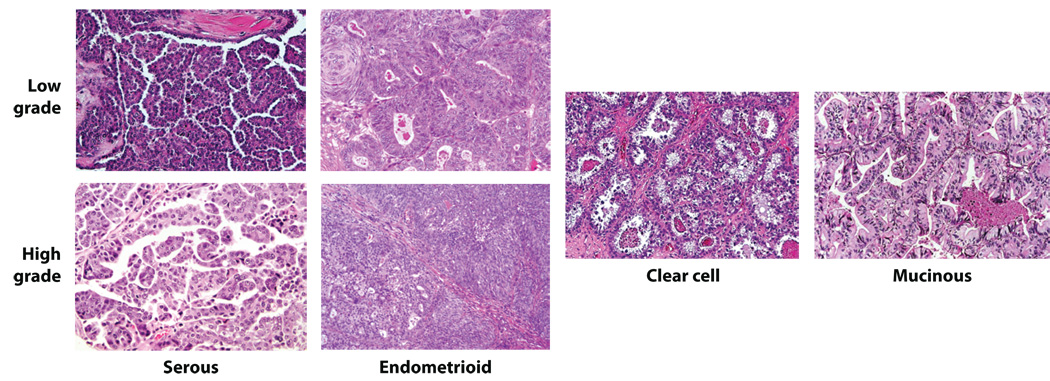
Approximately 90% of primary malignant ovarian tumors are epithelial (carcinomas), and are thought by most investigators to arise from the ovarian surface epithelium (OSE) or more likely from surface epithelial inclusion cysts (2, 3). Some investigators have suggested they may develop from the secondary Müllerian system, which includes paraovarian and paratubal cysts, the rete ovarii, endosalpingiosis, and endometriosis (4). The classification of ovarian epithelial tumors currently used by pathologists is based entirely on tumor cell morphology. The four major types of epithelial tumors (serous, endometrioid, clear cell, and mucinous) bear strong resemblance to the normal cells lining different organs in the female genital tract. For example, serous, endometrioid, and mucinous tumor cells exhibit morphological features similar to non-neoplastic epithelial cells in the fallopian tube, endometrium, and endocervix, respectively. Representative examples of serous, endometrioid, clear cell, and mucinous ovarian carcinomas are shown in Figure 1. The histological similarity of ovarian epithelial tumors to epithelia in other portions of the female genital tract is not surprising, given that all of these epithelia, as well as the cells lining the peritoneal cavity, are thought to be derived from a common embryological precursor, the coelomic mesothelium (5). Of note, provocative recent studies suggest the distal fallopian tube may actually be the site of origin of at least some serous carcinomas previously thought to arise in the ovary or pelvic peritoneum (6, 7). Once grouped by cell type, tumors can be further subdivided into those that are clearly benign (cystadenomas), those that are frankly malignant (carcinomas), and those that have features intermediate between these two (variably called “atypical proliferative” tumors, tumors of “low malignant potential” or tumors of “borderline” malignancy). The present clinical management of ovarian carcinoma patients is not significantly influenced by the histological subtype of the tumor, although accumulating clinical-pathological and molecular data suggest the major subtypes likely represent distinct disease entities as will be reviewed below.
Figure 1. Representative examples of the major histological types of ovarian carcinoma.
In addition to type of differentiation, ovarian carcinomas can be sub-classified based on degree of differentiation (tumor grade). Historically, the most commonly used grading systems have been those proposed by the International Federation of Gynecology and Obstetrics (FIGO), the World Health Organization (WHO), and the Gynecologic Oncology Group (GOG) (8). The FIGO system uses 3 grades based on architectural criteria, i.e., the proportion of glandular or papillary structures relative to areas of solid tumor growth. Grades 1, 2, and 3 correspond to <5%, 5–50%, and >50% solid growth, respectively. The WHO system incorporates both architectural and cytological features, but these are not assigned based on quantitative criteria and as a consequence, this system can be considered rather subjective. In the GOG system, the grading method varies depending on the histological type of the tumor. For example, endometrioid adenocarcinomas are graded using FIGO criteria, while clear cell carcinomas are not assigned a grade at all. More recently, a 3 grade system has been proposed that can be applied to all ovarian carcinomas (9), and two binary grading systems have been proposed for ovarian serous carcinomas, the most common type (10, 11). As we will discuss, review of both clinicopathological and molecular studies to date has led to a model in which ovarian carcinomas can be generally divided into two broad categories designated Type I and Type II tumors, akin to the division of endometrial carcinomas into two major types as recently reviewed by Di Cristofano and Ellenson (12). Tumor grade is an important, albeit not sole factor, distinguishing Type I from Type II tumors.
CLINICOPATHOLOGICAL FEATURES OF THE MAJOR TYPES OF OVARIAN CARCINOMAS
During the 1990’s, a number of advances were made in the histopathological classification of ovarian carcinomas (reviewed by Seidman et al.) (13). These include better recognition of patterns of metastatic carcinoma previously misinterpreted as primary ovarian tumors, establishment of improved criteria for distinguishing invasive from non-invasive endometrioid and mucinous ovarian carcinomas, and interpretation of carcinosarcomas (malignant mixed mesodermal/Müllerian tumors) as carcinomas with areas of “sarcomatous” differentiation (or epithelial-mesenchymal transition) rather than sarcomas. In addition, primary peritoneal serous carcinomas and ovarian serous carcinomas are now considered essentially interchangeable for the purposes of diagnosis and treatment. These and perhaps other factors have altered the current histologic type and stage distribution of ovarian carcinomas compared to earlier case series. Seidman and colleagues recently analyzed the histologic type and stage distribution of 220 consecutive ovarian and peritoneal carcinomas (13). In Seidman’s series, nearly 70% of tumors were serous and fewer than 5% of these were confined to one or both ovaries (Stage 1) at diagnosis. Serous carcinomas typically display papillary or solid growth with slit-like spaces. Nuclear atypia is usually marked and mitotic activity abundant. Endometrioid adenocarcinomas account for 10–20% of ovarian carcinomas in most older reports, but in the more recent series of Seidman and colleagues, only 7% were endometrioid. These tumors have morphological features similar to their endometrial counterparts, showing varying quantities of overt gland formation, sometimes accompanied by squamous differentiation. In contrast to the serous carcinomas, over 50% of endometrioid adenocarcinomas are confined to the ovaries at diagnosis and nuclear atypia is usually less pronounced. Approximately 10% of ovarian carcinomas in the Seidman series showed clear cell differentiation. Several growth patterns (e.g., solid, papillary, tubulocystic) for clear cell carcinoma have been recognized. Although nearly one-third are Stage 1 at diagnosis, some studies have noted a relatively unfavorable prognosis of these tumors, even when corrected for tumor stage (14, 15). Finally, it is worth noting that mucinous carcinomas comprised fewer than 3% of primary ovarian carcinomas in the Seidman series and were almost always confined to the ovary at diagnosis. Mucinous adenocarcinomas also show overt gland formation, but in contrast to endometrioid adenocarcinomas, the tumor cell cytoplasm is mucin-rich. It should be kept in mind that many previous clinical and molecular analyses of mucinous adenocarcinomas were almost certainly compromised by inadvertent inclusion of metastatic adenocarcinoma to the ovaries (frequently from the gastrointestinal tract) misclassified as primary ovarian carcinomas (16).
For many types of common adult solid tumors, such as those of the colon, breast, and uterine cervix, the stages of neoplastic progression are fairly well defined and reflected by morphologically recognizable entities that represent a continuum including normal epithelium, preinvasive lesions, invasive carcinoma, and metastatic disease. In contrast, our understanding of the progression of ovarian carcinoma is incomplete, perhaps because we have only recently begun thinking about the different histological types of ovarian carcinomas as largely distinct disease entities. As will be described below, molecular analyses of carefully classified tumors have begun to shed light on many aspects of ovarian cancer pathogenesis, including the identification of some of the precursor lesions that likely give rise to overtly malignant tumors.
OVERVIEW OF MOLECULAR GENETIC ALTERATIONS
It is well established that tumors develop and progress as the result of accumulated molecular genetic or genomic changes such as point mutation, gene amplification, deletion, and translocation. Analysis of these alterations has historically led to the identification of new cancer-associated genes including oncogenes, tumor suppressor genes and genes involved in DNA damage repair. Recent technological advances and the success of the human genome project have greatly facilitated attempts to reveal the molecular landscape of the cancer genome and the discovery of novel cancer-associated genes. For example, a high-throughput mutational analysis platform and sophisticated bioinformatics tools have been employed for large-scale mutational analyses of colorectal and breast cancers (17, 18). Furthermore, a number of methods have been developed to quantitatively measure DNA copy number changes in cancer genomes. These include digital karyotyping and array-based techniques such as single nucleotide polymorphism (SNP) array, array comparative genomic hybridization (aCGH) and representational oligonucleotide microarray analysis (ROMA). Briefly, digital karyotyping counts sequence tags from specific loci distributed throughout the human genome and thus it provides a digital readout to precisely locate amplified and deleted chromosomal regions (19, 20). Array-based technologies, on the other hand, compare the content of cancer and reference genomes and localize amplified or deleted signals in chromosomal regions using array hybridization techniques. Collectively, these technologies provide an unprecedented mapping resolution that allows precise localization of the amplified and deleted chromosomal regions compared to conventional cytogenetic methods. High throughput array-based methods to evaluate gene expression in cancer specimens have also been applied to ovarian carcinomas. Such studies have shown that the different histological types of ovarian carcinoma are largely distinguishable based on their global gene expression profiles, although some overlap – particularly between serous carcinomas and high-grade endometrioid adenocarcinomas is present (21, 22, 23, 24). Over the past several years, a number of studies have evaluated molecular genetic alterations in ovarian epithelial tumors. Although relatively few changes appear to be characteristic of ovarian carcinomas in general, more recent studies have shown that certain alterations appear to be particularly characteristic of specific histological types. Yet others help to distinguish high-grade from low-grade carcinomas within certain histological types. In the following sections, we will summarize the most common molecular alterations that characterize each major type of ovarian carcinoma.
Serous type high-grade versus low-grade carcinomas
Because serous carcinomas comprise the majority of ovarian carcinomas, most published studies of generic “ovarian cancer” or “ovarian carcinoma”, unless otherwise indicated, likely included mainly serous carcinomas. Although recent clinical and molecular studies of ovarian cancer are more likely to take histological type into account, they are also usually weighted toward serous carcinomas, since these tumors are most prevalent. As a consequence, molecular alterations in typical (high-grade, i.e, grade 2 and grade 3) serous carcinomas as well as a spectrum of benign, borderline, and low-grade malignant ovarian serous neoplasms have been studied rather extensively. A recent study has shown no significant biological or clinical difference between moderately differentiated (grade 2) and poorly differentiated (grade 3) serous carcinomas (25). In the past, it was generally assumed that serous tumors likely progress from benign serous cystadenoma, to serous borderline tumor (SBT, also known as serous tumor of low malignant potential or atypical proliferative serous tumor), to low-grade serous carcinoma, and then finally to high-grade serous carcinoma. However, clinicopathological observations and recent molecular genetic studies challenge this paradigm by clearly demonstrating two distinct pathways leading to the development of low-grade versus high-grade serous carcinoma.
Mutational analyses
Although substantial efforts have been devoted to identifying somatic mutations in sporadic serous carcinomas, only a few genes have been found to be frequently mutated in ovarian serous tumors. For example, TP53 is mutated in 50% or more high-grade serous carcinomas (26, 27), and activating mutations of KRAS or BRAF are present in over half of low-grade serous carcinomas and SBTs (28). Mutations in several other tumor suppressor genes and oncogenes such as BRCA1/2, PTEN, and PIK3CA have also been reported in ovarian serous carcinomas, but their mutation frequency is generally low (< 10%) (27, 29, 30). Although early studies identified rather frequent HER2/neu (ERBB2) amplification/copy number gains in ovarian serous carcinomas (31), a recent analysis using comprehensive genome-wide digital karyotyping technologies failed to identify high levels of ERBB2 gene amplification in 33 high-grade or 10 low-grade serous carcinomas (32). Therefore, in the following discussion we will focus mainly on mutations in TP53, KRAS and BRAF in serous carcinomas.
In contrast to low-grade serous carcinoma where mutations in TP53 are rare, approximately 50% or more of advanced stage, presumably high-grade, serous carcinomas have mutant TP53 (33, 34, 35). The TP53 mutation frequency was found to be even higher (~80%) in high-grade serous carcinoma when purified tumor samples were used for sequence analysis (36). In their study of early (stage I) serous carcinomas Leitao and colleagues identified overexpression of p53 and mutation of TP53 in well over half of the cases, suggesting that TP53 mutation is an early event in the development of high-grade serous carcinomas (37). On a related note, a small series of intraepithelial serous carcinomas in the fallopian tube with co-existing ovarian serous carcinomas were recently found to share identical TP53 mutations, suggesting a common origin of tumors in the two sites and providing further evidence of a role for TP53 mutations early in serous carcinoma pathogenesis (6).
