Abstract
Background
Conflicting results have been reported on the association of plasma total homocysteine (tHcy) and cholesterol levels in Alzheimer disease (AD). The objective of this study was to determine the relationship between cognitive performance and plasma levels of tHcy and its biological determinants folate and vitamin B12, and lipids in clinically diagnosed AD patients.
Methods
A cross-sectional database review was performed on two separate groups of patients (n = 191). Mini-Mental State Exam (MMSE) scores, plasma levels of tHcy, vitamin B12, folate, cholesterol, and triglycerides were analyzed.
Results
The MMSE scores were inversely correlated with age, plasma levels of tHcy and LDL cholesterol. However, only the inverse relationship between MMSE scores and LDL cholesterol levels persisted after adjustment for age, sex, and status of statin treatment. Plasma tHcy levels increased significantly with age and were inversely related to vitamin B12 and folate levels, which modified the relationship between MMSE scores and plasma tHcy levels.
Conclusions
The plasma tHcy levels appeared to relate more to aging than to cognition. Cognitive performance was inversely associated with plasma LDL cholesterol levels in AD patients. Our findings provide further evidence that high LDL cholesterol levels may play a role in the pathogenesis of AD.
Key Words: Cognition, Homocysteine, Cholesterol, Alzheimer disease
Introduction
Alzheimer disease (AD) is a progressive neurodegenerative disease. The neuropathology of AD is characterized by deposition of amyloid-β peptide (Aβ) in senile plaques, formation of neurofibrillary tangles composed of hyper-phosphorylated tau protein, and cortical neuronal loss. Typically, patients with AD start with amnestic memory loss and progress to impairment of other mental abilities and functional impairment in activities of daily living. The pathogenic mechanisms that lead to the development of AD, however, are not fully understood. One of the main hypotheses is that β-amyloidosis (production and deposition of the Aβ) plays a crucial role in the pathogenesis of AD [1]. This hypothesis is supported by discoveries of causative mutations in the gene encoding amyloid-β precursor protein (APP) and in genes of presenilin-1 and −2 in early-onset familial AD [2]. All of these mutations cause increased production of highly amyloidogenic Aβ peptides that are deposited in the senile plaques.
Increasing evidence suggests that cardiovascular risk factors may play important roles in the development of AD. The apolipoprotein (apo) E4 allele, associated with hypercholesterolemia and coronary artery disease, is a strong risk factor for AD [3]. Recent epidemiological data have indicated an apparent reduction of AD prevalence in people treated with statins, a class of cholesterol-lowering drugs [4,5,6]. Prospective studies, however, have produced mixed results on the effect of statins on the development of AD [7,8,9,10]. Furthermore, several observational epidemiological studies examining the association between plasma cholesterol levels and AD have yielded conflicting results [11,12,13,14]. Another risk factor for vascular disease, elevated plasma total homocysteine (tHcy) levels [15, 16], has also been implicated as a strong and independent risk factor for the development of dementia and AD [17]. Other studies, however, found no association between plasma tHcy levels and AD [18, 19]. In the present study, we examined the association of plasma levels of tHcy and its biological determinants folate and vitamin B12 with cognitive performance in clinically diagnosed AD patients as well as the correlations between cognitive function and plasma lipid levels in these patients.
Methods
Patients
Cross-sectional database review was performed on two separate groups of patients: one from the Kirklin Clinic at the University of Alabama at Birmingham (UAB) and another one from the Cognitive Neurology Clinics at Emory University. The procedures were approved by the Institutional Review Board (IRB) at UAB and Emory University, respectively.
The UAB group was generated from database review on 971 patients seen in a neurology clinic with a predominant emphasis on memory disorders at the Kirklin Clinic during January 2000 to May 2002. The majority of the patients were female (60%, n = 584) and white (78%, n = 761). All patients received a thorough neurological evaluation. Most patients (>80%) had some kind of memory complaint. Those without a cognitive complaint did not have a thorough mental status evaluation. Those with a memory complaint also received a cognitive evaluation. The cognitive evaluation includes a Mini-Mental State Exam (MMSE) [20] as well as history, exam, laboratory evaluation and neuroimaging. The history was focused on memory loss and cognitive and functional abilities. The exam included a bedside assessment of memory, language, visuospatial function, judgment and insight, mood, thought content, and thought processes. The assessment of memory included short- and long-term memory and response to cuing. It also included general fund of knowledge questions such as ‘Who is the president of the United States’. The neurological exam included cranial nerves, motor, sensory, gait and coordination, and reflexes. Laboratory (blood) work was performed to rule out treatable causes of cognitive decline. Neuro-imaging was usually MRI of the head, but occasionally included CT scan only. Neuropsychological testing was also obtained on any cases that were not straightforward or were thought to represent mild cognitive impairment (MCI) [21]. Patients with MCI were excluded from this analysis. Only patients with a diagnosis of probable or possible AD according to NINCDS-ADRDA criteria [22] were studied. These constituted 139 patients (65 male and 74 female).
