Abstract
Eyeblink conditioning using a conditioned stimulus (CS) from one sensory modality (e.g., an auditory CS) is greatly enhanced when the subject is previously trained with a CS from a different sensory modality (e.g., a visual CS). The enhanced acquisition to the second modality CS results from cross modal savings. The current study was designed to examine the role of the cerebellum in establishing cross modal savings in eyeblink conditioning with rats. In the first experiment rats were given paired or unpaired presentations with a CS (tone or light) and an unconditioned stimulus (US). All rats were then given paired training with a different modality CS. Only rats given paired training showed cross modal savings to the second modality CS. Experiment 2 showed that cerebellar inactivation during initial acquisition to the first modality CS completely prevented savings when training was switched to the second modality CS. Experiment 3 showed that cerebellar inactivation during initial cross modal training also prevented savings to the second modality stimulus. These results indicate that the cerebellum plays an essential role in establishing cross modal savings of eyeblink conditioning.
Keywords: associative learning, classical conditioning, eyelid conditioning, interpositus nucleus, cerebellar cortex
In a typical eyeblink conditioning experiment, a brief conditioned stimulus (CS), such as a tone or a light, is paired with an unconditioned stimulus (US). Before training, the US elicits an unconditioned eyeblink response (eyelid closure or nictitating membrane (NM) movement). After a sufficient number of CS-US pairings, conditioned eyeblink responses (CRs) occur to presentations of the CS and precede the onset of the US (Gormezano, Schneiderman, Deaux, & Fuentes, 1962; Schneiderman, Fuentes, & Gormezano, 1962).
The brain areas necessary for acquiring delay eyeblink conditioning are located within the cerebellum and its interconnected brainstem nuclei (Christian & Thompson, 2003). In the cerebellum, the anterior cerebellar interpositus nucleus (IPN) and cerebellar cortex (CCTX) are known to be necessary for acquisition, retention and timing of conditioned eyeblink responses (Attwell, Cooke, & Yeo, 2002; Attwell, Ivarsson, Millar, & Yeo, 2002; Attwell, Rahman, & Yeo, 2001; Bao, Chen, Kim, & Thompson, 2002; Clark & Lavond, 1993; Freeman, Halverson, & Poremba, 2005; Krupa & Thompson, 1997; Krupa, Thompson, & Thompson, 1993; McCormick, Clark, Lavond, & Thompson, 1982; McCormick & Thompson, 1984; Ohyama, Nores, Medina, Riusech, & Mauk, 2006; Perrett, Ruiz, & Mauk, 1993). CS and US inputs are relayed to the cerebellum from separate brainstem nuclei. CS information is sent to the cerebellum via pontine mossy fiber projections (Hesslow, Svensson, & Ivarsson, 1999; Steinmetz, Lavond, & Thompson, 1989; Steinmetz, Logan, Rosen, Thompson, Lavond, & Thompson, 1987; Steinmetz, Rosen, Chapman, Lavond, & Thompson, 1986). US information is sent to the cerebellum via climbing fiber input that originates from the inferior olive (Mauk, Steinmetz, & Thompson, 1986). Converging activation from the mossy fiber and climbing fiber pathways in the cerebellum induces plasticity for acquiring and storing the eyeblink CR memory.
Although substantial research has revealed the neuronal mechanisms underlying delay eyeblink conditioning, little attention has been given to the neurobiology of cross modal savings, even though it has received considerable attention in the behavioral literature (e. g., Holt & Kehoe, 1985; Kehoe, 1988; Kehoe & Holt, 1984; Kehoe, Horne, & Macrae, 1995; Kehoe, Morrow, & Holt, 1984; Kehoe & Napier, 1991; Schreurs & Kehoe, 1987). Cross modal savings occurs when a CS-US association is first acquired using one modality CS (e.g., a tone) and subsequent learning with a different modality CS (e.g., a light) is enhanced. The theoretical connectionist network model proposed by Kehoe (1988) offers an explanation for cross modal savings. Briefly, this model proposes that learning with the cross modal stimulus is enhanced because it capitalizes upon previous training with the other modality stimulus. Cross modal savings is a form of general transfer, a “learning-to-learn” phenomenon, because the memory underlying the CR is able to generalize from one sensory modality to another (Harlow, 1949; Kehoe, 1988; Kehoe & Holt, 1984; Kehoe et al., 1995). Importantly, the CR does not immediately generalize across sensory modalities and additional training with the new modality CS is necessary to reacquire the CR. This finding supports the hypothesis that the CS-US association formed during initial acquisition enhances learning to the new modality CS, rather than producing primary stimulus generalization (Kehoe & Holt, 1984).
Since plasticity in the cerebellum (i.e., the IPN and/or the CCTX) has been shown to be necessary for the formation of the memory underlying delay eyeblink conditioning, it is hypothesized that cerebellar plasticity is also involved in establishing the memory necessary for cross modal savings. Additional support for this hypothesis can be found in a computational model of eyeblink conditioning proposed by Mauk and colleagues (Mauk & Donegan, 1997; Medina, Garcia, & Mauk, 2001; Medina, Garcia, Nores, Taylor, & Mauk, 2000; Medina & Mauk, 1999; Medina & Mauk, 2000). Computer simulations of this model indicated that savings of eyeblink conditioning results from synaptic plasticity in the cerebellar IPN (Medina et al., 2001). Medina et al. (2001) tested this hypothesis experimentally in rabbits. They first gave rabbits paired presentations of a tone CS and US to establish eyeblink conditioning. Then, rabbits were given extensive extinction training with tone CS-alone presentations that effectively extinguished eyeblink CRs. According to the computational model the decrease in responding after extinction training results primarily from changes in synaptic plasticity in the CCTX. Thus, they predicted that removal of the CCTX influence on the IPN after extinction training would produce a recovery in CRs. To test this prediction, picrotoxin (GABAA antagonist) was infused into the IPN to block the synaptic input that comes from Purkinje cells in the CCTX. Tone-alone presentations were then used to assess possible savings. The rabbits showed a recovery in CRs, which confirmed the hypothesis that savings for the eyeblink CR was located within the IPN, and that the extinction of the response resulted from synaptic changes within the CCTX (Medina et al., 2001). It is possible synaptic plasticity in the cerebellum, and in particular the IPN, is the important underlying mechanism of savings responsible for enhancing learning to a cross modal stimulus during transfer training.
An alternative hypothesis is that regions outside the cerebellum, such as the hippocampus, amygdala, perirhinal cortex, or prefrontal cortex may be necessary for establishing cross modal savings. These regions have been shown to modulate acquisition of the eyeblink CR and are also important for forming higher-order associations such as trace and contextual conditioning (Campolattaro & Freeman, 2006a; 2006b; Chachich & Powell, 1998; Christian & Thompson, 2003; Kim, Clark, & Thompson, 1995; Lee & Kim, 2004; Nicholson & Freeman, 2000; Solomon, Vander Schaaf, Thompson, & Weisz, 1986; Weible, McEchron, & Disterhoft, 2000; Weiss, Bouwmeester, Power, & Disterhoft, 1999; Weisz, Harden, & Xiang, 1992). Additionally, regions such as the amygdala, perirhinal cortex, and prefrontal cortex receive and/or integrate information from multiple stimulus modalities (Doron & Ledoux, 1999; Furtak, Wei, Agster, & Burwell, 2007; Hoover & Vertes, 2007). Multisensory processing in these regions may be important for enhancing learning during cross modal eyeblink conditioning.