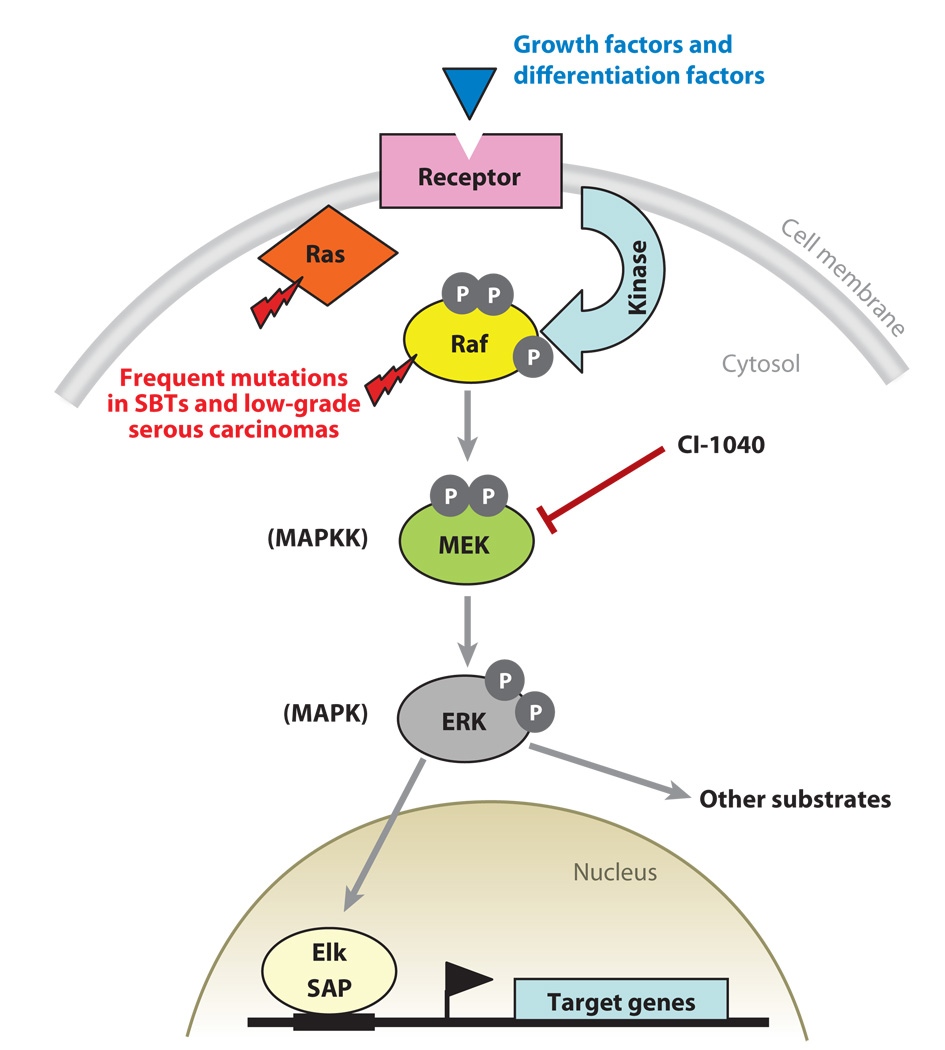
Activating mutations in KRAS and one of its downstream effectors, BRAF, have been identified in a variety of human cancers and mutations of either KRAS or BRAF lead to constitutive activation of MAPK signaling (Figure 2) (38). Molecular genetic studies have highlighted the importance of the Ras/Raf/MEK/MAPK signaling pathway in the pathogenesis of low-grade ovarian serous carcinomas. Frequent KRAS mutations in SBT were first reported by Mok et al. (39). Several subsequent studies verified the original finding and further demonstrated that mutations in KRAS and BRAF characterize both SBTs and low-grade serous carcinomas (28, 40, 41, 42). Specifically, activating mutations in codon 12 and less commonly in codon 13 of KRAS or in codon 600 of BRAF occur in approximately two thirds of SBTs and low-grade serous carcinomas. Mutations in KRAS and BRAF are mutually exclusive insofar as tumors with mutant KRAS do not have mutant BRAF and vice versa. Furthermore, a 12-bp insertion mutation of ERBB2 (HER2/neu), which activates an upstream regulator of K-Ras, has been found in 9% of SBTs and these mutations are only observed in tumors lacking mutant KRAS and BRAF (30, 43). In contrast, KRAS and BRAF mutations are very uncommon in high-grade serous carcinomas (28). These data provide compelling evidence indicating that KRAS and BRAF mutations are largely confined to low-grade serous carcinomas and SBTs and suggest that SBTs are likely precursors of low-grade serous carcinomas, but not the more common high-grade serous carcinomas. KRAS and BRAF mutations are lacking in isolated serous cystadenomas, putative precursors of SBTs (44). However, identical KRAS or BRAF mutations were detected the SBTs and adjacent cystadenoma epithelium in serous cystadenomas associated with small SBTs (45). Collectively, these findings suggest mutations of KRAS and BRAF are early events associated with serous tumor initiation and that a small subset of serous cystadenomas which acquire KRAS or BRAF mutations may progress to SBT.
Figure 2. Schematic illustration of the RAS-RAF-MEK-ERK (MAPK) signaling pathway.
This cell signaling pathway is important for the cellular response to a variety of growth and differentiation factors. Aberration of this pathway in ovarian SBTs and low-grade serous carcinomas is mainly due to activating mutations of KRAS and BRAF, which result in constitutive activation of MAPK-mediated signaling in these tumors. Activated MAPK signaling alters expression of downstream target genes, including up-regulation of cyclin D1. Several MEK small compound inhibitors such as CI-1040 have been generated. These inhibitors effectively suppress MAPK activation and hold promise for treatment of patients with advanced stage low-grade serous carcinomas.
Overall, 70–80% of low-grade serous carcinomas and SBTs express active MAPK (46). Insights into the biological significance of activated MAPK signaling in SBT and low-grade serous carcinoma have been provided by Pohl et al. who applied serial analysis of gene expression (SAGE) to identify genes regulated by activated MAPK in low-grade serous carcinoma cells with mutant BRAF (47). The transcriptome of these cells was compared to that of cells treated by CI-1040, a compound that selectively inhibits MEK (Figure 2) (48). The most striking changes after MEK inhibition were down-regulation of cyclin D1, COBRA1 and transglutaminase-2, and up-regulation of TRAIL, thrombospondin-1, optineurin and palladin. Among all the differentially expressed genes, cyclin D1 demonstrated the greatest change in gene expression. Overexpression of cyclin D1 has been associated with low-grade ovarian carcinomas, a finding consistent with the view that cyclin D1 is a downstream target of active MAPK which is constitutively expressed in most low-grade ovarian tumors because of frequent activating mutations in KRAS and BRAF (49, 50, 51). Although activating mutations of KRAS and BRAF are required to maintain cellular proliferation and survival, in non-transformed normal cells such mutations may induce cell cycle arrest and cellular senescence, a phenomenon known as “oncogene-induced senescence” (52). The molecular genetic event(s) that help early tumor cells escape this effect during the development of low-grade serous carcinoma are as yet unknown. Finally, it should be noted that activated MAPK signaling has been observed in a substantial fraction (41%) of high-grade serous carcinomas as well, presumably through mechanisms other than activating mutations of KRAS and BRAF (46, 53).
DNA copy number changes
Several studies have analyzed global DNA copy number alterations specifically in high-grade and low-grade serous carcinomas (32, 54, 55, 56, 57). A general conclusion that can be drawn from these studies is that high-grade serous carcinomas are characterized by more diffuse and higher levels of sub-chromosomal gains and losses than low-grade serous carcinomas and SBTs, suggesting that chromosomal instability is more pronounced in high-grade serous carcinomas than in low-grade serous carcinomas and SBTs. Based on SNP array analysis of DNA copy number alterations in purified ovarian tumor samples, Nakayama and colleagues found the most frequently amplified sub-chromosomal regions harbor the CCNE1 (cyclin E1), AKT2, NOTCH3, RSF1, and PIK3CA loci (32). Dual color fluorescence in situ hybridization (FISH) was performed to validate the findings in a sizeable independent set of serous carcinomas. The results showed high level DNA copy number gains/amplification of CCNE1, NOTCH3, RSF1, AKT2, and PIK3CA loci in 36.1%, 32.1%, 15.7%, 13.6%, and 10.8% of high-grade serous carcinomas, respectively. In contrast, low-grade serous tumors did not show high level amplification at any of the above loci, and only 2 of 24 low-grade tumors showed low copy number gains at the NOTCH3 locus.
The gene amplifications noted above undoubtedly have both biological and clinical significance. For example, deregulation of the PI3K/Pten signaling pathway, for example through amplification of PIK3CA which encodes the catalytic subunit of PI3K, has been shown to play a causal role in invasion, metastasis and chemoresistance in ovarian cancer (58, 59, 60). CCNE1 gene amplification and overexpression contributes to oncogenesis and genetic instability, particularly in the presence of mutant p53, which is present in most high-grade serous carcinomas (61, 62, 63, 64). Moreover, expression of low molecular weight cyclin E is associated with worse clinical outcome in ovarian cancer patients (65, 66). The functional consequences of other gene amplifications in ovarian serous carcinomas are just beginning to be elucidated. RSF1, a chromatin remodeling gene, is located in a chromosome 11q13.5 amplicon. Overexpression of Rsf-1 stimulated cell proliferation, while Rsf-1 knockdown inhibited cell growth in ovarian cancer cells harboring RSF1 amplification (67). Patients with 11q13.5 amplification or Rsf-1 over-expression had a significantly shorter overall survival than those without. Rsf-1 up-regulation conferred resistance to paclitaxel and carboplatin in vitro, a finding that may explain the association of Rsf-1 expression with worse clinical outcome. Amplification of the NOTCH3 locus at 19p13.12 has been validated by several independent methods including SNP array, digital karyotyping, quantitative PCR and dual-color FISH analysis (68). Inactivation of Notch3 by either γ-secretase inhibitors or Notch3-specific siRNAs suppressed cell proliferation and induced apoptosis in cell lines that overexpressed Notch3 but not in those with minimal Notch3 expression. These results indicate that Notch3 is required for proliferation and survival of Notch3-amplified tumors and suggest inactivation of Notch3 as a potential therapeutic approach for a subset ovarian high-grade serous carcinomas.
In contrast to high-grade serous carcinoma, discrete regions of chromosomal gains have not been reproducibly detected in low-grade serous carcinoma or its putative precursor lesion, SBT. However, a few sub-chromosomal regions show frequent deletion in low-grade serous carcinomas. Kuo and colleagues applied high resolution SNP arrays on purified tumor cells from low-grade serous carcinomas and SBTs, and found that heterozygous chromosome 1p losses characterized many low-grade serous carcinomas but rarely SBTs, suggesting that inactivation of potential tumor suppressor genes in this region may propel progression from SBT to low-grade serous carcinoma (I-M Shih laboratory, unpublished studies). In support of this notion, at least two potential tumor suppressors, CHD5 and miR-34, have recently been identified within the deleted region on chromosome 1p36 (69). Chd5 was recently characterized as a tumor suppressor that controls cell proliferation, apoptosis and senescence via the p19(Arf)/p53 pathway (70). miR-34 is a p53-regulated microRNA that mimics p53’s effects on growth arrest and apoptosis (71, 72). Further studies are required to determine possible roles for CHD5 or miR-34 inactivation in the pathogenesis of low-grade ovarian serous carcinomas.
Gene expression
Transcriptome-wide gene expression profiling using SAGE and gene expression microarrays has been applied to ovarian tumors including high-grade serous (G2 and G3) carcinomas, low-grade (G1 or well-differentiated) carcinomas, and SBTs (21, 55, 73, 74, 75, 76). A major finding of several of these studies is that low-grade and high-grade serous carcinomas are distinguishable based on their gene expression profiles. In those studies that included SBTs, the SBTs always clustered with low-grade rather than high-grade serous carcinomas. This finding provides further evidence that SBTs and low-grade serous carcinomas are closely related and both tumors are distinct from high-grade serous carcinomas.
A number of genes shown to be differentially expressed between high-grade and low-grade serous carcinomas are involved in a number of important cellular properties, including cell cycle regulation, apoptosis, tumor invasion and control of local immunity (75, 77). Ovarian tumor progression is accompanied by the acquisition of novel gene products and these new tumor-associated antigens elicit a host immune response that exerts selective pressure upon the emerging tumor clones. One of the mechanisms by which ovarian cancer cells may evade immune surveillance is by up-regulating human leukocyte antigen-G (HLA-G) expression. HLA-G is overexpressed in most high-grade serous carcinomas but rarely in low-grade serous carcinomas and SBTs (78). HLA-G is a non-classical MHC class I molecule that contributes to inactivation of the local immune response within the tumor microenvironment (79). The aggressive behavior of high-grade serous carcinoma may be related, at least in part, to its ability to escape immune surveillance more effectively.
In contrast, only a few known genes appear to be preferentially expressed in low-grade serous carcinoma and SBT. High levels of p21/WAF1, with concomitant expression of cell growth suppressors, gadd34 and BTG-2, have been demonstrated in these tumors (55, 80). The biological significance of p21 up-regulation in SBT and low-grade serous carcinoma remains speculative. p21 may contribute to negative cell cycle regulation in response to a variety of environmental cues or to oncogenic stress, since most SBTs and low-grade serous carcinomas have activating mutations of KRAS, BRAF or ERBB2. Cyclin D1 is also more frequently expressed in low-grade tumors as compared to high-grade carcinomas (49, 50, 51). As previously discussed, this finding is consistent with the view that cyclin D1 is a downstream target of active MAPK which is constitutively expressed in most low-grade ovarian tumors because of frequent activating mutations in KRAS or BRAF.
Although somatic mutations of BRCA1 and BRCA2 are known to be rather uncommon in sporadic ovarian carcinomas, accumulated studies suggest these genes may be inactivated, particularly in serous carcinomas, through mechanisms other than mutation (81). Horiuchi and colleagues found reduced expression of BRCA1 transcripts in serous carcinomas, often associated with LOH at the BRCA1 locus (82). Hypermethylation of the BRCA1 promoter accompanied by loss of Brca1 protein expression has been observed in 15–31% of sporadic ovarian carcinomas (83). Moreover, high-grade serous carcinomas with genetic vs. epigenetic inactivation of BRCA1 have recently been shown to have distinct molecular abnormalities involving the PI3K/Pten signaling pathway (84). Specifically, tumors with BRCA1 mutations typically had decreased PTEN mRNA levels while those with epigenetic loss of BRCA1 had copy number gains of PIK3CA. Finally, WT1 (Wilms’ tumor 1) encodes a protein that plays important roles in genitourinary tract development. Several studies have noted preferential expression of WT1 in serous carcinomas (both low-grade and high-grade) compared to the other histologic types of ovarian carcinoma (85, 86).
Endometrioid type high-grade versus low-grade carcinomas
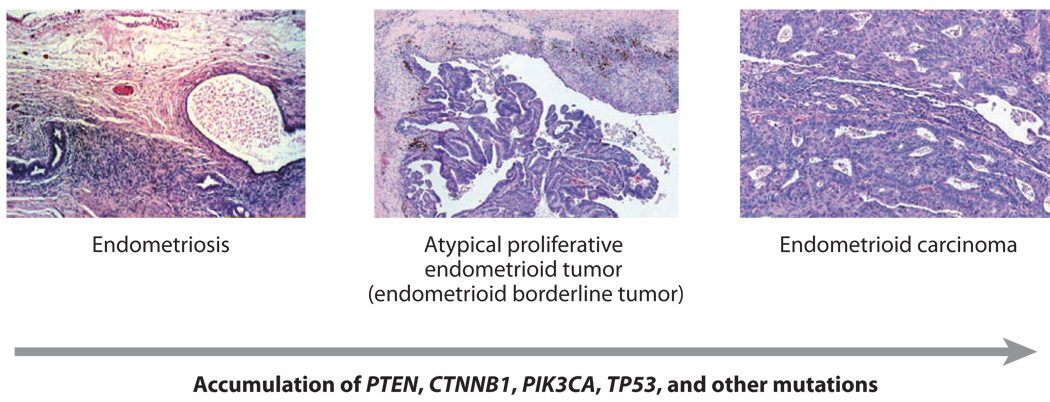
The association of ovarian endometrioid carcinoma with endometriosis has long been recognized (87, 88, 89, 90). This association, along with the identification of genetic alterations in endometriotic lesions and the observation of a morphological transition from endometriosis to carcinoma in over one-third of cases have led many to consider endometriosis a likely precursor of endometrioid carcinoma (2, 91, 92). In their analysis of benign ovarian endometrioid tumors and well differentiated endometrioid carcinomas, Bell and Kurman found frequent coexistence of endometriosis, benign endometrioid neoplasms such as endometrioid adenofibroma or endometrioid tumor of borderline or low malignant potential, and well differentiated endometrioid carcinoma (93). These findings provide support for an ovarian endometrioid adenoma-carcinoma progression (i.e., endometriosis to benign endometrioid neoplasm to well differentiated carcinoma) (Figure 3), similar to that proposed for progression of low-grade serous carcinoma from SBT.