The Emory participants were selected based on the review of patients seen in the Memory Disorders Clinic between September 2003 and August 2004. A total of 194 new patients were screened during this period (mean age 72.2 years, 19.2% non-Caucasian, 61.7% female). Similar clinical assessment was conducted as described for the UAB group, except that all patients seen at Emory received a standard neuropsychological test battery consisting of the MMSE, WMS-R Logical Memory, CERAD list learning task, Category Fluency (Animal, Vegetables), Phonemic Fluency (FAS), 30-Item Boston Naming Test, Clock Drawing, Digit Span, WAIS-R Digit Symbol and Similarities, Judgment of Line Orientation, Brief Visuospatial Memory Test, Trail-Making Test, and Geriatric Depression Scale. A total of 52 patients (mean age 73.4 years, 20% non-Caucasian, 54% female) with available tHcy data and diagnosed with probable or possible AD according to NINCDS-ADRDA criteria [22] were included in the analysis.
Measurements of Plasma Parameters
Plasma levels of homocysteine, vitamin B12, folate, and lipids (total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides) were mostly obtained at the CLIA approved laboratory at the Kirklin Clinic (UAB) and the Cognitive Neurology Clinics (Emory). However, when recent data were available from referring physicians, the latter values were used. Mean values are reported in table 1.
Table 1.
Characteristics of the patients with AD
| Characteristic | Patients (n) |
|---|---|
| Age, mean ± SD, years | 72.6±9.2 (191) |
| Sex, % female | 53.4 (102) |
| Ethnic | |
| % Caucasian | 82.2 (157) |
| % African-American | 16.8 (32) |
| % Others | 2.1 (4) |
| MMSE scores (max. 30), mean ± SD | 23.3±5.6 (191) |
| Plasma variable, mean ± SD | |
| Total homocysteine, μmol/l | 10.6±3.6 (178) |
| Vitamin B12, pg/ml | 515.7±289.4 (168) |
| Folate, ng/ml | 17.1±4.2 (144) |
| Total cholesterol, mg/dl | 212.1±45.7 (137) |
| LDL cholesterol, mg/dl | 126.1±37.3 (132) |
| HDL cholesterol, mg/dl | 53.8±17.4 (133) |
| Triglyceride, mg/dl | 167.7±109.8 (134) |
Statistical Analysis
Data were expressed as mean ± SD. Comparisons between groups were performed by two-tailed Student's t test (for normal distributed data) or by Mann-Whitney rank-sum test (for non-normal distributed data). Simple correlations were determined by Pearson product moment correlation analysis. Associations between MMSE scores (dependent variables) and demographic (i.e. age and sex) and biochemical variables (i.e. plasma tHcy, vitamin B12, folate, and lipids) were evaluated by multiple linear regression analysis. The SigmaStat software (SPSS Science, Chicago, Ill., USA) was used for all statistical analyses. p < 0.05 was considered statistically significant.
Results
Characteristics of Study Populations
The characteristics of recruited patients are shown in table 1. In total of 191 AD patients with complete MMSE data, plasma total homocysteine data were available for 178 (93%) patients. Vitamin B12 and folate data were available for 168 (88%) and 144 (75%) patients, respectively. Plasma lipid profiles (total cholesterol, LDL cholesterol, HDL cholesterol, and total triglyceride) were available for 137 (72%), 132 (69%), and 133 (70%) patients, respectively. The mean plasma tHcy concentration for these patients (10.6 ± 3.6 μmol/l) was within the normal range. Only about 15% (27 of 178) patients had plasma tHcy levels over 14 μmol/l. Our patients seemed to have had sufficient supplementation of vitamin B12 and folate in the diet as shown by their mean plasma vitamin B12 and folate levels. About 95% (159 of 168) patients had plasma vitamin B12 levels within or higher than the normal range. Furthermore, about 70% (101 of 144) patients had plasma folate levels over the normal maximum value of 15 ng/ml. The plasma lipid (cholesterol and triglyceride) levels were modestly elevated in this group of patients. In addition, about 19% (36 of 191) patients were receiving different statin treatment (Lipitor, Zocor, Pravachol, or Mevacor).