Even though the cerebellar IPN and CCTX are critical for the initial acquisition of excitatory eyeblink conditioning, some evidence suggests that an intact cerebellum is not necessary for establishing more complex forms of eyeblink conditioning. For example, Welsh and Harvey (1991) found that unilateral inactivation of the cerebellar IPN with lidocaine prevented expression, but not learning to a light CS in rabbits that were first given eyeblink conditioning with a tone CS. The finding that transfer of learning to the light CS was not affected by inactivation of the cerebellar IPN might suggest that acquisition to the light CS resulted from non-cerebellar processing. However, it is possible that the infusions used in this study were too small or were placed outside the critical region of the IPN to affect learning to the light CS (Christian & Thompson, 2003). Similar to the findings in the Welsh and Harvey (1991) study, Freeman et al. (2005) found evidence for learning without an intact cerebellum. In the Freeman et al. (2005) study, rats were given excitatory eyeblink conditioning with a tone CS followed by discrimination training between a tone CS that was paired with a US and a compound light/tone CS that was not paired with a US. This type of training was used to establish the light CS as a conditioned inhibitor. During the discrimination training phase rats were given infusions of muscimol into the cerebellar hemisphere that was ipsilateral to US. The spread of muscimol within the cerebellum reached both the IPN and CCTX. Combined inactivation of the IPN and CCTX prevented expression, but not acquisition of discrimination between the tone and compound CSs. Additionally, inhibitory learning to the light CS, which was assessed with a retardation test, was also not affected. The results from these studies suggest that non-cerebellar mechanisms could play a role in cross modal eyeblink conditioning.
The current study was designed to examine the role of the cerebellum in establishing cross modal eyeblink conditioning with rats. The first experiment used the necessary behavioral control conditions to show that cross modal savings relies upon associative learning, rather than non-associative factors. The subsequent experiments used pharmacological inactivation to assess the role of the cerebellum in establishing cross modal savings. The second experiment used cerebellar inactivation with muscimol during acquisition with the first modality CS to determine whether cerebellar plasticity is necessary to observe savings to the second modality CS. In the third experiment, cerebellar inactivation was given during initial acquisition with the second modality CS in order to determine whether additional cerebellar plasticity is required for establishing cross modal savings.
Experiment 1
The aim of this experiment was to examine cross modal savings with rats, which has been previously observed in NM conditioning with rabbits (Holt & Kehoe, 1985; Kehoe, 1988; Kehoe & Holt, 1984; Kehoe et al., 1995; Kehoe, Morrow, & Holt, 1984; Kehoe & Napier, 1991; Schreurs & Kehoe, 1987). Paired and unpaired training conditions were used to show that cross modal savings results from associative learning, and not from non-associative factors such as sensitization or pseudo-conditioning. Rats were given two phases of training. In phase 1, they received five daily 100-trial sessions of either paired or unpaired training with a CS (tone or light) and periorbital shock US. In phase 2, all rats were given two sessions of paired training with the CS modality not used during phase 1.
Method
Subjects
Subjects were 16 male Long-Evans rats (200–250g), approximately 150 days old at the beginning of the experiment. The rats were housed in Spence Laboratories of Psychology at the University of Iowa with a 12-hr light-dark cycle, with light onset at 07:00am.
Surgery
One week prior to training, rats were removed from their home cage and anesthetized by an i.p. injection of sodium pentobarbital (80 mg/kg). An i.p. injection of atropine sulfate (0.45 mg/kg) was administered to reduce respiratory tract secretions. The rats were fitted with differential electromyograph (EMG) electrodes placed in the left upper eyelid muscle (orbicularis oculi) and a ground electrode was attached to a stainless steel skull screw. The EMG electrode leads terminated in gold pins held in a plastic connector, which was secured to the skull with dental acrylic. A bipolar stimulating electrode (for delivering the shock US) was implanted subdermally, immediately caudal to the left eye that was secured to the skull with dental acrylic.
Conditioning Apparatus
The conditioning apparatus consisted of four small-animal sound attenuation chambers (BRS/LVE, Laurel, MD). Within each sound attenuation chamber was a smallanimal operant chamber (BRS/LVE, Laurel, MD) where the rats were kept during conditioning. One wall of the operant chamber was fitted with two speakers. The back wall of the sound attenuating chamber was equipped with a small house light and an exhaust fan. A light bulb (for delivering the light CS) was located on the back wall of the sound attenuating chamber, positioned directly behind the operant chamber. The electrode leads from the rat’s headstage were connected to peripheral equipment and a desktop computer. Computer software controlled the delivery of stimuli and the recording of eyelid EMG activity (JSA Designs, Raleigh, NC). The shock unconditioned stimulus (1–2 mA, DC constant current) was delivered through a stimulus isolator (Model number 365A, World Precision Instruments, Sarasota FL). EMG activity was recorded differentially, filtered (500–5000Hz) and integrated by equipment (JSA Designs, Raleigh, NC) as described in other reports (Freeman et al., 2005; Nicholson & Freeman, 2002).
Conditioning Procedures
All rats recovered from surgery for one week before beginning training. For all rats in this study, the CSs were a 2-kHz tone and a 6 W light (counterbalanced), and the US was a 1–2.0 mA periorbital shock. The duration of each CS was 400 ms, where the onset of the 25 ms US coincided with the offset of the CS. This experiment consisted of two phases. Rats first received five 100-trial sessions of either paired (n = 8) or unpaired (n = 8) training with the first modality CS and the US. Next, all rats were given two 100-trial sessions of paired training with the second modality CS.
Conditioned Responses
Conditioned responses (CRs) were defined as electromyography (EMG) activity that exceeded a threshold of 0.4 units (amplified and integrated units in volts) above the baseline mean during the CS period after 80 ms. CRs during CS-US trials were defined as responses obtained after the baseline period, but before the onset of the US.
Results and Discussion
This experiment demonstrated that robust cross modal savings occurs in eyeblink conditioning with rats, which is similar to the findings previously described with rabbits (Holt & Kehoe, 1985; Kehoe, 1988; Kehoe & Holt, 1984; Kehoe et al., 1995; Kehoe, Morrow, & Holt, 1984; Kehoe & Napier, 1991; Schreurs & Kehoe, 1987). Rats that received paired training during phase 1 acquired a high percentage of eyeblink CRs to the first modality CS reaching asymptotic responding (~85%) by the third training session. The CR percentages for rats that received unpaired training were consistently low (~10%) throughout phase 1. Acquisition to the second modality CS during cross modal training was only enhanced in the rats that were given paired training in phase 1. These rats had ~60% CRs on the first transfer session compared to ~20% CRs observed in the unpaired group. This finding demonstrates that cross modal savings results from associative learning, and not from non-associative factors (Figure 1).
Figure 1.
Cross modal eyeblink conditioning. Mean (± SEM) conditioned response (CR) percentage rats given paired (black circles) or unpaired (white circles) training with CS1 (sessions 1–5) and paired training with CS2 (sessions 6–7). The dashed line separates the sessions for acquisition (CS1) and cross modal training (CS2).