Figure 3. Histological and molecular genetic progression of ovarian endometrioid carcinoma.
Low-grade endometrioid carcinomas often arise from endometrioid borderline tumors, which in turn may arise from endometriosis. This step-wise histopathological progression is often accompanied by accumulation of mutations predicted to deregulate canonical Wnt signaling (usually CTNNB1) and/or PI3K/Pten signaling (PTEN, PIK3CA). TP53 mutations are usually observed in high-grade endometrioid carcinomas in the absence of Wnt and PI3K/Pten pathway defects.
Mutational analyses
The ovarian endometrioid carcinomas share many molecular genetic features with their uterine counterparts. Indeed, mutations in several of the same tumor suppressor genes, oncogenes, and genes involved in DNA repair have been observed in both endometrial and ovarian endometrioid carcinomas. The molecular genetics of endometrial carcinomas have been recently reviewed by Di Cristofano and Ellenson (12).
The canonical Wnt (i.e., Wnt/β-catenin/Tcf, hereafter Wnt/β-cat) signaling pathway is involved in the regulation of several important cellular processes, including cell fate determination, proliferation, motility, and survival. In this pathway, β-catenin is a key effector that is stabilized as a consequence of selected Wnt ligands binding to their cell surface receptors. β-catenin-mediated signaling is deregulated in 16–38% of human ovarian endometrioid carcinomas, usually on the basis of activating mutations of CTNNB1, the gene that encodes β-catenin, and rarely because of inactivating mutations in genes encoding negative regulators of β-catenin such as APC, AXIN1, or AXIN2 (24, 94, 95, 96, 97). Notably, CTNNB1 mutations are very uncommon in the other major types of ovarian carcinoma (94). Several studies have noted the association of CTNNB1 mutation with squamous differentiation, low tumor grade, and favorable outcome (24, 27, 98, 99)
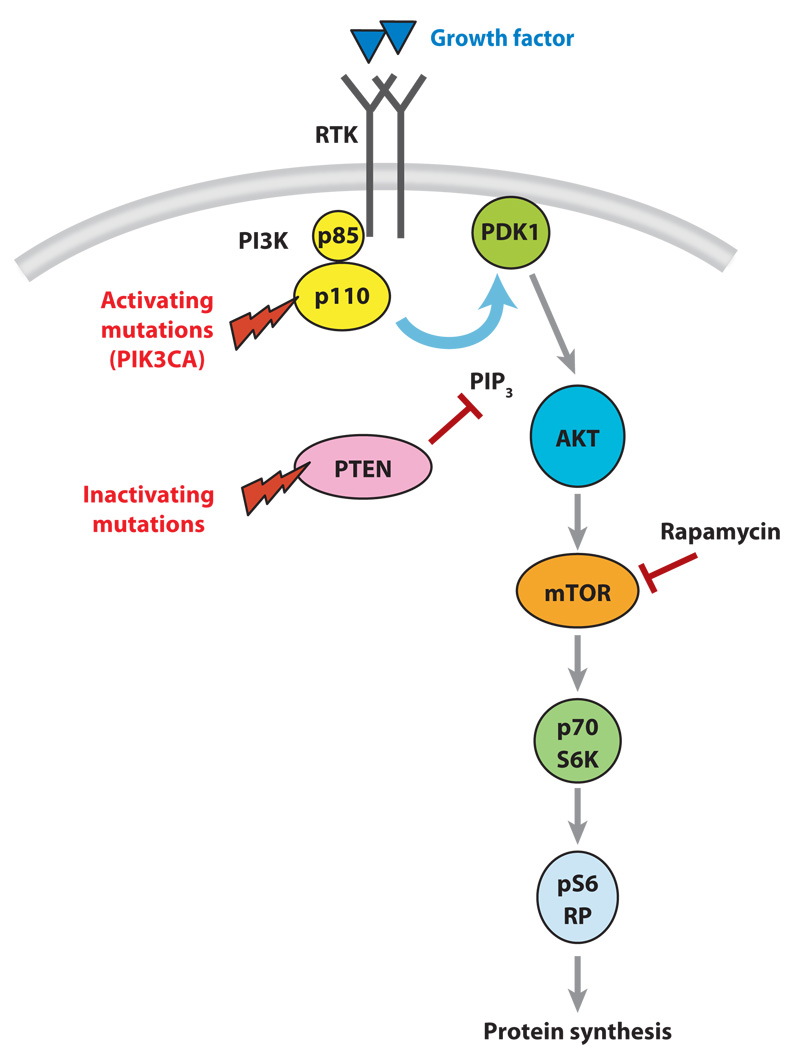
Inactivating mutations of the tumor suppressor gene PTEN have been reported in 14–21% of ovarian endometrioid carcinomas, and like CTNNB1, PTEN mutations are rare in the other major types of ovarian carcinomas (100, 101, 101). Inactivation of Pten, the lipid phosphatase that converts PIP3 to PIP2, is one mechanism by which activation of phosphotidylinositol 3-kinase (PI3K) signaling occurs in human tumors (Figure 4) (102). An alternative mechanism by which PI3K/Pten signaling is activated in endometrioid adenocarcinomas is through activating mutations of PIK3CA, which encodes the p110 catalytic subunit of PI3K. Most reported PIK3CA mutations are clustered in exons 9 and 20, and PIK3CA mutations in these exons have been identified in 20% of ovarian endometrioid and clear cell carcinomas but in only 2% of ovarian serous carcinomas (30, 103). Interestingly, Oda and colleagues reported a high frequency of concomitant PIK3CA and PTEN mutations in uterine endometrioid adenocarcinomas, and functional studies suggested that mutation of both genes may have an additive effect on PI3K pathway activation (104). PIK3CA and PTEN mutations also co-occur in a subset of ovarian endometrioid carcinomas and in both the ovary and the endometrium, PIK3CA mutations are associated with adverse prognostic indicators (27, 105). In a recent mutational analysis of 72 primary ovarian endometrioid carcinomas, Wu and colleagues found that mutational defects in canonical Wnt signaling were significantly associated with mutations predicted to deregulate PI3K/Pten signaling (24). Tumors with these mutations tended to be low-grade and low-stage. Hence, defects in these two signaling pathways appear to be particularly characteristic of this subtype of ovarian cancer, a finding with implications for future therapeutic strategies that include molecularly targeted agents.
Figure 4. Schematic illustration of one aspect of PI3K/Pten signaling.
Activating mutations of PIK3CA or inactivating mutations of PTEN result in activation of Akt-mediated signaling to mTOR and other downstream effectors that affect protein synthesis. Rapamycin is a well known inhibitor of mTOR. GF, growth factor; RTK, receptor tyrosine kinase.
Activating mutations in KRAS and BRAF have been reported in ovarian endometrioid carcinomas, but the frequency of mutations in these genes is rather low at less than 7% (24, 41, 106, 107, 108). Microsatellite instability has been observed in 13–20% of ovarian endometrioid carcinomas, and is usually associated with loss of hMLH1 or hMSH2 expression (101, 109). Both of these types of alterations are observed with greater frequency in endometrioid carcinomas arising in the endometrium.
In contrast, TP53 mutations are common in both uterine and ovarian endometrioid carcinomas. TP53 mutations have been reported in upwards of 60% of endometrioid carcinomas arising in the ovary overall, and in a greater percentage of high-grade tumors (110). In the aforementioned series of 72 endometrioid carcinomas, Wu and colleagues documented TP53 mutations in 32 tumors; five additional tumors showed diffuse and strong nuclear accumulation of p53 protein, presumably because of missense mutations outside of the exons sequenced. TP53 mutations were significantly associated with high tumor grade and were uncommon in tumors with documented Wnt/β-cat and/or PI3K/Pten signaling defects. Akin to the serous carcinomas, the molecular findings support the division of ovarian endometrioid carcinomas into two subgroups. Low-grade (FIGO grade 1) tumors are characterized by mutations that deregulate the canonical Wnt/β-cat and PI3K/Pten signaling pathways and typically lack TP53 mutations. High-grade (FIGO grade 2 and 3) tumors often harbor mutations of TP53 and lack Wnt/β-cat or PI3K/Pten signaling pathway defects.
DNA copy number changes
Given their reduced abundance compared to the serous carcinomas, comprehensive studies of DNA copy number changes specifically in ovarian endometrioid carcinomas are rather few. Using conventional CGH methods, Tapper and colleagues identified gains of chromosome 1q in 5 of 8 cases, but the genes presumably targeted by these gains were not identified (111). These investigators also evaluated several serous and mucinous carcinomas and found divergence of DNA copy number changes between the three tumor types, providing additional molecular evidence for each type as a distinct disease entity. More recently, Mayr et al., used both conventional and aCGH to identify frequent gains at the JUNB, KRAS2, MYCN, ESR, and CCND2 loci in six endometrioid carcinomas (56). Clearly, comprehensive analysis of a larger number of cases is needed to help identify additional oncogenes and tumor suppressor genes that participate in the molecular pathogenesis of this group of ovarian cancers.
Gene expression
A number of investigators have used comprehensive high-throughput technologies to profile gene expression in ovarian cancers, including endometrioid carcinomas (21, 22, 24, 112). Perhaps one of the more informative studies is that of Wu and colleagues, who used high density oligonucleotide microarrays to analyze global gene expression in 41 serous, 37 endometrioid, 13 mucinous and 8 clear cell ovarian carcinomas (24). Notably, all of the endometrioid tumors were annotated with data on the mutational status of the TP53, CTNNB1, PTEN, PIK3CA and KRAS genes. Although the gene expression profiles of both clear cell and mucinous carcinomas were found to be largely distinct from each other and from serous carcinomas, substantial overlap between the expression profiles of endometrioid and serous carcinomas was identified. When the mutational status of the above genes was taken into account, it was noted that endometrioid tumors with gene expression profiles similar to serous carcinomas were usually high-grade and harbored TP53 mutations, while the endometrioid tumors with expression patterns distinct from the serous carcinomas tended to be low-grade and harbor mutations of CTNNB1, PTEN, and/or PIK3CA. Once again, the molecular data support the division of endometrioid carcinomas into two major groups based at least in part, on tumor grade. Shared genetic alterations such as TP53 mutation may be responsible for similarities in global gene expression pattern between high-grade endometrioid carcinomas and typical (high-grade) serous carcinomas. Some of the overlap in gene expression between serous carcinomas and high-grade endometrioid carcinomas may be due to pathologists’ inability to reliably distinguish between the two based solely on morphological criteria. Further studies are needed to address this issue and to determine whether moderately differentiated (i.e., FIGO grade 2) endometrioid carcinomas have molecular features more in keeping with grade 3 versus grade 1 tumors.
Clear cell and mucinous carcinoma
The molecular changes in ovarian clear cell and mucinous tumors have not been as extensively studied as in serous and endometrioid tumors. This is probably due to the relative rarity of clear cell and especially primary mucinous carcinomas, now that metastatic mucinous adenocarcinomas to the ovary are being more rigorously excluded. As a consequence, most studies in the published literature have analyzed only limited numbers of clear cell and mucinous carcinomas and therefore the true prevalence of many specific molecular alterations in these tumor types is unknown. Nevertheless, some important conclusions can be drawn regarding the role of a few molecular alterations in the pathogenesis of these tumors. As with endometrioid carcinomas, there is a close association between endometriosis and clear cell carcinoma (88, 89, 90). Indeed in several studies, the association with endometriosis was more impressive for clear cell than for endometrioid cancers.
Mutation analyses
Mutations in KRAS, BRAF, and TP53 are present in some clear cell carcinomas but their frequency is generally low (27, 41). For example, TP53 mutation and BRAF mutation were found in 8.3% and 6.3% of clear cell carcinomas, respectively. Mutations predicted to deregulate PI3K/Pten signaling are more common in clear cell carcinomas, with PIK3CA mutations reported in 20–25% and PTEN mutations in 8% of tumors in a few small series (27, 103, 113). Interestingly, Sato et al. demonstrated somatic mutations of PTEN in 21% of ovarian endometriotic cysts, indicative of shared molecular alterations between clear cell and endometrioid carcinomas of the ovary and their putative precursor lesion (113). Among 7 clear cell carcinomas with synchronous endometriosis, LOH at the PTEN locus in both the carcinoma and associated endometriosis was detected in 3 cases. One case displayed LOH only in the carcinoma, and 3 cases lacked LOH at PTEN in both carcinoma and associated endometriosis. In no cases were there LOH events involving PTEN in the endometriosis only. The findings suggest that inactivation of the PTEN tumor suppressor gene, when it occurs, is a relatively early event in the development of ovarian clear cell carcinoma. Although studies to date are rather limited, the clear cell carcinomas do not appear to share many other changes with endometrioid carcinomas, as canonical Wnt signaling pathway defects and microsatellite instability have not been observed with significant frequency in the clear cell tumors (27, 114, 115).
In primary ovarian mucinous carcinomas, KRAS mutation is a very common molecular alteration - occurring in upwards of 75% of ovarian mucinous carcinomas (106, 116, 117, 118). Mucinous adenocarcinomas often contain areas indistinguishable from mucinous cystadenoma and mucinous borderline tumor and progression from benign to borderline and from borderline to malignant neoplasia has been proposed. Interestingly, identical KRAS mutations have been detected in mucinous carcinomas and adjacent mucinous cystadenoma and borderline tumor (119), a molecular finding supporting this morphological continuum of tumor progression in ovarian mucinous neoplasms.
DNA copy number changes
At least two independent reports describe the use of classical CGH to study DNA copy number changes in clear cell carcinomas (120, 121). In their analysis of 18 tumors, Dent and colleagues found that chromosome 9p21 deletion was the most common copy number alteration, followed by losses on chromosomes 1p, 11q and 10q (including the PTEN locus at 10q23.3). DNA copy number gains were generally not detected (121). In contrast, in their analysis of 12 ovarian clear cell carcinomas, Suehiro and colleagues identified increased DNA copy number at several chromosomal regions as well as frequent losses of chromosome 19p (120). The discordant findings between the two studies suggest that clear cell carcinomas may be quite heterogeneous and analysis of large numbers of tumor samples using more advanced technologies with higher resolution may be needed to reliably identify common alterations of DNA copy number in clear cell carcinomas. Toward this end, Kuo et al. applied high density SNP arrays to analyze a total of 12 purified ovarian clear cell carcinomas (IM Shih laboratory, unpublished data). The results demonstrate that on a genome-wide scale, the extent and level of DNA copy number gains and losses are less pronounced in clear cell carcinoma than in high-grade serous carcinomas. The most common changes in clear cell carcinomas include amplifications of regions on chromosomes 8q and 20q, and deletions on chromosome 9q; however, these events are not unique to clear cell carcinoma as they are also observed in high-grade serous carcinomas. Genome-wide analyses of DNA copy number changes in primary ovarian mucinous carcinomas have not yet been reported.