Comparisons between Male and Female Patients
As AD is thought to be more prevalent in females than in males [23], variables were compared between the two groups of patients. In all variables obtained, significant differences were found only in plasma cholesterol levels between male and female AD patients (table 2). Female patients had significantly higher plasma levels of total, LDL, and HDL cholesterol than male patients did. However, the ratio of plasma total to HDL cholesterol was higher in male patients than that in female patients (4.5 ± 1.3 vs. 3.9 ± 1.0; p = 0.006).
Table 2.
Comparisons between male and female AD patients
| Variable | Male (n) | Female (n) | p |
|---|---|---|---|
| Age | 73.1±8.8 (89) | 72.1±9.5 (102) | 0.458 |
| MMSE scores | 23.8±5.1 (89) | 22.9±6.1 (102) | 0.426 |
| Total homocysteine, μmol/l | 11.0±3.8 (83) | 10.2±3.4 (95) | 0.095 |
| Vitamin B12, pg/ml | 516.4±313.4 (78) | 515.1±268.5 (90) | 0.686 |
| Folate, ng/ml | 16.8±4.3 (68) | 17.3±4.1 (76) | 0.513 |
| Total cholesterol, mg/dl | 198.0±43.2 (63) | 224.1±44.7 (74) | <0.001∗∗∗ |
| LDL cholesterol, mg/dl | 116.3±34.7 (62) | 134.7±37.7 (70) | 0.004∗∗ |
| HDL cholesterol, mg/dl | 46.3±15.3 (62) | 60.3±16.6 (71) | <0.001∗∗∗ |
| Triglyceride, mg/dl | 171.7±110.1 (62) | 164.2±110.3 (72) | 0.463 |
Values are means ± SD
p < 0.01
p < 0.001.
Correlations between MMSE Scores and Plasma Variables
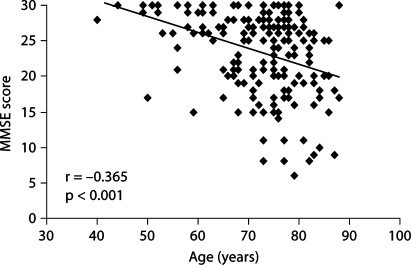
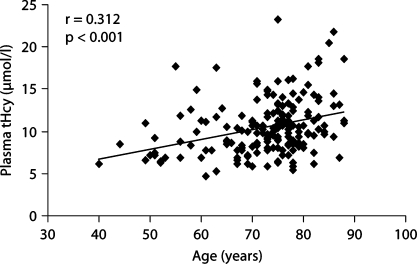
Before the relationships between MMSE scores and plasma variables were determined, the correlation between MMSE scores and age was analyzed. As expected, MMSE score were inversely related to age (r = −0.365; p < 0.001) (fig. 1). Simple correlations between MMSE scores and plasma levels of tHcy, vitamin B12, folate, and plasma lipid levels are shown in table 3. Plasma levels of tHcy were inversely correlated with MMSE score (r = −0.169; p = 0.024). However, the inverse relationship between MMSE scores and plasma tHcy levels became nonsignificant in a multiple regression analysis after adjustment for age and sex (β = −0.113; p = 0.328) or for plasma levels of vitamin B12 and folate (β = −0.208; p = 0.136). We found that plasma tHcy levels increased significantly with age (r = 0.312; p < 0.001) (fig. 2). Plasma levels of vitamin B12 and folate were inversely related to plasma tHcy levels (r = −0.211; p = 0.008 and r = −0.191; p = 0.024, respectively). In addition, plasma levels of vitamin B12 and folate were correlated positively (r = 0.213; p = 0.013).
Fig. 1.
Correlation between MMSE scores and age in AD patients (n = 191). The MMSE score is inversely correlated with age (r = −0.365; p < 0.001).
Table 3.