CR percentages from phase 2 were inspected using 10-trial blocks to obtain a detailed temporal analysis for the rate of acquisition. The percentage of CRs for rats given paired training during phase 1 initially occurred at a low level (~10%) on the first block of training, but rapidly increased during the subsequent blocks to ~80% by the 9th block (Figure 2). Acquisition of CRs in rats in the unpaired control group occurred at a slower rate reaching ~70% by the 20th block (Figure 2). The rate of acquisition for rats given unpaired training was also identical to the rate observed in during phase 1 in the group given paired training. It was possible that unpaired training produced inhibition to the first modality CS through learned irrelevance (Rush, Robinette, & Stanton, 2001; Allen, Chelius, Masand, Gluck, Myers, & Schnirman, 2002). However, any inhibition that may have been acquired to the first modality CS did not generalize to impair learning to the second modality CS (Figure 2).
Figure 2.
Mean (± SEM) CR percentage during cross modal training with CS2 in 10-trial blocks for rats that previously received paired (paired-paired; black circles) or unpaired (unpaired-paired; white circles) training with CS1. CR percentages from rats that received paired training during phase 1 (paired CS1; gray circles) are included to show de novo acquisition. The dashed line separates the two sessions of cross modal training.
No statistical differences were found for CS modality type (light vs tone) during acquisition to CS1 or during cross modal transfer to CS2. Analyses were, therefore, collapsed across the stimulus modality factor. A repeated measures ANOVA using group and session factors confirmed that rats given paired training during phase 1 acquired significantly higher CR percentages than rats given unpaired training, F(4,56) = 40.02, p < .001. A follow-up test (Tukey’s honestly significant difference (HSD)) revealed that rats given paired training showed significantly more CRs on sessions 2–5 relative to the unpaired control rats, p < .01 (Figure 1). The data from one rat in each conditioning group was not used for analysis of the phase 2 because they did not complete the second session. A repeated measures ANOVA performed on the stimulus modality, group, and session factors for phase 2 data revealed a significant three-way interaction, F(4,48) = 3.63, p < .001. A follow-up test (HSD) showed this interaction was due to faster acquisition during phase 2 for rats that were initially given paired training (Figure 1). A repeated measures ANOVA revealed a significant interaction between the group and block factors for performance during the cross modal training, F(19,228) = 2.24, p < .01. A follow-up test (HSD) revealed that the rats given paired training during phase 1 produced significantly more eyeblink CRs on blocks 3–18, p < .05, than rats initially given unpaired training (Figure 2). There were no differences between CR percentages in phase 2 for rats in the unpaired control group and de novo acquisition for rats that received paired training during phase 1.
Experiment 2
Inactivation of the cerebellar hemisphere ipsilateral to the trained eye prevents acquisition and behavioral savings of auditory eyeblink conditioning (Clark & Lavond, 1993; Freeman et al., 2005; Krupa & Thompson, 1997; Krupa et al, 1993), which suggests that plasticity in the cerebellum is necessary for learning to occur. The present experiment used muscimol inactivation of the cerebellar IPN and CCTX (Freeman et al., 2005) to test the hypothesis that they are necessary for cross modal eyeblink conditioning. It is possible, however, that regions other than the cerebellum such as the hippocampus, amygdala, perirhinal cortex and/or prefrontal cortex may be capable of establishing the association underlying cross modal savings. In this experiment, rats were given unilateral infusions of muscimol (GABAA agonist) or saline before each of five 100-trial sessions of paired training with first modality CS and the US. Both groups then received two 100-trial drug-free sessions of paired training with the second modality CS to assess cross modal savings.
Method
Surgery
Except for the following changes, the surgery was performed as described in experiment 1. After the onset of anesthesia, a 23 gauge guide cannula (15.0 mm) was chronically implanted immediately above the left IPN in each rat. The coordinates for implanting the guide cannula were AP −11.5 and ML +2.3 from bregma, and DV −5.6 from the surface of the skull. A separate 30 gauge cannula (16.0 mm) was placed within the guide cannula. The guide cannula was fixed to the skull with dental acrylic.
Conditioning procedure and cerebellar inactivation
This experiment consisted of three phases. In phase 1, all rats received five 100-trial sessions with the first modality CS. Sixty minutes before the start of each session, rats received either a 1.0 µL infusion of 10 mM muscimol (n = 12) or 0.1M phosphate buffered saline (PBS, n = 10) into the left interpositus nucleus. Infusions were delivered via a 30 gauge cannula (15.5 mm) connected to polyethelene tubing, which was connected to a Hamilton syringe mounted on an infusion pump (Harvard Apparatus). Infusions were delivered for 60 sec at a rate of 30 µL/hour. Phase 2 consisted of 20 CS alone trials with the same stimulus modality used in phase 1. This phase was necessary to determine whether muscimol infusions blocked learning, and not just expression of the eyeblink CR during phase 1. Extinction trials were used in phase 2 to prevent any acquisition to the first CS from occurring in the absence of muscimol. Two drug-free 100-trial sessions of cross modal training were then given in phase 3.
Histology
Rats were deeply anesthetized with an overdose of sodium pentobarbital (90 mg/kg) and transcardially perfused with 0.9 % saline followed by 3% formalin. The brains were removed from the skull and post-fixed in 0.1M phosphate buffered sugared PBS, and subsequently sectioned at 50 µm on a sliding microtome (American Optical, Buffalo, NY). Cerebellar sections were mounted on slides, stained with thionin, and examined for cannula placement.
Some of the rats (n = 6) were selected to receive infusions of fluorescent muscimol (BODIPY TMR-X Muscimol, Molecular Probes) so that the spread of muscimol in the cerebellum could be determined. The fluorescent muscimol was infused as described in the methods above. Rats were anesthetized and perfused with 4% paraformaldehyde approximately 40–60 minutes after receiving the infusion. The brains were removed and allowed to soak in a 30% sucrose buffered solution for 72 hours. The brains were then sliced on a freezing microtome at 50 µm and mounted on slides. The location of fluorescently labeled tissue was assessed using a microscope equipped with a yellow-orange florescent (572 nm wavelength) filter, and photographed with exposure times that maximized image clarity (Campolattaro & Freeman, 2008).