Gene expression
Several comprehensive gene expression analyses of ovarian carcinomas have noted that clear cell carcinomas are readily distinguishable from the other histological types of ovarian carcinomas based on their global gene expression profiles (21, 22, 112). A number of genes have been reported to be preferentially expressed in ovarian clear cell carcinomas and in some cases, in associated endometriosis. These include hepatocyte nuclear factor-1beta (HNF-1β), glutathione peroxidase 3, plasma membrane-associated sialidase (NEU3), osteopontin, nicotinamide N-methyltransferase, RNA-binding protein with multiple splicing, annexin A4, tissue factor pathway inhibitor 2 and MAP3k5/ask1, dipeptidyl peptidase IV (CD26), FXYD domain-containing ion transport regulator 2, and genes involved in nucleotide excision repair, ERCC1 and XPB (21, 73, 74, 112, 122, 123, 124, 125). Among these overexpressed genes, HNF-1β appears to be a relatively specific marker to ovarian clear cell carcinoma (125). HNF-1β is a nuclear homeodomain protein that participates in regulating gene expression in liver and several other tissues including secretory endometrium. Given that HNF-1 is essential in controlling multiple genes involved in glucose/glycogen metabolism, HNF-1β up-regulation may be in part responsible for the characteristic cytological feature (i.e., the glycogen-rich, clear-appearing cytoplasm) of clear cell carcinoma. Furthermore, many genes relatively specific to clear cell carcinoma are also regulated by HNF-1β (126, 127). Knockdown of HNF-1β expression in clear cell carcinoma cells induces apoptosis, suggesting that HNF-1β is essential for survival of cancer cells (125).
Not unexpectedly, the pattern of gene expression of mucinous carcinomas is largely distinguishable from serous, endometrioid, and clear cell carcinomas based on comprehensive gene expression profiling (21, 128). Using high density oligonucleotide microarrays to profile gene expression in 3 primary mucinous ovarian carcinomas and 4 mucinous borderline tumors compared to 31 serous carcinomas, 3 serous borderline tumors and 8 endometrioid carcinomas, Heinzelmann-Schwarz and colleagues identified a characteristic gene expression profile associated with mucinous ovarian tumors (128). Ovarian mucinous tumors express several mucin genes (MUC2, MUC3 and MUC17) that are characteristic of mucinous carcinomas irrespective of their tissue origins. Because primary ovarian mucinous tumors often display intestinal type differentiation, it is not surprising that additional genes with preferential expression in ovarian mucinous tumors encode markers of intestinal differentiation such as the caudal type homeobox transcription factors CDX1 and CDX2, and LGALS4 (galectin 40), which encodes an intestinal cell surface adhesion molecule that is overexpressed in intestinal carcinomas. Immunohistochemical analysis of LGALS4 expression in a spectrum of ovarian mucinous neoplasms showed that LGALS4 is not detectable in normal ovarian surface epithelium, but is expressed at high levels in mucinous cystadenomas, borderline tumors, and carcinomas, indicating that increased LGALS4 expression occurs early during mucinous ovarian tumor progression (128).
MOUSE MODELS OF OVARIAN CANCER
Genetically engineered mouse (GEM) models of each major subtype of ovarian cancer will undoubtedly prove useful for improving knowledge of ovarian cancer biology and for preclinical testing of signal transduction inhibitors as novel therapeutics. Historically, most animal models of ovarian cancer were based on xenografting human ovarian cancer cells into immunodeficient mice. Such models have limitations, including incomplete recapitulation of tumor-host interactions and inability to replicate early stages of tumor development. Recently described GEM models of ovarian cancer appear to overcome some weaknesses of the xenograft models as tumors arise orthotopically in immunologically intact animals and more closely mimic the behavior of human ovarian cancers. For example, Orsulic et al. employed an approach involving ex vivo genetic modification of primary murine ovarian surface epithelium (MOSE) (129). MOSE cells were removed from p53−/− transgenic mice expressing the cell surface avian retroviral receptor (ARR) under the control of the keratin-5 promoter and cultured in vitro for several generations. The cultured MOSE were infected, via the ARR, with replication-competent avian leucosis-derived vectors carrying oncogenes, such as c-MYC, KRAS, or AKT. MOSE cells expressing various combinations of oncogenes were then surgically transplanted under the ovarian bursa of recipient mice. The addition of any two of the oncogenes was sufficient to induce tumor formation when infected cells were injected at subcutaneous, intraperitoneal, or ovarian sites. In another model, Connolly et al. employed a potentially simpler strategy utilizing a promoter element from the murine Müllerian Inhibitory Substance II Receptor (MISIIR) gene to drive expression of SV40 T-Ag (large and small) in MOSE (130). In this model, approximately 50% of female mice develop bilateral poorly differentiated ovarian carcinomas. Flesken-Nikitin and colleagues used an alternative approach to conditionally inactivate the p53 and Rb tumor suppressors in the mouse ovarian surface epithelium (131). A single ovarian intrabursal injection of recombinant adenovirus expressing Cre recombinase (AdCre) in female mice homozygous for conditional (floxed) p53 and Rb alleles consistently led to development of ovarian carcinomas. Mice from all three models develop poorly differentiated papillary carcinomas of the ovary with greatest resemblance to human serous carcinomas. The tumors disseminate intra-peritoneally and are frequently accompanied by bloody ascites.
Given the morphological and molecular heterogeneity of ovarian carcinomas described above, a goal for researchers in the field is the development of mouse models that recapitulate each major histological type of ovarian cancer. Toward that end, Dinulescu et al. described the use of AdCre to conditionally activate an oncogenic KRas allele in the MOSE (132). When AdCre was injected into the ovarian bursa, the animals developed ovarian and peritoneal implants histologically reminiscent of human endometriosis. When the same approach was used to coordinately activate KRas and inactivate Pten in the MOSE, ovarian carcinomas resembling endometrioid adenocarcinomas developed. However, as described above, KRAS mutations in human ovarian endometrioid carcinomas are rather uncommon, and data supporting cooperation between mutant KRAS and PTEN loss in human tumors are lacking (24). In an attempt to more closely recapitulate findings in human endometrioid carcinomas, and in light of the data identifying frequent co-occurrence of canonical Wnt and PI3K/Pten/Akt signaling defects in the human tumors, Wu and colleagues employed ovarian bursal injection of AdCre to conditionally inactivate the Pten and Apc tumor suppressor genes in the ovarian surface epithelium of Apcflox/flox;Ptenflox/flox mice (24). The injected ovaries uniformly and rapidly developed tumors with similar morphology to human ovarian endometrioid cancers (Figure 5A, 5B). In addition, the mouse tumors exhibited biological behavior and gene expression patterns similar to their human tumor counterparts with comparable signaling pathway defects.
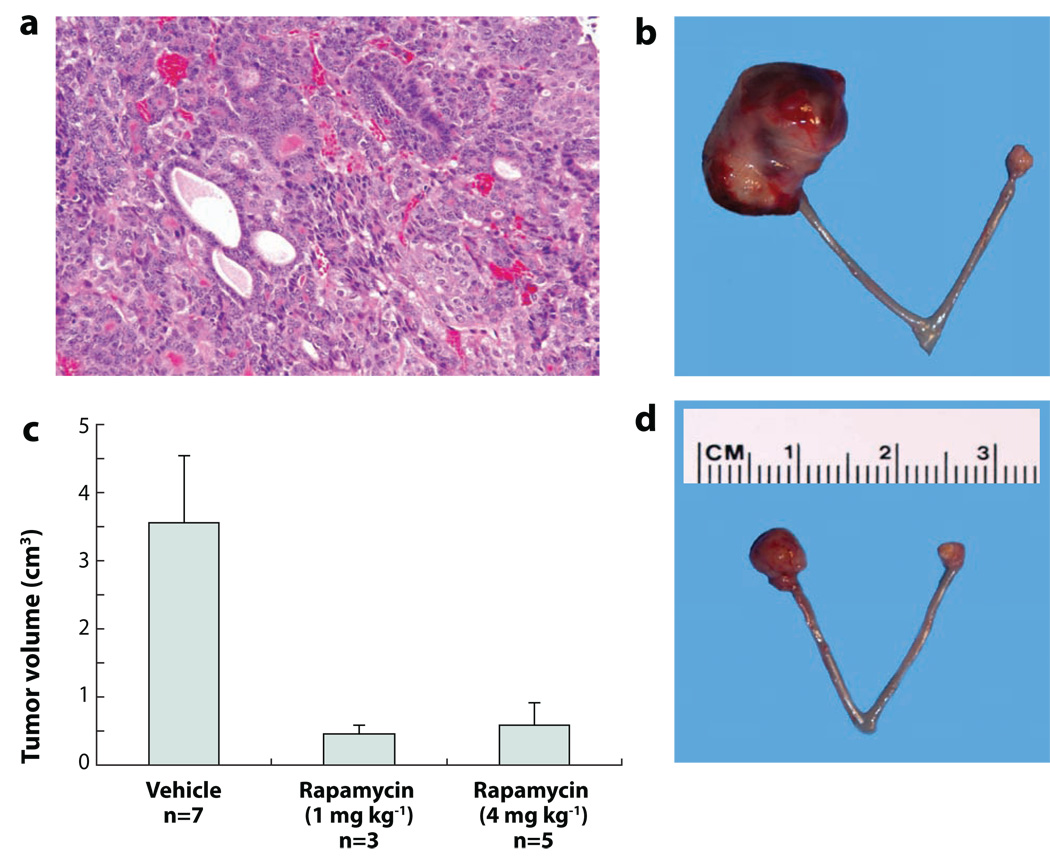
Figure 5. Genetically engineered mouse models of ovarian cancer recapitulate their human counterparts and respond to molecularly-targeted therapeutics.
A) Hematoxylin & eosin stained section of endometrioid carcinoma-like mouse tumor. B) Representative ovarian carcinoma in right ovary of Apcflox/flox;Ptenflox/flox mouse 11 wks after ovarian bursal injection of adenovirus expressing Cre recombinase (AdCre). C) Rapamycin inhibits tumor growth. Animals with small ovarian carcinomas (6 wks after AdCre injection) were treated twice weekly for 4 wks with vehicle or two different doses of rapamycin. D) Representative ovarian carcinoma in right ovary after 4 wks of treatment with rapamycin.
It is expected that mouse models of ovarian cancer will yield new insights into ovarian cancer pathogenesis, particularly with respect to putative precursor lesions and mechanisms by which tumors develop and progress. In addition, mouse models that faithfully recapitulate their human tumor counterparts may prove especially useful for pre-clinical testing of novel therapeutics that target specific molecular defects in the tumor cells. Proof-of-principle experiments have been performed that support this latter goal. As described above, mice with conditional deregulation of canonical Wnt and PI3K/Pten signaling due to Apc and Pten inactivation, respectively, consistently develop endometrioid-like carcinomas. Rapamycin is well known as an inhibitor of mTOR, a downstream effector of activated Akt (Figure 4) (102, 133). Moreover, activation of canonical Wnt signaling has also been shown to up-regulate mTOR without involving β-catenin-dependent transcription (134). A number of clinical trials have examined effects of rapamycin and structurally related compounds on several different types of human cancers and shown efficacy in some studies (135). Intrabursal injection of AdCre was used to induce small tumors in the ovaries of Apcflox/flox;Ptenflox/flox mice, and then animals were treated for one month with rapamycin. When compared to animals treated with vehicle alone, the rapamycin-treated animals showed marked and statistically significant inhibition of tumor growth (KR Cho laboratory, Figure 5C, 5D). GEM models such as the ones described above, can be further developed to allow monitoring of ovarian tumor development and progression in live animals. The ability to non-invasively and quantitatively image ovarian tumors in live animals would significantly enhance our understanding of the pharmacokinetics and bioavailability of specific drugs, would provide a powerful tool to validate drug-target interactions of new compounds, and would aid in designing the most rational and successful combination therapies for evaluation in future clinical trials. The aforementioned GEM model of ovarian endometrioid carcinomas has been further engineered to include a conditional luciferase reporter allele. Kaelin and colleagues generated a Cre-inducible form of firefly luciferase knocked into the ubiquitously expressed ROSA26 locus (136). These mice were crossed with Apcflox/flox;Ptenflox/flox mice to generate Apcflox/flox;Ptenflox/flox;ROSA26L-S-L-Luc/+ mice. After AdCre injection, ovarian tumor growth can be monitored longitudinally over time in living animals (KR Cho laboratory, unpublished data). Animal models such as this are expected to accelerate the translation of promising new therapies from the laboratory to the clinic.
NEW PROPOSED CLASSIFICATION OF OVARIAN EPITHELIAL TUMORS
The collective analyses of the clinicopathological and molecular features of the major types of ovarian carcinomas described above have yielded some unifying themes which provide the basis for a proposed model of ovarian carcinoma development and progression. It has been proposed that surface epithelial tumors can be divided into two broad categories designated Type I and Type II tumors based on their pattern of tumor progression and molecular genetic changes (137, 138). In this model, Type I and Type II refer to tumorigenic pathways and are not specific histopathologic terms (Table 1). The division of serous carcinomas between Type I and Type II categories is illustrated in Figure 6. Type I tumors include low-grade serous carcinoma, low-grade endometrioid carcinoma, mucinous carcinoma, and a subset of clear cell carcinomas, which develop in a stepwise fashion from well-recognized precursors, in most cases, borderline tumors. The borderline tumors, in turn, appear to develop from the ovarian surface epithelium or inclusion cysts in the case of serous and mucinous tumors and from endometriosis in the case of endometrioid and clear cell tumors. For these tumors, the stepwise progression of borderline tumor to carcinoma closely approximates the well characterized “adenoma-carcinoma” sequence in colorectal carcinoma. In general, most Type I tumors are slow growing as evidenced by the observation that they are generally large and often confined to the ovary at diagnosis. In contrast, the Type II tumors are high-grade and almost always have spread beyond the ovaries at presentation. Type II carcinomas include high-grade serous carcinoma, high-grade endometrioid carcinoma, undifferentiated carcinoma, probably some clear cell carcinomas, and malignant mixed mesodermal tumor (carcinosarcoma). Other than their association with endometriosis which is in keeping with the Type I pathway, the clinicopathologic and molecular features allowing distinction of Type I from Type II clear cell carcinomas are yet to be defined. Type II carcinomas presumably evolve rapidly, disseminate early in their clinical course and are highly aggressive not only because of the malignant nature of the cancer cells but also because the anatomic locations in which they arise (i.e., ovary or perhaps fallopian tube) provide immediate access to adjacent pelvic organs and the peritoneal cavity. In contrast to Type I tumors, Type II tumors are rarely associated with morphologically recognizable precursor lesions; however, precursor lesions of the Type II tumors may arise from “dysplasia” in inclusion cysts or serous intraepithelial carcinoma in the fallopian tubes (6, 139, 140). These precursor lesions may be difficult to recognize because they presumably undergo rapid transit from the occult lesion to a clinically diagnosed carcinoma. Type I and Type II tumors have very different molecular profiles. Chromosomal instability levels, as reflected by genome-wide changes in DNA copy number, are much higher in Type II tumors than in Type I tumors. Type I tumors often harbor somatic mutations of genes encoding protein kinases including KRAS, BRAF, PIK3CA and ERRB2, and other signaling molecules including CTNNB1 and PTEN. In contrast, Type II tumors generally lack these mutations but are characterized by a high frequency of TP53 mutations which are rare in Type I tumors. The division of ovarian cancer into two broad groups, Type I and Type II, continues to emphasize the heterogeneity of ovarian cancers, but also provides a morphological and molecular framework for future studies aimed at improving our understanding of ovarian cancer pathogenesis. This, in turn, will have significant implications for improving the detection and treatment of ovarian cancer as discussed in the concluding section. This simplified classification system will undoubtedly continue to evolve, particularly as the molecular changes that characterize the less common types of ovarian carcinomas (i.e., mucinous, clear cell, undifferentiated) become more clearly defined.