Association between MMSE scores and plasma variables in AD patients
| Plasma variables | Patients (n) | Correlation coefficient (r)a | p | Regression coefficient (β)b | p |
|---|---|---|---|---|---|
| Total homocysteine, μmol/l | 178 | −0.169 | 0.024∗ | −0.113 | 0.328 |
| Vitamin B12, pg/ml | 168 | −0.094 | 0.223 | −0.002 | 0.279 |
| Folate, ng/ml | 144 | 0.042 | 0.614 | 0.128 | 0.231 |
| Total cholesterol, mg/dl | 137 | −0.099 | 0.249 | −0.009 | 0.384 |
| LDL cholesterol, mg/dl | 132 | −0.183 | 0.035∗ | −0.031 | 0.020∗ |
| HDL cholesterol, mg/dl | 133 | 0.028 | 0.754 | 0.035 | 0.250 |
| Triglyceride, mg/dl | 134 | 0.030 | 0.733 | −0.0002 | 0.972 |
Pearson product moment correlation analysis with no adjustment.
Multiple linear regression analysis with adjustment for age and sex. Dependent variable: MMSE scores.
p < 0.05.
Fig. 2.
Correlation between plasma total homocysteine levels and age in AD patients (n = 178). Plasma tHcy levels increase significantly with age (r = 0.312; p < 0.001).
Plasma levels of total cholesterol, HDL cholesterol, and triglycerides were not associated with MMSE scores either by the simple correlation analysis or by the multiple regression analysis after adjustment for age and sex. However, there was an inverse correlation between MMSE scores and plasma LDL cholesterol levels (table 3). This inverse relationship persisted in the multiple linear regression analysis after adjustment for age and sex (β = −0.031; p = 0.02) (table 3). In addition, after adjustment for the status of statin treatment as well as for age and sex, the inverse association between MMSE scores and LDL cholesterol levels became more evident (β = −0.036, p = 0.007).
Discussion
The results of our study show that cognitive performance declines and plasma tHcy levels increase significantly with age in clinically diagnosed AD patients. Although an inverse correlation between MMSE scores and plasma tHcy was observed in these patients, this relationship became nonsignificant after adjustment for age. Others also have shown that age was a significant confounder in the relationship between memory scores and plasma tHcy levels [24]. Plasma levels of vitamin B12 and folate were not related to cognitive performance but were inversely related to plasma tHcy levels.
Elevated plasma tHcy levels, a risk factor for cardiovascular disease, have been shown to be associated with an increased risk for dementia and AD. Several reports indicated an inverse association between plasma tHcy levels and simultaneously assessed cognitive function [25,26,27,28]. Two case-control studies showed higher plasma tHcy levels in people with AD [29, 30]. They found that individuals who had elevated serum tHcy concentration (>14 μmol/l) were 4.5 times as likely to have AD as were those with low serum tHcy (<11 μmol/l). A prospective study also indicated that baseline homocysteine concentrations predicted the risk of incident AD and dementia over an 8-year period in the Framingham Heart Study population [17]. A recent study also reported that homocysteine is an independent risk factor for both dementia and cognitive impairment in a Latino population [31]. Other studies, however, found no significant association between plasma tHcy levels and AD [18, 19, 24] or cognitive function in either cross-sectional [32,33,34] or longitudinal [32] analyses. There have been no reports yet on randomized trials of folate and/or vitamin B supplementation in relation to dementia or AD as outcomes.
In the present study, no independent association was found between plasma tHcy levels and MMSE scores in the clinically diagnosed AD patients. However, it is worth to note that mean plasma tHcy concentration for these patients (10.6 ± 3.6 μmol/l) was within the normal range (6–14 μmol/l). Only about 15% of patients had plasma tHcy levels over 14 μmol/l. In other studies, the average plasma tHcy levels of patients were significantly higher [17, 30]. Plasma tHcy levels are influenced by vitamin B12 and folate intake. Vitamin B12 and folate are needed for the conversion of homocysteine to methionine [35]. One possible explanation for lower plasma tHcy levels in our patients could be due to the sufficient supplementation of vitamin B12 and folate in the diet as shown by their mean plasma vitamin B12 and folate levels (515.7 ± 289.4 pg/ml and 17.1 ± 4.2 ng/ml, respectively). While about 94% of patients had plasma vitamin B12 levels within or higher than the general range of 211–911 pg/ml, all patients had plasma folate levels within the normal range of 2.8–15 ng/ml. Furthermore, about 70% of patients had plasma folate levels over the normal maximum value of 15 ng/ml. Therefore, it is plausible that the correlation between plasma tHcy levels and MMSE scores was obscured in the present study by beneficial vitamin B12 and folate status. Indeed, it has been observed that the power of using plasma tHcy levels to predict impaired cognition decreases when the status of vitamin B12 and folate in the patient population improves [36].