Results and Discussion
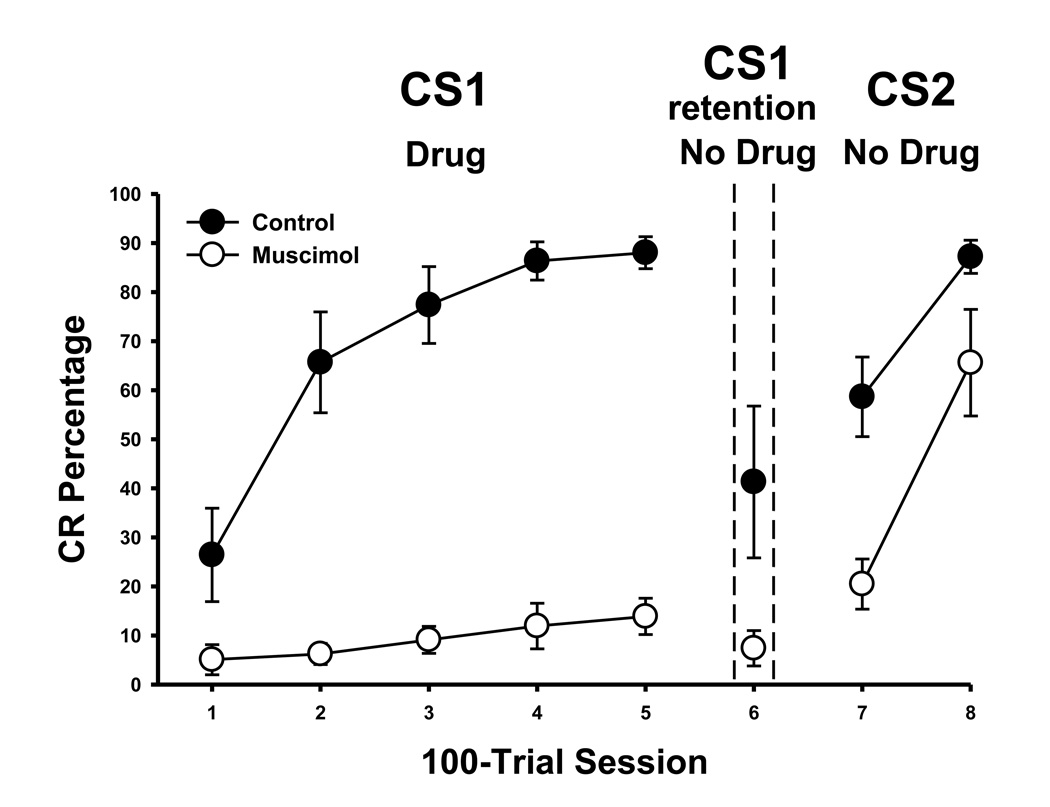
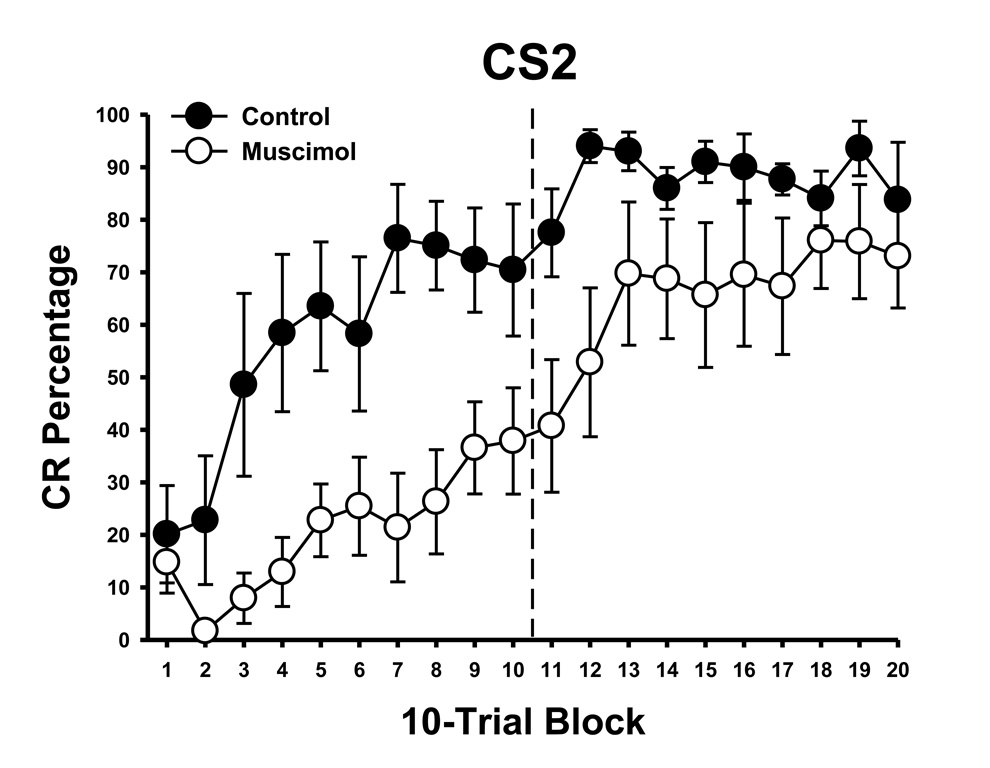
The findings of this experiment showed that the cerebellum is necessary for producing cross modal eyeblink conditioning. The results also replicated the previous finding that unilateral cerebellar inactivation is effective for blocking auditory eyeblink conditioning (Clark & Lavond, 1993; Freeman et al., 2005; Krupa & Thompson, 1997; Krupa et al, 1993), and extends those findings to include a visual CS. CRs for rats in the muscimol group remained consistently low (~10%) during acquisition to the first modality CS (Figure 3, left) relative to the control group which acquired high levels of CRs that were asymptotic (~85%) by the fourth session of training. Rats given muscimol infusions did not show CRs during the retention test in phase 2, confirming that muscimol infusions blocked learning during phase 1 and not only expression of eyeblink CRs (Figure 3, middle). The decrease in CRs in the control group was due to extinction during this test. Rats in the control group showed an enhanced rate of learning during cross modal training in phase 3, whereas acquisition for rats in the muscimol group occurred at a rate that was similar to de novo training (Figure 3, right). Figure 4 shows the rate of acquisition to the cross modal stimulus in 10-trial blocks. Control rats showed rapid acquisition during this phase reaching ~70% by the end of the first session, whereas rats that received muscimol infusions during initial training acquired CRs to ~ 40%.
Figure 3.
Cerebellar inactivation during CS1 acquisition. Mean (± SEM) conditioned response (CR) percentage during CS1 acquisition (session 1–5), CS1 retention (session 6) and CS2 cross modal training (session 7–8) for control (black circles) and muscimol inactivated (white circles) rats. The dashed line separates the sessions for acquisition (CS1, Drug), retention testing (CS1 retention, No Drug), and cross modal training (CS2, No Drug).
Figure 4.
Mean (± SEM) CR percentage during cross modal training with CS2 in 10-trial blocks for rats that received muscimol (black circles) or control (white circles) training during phase 1. The dashed line separates the two sessions of cross modal training.
As in experiment 1, no statistical differences were found for CS modality type (light vs tone) during acquisition to CS1 or during cross modal transfer to CS2. Analyses were, therefore, collapsed across the stimulus modality factor. A repeated measures ANOVA revealed a significant interaction between the session and group factors for CR percentage in phase 1, F(4,80) = 27.3, p < .001. A follow up test showed that control rats acquired significantly more eyeblink CRs than rats in the muscimol group during all five sessions of phase 1 of training, ps < .01. A t-test confirmed that rats in the control group responded significantly more than rats in the muscimol group during the retention test, t(20) = 3.27, p < .01. A repeated measures ANOVA of the group and session factors revealed a significant group effect in phase 3 cross modal training, which was due to faster acquisition in the control group, F(1,20) = 59.84, p < .001. Analysis of the block data in the phase 3 revealed a significant interaction between the group and session factors, F(19, 380) = 2.69, p < .001. A follow up test (HSD) showed that this interaction was due to faster acquisition in the control group on blocks 2–17, p < .05.