Table 1.
Common Precursor Lesions and Molecular Features of Type I and Type II Carcinomas
| Type I tumors | Common Precursors | Most Frequent Mutations | Chromosomal Instability (CIN)a |
|---|---|---|---|
| Low-grade serous CAb | Serous borderline tumor | KRAS, BRAF | Low |
| Low-grade endometrioid CA | Endometriosis | CTNNB1, PTEN | Low |
| Most clear cell CAc | Endometriosis | PIK3CA | Low |
| Mucinous CA | Mucinous borderline tumor | KRAS | Low |
| Type II tumors | |||
| High-grade serous CA | Not recognizedd | TP53 | High |
| High-grade endometrioid CA | Not recognized | TP53 | High |
| Clear cell CAc | Not recognized | ? | ? |
| Undifferentiated CA | Not recognized | ? | ? |
| Carcinosarcoma | Not recognized | TP53 | ? |
Low vs. High chromosomal instability (CIN) refers to comparison between low-grade and high-grade carcinomas within same histologic type
CA: Carcinoma
Criteria for classification of clear cell CA subsets into Type I vs. Type II categories are uncertain. It is thought that most clear cell carcinomas behave like Type I tumors while some clear cell carcinomas, presumably high-grade, may be Type II tumors.
Some high-grade serous CAs may be associated with tubal intraepithelial serous carcinoma
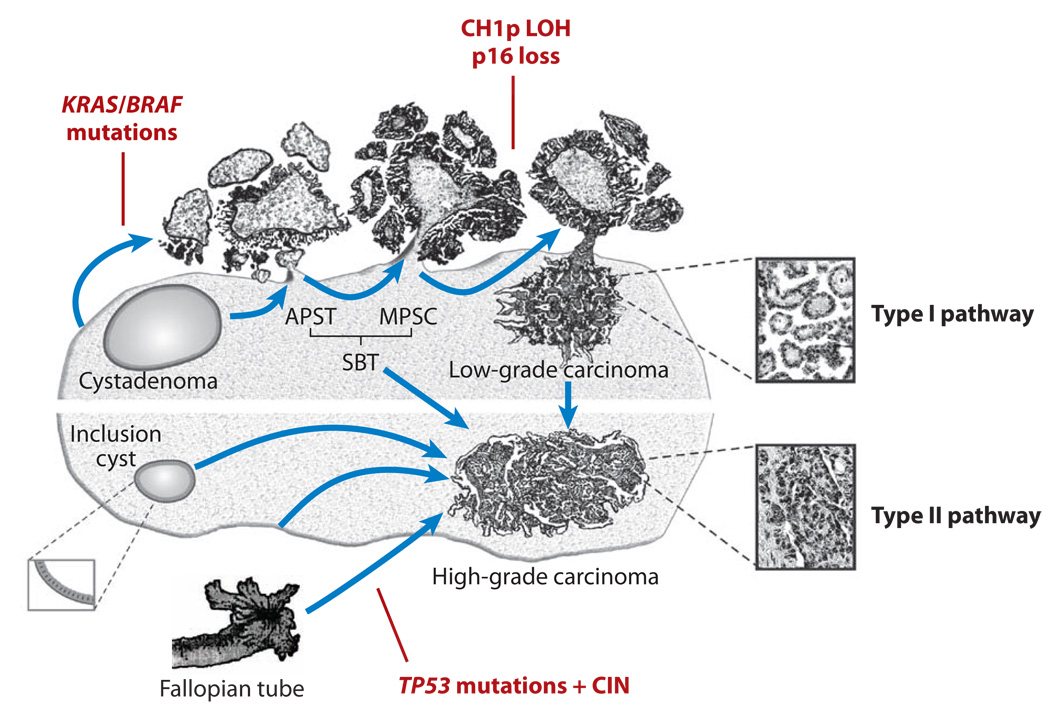
Figure 6. Schematic illustration of Type I and Type II ovarian serous carcinoma pathogenesis.
Development of ovarian high-grade and low-grade serous carcinomas involves two distinct pathways. Low-grade (Type I) carcinomas arise from serous borderline tumors (SBTs), which in turn develop from serous cystadenomas. This stepwise tumor progression in the low-grade pathway contrasts with the rapid progression pathway of high-grade (Type II) carcinomas, for which precursor lesions are not well recognized. High-grade carcinomas may arise from ovarian surface inclusions, peritoneal mesothelium or the distal portion (fimbriae) of the fallopian tube. These carcinomas disseminate to pelvic and peritoneal organs early in their progression.
IMPLICATIONS FOR EARLY DETECTION, DIAGNOSIS AND TREATMENT
A currently held view of ovarian tumorigenesis is that carcinoma begins in the ovary (or for some serous carcinomas, in the distal fallopian tube), undergoes sequential stages of tumor progression in situ, and then spreads to the pelvic and abdominal cavities before metastasizing to more distant sites. Based on this view, advances in early detection strategies to identify ovarian cancer precursor lesions or early stage carcinomas should theoretically have a major impact on mortality and survival in patients. However, findings from many of the studies reviewed above challenge this paradigm and suggest that new insights and approaches should be taken into account in developing novel detection techniques and therapeutic interventions for ovarian cancer. The new paradigm and approaches for early detection and treatment of ovarian cancer have recently been reviewed by Kurman and colleagues (141).
First, the focus on detecting early stage disease should perhaps be shifted to detection of “low volume” disease. Current screening methods aimed at detecting stage I disease have not been effective as no prospective, randomized trials of any ovarian cancer screening test(s) known so far have led to a decrease in mortality. Tumors that are amenable to current strategies for early detection are usually those belonging to the Type I group, which remain confined to the ovary for a considerable period of time and often present as large tumor masses. Unfortunately, these account only for a minority (<25%) of malignant ovarian epithelial tumors. The majority of what is considered “ovarian cancer” belongs to the Type II category. These tumors are rarely confined to the ovary at diagnosis, presumably because they evolve rapidly and spread to extraovarian sites early in their development. Moreover, as noted earlier, a significant number of Type II “ovarian carcinomas”, especially the high-grade serous carcinomas, may develop from the peritoneum and fallopian tube, and involve the ovary secondarily (140). By definition, such tumors are advanced stage early in the course of their progression. Indeed, when thorough staging procedures are performed, stage I Type II tumors are rare (13). A more useful endpoint for the early detection of ovarian carcinoma may thus be tumor volume and not stage of disease. Perhaps the identification of stage III or IV tumors before the onset of symptoms would significantly improve the clinical outcome for patients who would otherwise be diagnosed with bulky disease. For Type II ovarian carcinomas, it is well recognized that the most important prognostic indicator is not stage, but the volume of residual disease following cytoreductive surgery (142, 143). The smaller the tumor volume at diagnosis, the more effective chemotherapy is likely to be. Because mutations of TP53 are currently the most common molecular genetic change in Type II tumors (36), it is possible that tumor DNA containing mutant TP53 DNA or polypeptides released from these tumors can be detected in body fluids. Accordingly, tests that detect mutant TP53 or autoantibodies that react to mutant p53 protein in the blood could prove to be very useful for detecting small volume disease in high-risk patients.
The readily recognizable aberrations of specific signaling pathways in many Type I tumors including low-grade serous carcinomas, low-grade endometrioid carcinomas and mucinous carcinomas suggest that inhibitors targeting specific components of these pathways may be effective for treating patients with advanced stage disease that is refractory to current chemotherapy. For example, MEK inhibitors such as CI-1040 and Quercetin from red wine extract deserve consideration as therapeutic agents for patients with low-grade serous carcinomas (144). This notion is supported by studies of Pohl et al., who demonstrated that treatment of ovarian serous carcinoma cells harboring KRAS or BRAF mutations with a MEK inhibitor, CI-1040, resulted in significant growth inhibition in vitro as compared to tumor cells with wild-type KRAS and BRAF (47). Since low-grade serous carcinoma and its presumptive precursor SBT have a high frequency of mutations in KRAS and BRAF, future clinical trials should help to determine if MEK inhibitors can prolong disease-free interval and overall survival in patients with advanced stage low-grade serous carcinomas. Similarly, the observation that growth of murine ovarian carcinomas with PI3K/Pten signaling pathway defects is profoundly inhibited by drugs that target this pathway provides further support for testing such agents in women with disseminated low grade (Type I) endometrioid carcinomas.
Finally, the high level of genetic instability that characterizes the majority of ovarian epithelial cancers (i.e., Type II tumors), presents a tremendous challenge with respect to the development of effective screening programs and therapeutic strategies that target the particular molecular defects present in a given patient’s tumor. The lack of shared aberrations of cellular signaling pathways (except those mediated by p53) in high-grade serous and endometrioid carcinomas makes it difficult to design target-based therapies for these tumors at present. Clearly, a major goal of ongoing work in the field is to identify additional “hallmark” genetic alterations that characterize the Type II tumors. As our understanding of the molecular pathogenesis of each major type of ovarian carcinoma improves, the design of “personalized” therapeutic regimens based on the particular alterations present in a given patient’s tumor may become a reality. It is reasonable to hope that drugs targeting specific molecular defects in tumor cells could be used alone or in combination with existing treatment modalities to substantially improve the clinical outcome for women with ovarian cancer.
SUMMARY POINTS
Ovarian carcinomas are a heterogeneous group of neoplasms based on their clinical, histopathological, and molecular features. Ovarian cancer research should take this into account.
Although pathologists currently employ a morphology-based classification system for ovarian carcinomas, a simplified classification that incorporates both clinicopathological and molecular features has been proposed. This simplified classification system separates ovarian carcinomas into two broad categories (Type I and Type II) based on their pattern of clinical behavior, tumor progression and molecular genetic alterations.
Serous carcinomas comprise the majority of ovarian carcinomas, and most serous carcinomas are high grade and harbor TP53 mutations (Type II).
Low grade serous and endometrioid carcinomas (both Type I) progress in a stepwise fashion from well defined precursor lesions and usually harbor gene mutations predicted to deregulate specific cell signaling pathways. Drugs that target these pathways may prove useful for treating women with disseminated Type I tumors.
The high level of chromosomal instability and relative paucity of known “hallmark” sequence mutations (other than TP53) that characterize the majority of ovarian carcinomas present a tremendous challenge with respect to developing effective screening programs or novel therapeutic strategies that target specific molecular defects in a given patient’s tumor.
Current screening methods aimed at detecting stage I disease have been generally disappointing. Tumors that are amenable to current strategies for early detection are usually those belonging to the Type I group. The more common and aggressive Type II tumors rarely present as early-stage disease. Thus, the focus on detecting early stage disease should perhaps be shifted to detection of “low volume” disease in Type II carcinomas.
Genetically engineered mouse models of each major type of ovarian carcinoma are being developed. Those that most closely mimic the molecular features and biological behavior of their human counterparts will likely prove useful for improving our understanding of ovarian cancer pathogenesis and for pre-clinical testing of molecularly targeted therapeutics.