Our study indicated that plasma cholesterol levels, especially atherogenic LDL cholesterol levels, had an inverse relationship with MMSE scores in AD patients. Although some studies found no association between plasma cholesterol levels and the risk for AD [11, 14], increasing lines of evidence have suggested that cholesterol metabolism plays an important role in the pathogenesis of AD. Epidemiologically, an increase in prevalence of cerebral senile plaques is found in cognitively intact individuals with heart disease compared to age-matched controls with no heart disease [37]. The apolipoprotein (apo) E4 allele is a strong risk factor, which is associated with hypercholesterolemia and an increased risk for cardiovascular disease [3]. Hypercholesterolemia has been shown as an independent risk factor for AD [13]. Levels of atherogenic plasma lipoprotein components, LDL cholesterol and apoB, are increased in AD patients and correlate with brain Aβ levels [38, 39]. Levels of atheroprotective plasma lipoprotein components, HDL cholesterol and apoA-I, are decreased in AD patients [40]. An apparent reduction of AD prevalence is found in people taking cholesterol-lowering drugs [4,5,6]. In vitro evidence also supports the role of cholesterol in modulating proteolytic processing of APP and/or subsequent amyloid formation and deposition. The secretion of a neuroprotective fragment of APP from cultured cells has been shown to decrease following an increase in the cholesterol content of the cells [41]. When cellular cholesterol levels are reduced with a cholesterol-lowering drug, the production of Aβ is inhibited due to changes in enzyme activities that are involved in the proteolytic processing of APP [42,43,44,45]. Experimentally, diet-induced hypercholesterolemia causes Aβ deposits in the brain of rabbits [46] and accelerates cerebral Aβ deposition in APP transgenic mice [47,48,49]. We have also shown that an atherogenic diet exacerbates cerebral β-amyloidosis and learning deficits as well as induces hypercholesterolemia and aortic atherosclerosis in a transgenic mouse model of AD [50]. The aortic atherosclerotic lesion area positively correlated with cerebral Aβ deposits in the transgenic mice on both atherogenic and normal diets[50]. In addition, we have shown that lack of the LDL receptor, which causes hypercholesterolemia, aggravates cerebral β-amyloidosis and learning deficits in a mouse model of AD [51]. Recently, we have demonstrated that a statin drug enhances learning and memory in normal aged mice as well as in mice with AD-like pathology [52].
In conclusion, this study shows cognitive performance deteriorates and plasma tHcy levels increase with age in clinically diagnosed AD patients. The plasma tHcy levels appeared to relate more to aging than to cognitive performance. Cognitive performance was inversely associated with plasma levels of LDL cholesterol independent of age and sex in these patients. Our data support the notion that plasma and/or cellular cholesterol homeostasis plays a significant role in the development of AD.
Acknowledgements
This study was supported in part by grants from the National Institutes of Health (AG031846, AG025949, and AG16582), the Alzheimer's Association (IIRG-05–13139), and an anonymous philanthropic foundation.