Histological examination showed that all infusion cannula tips were located within or immediately dorsal to the IPN. An example of the typical cannula placement is shown in figure 5A. Inspection of the brain sections for rats infused with fluorescent muscimol at the end of training showed that the muscimol infusions were contained within the left cerebellum with a lateral spread of ~0.5–1.0 mm to include both the left anterior and posterior IPN and overlying CCTX (HVI and lateral portions of anterior lobe; Figure 5B). The spread of fluorescent muscimol in the current study was similar to the findings reported by a previous study that used 2-DG autoradiography quantification methods to assess the spread of muscimol inactivation (Freeman et al., 2005).
Figure 5.
Cerebellar histology. A. A photograph depicting the location of a cannula tip placement for one rat in the cerebellar interpositus nucleus (IPN). The arrow indicates the location of the cannula tip. B. A photograph that shows a representative example of the spread of fluorescent muscimol (gray areas) to the cerebellar IPN, overlying cortex (HVI), and lateral anterior lobe (ANT).
Experiment 3
Experiment 2 showed that a functional cerebellum is necessary for cross modal savings of eyeblink conditioning. However,Welsh and Harvey (1991) found that unilateral inactivation of the cerebellar IPN with lidocaine prevented expression, but not learning to a light CS in rabbits that were first trained with a tone CS. This finding might suggest that additional learning with a new modality CS does not depend upon additional plasticity in the IPN. It was possible that cross modal savings for eyeblink conditioning occurs in other brain regions after a subject receives initial training, perhaps due to cerebellar feedback (Clark, McCormick, Lavond, & Thompson, 1984). Experiment 3 was designed to examine the effect of cerebellar inactivation on cross modal eyeblink conditioning in rats after they were trained with the first modality CS. The present experiment used muscimol infusions that were large enough to inactivate both the IPN and overlying regions of the CCTX, unlike the Welsh and Harvey (1991) study that only partially inactivated the IPN. After five sessions of training with the first modality CS, rats were given a unilateral muscimol or saline infusion before each of the first two sessions of cross modal training. Both groups of rats were then given two additional infusion-free sessions of cross modal training to assess savings.
Method
Excepted for the following changes, the surgery, conditioning procedures, and histology were the same as described in experiment 2. Rats in the muscimol group (n = 12) were anesthetized by isoflurane (1.5–2.0%) during surgery, whereas the controls rats (n = 12) were taken from a prior experiment that used sodium pentobarbital anesthesia. Acquisition of eyeblink conditioning in the group was nearly identical indicating that anesthesia type had no impact on the results (Figure 6). This experiment consisted of two phases. In phase 1, all rats were given five 100-trial sessions of CS training with the first modality CS. In phase 2, rats received infusions of muscimol or PBS and were given cross modal training with the second modality CS. An additional two infusion-free sessions of cross modal training were given to assess cross modal savings. These additional sessions were necessary because muscimol infusions were expected to block CR expression during the first two sessions of cross modal training.
Figure 6.
Cerebellar inactivation during phase 2 acquisition. Mean (± SEM) conditioned response CR during acquisition (session 1–5) and cross modal training (session 6–9) for control (black circles) and inactivated (white circles) rats. The dashed line separates the sessions for acquisition (CS1 Drug) and cross modal training sessions (CS2 Drug and CS2 No Drug).
Results and Discussion
Unilateral cerebellar inactivation during cross modal training prevented cross modal facilitation of eyeblink conditioning. Rats in the muscimol and control group acquired high levels of CRs that were asymptotic (~85%) by the fourth session of training during acquisition to the first modality CS (Figure 6, left). Rats given muscimol infusions in the cerebellum during the first two sessions of cross modal training produced few CRs (~10%), whereas rats that were given control infusions showed an enhanced rate of learning during these sessions (~70 and 90% respectfully, Figure 6, middle). Rats in the muscimol group required additional training with the second modality CS to match the percentages of eyeblink CRs observed in the control group (Figure 6, right), which indicates that learning to the cross modal CS was significantly impaired when the cerebellum was inactivated. Muscimol infusions in the cerebellum were successful in blocking cross modal facilitation of conditioning to the second modality CS even though rats were well-trained with the first modality CS. Additionally, the rate of acquisition, and overall CR percentages, during the initial cross modal savings test for rats in the control group (sessions 6–7) were the same as those observed during the drug-free sessions of cross modal training (sessions 8–9) for rats in the muscimol group (Figure 7). The rate transfer to the second modality CS for this group was slightly faster than the rate of transfer observed in experiments 1 and 2 (muscimol group in Figure 7 versus control group in Figure 4 or the paired-paired group in Figure 2). However, a repeated measures ANOVA using the block and group factors did not reveal any significant differences in CR percentages during acquisition of the second CS across these experiments.
Figure 7.
Mean (± SEM) CR percentage during phase 2 training (sessions 8–9) in 10-trial blocks for rats that received muscimol (black circles) or control (white circles) training during cross modal training (session 6–7). The dashed line separates the two sessions of cross modal training.
As in experiments 1 and 2, no statistical differences were found for CS modality type (light vs tone) during acquisition to CS1 or during cross modal transfer to CS2. Analyses were, therefore, collapsed across the stimulus modality factor. No statistical differences in CR percentage were found during acquisition to the first modality CS between rats in the muscimol and control groups (Figure 6, left). A repeated measures ANOVA confirmed that rats infused with muscimol produced significantly fewer CRs during the first two sessions of cross modal training than rats given saline infusions, F(1,22) = 15.32, p < .001 (Figure 6, middle). A follow up test (HSD) showed that these differences occurred on both sessions (6–7) of the initial cross modal savings test, p < .01. No statistical differences were found for CR percentage on sessions 6–7 for rats in the control group verses sessions 8–9 for rats in the muscimol group (Figure 6, right). Analysis of the block data for the drug-free sessions of cross modal training revealed a significant interaction between the group and session factors, F(19,418) = 10.52, p < .001. A follow up test (HSD) showed that this interaction was due to significantly fewer CRs for rats in muscimol group on blocks 1–5, p < .01 (Figure 7).
The current experiment showed that unilateral inactivation in the cerebellar hemisphere ipsilateral to the trained eye prevents savings to the second modality CS. This finding differs from a previous experiment that found that unilateral inactivation of the cerebellar IPN effects only expression, but not learning, of cross modal eyeblink conditioning (Welsh & Harvey, 1991). The current findings support the hypothesis that the ipsilateral cerebellum is necessary for acquisition and expression of cross modal eyeblink conditioning.
General Discussion
The present series of experiments demonstrated that cross modal savings occurs robustly in eyeblink conditioning with rats, and that enhanced acquisition to the second modality CS depends upon processing in the cerebellum. Experiment 1 used a control condition to show that cross modal eyeblink conditioning in rats relies on associative learning and not non-associative factors such as sensitization or pseudo-conditioning. This experiment also showed that cross modal eyeblink conditioning in rats is similar to the findings with rabbits (Holt & Kehoe, 1985; Kehoe, 1988; Kehoe & Holt, 1984; Kehoe, Horne, & Macrae, 1995; Kehoe, Morrow, & Holt, 1984; Kehoe & Napier, 1991; Schreurs & Kehoe, 1987). Experiment 2 showed that cross modal savings was prevented when the ipsilateral cerebellum was inactivated during acquisition with the first modality CS, indicating that learning induced cerebellar plasticity is important for establishing cross modal savings. Experiment 3 showed that inactivation of the ipsilateral cerebellum also impaired acquisition and prevented savings to the second modality CS when muscimol was administered during initial cross modal training in rats that were already trained with the first CS. Additional training during the drug-free cross modal training sessions was necessary for the inactivated rats to establish a percentage of CRs that matched the control group.