Glossary
IMPORTANT ACRONYMS
- aCGH
array comparative genomic hybridization
- AdCre
adenovirus expressing Cre recombinase
- FIGO
International Federation of Gynecology and Obstetrics
- FISH
fluorescence in situ hybridization
- GEM
genetically engineered mouse
- MOSE
murine ovarian surface epithelium
- OSE
ovarian surface epithelium
- SBT
serous borderline tumor, also known as serous tumor of low malignant potential or atypical proliferative serous tumor
- SNP
single nucleotide polymorphism
MINI-GLOSSARY KEY TERMS
- Müllerian system
includes organs derived from the Müllerian ducts during embryogenesis (fallopian tubes, uterus, and vagina)
- Endometriosis
endometrial tissue outside of the endometrium and myometrium
- Chromosomal instability
increased tendency to acquire chromosomal aberrations when chromosome replication, repair, or segregation are dysfunctional
- Microsatellite instability
increased tendency to acquire alterations in the number of microsatellite repeats (short, repeated DNA sequences) when DNA mismatch repair is dysfunctional
- Conditional (floxed) alleles
alleles with strategically located loxP recognition sequences allowing deletion of DNA between flanking loxP (flox) sites when Cre recombinase is expressed
- DNA copy number change
somatic increase or decrease of tumor DNA dosage in defined regions of chromosomes compared to normal DNA in the same regions
LITERATURE CITED
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Feeley KM, Wells M. Precursor lesions of ovarian epithelial malignancy. Histopathology. 2001;38:87–95. doi: 10.1046/j.1365-2559.2001.01042.x. [DOI] [PubMed] [Google Scholar]
- 3.Bell DA. Origins and molecular pathology of ovarian cancer. Mod Pathol. 2005;18 Suppl 2:S19–S32. doi: 10.1038/modpathol.3800306. [DOI] [PubMed] [Google Scholar]
- 4.Dubeau L. The cell of origin of ovarian epithelial tumors and the ovarian surface epithelium dogma: does the emperor have no clothes? Gynecol Oncol. 1999;72:437–442. doi: 10.1006/gyno.1998.5275. [DOI] [PubMed] [Google Scholar]
- 5.Kurman RJ. Blaustein's pathology of the female genital tract. 5th ed. New York: Springer-Verlag; 2002. [Google Scholar]
- 6.Kindelberger DW, Lee Y, Miron A, Hirsch MS, Feltmate C, Medeiros F, Callahan MJ, Garner EO, Gordon RW, Birch C, Berkowitz RS, Muto MG, Crum CP. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–169. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 7.Crum CP, Drapkin R, Kindelberger D, Medeiros F, Miron A, Lee Y. Lessons from BRCA: the tubal fimbria emerges as an origin for pelvic serous cancer. Clin Med Res. 2007;5:35–44. doi: 10.3121/cmr.2007.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silverberg SG. Histopathologic grading of ovarian carcinoma: a review and proposal. Int J Gynecol Pathol. 2000;19:7–15. doi: 10.1097/00004347-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu Y, Kamoi S, Amada S, Akiyama F, Silverberg SG. Toward the development of a universal grading system for ovarian epithelial carcinoma: testing of a proposed system in a series of 461 patients with uniform treatment and follow-up. Cancer. 1998;82:893–901. doi: 10.1002/(sici)1097-0142(19980301)82:5<893::aid-cncr14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 10.Malpica A, Deavers MT, Lu K, Bodurka DC, Atkinson EN, Gershenson DM, Silva EG. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol. 2004;28:496–504. doi: 10.1097/00000478-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Seidman JD, Horkayne-Szakaly I, Cosin JA, Ryu HS, Haiba M, Boice CR, Yemelyanova AV. Testing of two binary grading systems for FIGO stage III serous carcinoma of the ovary and peritoneum. Gynecol Oncol. 2006;103:703–708. doi: 10.1016/j.ygyno.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 12.Di Cristofano A, Ellenson LH. Endometrial Carcinoma. Annu Rev Pathol. 2007;2:57–85. doi: 10.1146/annurev.pathol.2.010506.091905. [DOI] [PubMed] [Google Scholar]
- 13.Seidman JD, Horkayne-Szakaly I, Haiba M, Boice CR, Kurman RJ, Ronnett BM. The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int J Gynecol Pathol. 2004;23:41–44. doi: 10.1097/01.pgp.0000101080.35393.16. [DOI] [PubMed] [Google Scholar]
- 14.Tammela J, Geisler JP, Eskew PN, Jr, Geisler HE. Clear cell carcinoma of the ovary: poor prognosis compared to serous carcinoma. Eur J Gynaecol Oncol. 1998;19:438–440. [PubMed] [Google Scholar]
- 15.Sugiyama T, Kamura T, Kigawa J, Terakawa N, Kikuchi Y, Kita T, Suzuki M, Sato I, Taguchi K. Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer. 2000;88:2584–2589. [PubMed] [Google Scholar]
- 16.Hart WR. Mucinous tumors of the ovary: a review. Int J Gynecol Pathol. 2005;24:4–25. [PubMed] [Google Scholar]
- 17.Wood LD, Parsons DW, Jones S, Lin J, Sjoblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J, Silliman N, Szabo S, Dezso Z, Ustyanksky V, Nikolskaya T, Nikolsky Y, Karchin R, Wilson PA, Kaminker JS, Zhang Z, Croshaw R, Willis J, Dawson D, Shipitsin M, Willson JK, Sukumar S, Polyak K, Park BH, Pethiyagoda CL, Pant PV, Ballinger DG, Sparks AB, Hartigan J, Smith DR, Suh E, Papadopoulos N, Buckhaults P, Markowitz SD, Parmigiani G, Kinzler KW, Velculescu VE, Vogelstein B. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Shen D, Parsons DW, Bardelli A, Sager J, Szabo S, Ptak J, Silliman N, Peters BA, van der Heijden MS, Parmigiani G, Yan H, Wang TL, Riggins G, Powell SM, Willson JK, Markowitz S, Kinzler KW, Vogelstein B, Velculescu VE. Mutational analysis of the tyrosine phosphatome in colorectal cancers. Science. 2004;304:1164–1166. doi: 10.1126/science.1096096. [DOI] [PubMed] [Google Scholar]
- 19.Wang TL, Maierhofer C, Speicher MR, Lengauer C, Vogelstein B, Kinzler KW, Velculescu VE. Digital karyotyping. Proc Natl Acad Sci U S A. 2002;99:16156–16161. doi: 10.1073/pnas.202610899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leary RJ, Cummins J, Wang TL, Velculescu VE. Digital karyotyping. Nat Protoc. 2007;2:1973–1986. doi: 10.1038/nprot.2007.276. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz DR, Kardia SL, Shedden KA, Kuick R, Michailidis G, Taylor JM, Misek DE, Wu R, Zhai Y, Darrah DM, Reed H, Ellenson LH, Giordano TJ, Fearon ER, Hanash SM, Cho KR. Gene expression in ovarian cancer reflects both morphology and biological behavior, distinguishing clear cell from other poor-prognosis ovarian carcinomas. Cancer Res. 2002;62:4722–4729. [PubMed] [Google Scholar]
- 22.Schaner ME, Ross DT, Ciaravino G, Sorlie T, Troyanskaya O, Diehn M, Wang YC, Duran GE, Sikic TL, Caldeira S, Skomedal H, Tu IP, Hernandez-Boussard T, Johnson SW, O'Dwyer PJ, Fero MJ, Kristensen GB, Borresen-Dale AL, Hastie T, Tibshirani R, van de Rijn M, Teng NN, Longacre TA, Botstein D, Brown PO, Sikic BI. Gene expression patterns in ovarian carcinomas. Mol Biol Cell. 2003;14:4376–4386. doi: 10.1091/mbc.E03-05-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marquez RT, Baggerly KA, Patterson AP, Liu J, Broaddus R, Frumovitz M, Atkinson EN, Smith DI, Hartmann L, Fishman D, Berchuck A, Whitaker R, Gershenson DM, Mills GB, Bast RC, Jr, Lu KH. Patterns of gene expression in different histotypes of epithelial ovarian cancer correlate with those in normal fallopian tube, endometrium, and colon. Clin Cancer Res. 2005;11:6116–6126. doi: 10.1158/1078-0432.CCR-04-2509. [DOI] [PubMed] [Google Scholar]
- 24.Wu R, Hendrix-Lucas N, Kuick R, Zhai Y, Schwartz DR, Akyol A, Hanash S, Misek DM, Katabuchi H, Williams BO, Fearon ER, Cho KR. Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/B-catenin and PI3K/Pten signaling pathways. Cancer Cell. 2007;11:321–333. doi: 10.1016/j.ccr.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Vang R, Shih IeM, Salani R, Sugar E, Ayhan A, Kurman RJ. Subdividing ovarian and peritoneal serous carcinoma into moderately- and poorly-differentiated does not have biologic validity based on molecular genetic and in vitro drug resistance data. Am J Surg Pathol. 2008 doi: 10.1097/PAS.0b013e31816fd555. (in press) [DOI] [PubMed] [Google Scholar]
- 26.Singer G, Stohr R, Cope L, Dehari R, Hartmann A, Cao DF, Wang TL, Kurman RJ, Shih IeM. Patterns of p53 mutations separate ovarian serous borderline tumors and low- and high-grade carcinomas and provide support for a new model of ovarian carcinogenesis: a mutational analysis with immunohistochemical correlation. Am J Surg Pathol. 2005;29:218–224. doi: 10.1097/01.pas.0000146025.91953.8d. [DOI] [PubMed] [Google Scholar]
- 27.Willner J, Wurz K, Allison KH, Galic V, Garcia RL, Goff BA, Swisher EM. Alternate molecular genetic pathways in ovarian carcinomas of common histological types. Hum Pathol. 2007 doi: 10.1016/j.humpath.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Singer G, Oldt R, 3rd, Cohen Y, Wang BG, Sidransky D, Kurman RJ, Shih IeM. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst. 2003;95:484–486. doi: 10.1093/jnci/95.6.484. [DOI] [PubMed] [Google Scholar]
- 29.Merajver SD, Pham TM, Caduff RF, Chen M, Poy EL, Cooney KA, Weber BL, Collins FS, Johnston CM, Frank TS. Somatic mutations in the BRCA1 gene in sporadic ovarian tumours. Nat.Genet. 1995;9:439–443. doi: 10.1038/ng0495-439. [DOI] [PubMed] [Google Scholar]
- 30.Nakayama K, Nakayama N, Kurman RJ, Cope L, Pohl G, Samuels Y, Velculescu VE, Wang TL, Shih IeM. Sequence mutations and amplification of PIK3CA and AKT2 genes in purified ovarian serous neoplasms. Cancer Biol Ther. 2006;5:779–785. doi: 10.4161/cbt.5.7.2751. [DOI] [PubMed] [Google Scholar]
- 31.Afify AM, Werness BA, Mark HF. HER-2/neu oncogene amplification in stage I and stage III ovarian papillary serous carcinoma. Exp Mol Pathol. 1999;66:163–169. doi: 10.1006/exmp.1999.2255. [DOI] [PubMed] [Google Scholar]
- 32.Nakayama K, Nakayama N, Jinawath N, Salani R, Kurman RJ, Shih IeM, Wang TL. Amplicon profiles in ovarian serous carcinomas. Int J Cancer. 2007;120:2613–2617. doi: 10.1002/ijc.22609. [DOI] [PubMed] [Google Scholar]
- 33.Kupryjanczyk J, Thor AD, Beauchamp R, Merritt V, Edgerton SM, Bell DA, Yandell DW. p53 gene mutations and protein accumulation in human ovarian cancer. Proc Natl Acad Sci U S A. 1993;90:4961–4965. doi: 10.1073/pnas.90.11.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wen WH, Reles A, Runnebaum IB, Sullivan-Halley J, Bernstein L, Jones LA, Felix JC, Kreienberg R, el-Naggar A, Press MF. p53 mutations and expression in ovarian cancers: correlation with overall survival. Int J Gynecol Pathol. 1999;18:29–41. doi: 10.1097/00004347-199901000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Chan WY, Cheung KK, Schorge JO, Huang LW, Welch WR, Bell DA, Berkowitz RS, Mok SC. Bcl-2 and p53 protein expression, apoptosis, and p53 mutation in human epithelial ovarian cancers. Am J Pathol. 2000;156:409–417. doi: 10.1016/S0002-9440(10)64744-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salani R, Kurman RJ, Giuntoli R, 2nd, Gardner G, Bristow R, Wang TL, Shih IM. Assessment of TP53 mutation using purified tissue samples of ovarian serous carcinomas reveals a higher mutation rate than previously reported and does not correlate with drug resistance. Int J Gynecol Cancer. 2007 doi: 10.1111/j.1525-1438.2007.01039.x. [DOI] [PubMed] [Google Scholar]
- 37.Leitao MM, Soslow RA, Baergen RN, Olvera N, Arroyo C, Boyd J. Mutation and expression of the TP53 gene in early stage epithelial ovarian carcinoma. Gynecol Oncol. 2004;93:301–306. doi: 10.1016/j.ygyno.2004.01.043. [DOI] [PubMed] [Google Scholar]
- 38.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 39.Mok SC, Bell DA, Knapp RC, Fishbaugh PM, Welch WR, Muto MG, Berkowitz RS, Tsao SW. Mutation of K-ras protooncogene in human ovarian epithelial tumors of borderline malignancy. Cancer Res. 1993;53:1489–1492. [PubMed] [Google Scholar]
- 40.Sieben NL, Macropoulos P, Roemen GM, Kolkman-Uljee SM, Jan Fleuren G, Houmadi R, Diss T, Warren B, Al Adnani M, De Goeij AP, Krausz T, Flanagan AM. In ovarian neoplasms, BRAF, but not KRAS, mutations are restricted to low-grade serous tumours. J Pathol. 2004;202:336–340. doi: 10.1002/path.1521. [DOI] [PubMed] [Google Scholar]
- 41.Mayr D, Hirschmann A, Lohrs U, Diebold J. KRAS and BRAF mutations in ovarian tumors: A comprehensive study of invasive carcinomas, borderline tumors and extraovarian implants. Gynecol Oncol. 2006;103:883–887. doi: 10.1016/j.ygyno.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 42.Ueda M, Toji E, Noda S. Germ line and somatic mutations of BRAF V599E in ovarian carcinoma. Int J Gynecol Cancer. 2007;17:794–797. doi: 10.1111/j.1525-1438.2006.00853.x. [DOI] [PubMed] [Google Scholar]
- 43.Wang SE, Narasanna A, Perez-Torres M, Xiang B, Wu FY, Yang S, Carpenter G, Gazdar AF, Muthuswamy SK, Arteaga CL. HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell. 2006;10:25–38. doi: 10.1016/j.ccr.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 44.Cheng EJ, Kurman RJ, Wang M, Oldt R, Wang BG, Berman DM, Shih IeM. Molecular genetic analysis of ovarian serous cystadenomas. Lab Invest. 2004;84:778–784. doi: 10.1038/labinvest.3700103. [DOI] [PubMed] [Google Scholar]
- 45.Ho CL, Kurman RJ, Dehari R, Wang TL, Shih IeM. Mutations of BRAF and KRAS precede the development of ovarian serous borderline tumors. Cancer Res. 2004;64:6915–6918. doi: 10.1158/0008-5472.CAN-04-2067. [DOI] [PubMed] [Google Scholar]
- 46.Hsu CY, Bristow R, Cha MS, Wang BG, Ho CL, Kurman RJ, Wang TL, Shih IeM. Characterization of active mitogen-activated protein kinase in ovarian serous carcinomas. Clin Cancer Res. 2004;10:6432–6436. doi: 10.1158/1078-0432.CCR-04-0893. [DOI] [PubMed] [Google Scholar]