References
- 1.Selkoe DJ. The genetics and molecular pathology of Alzheimer's disease: roles of amyloid and the presenilins. Neurol Clin. 2000;18:903–922. doi: 10.1016/s0733-8619(05)70232-2. [DOI] [PubMed] [Google Scholar]
- 2.Hardy J. Amyloid, the presenilins and Alzheimer's disease. Trends Neurosci. 1997;20:154–159. doi: 10.1016/s0166-2236(96)01030-2. [DOI] [PubMed] [Google Scholar]
- 3.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 4.Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol. 2000;57:1439–1443. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- 5.Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet. 2000;356:1627–1631. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- 6.Zamrini E, McGwin G, Roseman JM. Association between statin use and Alzheimer's disease. Neuroepidemiology. 2004;23:94–98. doi: 10.1159/000073981. [DOI] [PubMed] [Google Scholar]
- 7.Simons M, Schwarzler F, Lutjohann D, von Bergmann K, Beyreuther K, Dichgans J, Wormstall H, Hartmann T, Schulz JB. Treatment with simvastatin in normocholesterolemic patients with Alzheimer's disease: a 26-week randomized, placebo-controlled, double-blind trial. Ann Neurol. 2002;52:346–350. doi: 10.1002/ana.10292. [DOI] [PubMed] [Google Scholar]
- 8.Sparks DL, Sabbagh MN, Connor DJ, Lopez J, Launer LJ, Browne P, Wasser D, Johnson-Traver S, Lochhead J, Ziolwolski C. Atorvastatin for the treatment of mild to moderate Alzheimer disease: preliminary results. Arch Neurol. 2005;62:753–757. doi: 10.1001/archneur.62.5.753. [DOI] [PubMed] [Google Scholar]
- 9.Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Meinders AE, Norrie J, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey C, Westendorp RG. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 10.Collins R, Armitage J, Parish S, Sleight P, Peto R. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 11.Tan ZS, Seshadri S, Beiser A, Wilson PW, Kiel DP, Tocco M, D'Agostino RB, Wolf PA. Plasma total cholesterol level as a risk factor for Alzheimer disease: the Framingham Study. Arch Intern Med. 2003;163:1053–1057. doi: 10.1001/archinte.163.9.1053. [DOI] [PubMed] [Google Scholar]
- 12.Evans RM, Emsley CL, Gao S, Sahota A, Hall KS, Farlow MR, Hendrie H. Serum cholesterol, APOE genotype, and the risk of Alzheimer's disease: a population-based study of African Americans. Neurology. 2000;54:240–242. doi: 10.1212/wnl.54.1.240. [DOI] [PubMed] [Google Scholar]
- 13.Notkola IL, Sulkava R, Pekkanen J, Erkinjuntti T, Ehnholm C, Kivinen P, Tuomilehto J, Nissinen A. Serum total cholesterol, apolipoprotein E epsilon 4 allele, and Alzheimer's disease. Neuroepidemiology. 1998;17:14–20. doi: 10.1159/000026149. [DOI] [PubMed] [Google Scholar]
- 14.Romas SN, Tang MX, Berglund L, Mayeux R. APOE genotype, plasma lipids, lipoproteins, and AD in community elderly. Neurology. 1999;53:517–521. doi: 10.1212/wnl.53.3.517. [DOI] [PubMed] [Google Scholar]
- 15.Clarke R, Daly L, Robinson K, Naughten E, Cahalane S, Fowler B, Graham I. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med. 1991;324:1149–1155. doi: 10.1056/NEJM199104253241701. [DOI] [PubMed] [Google Scholar]
- 16.Boushey CJ, Beresford SA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA. 1995;274:1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 17.Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D'Agostino RB, Wilson PW, Wolf PA. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 18.Nilsson K, Gustafson L, Hultberg B. Relation between plasma homocysteine and Alzheimer's disease. Dement Geriatr Cogn Disord. 2002;14:7–12. doi: 10.1159/000058327. [DOI] [PubMed] [Google Scholar]
- 19.Mizrahi EH, Jacobsen DW, Debanne SM, Traore F, Lerner AJ, Friedland RP, Petot GJ. Plasma total homocysteine levels, dietary vitamin B6 and folate intake in AD and healthy aging. J Nutr Health Aging. 2003;7:160–165. [PubMed] [Google Scholar]