Harvey and Welsh (1991). found that unilateral inactivation of the IPN did not affect cross modal savings to a light CS in rabbits that were well-trained with a tone CS. It is possible that the infusions in the Harvey and Welsh (1991)study, which were contained within the IPN, were either too small or placed outside the critical region of the IPN to block cross modal savings (Christian & Thompson, 2003). A more recent study by Yeo, Lobo, and Baum (1997) showed that infusions of muscimol into the IPN produced a large, but not complete, impairment in the transfer of CR timing from one ISI to another. In that study, rabbits were first trained to a tone CS until they acquired CRs that had peak latencies accurately timed for a particular ISI. The rabbits were then given muscimol or control infusions into the IPN and trained with a different ISI. During subsequent drug-free sessions, they found that rabbits given unilateral IPN muscimol infusions that were determined to have spread to the IPN and CCTX (lobule HVI, ansiform lobule, and anterior lobe) were impaired during acquisition to the new ISI. This finding, as well as the data from the current experiments, supports the hypothesis that inactivation of the IPN and CCTX is necessary to impair new learning during transfer training.
The current study used muscimol infusion volumes (1.0µL) that were large enough to inactivate both the IPN and overlaying CCTX, which had a strong effect on cross modal savings. There are some findings that support the hypothesis that the CCTX is important for establishing cross modal eyeblink conditioning. Garcia, Steele, and Mauk (1999) showed that unilateral CCTX lesions in rabbits after they were first given eyeblink conditioning with one CS modality impaired acquisition to a new CS, indicating that the CCTX may play an important role in establishing savings for transfer between tone and vibrotactical CSs. It is also possible that a functional CCTX is important for transfer between the tone and visual CSs used in the current study, which is a hypothesis supported by the finding that some Purkinje cells in lobules HVI and HVII in the CCTX are responsive to both of these stimulus modalities (Caan, Delgado-Garcia, Stein, & Wattam-Bell, 1976). Purkinje cells may help integrate CS information that is sent to the cerebellum from the different CS modalities. On the other hand, the aforementioned study by Medina et al. (2001) indicted that the IPN could be the site of plasticity underlying savings, and possibly cross modal savings. Further experiments will be necessary to determine the independent roles of the IPN and CCTX in establishing savings between tone and visual CSs.
The connectionist network model proposed by Kehoe (1988) offers an explanation for the cross modal savings observed in the present experiments conducted with rats, as well as previous experiments that used rabbits (Holt & Kehoe, 1985; Kehoe, 1988; Kehoe & Holt, 1984; Kehoe, Horne, & Macrae, 1995; Kehoe, Morrow, & Holt, 1984; Kehoe & Napier, 1991; Schreurs & Kehoe, 1987). The basic model is composed of three input units: a CS1 (tone CS), a CS2 (light CS), and a US. These inputs converge on a unit called X. The X unit projects to a response unit (R), which is responsible for driving the CR. The US also projects to unit R to produce a UR. According to this model, the X-R connection becomes stronger as a function of CS1-US pairings converging on X. The X-R connection becomes vulnerable to control by CS1 as a consequence of CS1 and US converging on X. That is, activation of CS1 will eventually drive X-R to produce an eyeblink CR (Kehoe, 1988; Kehoe et al., 1995). The mechanism for cross modal savings in this model is the enduring strength of the X-R connection that is established through CS1-US pairings. Acquisition to CS2 (the cross modal CS) is facilitated because the X-R connection has been previously modified through CS1-US pairings.
There are parallels between the hypothetical units proposed in the Kehoe model of learning and the physiological models for cerebellar learning (i.e., Christian & Thompson, 2003; Mauk & Donagan 1997). The CSs (T and L) and US (US) input units converging on the hidden X unit roughly correspond to the convergence of pontine and inferior olive projections to the cerebellum. The connection between the X and output response unit R corresponds to the connections between the cerebellum and the red nucleus, which drives the CR through via the facial nucleus that innervates the eyelid muscles. The red nucleus is necessary for the production, but not acquisition or storage of a conditioned eyeblink response (Clark & Lavond, 1993). The connection between the US and R units mirror the relationship between an actual US’s influence on the facial nucleus to produce a UR. In sum, both models offer similar circuitries for the production of both the UR and CR.
As noted above, the Kehoe (1988) model states that the X-R connection undergoes modification to store the associative relationship between the CS and US. Here, the Kehoe (1988) model of conditioning does not completely correspond to physiological mechanisms of eyeblink conditioning (Christian & Thompson, 2003). However, if it is assumed that modification of the X-R connection is induced by X, then the correspondence between the X-R modification hypothesis and learning-related changes within the cerebellum can offer similar accounts for the mechanism of cross modal savings. A possible explanation that may account for cross modal savings is the ability of the new modality CS to capitalize on the learning-induced changes that occurred within cerebellar neurons from training with the first modality CS (Medina et al., 2001). For example, learning may be accelerated because the new modality CS benefits from the increased number of excitatory synapses in the IPN that are formed during initial training (Kleim, Freeman, Bruneau et al., 2002). Learning may also be enhanced because the new modality CS takes advantage of increased intrinsic excitability of cells within the cerebellar IPN (Aizenmen & Linden, 2000; Ohyama, Nores, & Mauk, 2003) or CCTX (Schreurs & Alkon, 1993; Schreurs, Gusev, Tomsic, Alkon & Shi, 1998) that develops after initial training.
It is possible that the enhanced rate of transfer also benefits from increased salience of the second modality CS due to a reduction in contextual associations that initially compete for associative strength. During phase 1, the CS, US, and background contextual cues are all novel features of the training environment. As training progresses the CS-US association is strengthened whereas context-US associations are reduced (Mackintosh, 1975). During the transfer phase, the new modality CS is very salient relative to the neutralized features of the training context and therefore may have enhanced learning. The present experiments do not address this hypothesis directly, but it is possible that shifting the context immediately before the start of transfer training will decrease the salience, and the amount of savings, to the new modality CS (Kehoe & Holt, 1984; Kehoe Morrow, & Holt, 1984).