- 47.Pohl G, Ho CL, Kurman RJ, Bristow R, Wang TL, Shih IeM. Inactivation of the mitogen-activated protein kinase pathway as a potential target-based therapy in ovarian serous tumors with KRAS or BRAF mutations. Cancer Res. 2005;65:1994–2000. doi: 10.1158/0008-5472.CAN-04-3625. [DOI] [PubMed] [Google Scholar]
- 48.Allen LF, Sebolt-Leopold J, Meyer MB. CI-1040 ( PD184352), a targeted signal transduction inhibitor of MEK (MAPKK) Semin Oncol. 2003;30:105–116. doi: 10.1053/j.seminoncol.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 49.Worsley SD, Ponder BA, Davies BR. Overexpression of cyclin D1 in epithelial ovarian cancers. Gynecol Oncol. 1997;64:189–195. doi: 10.1006/gyno.1996.4569. [DOI] [PubMed] [Google Scholar]
- 50.Sui L, Tokuda M, Ohno M, Hatase O, Hando T. The concurrent expression of p27(kip1) and cyclin D1 in epithelial ovarian tumors. Gynecol Oncol. 1999;73:202–209. doi: 10.1006/gyno.1999.5373. [DOI] [PubMed] [Google Scholar]
- 51.Gilks CB. Subclassification of ovarian surface epithelial tumors based on correlation of histologic and molecular pathologic data. Int J Gynecol Pathol. 2004;23:200–205. doi: 10.1097/01.pgp.0000130446.84670.93. [DOI] [PubMed] [Google Scholar]
- 52.Michaloglou C, Vredeveld LC, Mooi WJ, Peeper DS. BRAF(E600) in benign and malignant human tumours. Oncogene. 2008;27:877–895. doi: 10.1038/sj.onc.1210704. [DOI] [PubMed] [Google Scholar]
- 53.Davidson B, Givant-Horwitz V, Lazarovici P, Risberg B, Nesland JM, Trope CG, Schaefer E, Reich R. Matrix metalloproteinases (MMP), EMMPRIN (extracellular matrix metalloproteinase inducer) and mitogen-activated protein kinases (MAPK): co-expression in metastatic serous ovarian carcinoma. Clin Exp Metastasis. 2003;20:621–631. doi: 10.1023/a:1027347932543. [DOI] [PubMed] [Google Scholar]
- 54.Schraml P, Schwerdtfeger G, Burkhalter F, Raggi A, Schmidt D, Ruffalo T, King W, Wilber K, Mihatsch MJ, Moch H. Combined array comparative genomic hybridization and tissue microarray analysis suggest PAK1 at 11q13.5-q14 as a critical oncogene target in ovarian carcinoma. Am J Pathol. 2003;163:985–992. doi: 10.1016/S0002-9440(10)63458-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meinhold-Heerlein I, Bauerschlag D, Hilpert F, Dimitrov P, Sapinoso LM, Orlowska-Volk M, Bauknecht T, Park TW, Jonat W, Jacobsen A, Sehouli J, Luttges J, Krajewski M, Krajewski S, Reed JC, Arnold N, Hampton GM. Molecular and prognostic distinction between serous ovarian carcinomas of varying grade and malignant potential. Oncogene. 2005;24:1053–1065. doi: 10.1038/sj.onc.1208298. [DOI] [PubMed] [Google Scholar]
- 56.Mayr D, Kanitz V, Anderegg B, Luthardt B, Engel J, Lohrs U, Amann G, Diebold J. Analysis of gene amplification and prognostic markers in ovarian cancer using comparative genomic hybridization for microarrays and immunohistochemical analysis for tissue microarrays. Am J Clin Pathol. 2006;126:101–109. doi: 10.1309/n6x5mb24bp42kp20. [DOI] [PubMed] [Google Scholar]
- 57.Nowee ME, Snijders AM, Rockx DA, de Wit RM, Kosma VM, Hamalainen K, Schouten JP, Verheijen RH, van Diest PJ, Albertson DG, Dorsman JC. DNA profiling of primary serous ovarian and fallopian tube carcinomas with array comparative genomic hybridization and multiplex ligation-dependent probe amplification. J Pathol. 2007;213:46–55. doi: 10.1002/path.2217. [DOI] [PubMed] [Google Scholar]
- 58.Arboleda MJ, Lyons JF, Kabbinavar FF, Bray MR, Snow BE, Ayala R, Danino M, Karlan BY, Slamon DJ. Overexpression of AKT2/protein kinase Bbeta leads to up-regulation of beta1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer Res. 2003;63:196–206. [PubMed] [Google Scholar]
- 59.Dan HC, Sun M, Kaneko S, Feldman RI, Nicosia SV, Wang HG, Tsang BK, Cheng JQ. Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP) J Biol Chem. 2004;279:5405–5412. doi: 10.1074/jbc.M312044200. [DOI] [PubMed] [Google Scholar]
- 60.Meng Q, Xia C, Fang J, Rojanasakul Y, Jiang BH. Role of PI3K and AKT specific isoforms in ovarian cancer cell migration, invasion and proliferation through the p70S6K1 pathway. Cell Signal. 2006;18:2262–2271. doi: 10.1016/j.cellsig.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 61.Bortner DM, Rosenberg MP. Induction of mammary gland hyperplasia and carcinomas in transgenic mice expressing human cyclin E. Mol Cell Biol. 1997;17:453–459. doi: 10.1128/mcb.17.1.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bedrosian I, Lu KH, Verschraegen C, Keyomarsi K. Cyclin E deregulation alters the biologic properties of ovarian cancer cells. Oncogene. 2004;23:2648–2657. doi: 10.1038/sj.onc.1207408. [DOI] [PubMed] [Google Scholar]
- 63.Spruck CH, Won KA, Reed SI. Deregulated cyclin E induces chromosome instability. Nature. 1999;401:297–300. doi: 10.1038/45836. [DOI] [PubMed] [Google Scholar]
- 64.Minella AC, Swanger J, Bryant E, Welcker M, Hwang H, Clurman BE. p53 and p21 form an inducible barrier that protects cells against cyclin E-cdk2 deregulation. Curr Biol. 2002;12:1817–1827. doi: 10.1016/s0960-9822(02)01225-3. [DOI] [PubMed] [Google Scholar]
- 65.Farley J, Smith LM, Darcy KM, Sobel E, O'Connor D, Henderson B, Morrison LE, Birrer MJ. Cyclin E expression is a significant predictor of survival in advanced, suboptimally debulked ovarian epithelial cancers: a Gynecologic Oncology Group study. Cancer Res. 2003;63:1235–1241. [PubMed] [Google Scholar]
- 66.Davidson B, Skrede M, Silins I, Shih IeM, Trope CG, Florenes VA. Low-molecular weight forms of cyclin E differentiate ovarian carcinoma from cells of mesothelial origin and are associated with poor survival in ovarian carcinoma. Cancer. 2007;110:1264–1271. doi: 10.1002/cncr.22918. [DOI] [PubMed] [Google Scholar]
- 67.Shih IeM, Sheu JJ, Santillan A, Nakayama K, Yen MJ, Bristow RE, Vang R, Parmigiani G, Kurman RJ, Trope CG, Davidson B, Wang TL. Amplification of a chromatin remodeling gene, Rsf-1/HBXAP, in ovarian carcinoma. Proc Natl Acad Sci U S A. 2005;102:14004–14009. doi: 10.1073/pnas.0504195102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park JT, Li M, Nakayama K, Mao TL, Davidson B, Zhang Z, Kurman RJ, Eberhart CG, Shih IeM, Wang TL. Notch3 gene amplification in ovarian cancer. Cancer Res. 2006;66:6312–6318. doi: 10.1158/0008-5472.CAN-05-3610. [DOI] [PubMed] [Google Scholar]
- 69.Okawa ER, Gotoh T, Manne J, Igarashi J, Fujita T, Silverman KA, Xhao H, Mosse YP, White PS, Brodeur GM. Expression and sequence analysis of candidates for the 1p36.31 tumor suppressor gene deleted in neuroblastomas. Oncogene. 2008;27:803–810. doi: 10.1038/sj.onc.1210675. [DOI] [PubMed] [Google Scholar]
- 70.Bagchi A, Papazoglu C, Wu Y, Capurso D, Brodt M, Francis D, Bredel M, Vogel H, Mills AA. CHD5 is a tumor suppressor at human 1p36. Cell. 2007;128:459–475. doi: 10.1016/j.cell.2006.11.052. [DOI] [PubMed] [Google Scholar]
- 71.Bommer GT, Gerin I, Feng Y, Kaczorowski AJ, Kuick R, Love RE, Zhai Y, Giordano TJ, Qin ZS, Moore BB, MacDougald OA, Cho KR, Fearon ER. p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol. 2007;17:1298–1307. doi: 10.1016/j.cub.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 72.He X, He L, Hannon GJ. The guardian's little helper: microRNAs in the p53 tumor suppressor network. Cancer Res. 2007;67:11099–11101. doi: 10.1158/0008-5472.CAN-07-2672. [DOI] [PubMed] [Google Scholar]
- 73.Hough CD, Sherman-Baust CA, Pizer ES, Montz FJ, Im DD, Rosenshein NB, Cho KR, Riggins GJ, Morin PJ. Large-scale serial analysis of gene expression reveals genes differentially expressed in ovarian cancer. Cancer Res. 2000;60:6281–6287. [PubMed] [Google Scholar]
- 74.Hough CD, Cho KR, Zonderman AB, Schwartz DR, Morin PJ. Coordinately up-regulated genes in ovarian cancer. Cancer Res. 2001;61:3869–3876. [PubMed] [Google Scholar]
- 75.Bonome T, Lee JY, Park DC, Radonovich M, Pise-Masison C, Brady J, Gardner GJ, Hao K, Wong WH, Barrett JC, Lu KH, Sood AK, Gershenson DM, Mok SC, Birrer MJ. Expression profiling of serous low malignant potential, low-grade, and high-grade tumors of the ovary. Cancer Res. 2005;65:10602–10612. doi: 10.1158/0008-5472.CAN-05-2240. [DOI] [PubMed] [Google Scholar]
- 76.Gilks CB, Vanderhyden BC, Zhu S, van de Rijn M, Longacre TA. Distinction between serous tumors of low malignant potential and serous carcinomas based on global mRNA expression profiling. Gynecol Oncol. 2005;96:684–694. doi: 10.1016/j.ygyno.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 77.Landen CN, Jr, Birrer MJ, Sood AK. Early events in the pathogenesis of epithelial ovarian cancer. J Clin Oncol. 2008;26:995–1005. doi: 10.1200/JCO.2006.07.9970. [DOI] [PubMed] [Google Scholar]
- 78.Singer G, Rebmann V, Chen YC, Liu HT, Ali SZ, Reinsberg J, McMaster MT, Pfeiffer K, Chan DW, Wardelmann E, Grosse-Wilde H, Cheng CC, Kurman RJ, Shih IeM. HLA-G is a potential tumor marker in malignant ascites. Clin Cancer Res. 2003;9:4460–4464. [PubMed] [Google Scholar]
- 79.Sheu JJ, Shih IeM. Clinical and biological significance of HLA-G expression in ovarian cancer. Semin Cancer Biol. 2007;17:436–443. doi: 10.1016/j.semcancer.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vassilopoulos I, Korkolopoulou P, Konstantinidou AE, Patsouris E, Eftichiadis C, Thymara I, Perdiki M, Pavlakis K, Agapitos E, Davaris PS. Evaluation of the cyclin-dependent kinase inhibitor p21Cip1 in epithelial ovarian tumors of low malignant potential and adenocarcinomas. Histol Histopathol. 2003;18:761–770. doi: 10.14670/HH-18.761. [DOI] [PubMed] [Google Scholar]
- 81.Cannistra SA. BRCA-1 in Sporadic Epithelial Ovarian Cancer: Lessons Learned from the Genetics of Hereditary Disease. Clin Cancer Res. 2007;13:7225–7227. doi: 10.1158/1078-0432.CCR-07-2222. [DOI] [PubMed] [Google Scholar]
- 82.Wang C, Horiuchi A, Imai T, Ohira S, Itoh K, Nikaido T, Katsuyama Y, Konishi I. Expression of BRCA1 protein in benign, borderline, and malignant epithelial ovarian neoplasms and its relationship to methylation and allelic loss of the BRCA1 gene. J Pathol. 2004;202:215–223. doi: 10.1002/path.1507. [DOI] [PubMed] [Google Scholar]
- 83.Baldwin RL, Nemeth E, Tran H, Shvartsman H, Cass I, Narod S, Karlan BY. BRCA1 promoter region hypermethylation in ovarian carcinoma: a population-based study. Cancer Res. 2000;60:5329–5333. [PubMed] [Google Scholar]
- 84.Press JZ, De Luca A, Boyd N, Young S, Troussard A, Ridge Y, Kaurah P, Kalloger SE, Blood KA, Smith M, Spellman PT, Wang Y, Miller DM, Horsman D, Faham M, Gilks CB, Gray J, Huntsman DG. Ovarian carcinomas with genetic and epigenetic BRCA1 loss have distinct molecular abnormalities. BMC Cancer. 2008;8:17. doi: 10.1186/1471-2407-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Acs G, Pasha T, Zhang PJ. WT1 is differentially expressed in serous, endometrioid, clear cell, and mucinous carcinomas of the peritoneum, fallopian tube, ovary, and endometrium. Int J Gynecol Pathol. 2004;23:110–118. doi: 10.1097/00004347-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 86.O'Neill CJ, Deavers MT, Malpica A, Foster H, McCluggage WG. An immunohistochemical comparison between low-grade and high-grade ovarian serous carcinomas: significantly higher expression of p53, MIB1, BCL2, HER-2/neu, and C-KIT in high-grade neoplasms. Am J Surg Pathol. 2005;29:1034–1041. [PubMed] [Google Scholar]
- 87.DePriest PD, Banks ER, Powell DE, van Nagell JR, Jr, Gallion HH, Puls LE, Hunter JE, Kryscio RJ, Royalty MB. Endometrioid carcinoma of the ovary and endometriosis: the association in postmenopausal women. Gynecol Oncol. 1992;47:71–75. doi: 10.1016/0090-8258(92)90079-x. [DOI] [PubMed] [Google Scholar]
- 88.Fukunaga M, Nomura K, Ishikawa E, Ushigome S. Ovarian atypical endometriosis: its close association with malignant epithelial tumours. Histopathology. 1997;30:249–255. doi: 10.1046/j.1365-2559.1997.d01-592.x. [DOI] [PubMed] [Google Scholar]
- 89.Yoshikawa H, Jimbo H, Okada S, Matsumoto K, Onda T, Yasugi T, Taketani Y. Prevalence of endometriosis in ovarian cancer. Gynecol Obstet Invest. 2000;50 Suppl 1:11–17. doi: 10.1159/000052873. [DOI] [PubMed] [Google Scholar]
- 90.Erzen M, Rakar S, Klancnik B, Syrjanen K, Klancar B. Endometriosis-associated ovarian carcinoma (EAOC): an entity distinct from other ovarian carcinomas as suggested by a nested case-control study. Gynecol Oncol. 2001;83:100–108. doi: 10.1006/gyno.2001.6382. [DOI] [PubMed] [Google Scholar]
- 91.Sainz de la Cuesta R, Eichhorn JH, Rice LW, Fuller AF, Jr, Nikrui N, Goff BA. Histologic transformation of benign endometriosis to early epithelial ovarian cancer. Gynecol Oncol. 1996;60:238–244. doi: 10.1006/gyno.1996.0032. [DOI] [PubMed] [Google Scholar]
- 92.Thomas EJ, Campbell IG. Molecular genetic defects in endometriosis. Gynecol Obstet Invest. 2000;50 Suppl 1:44–50. doi: 10.1159/000052878. [DOI] [PubMed] [Google Scholar]
- 93.Bell KA, Kurman RJ. A clinicopathologic analysis of atypical proliferative (borderline) tumors and well-differentiated endometrioid adenocarcinomas of the ovary. Am J Surg Pathol. 2000;24:1465–1479. doi: 10.1097/00000478-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 94.Wright K, Wilson P, Morland S, Campbell I, Walsh M, Hurst T, Ward B, Cummings M, Chenevix-Trench G. beta-catenin mutation and expression analysis in ovarian cancer: exon 3 mutations and nuclear translocation in 16% of endometrioid tumours. Int J Cancer. 1999;82:625–629. doi: 10.1002/(sici)1097-0215(19990827)82:5<625::aid-ijc1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 95.Sagae S, Kobayashi K, Nishioka Y, Sugimura M, Ishioka S, Nagata M, Terasawa K, Tokino T, Kudo R. Mutational analysis of beta-catenin gene in Japanese ovarian carcinomas: frequent mutations in endometrioid carcinomas. Jpn J Cancer Res. 1999;90:510–515. doi: 10.1111/j.1349-7006.1999.tb00777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu R, Zhai Y, Fearon ER, Cho KR. Diverse Mechanisms of beta-Catenin Deregulation in Ovarian Endometrioid Adenocarcinomas. Cancer Res. 2001;61:8247–8255. [PubMed] [Google Scholar]