- 20.Folstein M, Folstein S, McHugh P. Mini-Mental State: A practical guide for grading the cognitive state of the patient for the physician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Petersen R, Smith G, Waring S, Ivnik R, Tangalos E, Kokmen E. Mild cognitive impairment. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 22.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of the Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 23.Jorm AF, Korten AE, Henderson AS. The prevalence of dementia: a quantitative integration of the literature. Acta Psychiatr Scand. 1987;76:465–479. doi: 10.1111/j.1600-0447.1987.tb02906.x. [DOI] [PubMed] [Google Scholar]
- 24.Luchsinger JA, Tang MX, Shea S, Miller J, Green R, Mayeux R. Plasma homocysteine levels and risk of Alzheimer disease. Neurology. 2004;62:1972–1976. doi: 10.1212/01.wnl.0000129504.60409.88. [DOI] [PubMed] [Google Scholar]
- 25.Bell IR, Edman JS, Selhub J, Morrow FD, Marby DW, Kayne HL, Cole JO. Plasma homocysteine in vascular disease and in nonvascular dementia of depressed elderly people. Acta Psychiatr Scand. 1992;86:386–390. doi: 10.1111/j.1600-0447.1992.tb03285.x. [DOI] [PubMed] [Google Scholar]
- 26.Riggs KM, Spiro A, 3rd, Tucker K, Rush D. Relations of vitamin B-12, vitamin B-6, folate, and homocysteine to cognitive performance in the Normative Aging Study. Am J Clin Nutr. 1996;63:306–314. doi: 10.1093/ajcn/63.3.306. [DOI] [PubMed] [Google Scholar]
- 27.Lehmann M, Gottfries CG, Regland B. Identification of cognitive impairment in the elderly: homocysteine is an early marker. Dement Geriatr Cogn Disord. 1999;10:12–20. doi: 10.1159/000017092. [DOI] [PubMed] [Google Scholar]
- 28.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Hyperhomocysteinemia associated with poor recall in the third National Health and Nutrition Examination Survey. Am J Clin Nutr. 2001;73:927–933. doi: 10.1093/ajcn/73.5.927. [DOI] [PubMed] [Google Scholar]
- 29.McCaddon A, Davies G, Hudson P, Tandy S, Cattell H. Total serum homocysteine in senile dementia of Alzheimer type. Int J Geriatr Psychiatry. 1998;13:235–239. doi: 10.1002/(sici)1099-1166(199804)13:4<235::aid-gps761>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 30.Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol. 1998;55:1449–1455. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
- 31.Haan MN, Miller JW, Aiello AE, Whitmer RA, Jagust WJ, Mungas DM, Allen LH, Green R. Homocysteine, B vitamins, and the incidence of dementia and cognitive impairment: results from the Sacramento Area Latino Study on Aging. Am J Clin Nutr. 2007;85:511–517. doi: 10.1093/ajcn/85.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalmijn S, Launer LJ, Lindemans J, Bots ML, Hofman A, Breteler MM. Total homocysteine and cognitive decline in a community-based sample of elderly subjects: the Rotterdam Study. Am J Epidemiol. 1999;150:283–289. doi: 10.1093/oxfordjournals.aje.a010000. [DOI] [PubMed] [Google Scholar]
- 33.Ravaglia G, Forti P, Maioli F, Zanardi V, Dalmonte E, Grossi G, Cucinotta D, Macini P, Caldarera M. Blood homocysteine and vitamin B levels are not associated with cognitive skills in healthy normally ageing subjects. J Nutr Health Aging. 2000;4:218–222. [PubMed] [Google Scholar]
- 34.Ellinson M, Thomas J, Patterson A. A critical evaluation of the relationship between serum vitamin B, folate and total homocysteine with cognitive impairment in the elderly. J Hum Nutr Diet. 2004;17:371–383. doi: 10.1111/j.1365-277X.2004.00532.x. quiz 385–387. [DOI] [PubMed] [Google Scholar]
- 35.Selhub J, Jacques PF, Wilson PW, Rush D, Rosenberg IH. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. JAMA. 1993;270:2693–2698. doi: 10.1001/jama.1993.03510220049033. [DOI] [PubMed] [Google Scholar]
- 36.Miller JW, Green R, Ramos MI, Allen LH, Mungas DM, Jagust WJ, Haan MN. Homocysteine and cognitive function in the Sacramento Area Latino Study on Aging. Am J Clin Nutr. 2003;78:441–447. doi: 10.1093/ajcn/78.3.441. [DOI] [PubMed] [Google Scholar]
- 37.Sparks DL, Hunsaker JC, 3rd, Scheff SW, Kryscio RJ, Henson JL, Markesbery WR. Cortical senile plaques in coronary artery disease, aging and Alzheimer's disease. Neurobiol Aging. 1990;11:601–607. doi: 10.1016/0197-4580(90)90024-t. [DOI] [PubMed] [Google Scholar]