Mulitmodal processing within/or between the IPN, CCTX and pontine nucleus may be important for establishing cross modal savings. Information from auditory and visual CSs is sent to the cerebellum via mossy fibers that arise from the pontine nuclei (Bjaalie & Brodal, 1989; Campolattaro, Halverson, & Freeman, 2007; Parenti, Zappalà Serapide, Pantò & Cicirata, 2002; Steinmetz & Sengelaub, 1992; Watt & Mihailoff, 1983). A recent neuroanatomical tracing study with cats showed that visual and auditory modalities overlap within the rostral portion of the ventral pontine nucleus (Perales, Winer, & Priento, 2006). Although different sensory modalities overlap within the pons, it is not known if individual pontine neurons process both visual and auditory information. It is possible that CS modalities are segregated within the pontine nucleus, as damage in the lateral pontine nucleus has been shown to selectively impair eyeblink conditioning with an auditory CS, and not to a visual CS (Christian & Thompson, 2003; Steinmetz et al., 1987). However, lesions to mossy fibers that project CS information from the pontine nucleus to the cerebellum have been shown to abolish eyeblink conditioning to both auditory and visual CSs (Lewis, LoTurco, & Solomon, 1987). These findings suggest that auditory and visual CS information is segregated in the pontine nuclei and is then integrated within the cerebellum. Support for this hypothesis comes from a study by Tracy, Britton, and Steinmentz (2001) that found evidence of multimodal processing in cerebellar IPN neurons in rabbits given eyeblink conditioning with both auditory and visual CSs. Interestingly, IPN neurons and the behavioral eyeblink CR do not immediately generalize from one modality CS to another (Kehoe & Holt, 1984; Ohyama et al., 2003). That is, additional training with the new modality CS may be necessary for eliciting multimodal responses in IPN neurons. It is possible that multisensory integration in the cerebellum is important for enhancing learning during cross modal eyeblink conditioning.
In conclusion, muscimol inactivation of the cerebellar IPN and CCTX prevented enhanced learning to the cross modal stimulus when it was administered during training with either the first or second modality CS. The findings, therefore, support the hypothesis that a functionally intact cerebellum is necessary for establishing cross modal eyeblink conditioning. Future experiments will address how the different modality CS pathways (e.g., auditory and visual) interact with the cerebellum to produce cross modal savings of eyeblink conditioning.
Acknowledgments
This research was supported by National Institute for Mental Health grant MH080005 to JHF.
References
- Aizemen CD, Linden DJ. Rapid, synaptically driven increases in the intrinsic excitability of cerebellar deep nuclear neurons. Nature Neuroscience. 2000;3(2):109–111. doi: 10.1038/72049. [DOI] [PubMed] [Google Scholar]
- Allen MT, Chelius L, Masand V, Gluck MA, Myers CE, Schnirman G. Integrative Physiological & Behavioral Science. 2002;37(3):188–124. doi: 10.1007/BF02734181. [DOI] [PubMed] [Google Scholar]
- Attwell PJ, Cooke SF, Yeo CH. Cerebellar function in consolidation of a motor memory. Neuron. 2002;34(6):1011–1020. doi: 10.1016/s0896-6273(02)00719-5. [DOI] [PubMed] [Google Scholar]
- Attwell PJ, Ivarsson M, Millar L, Yeo CH. Cerebellar mechanisms in eyeblink conditioning. Annals of the New York Academy of Sciences. 2002;978:79–92. doi: 10.1111/j.1749-6632.2002.tb07557.x. [DOI] [PubMed] [Google Scholar]
- Attwell PJ, Rahman S, Yeo CH. Acquisition of eyeblink conditioning is critically dependent on normal function in cerebellar cortical lobule HVI. The Journal of Neuroscience. 2001;21(15):5715–5722. doi: 10.1523/JNEUROSCI.21-15-05715.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Chen L, Kim JJ, Thompson RF. Cerebellar cortical inhibition and classical eyeblink conditioning. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(3):1592–1597. doi: 10.1073/pnas.032655399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjaalie JG, Brodal P. Visual pathways to the cerebellum: segregation in the pontine nuclei of terminal fields from different visual cortical areas in the cat. Neuroscience. 1989;29(1):95–107. doi: 10.1016/0306-4522(89)90335-7. [DOI] [PubMed] [Google Scholar]
- Campolattaro MM, Freeman JF. Perirhinal cortex lesions impair feature-negative discrimination. Neurobiology of Learning & Memory. 2006a;86(2):205–213. doi: 10.1016/j.nlm.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolattaro MM, Freeman JF. Perirhinal cortex lesions impair simultaneous but not serial feature-positive discrimination learning. Behavioral Neuroscience. 2006b;120(4):970–975. doi: 10.1037/0735-7044.120.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolattaro MM, Freeman JF. Eyeblink conditioning in 12-day-old rats using pontine stimulation as the conditioned stimulus. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(23):8120–8123. doi: 10.1073/pnas.0712006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolattaro MM, Halverson HE, Freeman JF. Medial auditory thalamic stimulation as a conditioned stimulus for eyeblink conditioning in rats. Learning & Memory. 2007;14(3):152–159. doi: 10.1101/lm.465507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caan W, Delgado-Garcia J, Stein JF, Wattam-Bell J. Proceedings: Interaction of visual and auditory inputs to cerebellar purkinje cells in cat posterior vermis. The Journal of Physiology. 1976;258(1):20P–21P. [PubMed] [Google Scholar]
- Chachich M, Powell DA. Both medial prefrontal and amygdala central nucleus lesions abolish heart rate classical conditioning, but only prefrontal lesions impair reversal of eyeblink differential conditioning. Neuroscience Letters. 1998;257(3):151–154. doi: 10.1016/s0304-3940(98)00832-5. [DOI] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learning & Memory. 2003;10(6):427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Clark GA, McCormick DA, Lavond DG, Thompson RF. Effects of lesions of cerebellar nuclei on conditioned behavioral and hippocampal responses. Brain Research. 1984;29(1):125–136. doi: 10.1016/0006-8993(84)90658-9. [DOI] [PubMed] [Google Scholar]
- Clark RE, Lavond DG. Reversible lesions of the red nucleus during acquisition and retention of a classically conditioned behavior in rabbits. Behavioral Neuroscience. 1993;107(2):264–270. doi: 10.1037//0735-7044.107.2.264. [DOI] [PubMed] [Google Scholar]
- Doron NN, LeDoux JE. Organization of projections to the lateralamygdale from auditory and visual areas of the thalamus in the rat. The Journal of Comparative Neurology. 1999;412(3):383–409. [PubMed] [Google Scholar]
- Freeman JH, Jr, Halverson HE, Poremba A. Differential effects of cerebellar inactivation on eyeblink conditioned excitation and inhibition. The Journal of Neuroscience. 2005;25(4):889–895. doi: 10.1523/JNEUROSCI.4534-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtak SC, Wei S, Agster KL, Burwell RD. Functional neuroanatomy of the parahippicampal region in the rat: the perirhinal and postrhinal cortices. Hippocampus. 2007;17(9):709–722. doi: 10.1002/hipo.20314. [DOI] [PubMed] [Google Scholar]
- Garcia KS, Steele PM, Mauk MD. Cerebellar cortex lesions prevent acquisition of conditioned eyelid responses. The Journal of Neuroscience. 1999;19(24):10940–10947. doi: 10.1523/JNEUROSCI.19-24-10940.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormezano I, Schneiderman N, Deaux EG, Fuentes I. Nictitating membrane: classical conditioning and extinction in the albino rabbit. Science. 1962;138:33–34. doi: 10.1126/science.138.3536.33. [DOI] [PubMed] [Google Scholar]