- 97.Moreno-Bueno G, Gamallo C, Perez-Gallego L, de Mora JC, Suarez A, Palacios J. β-Catenin Expression Pattern, β-Catenin Gene Mutations, and Microsatellite Instability in Endometrioid Ovarian Carcinomas and Synchronous Endometrial Carcinomas. Diagn Mol Pathol. 2001;10:116–122. doi: 10.1097/00019606-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 98.Gamallo C, Palacios J, Moreno G, Calvo de Mora J, Suarez A, Armas A. beta-catenin expression pattern in stage I and II ovarian carcinomas: relationship with beta-catenin gene Mutations, Clinicopathological features, and clinical outcome. Amer J Path. 1999;155:527–536. doi: 10.1016/s0002-9440(10)65148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saegusa M, Okayasu I. Frequent nuclear beta-catenin accumulation and associated mutations in endometrioid-type endometrial and ovarian carcinomas with squamous differentiation. J Pathol. 2001;194:59–67. doi: 10.1002/path.856. [DOI] [PubMed] [Google Scholar]
- 100.Obata K, Morland SJ, Watson RH, Hitchcock A, Chenevix-Trench G, Thomas EJ, Campbell IG. Frequent PTEN/MMAC mutations in endometrioid but not serous or mucinous epithelial ovarian tumors. Cancer Res. 1998;58:2095–2097. [PubMed] [Google Scholar]
- 101.Catasus L, Bussaglia E, Rodrguez I, Gallardo A, Pons C, Irving JA, Prat J. Molecular genetic alterations in endometrioid carcinomas of the ovary: similar frequency of beta-catenin abnormalities but lower rate of microsatellite instability and PTEN alterations than in uterine endometrioid carcinomas. Hum Pathol. 2004;35:1360–1368. doi: 10.1016/j.humpath.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 102.Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 103.Campbell IG, Russell SE, Choong DY, Montgomery KG, Ciavarella ML, Hooi CS, Cristiano BE, Pearson RB, Phillips WA. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res. 2004;64:7678–7681. doi: 10.1158/0008-5472.CAN-04-2933. [DOI] [PubMed] [Google Scholar]
- 104.Oda K, Stokoe D, Taketani Y, McCormick F. High frequency of coexistent mutations of PIK3CA and PTEN genes in endometrial carcinoma. Cancer Res. 2005;65:10669–10673. doi: 10.1158/0008-5472.CAN-05-2620. [DOI] [PubMed] [Google Scholar]
- 105.Catasus L, Gallardo A, Cuatrecasas M, Prat J. PIK3CA mutations in the kinase domain (exon 20) of uterine endometrial adenocarcinomas are associated with adverse prognostic parameters. Mod Pathol. 2008;21:131–139. doi: 10.1038/modpathol.3800992. [DOI] [PubMed] [Google Scholar]
- 106.Enomoto T, Weghorst CM, Inoue M, Tanizawa O, Rice JM. K-ras activation occurs frequently in mucinous adenocarcinomas and rarely in other common epithelial tumors of the human ovary. Am J Pathol. 1991;139:777–785. [PMC free article] [PubMed] [Google Scholar]
- 107.Caduff RF, Svoboda-Newman SM, Bartos RE, Ferguson AW, Frank TS. Comparative analysis of histologic homologues of endometrial and ovarian carcinoma. Am J Surg Pathol. 1998;22:319–326. doi: 10.1097/00000478-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 108.Amemiya S, Sekizawa A, Otsuka J, Tachikawa T, Saito H, Okai T. Malignant transformation of endometriosis and genetic alterations of K-ras and microsatellite instability. Int J Gynaecol Obstet. 2004;86:371–376. doi: 10.1016/j.ijgo.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 109.Liu J, Albarracin CT, Chang KH, Thompson-Lanza JA, Zheng W, Gershenson DM, Broaddus R, Luthra R. Microsatellite instability and expression of hMLH1 and hMSH2 proteins in ovarian endometrioid cancer. Mod Pathol. 2004;17:75–80. doi: 10.1038/modpathol.3800017. [DOI] [PubMed] [Google Scholar]
- 110.Kolasa IK, Rembiszewska A, Janiec-Jankowska A, Dansonka-Mieszkowska A, Lewandowska AM, Konopka B, Kupryjanczyk J. PTEN mutation, expression and LOH at its locus in ovarian carcinomas. Relation to TP53, K-RAS and BRCA1 mutations. Gynecol Oncol. 2006;103:692–697. doi: 10.1016/j.ygyno.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 111.Tapper J, Butzow R, Wahlstrom T, Seppala M, Knuutila S. Evidence for divergence of DNA copy number changes in serous, mucinous and endometrioid ovarian carcinomas. Br J Cancer. 1997;75:1782–1787. doi: 10.1038/bjc.1997.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zorn KK, Bonome T, Gangi L, Chandramouli GV, Awtrey CS, Gardner GJ, Barrett JC, Boyd J, Birrer MJ. Gene expression profiles of serous, endometrioid, and clear cell subtypes of ovarian and endometrial cancer. Clin Cancer Res. 2005;11:6422–6430. doi: 10.1158/1078-0432.CCR-05-0508. [DOI] [PubMed] [Google Scholar]
- 113.Sato N, Tsunoda H, Nishida M, Morishita Y, Takimoto Y, Kubo T, Noguchi M. Loss of heterozygosity on 10q23.3 and mutation of the tumor suppressor gene PTEN in benign endometrial cyst of the ovary: possible sequence progression from benign endometrial cyst to endometrioid carcinoma and clear cell carcinoma of the ovary1. Cancer Res. 2000;60:7052–7056. [PubMed] [Google Scholar]
- 114.Fujita M, Enomoto T, Yoshino K, Nomura T, Buzard GS, Inoue M, Okudaira Y. Microsatellite instability and alterations in the hMSH2 gene in human ovarian cancer. Int J Cancer. 1995;64:361–366. doi: 10.1002/ijc.2910640602. [DOI] [PubMed] [Google Scholar]
- 115.King BL, Carcangiu ML, Carter D, Kiechle M, Pfisterer J, Pfleiderer A, Kacinski BM. Microsatellite instability in ovarian neoplasms. Br J Cancer. 1995;72:376–382. doi: 10.1038/bjc.1995.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mok CH, Bell DA, Knapp RC, Fishbaugh PM, Welch WR, Muto MG, Berkowitz RS, Tsao S. Mutation of K-ras protooncogene in human ovarian epithelial tumors of borderline malignancy. Cancer Res. 1993;53:1489–1492. [PubMed] [Google Scholar]
- 117.Ichikawa Y, Nishida M, Suzuki H, Yoshida S, Tsunoda H, Kubo T, Uchida K, Miwa M. Mutation of K-ras protooncogene is associated with histological subtypes in human mucinous ovarian tumors. Cancer Res. 1994;54:33–35. [PubMed] [Google Scholar]
- 118.Gemignani ML, Schlaerth AC, Bogomolniy F, Barakat RR, Lin O, Soslow R, Venkatraman E, Boyd J. Role of KRAS and BRAF gene mutations in mucinous ovarian carcinoma. Gynecol Oncol. 2003;90:378–381. doi: 10.1016/s0090-8258(03)00264-6. [DOI] [PubMed] [Google Scholar]
- 119.Cuatrecasas M, Villanueva A, Matias-Guiu X, Prat J. K-ras mutations in mucinous ovarian tumors: a clinicopathologic and molecular study of 95 cases. Cancer. 1997;79:1581–1586. doi: 10.1002/(sici)1097-0142(19970415)79:8<1581::aid-cncr21>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 120.Suehiro Y, Sakamoto M, Umayahara K, Iwabuchi H, Sakamoto H, Tanaka N, Takeshima N, Yamauchi K, Hasumi K, Akiya T, Sakunaga H, Muroya T, Numa F, Kato H, Tenjin Y, Sugishita T. Genetic aberrations detected by comparative genomic hybridization in ovarian clear cell adenocarcinomas. Oncology. 2000;59:50–56. doi: 10.1159/000012137. [DOI] [PubMed] [Google Scholar]
- 121.Dent J, Hall GD, Wilkinson N, Perren TJ, Richmond I, Markham AF, Murphy H, Bell SM. Cytogenetic alterations in ovarian clear cell carcinoma detected by comparative genomic hybridisation. Br J Cancer. 2003;88:1578–1583. doi: 10.1038/sj.bjc.6600896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang K, Gan L, Jeffery E, Gayle M, Gown AM, Skelly M, Nelson PS, Ng WV, Schummer M, Hood L, Mulligan J. Monitoring gene expression profile changes in ovarian carcinomas using cDNA microarray. Gene. 1999;229:101–108. doi: 10.1016/s0378-1119(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 123.Welsh JB, Zarrinkar PP, Sapinoso LM, Kern SG, Behling CA, Monk BJ, Lockhart DJ, Burger RA, Hampton GM. Analysis of gene expression profiles in normal and neoplastic ovarian tissue samples identifies candidate molecular markers of epithelial ovarian cancer. Proc Natl Acad Sci U S A. 2001;98:1176–1181. doi: 10.1073/pnas.98.3.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Reed E, Yu JJ, Davies A, Gannon J, Armentrout SL. Clear cell tumors have higher mRNA levels of ERCC1 and XPB than other histological types of epithelial ovarian cancer. Clin Cancer Res. 2003;9:5299–5305. [PubMed] [Google Scholar]
- 125.Tsuchiya A, Sakamoto M, Yasuda J, Chuma M, Ohta T, Ohki M, Yasugi T, Taketani Y, Hirohashi S. Expression profiling in ovarian clear cell carcinoma: identification of hepatocyte nuclear factor-1 beta as a molecular marker and a possible molecular target for therapy of ovarian clear cell carcinoma. Am J Pathol. 2003;163:2503–2512. doi: 10.1016/s0002-9440(10)63605-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Senkel S, Lucas B, Klein-Hitpass L, Ryffel GU. Identification of target genes of the transcription factor HNF1beta and HNF1alpha in a human embryonic kidney cell line. Biochim Biophys Acta. 2005;1731:179–190. doi: 10.1016/j.bbaexp.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 127.Tanaka T, Tomaru Y, Nomura Y, Miura H, Suzuki M, Hayashizaki Y. Comprehensive search for HNF-1beta-regulated genes in mouse hepatoma cells perturbed by transcription regulatory factor-targeted RNAi. Nucleic Acids Res. 2004;32:2740–2750. doi: 10.1093/nar/gkh597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Heinzelmann-Schwarz VA, Gardiner-Garden M, Henshall SM, Scurry JP, Scolyer RA, Smith AN, Bali A, Vanden Bergh P, Baron-Hay S, Scott C, Fink D, Hacker NF, Sutherland RL, O'Brien PM. A distinct molecular profile associated with mucinous epithelial ovarian cancer. Br J Cancer. 2006;94:904–913. doi: 10.1038/sj.bjc.6603003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Orsulic S, Li Y, Soslow RA, Vitale-Cross LA, Varmus HE. Induction of ovarian cancer by defined multiple genetic changes in a mouse model system. Cancer Cell. 2002;1:53–62. doi: 10.1016/s1535-6108(01)00002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Connolly DC, Bao R, Nikitin AY, Stephens KC, Poole TW, Hua X, Harris SS, Vanderhyden BC, Hamilton TC. Female Mice Chimeric for Expression of the Simian Virus 40 TAg under Control of the MISIIR Promoter Develop Epithelial Ovarian Cancer. Cancer Res. 2003;63:1389–1397. [PubMed] [Google Scholar]
- 131.Flesken-Nikitin A, Choi KC, Eng JP, Shmidt EN, Nikitin AY. Induction of carcinogenesis by concurrent inactivation of p53 and Rb1 in the mouse ovarian surface epithelium. Cancer Res. 2003;63:3459–3463. [PubMed] [Google Scholar]
- 132.Dinulescu DM, Ince TA, Quade BJ, Shafer SA, Crowley D, Jacks T. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med. 2005;11:63–70. doi: 10.1038/nm1173. [DOI] [PubMed] [Google Scholar]
- 133.Powis G, Ihle N, Kirkpatrick DL. Practicalities of drugging the phosphatidylinositol-3-kinase/Akt cell survival signaling pathway. Clin Cancer Res. 2006;12:2964–2966. doi: 10.1158/1078-0432.CCR-06-0617. [DOI] [PubMed] [Google Scholar]
- 134.Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 135.Rubio-Viqueira B, Hidalgo M. Targeting mTOR for cancer treatment. Curr Opin Investig Drugs. 2006;7:501–512. [PubMed] [Google Scholar]
- 136.Safran M, Kim WY, Kung AL, Horner JW, DePinho RA, Kaelin WG., Jr Mouse reporter strain for noninvasive bioluminescent imaging of cells that have undergone Cre-mediated recombination. Mol Imaging. 2003;2:297–302. doi: 10.1162/15353500200303154. [DOI] [PubMed] [Google Scholar]
- 137.Shih IeM, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164:1511–1518. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shih IeM, Kurman RJ. Molecular pathogenesis of ovarian borderline tumors: new insights and old challenges. Clin Cancer Res. 2005;11:7273–7279. doi: 10.1158/1078-0432.CCR-05-0755. [DOI] [PubMed] [Google Scholar]
- 139.Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, Garber J, Birch C, Mou H, Gordon RW, Cramer DW, McKeon FD, Crum CP. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211:26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 140.Crum CP, Drapkin R, Miron A, Ince TA, Muto M, Kindelberger DW, Lee Y. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19:3–9. doi: 10.1097/GCO.0b013e328011a21f. [DOI] [PubMed] [Google Scholar]
- 141.Kurman RJ, Visvanathan K, Roden R, Wu TC, Shih IM. Early detection and treatment of ovarian cancer: shifting from early stage to minimal volume of disease based on a new model of carcinogenesis. Am J Obstet Gynecol. 2008 doi: 10.1016/j.ajog.2008.01.005. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jacobs IJ, Skates SJ, MacDonald N, Menon U, Rosenthal AN, Davies AP, Woolas R, Jeyarajah AR, Sibley K, Lowe DG, Oram DH. Screening for ovarian cancer: a pilot randomised controlled trial. Lancet. 1999;353:1207–1210. doi: 10.1016/S0140-6736(98)10261-1. [DOI] [PubMed] [Google Scholar]
- 143.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 144.Lee KW, Kang NJ, Heo YS, Rogozin EA, Pugliese A, Hwang MK, Bowden GT, Bode AM, Lee HJ, Dong Z. Raf and MEK protein kinases are direct molecular targets for the chemopreventive effect of quercetin, a major flavonol in red wine. Cancer Res. 2008;68:946–955. doi: 10.1158/0008-5472.CAN-07-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]