- 38.Kuo YM, Emmerling MR, Bisgaier CL, Essenburg AD, Lampert HC, Drumm D, Roher AE. Elevated low-density lipoprotein in Alzheimer's disease correlates with brain abeta 1–42 levels. Biochem Biophys Res Commun. 1998;252:711–715. doi: 10.1006/bbrc.1998.9652. [DOI] [PubMed] [Google Scholar]
- 39.Caramelli P, Nitrini R, Maranhao R, Lourenco AC, Damasceno MC, Vinagre C, Caramelli B. Increased apolipoprotein B serum concentration in Alzheimer's disease. Acta Neurol Scand. 1999;100:61–63. doi: 10.1111/j.1600-0404.1999.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 40.Merched A, Xia Y, Visvikis S, Serot JM, Siest G. Decreased high-density lipoprotein cholesterol and serum apolipoprotein AI concentrations are highly correlated with the severity of Alzheimer's disease. Neurobiol Aging. 2000;21:27–30. doi: 10.1016/s0197-4580(99)00103-7. [DOI] [PubMed] [Google Scholar]
- 41.Bodovitz S, Klein WL. Cholesterol modulates alpha-secretase cleavage of amyloid precursor protein. J Biol Chem. 1996;271:4436–4440. doi: 10.1074/jbc.271.8.4436. [DOI] [PubMed] [Google Scholar]
- 42.Simons M, Keller P, De Strooper B, Beyreuther K, Dotti CG, Simons K. Cholesterol depletion inhibits the generation of beta-amyloid in hippocampal neurons. Proc Natl Acad Sci USA. 1998;95:6460–6464. doi: 10.1073/pnas.95.11.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frears ER, Stephens DJ, Walters CE, Davies H, Austen BM. The role of cholesterol in the biosynthesis of beta-amyloid. Neuroreport. 1999;10:1699–1705. doi: 10.1097/00001756-199906030-00014. [DOI] [PubMed] [Google Scholar]
- 44.Fassbender K, Simons M, Bergmann C, Stroick M, Lutjohann D, Keller P, Runz H, Kuhl S, Bertsch T, von Bergmann K, Hennerici M, Beyreuther K, Hartmann T. Simvastatin strongly reduces levels of Alzheimer's disease beta-amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc Natl Acad Sci USA. 2001;98:5856–5861. doi: 10.1073/pnas.081620098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kojro E, Gimpl G, Lammich S, Marz W, Fahrenholz F. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the alpha-secretase ADAM 10. Proc Natl Acad Sci USA. 2001;98:5815–5820. doi: 10.1073/pnas.081612998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sparks DL, Scheff SW, Hunsaker JC, 3rd, Liu H, Landers T, Gross DR. Induction of Alzheimer-like beta-amyloid immunoreactivity in the brains of rabbits with dietary cholesterol. Exp Neurol. 1994;126:88–94. doi: 10.1006/exnr.1994.1044. [DOI] [PubMed] [Google Scholar]
- 47.Refolo LM, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K, Pappolla MA. Hypercholesterolemia accelerates the Alzheimer's amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- 48.Shie FS, Jin LW, Cook DG, Leverenz JB, LeBoeuf RC. Diet-induced hypercholesterolemia enhances brain A beta accumulation in transgenic mice. Neuroreport. 2002;13:455–459. doi: 10.1097/00001756-200203250-00019. [DOI] [PubMed] [Google Scholar]
- 49.Levin-Allerhand JA, Lominska CE, Smith JD. Increased amyloid-levels in APPSWE transgenic mice treated chronically with a physiological high-fat high-cholesterol diet. J Nutr Health Aging. 2002;6:315–319. [PubMed] [Google Scholar]
- 50.Li L, Cao D, Garber DW, Kim H, Fukuchi K. Association of aortic atherosclerosis with cerebral beta-amyloidosis and learning deficits in a mouse model of Alzheimer's disease. Am J Pathol. 2003;163:2155–2164. doi: 10.1016/s0002-9440(10)63572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cao D, Fukuchi K, Wan H, Kim H, Li L. Lack of LDL receptor aggravates learning deficits and amyloid deposits in Alzheimer transgenic mice. Neurobiol Aging. 2006;27:1632–1643. doi: 10.1016/j.neurobiolaging.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 52.Li L, Cao D, Kim H, Lester R, Fukuchi K. Simvastatin enhances learning and memory independent of amyloid load in mice. Ann Neurol. 2006;60:729–739. doi: 10.1002/ana.21053. [DOI] [PubMed] [Google Scholar]