- Harlow HF. The formation of learning sets. Psychological Review. 1949;56(1):51–65. doi: 10.1037/h0062474. [DOI] [PubMed] [Google Scholar]
- Hesslow G, Svensson P, Ivarsson M. Learned movements elicited by direct stimulation of cerebellar mossy fiber afferents. Neuron. 1999;24(1):179–185. doi: 10.1016/s0896-6273(00)80831-4. [DOI] [PubMed] [Google Scholar]
- Holt PE, Kehoe EJ. Cross-modal transfer as a function of similarities between training task in classical conditioning of the rabbit. Animal Learning & Behavior. 1985;13(1):51–59. [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Structure and Function. 2007;212(2):149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Kehoe EJ. A layered network model of associative learning: learning to learn and configuration. Psychological Review. 1988;95(4):411–433. doi: 10.1037/0033-295x.95.4.411. [DOI] [PubMed] [Google Scholar]
- Kehoe EJ, Holt PE. Transfer across CS-US intervals and sensory modalities in classical conditioning of the rabbit. Animal Learning & Behavior. 1984;12(2):122–128. [Google Scholar]
- Kehoe EJ, Horne AJ, Macrae M. Learning to learn: real-time features and a connectionist model. Adaptive Behavior. 1995;3(3):235–271. [Google Scholar]
- Kehoe EJ, Morrow LD, Holt PE. General transfer across sensory modalities survives reductions in the original conditioned reflex in the rabbit. Animal Learning & Behavior. 1984;12(2):129–136. [Google Scholar]
- Kehoe EJ, Napier RM. In the blink of an eye: real-time stimulus factors in delay and trace conditioning of the rabbit's nictitating membrane response. The Quarterly Journal of Experimental Psychology. B, Comparative and Physiological Psychology. 1991;43(3):257–277. [PubMed] [Google Scholar]
- Kim JJ, Clark RE, Thompson RF. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behavioral Neuroscience. 1995;109(2):195–203. doi: 10.1037//0735-7044.109.2.195. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Freeman JH, Jr, Bruneau R, Nolan BC, Cooper NR, Zook A, Walters D. Synapse formation is associated with memory storage in the cerebellum. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(20):13228–13231. doi: 10.1073/pnas.202483399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupa DJ, Thompson JK, Thompson RF. Localization of a memory trace in the mammalian brain. Science. 1993;260(5110):989–991. doi: 10.1126/science.8493536. [DOI] [PubMed] [Google Scholar]
- Krupa DJ, Thompson RF. Reversible inactivation of the cerebellar interpositus nucleus completely prevents acquisition of the classically conditioned eye-blink response. Learning & Memory. 1997;3(6):545–556. doi: 10.1101/lm.3.6.545. [DOI] [PubMed] [Google Scholar]
- Lee T, Kim JJ. Differential effects of cerebellar, amygdalar, and hippocampal lesions on classical eyeblink conditioning in rats. The Journal of Neuroscience. 2004;24(13):3242–3250. doi: 10.1523/JNEUROSCI.5382-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JL, Lo Turco JJ, Solomon PR. Lesions of the middle cerebellar peduncle disrupt acquisition and retention of the rabbit's classically conditioned nictitating membrane response. Behavioral Neuroscience. 1987;101(2):151–157. doi: 10.1037//0735-7044.101.2.151. [DOI] [PubMed] [Google Scholar]
- Mauk MD, Donegan NH. A model of pavlovian eyelid conditioning based on the synaptic organization of the cerebellum. Learning & Memory. 1997;4(1):130–158. doi: 10.1101/lm.4.1.130. [DOI] [PubMed] [Google Scholar]
- Mauk MD, Steinmetz JE, Thompson RF. Classical conditioning using stimulation of the inferior olive as the unconditioned stimulus. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(14):5349–5353. doi: 10.1073/pnas.83.14.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Clark GA, Lavond DG, Thompson RF. Initial localization of the memory trace for a basic form of learning. Proceedings of the National Academy of Sciences of the United States of America. 1982;79(8):2731–2735. doi: 10.1073/pnas.79.8.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Thompson RF. Cerebellum: essential involvement in the classically conditioned eyelid response. Science. 1984;223(4633):296–299. doi: 10.1126/science.6701513. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. A theory of attention: variation in the associability of stimuli with reinforcement. Psychological Review. 1975;82(4):276–298. [Google Scholar]
- Medina JF, Garcia KS, Mauk MD. A mechanism for savings in the cerebellum. The Journal of Neuroscience. 2001;21(11):4081–4089. doi: 10.1523/JNEUROSCI.21-11-04081.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Garcia KS, Nores WL, Taylor NM, Mauk MD. Timing mechanisms in the cerebellum: testing predictions of a large-scale computer simulation. The Journal of Neuroscience. 2000;20(14):5516–5525. doi: 10.1523/JNEUROSCI.20-14-05516.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Mauk MD. Simulation of cerebellar motor learning: computational analysis of plasticity at the mossy fiber to deep nucleus synapse. The Journal of Neuroscience. 1999;19(16):7140–7151. doi: 10.1523/JNEUROSCI.19-16-07140.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Mauk MD. Computer simulation of cerebellar information processing. Nature Neuroscience. 2000;3 Suppl:1205–1211. doi: 10.1038/81486. [DOI] [PubMed] [Google Scholar]
- Nicholson DA, Freeman JH., Jr Lesions of the perirhinal cortex impair sensory preconditioning in rats. Behavioural Brain Research. 2000;112(1–2):69–75. doi: 10.1016/s0166-4328(00)00168-6. [DOI] [PubMed] [Google Scholar]
- Nicholson DA, Freeman JH., Jr Neuronal correlates of conditioned inhibition of the eyeblink response in the anterior interpositus nucleus. Behavioral Neuroscience. 2002;116(1):22–36. [PubMed] [Google Scholar]
- Ohyama T, Nores WL, Mauk MD. Stimulus generalization of conditioned eyelid responses produced without cerebellar cortex: implications for plasticity in the cerebellar nuclei. Learning & Memory. 2003;10(5):346–354. doi: 10.1101/lm.67103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Nores WL, Medina JF, Riusech FA, Mauk MD. Learning-induced plasticity in deep cerebellar nucleus. The Journal of Neuroscience. 2006;26(49):12656–12663. doi: 10.1523/JNEUROSCI.4023-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parenti R, Zappalà A, Serapide MF, Pantò MR, Cicirata F. Projections of the basilar pontine nuclei and nucleus reticularis tegmenti pontis to the cerebellar nuclei of the rat. The Journal of Comparative Neurology. 2002;452(2):115–127. doi: 10.1002/cne.10316. [DOI] [PubMed] [Google Scholar]
- Perales M, Winer JA, Prieto JJ. Focal projections of cat auditory cortex to the pontine nuclei. The Journal of Comparative Neurology. 2006;497(6):959–980. doi: 10.1002/cne.20988. [DOI] [PubMed] [Google Scholar]
- Perrett SP, Ruiz BP, Mauk MD. Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. The Journal of Neuroscience. 1993;13(4):1708–1718. doi: 10.1523/JNEUROSCI.13-04-01708.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AN, Robinette BL, Stanton ME. Ontogenetic differences in the effects of unpaired stimulus preexposure on eyeblink conditioning in the rat. Developmental Psychobiology. 2000;39(1):8–18. doi: 10.1002/dev.1023. [DOI] [PubMed] [Google Scholar]
- Schneiderman N, Fuentes I, Gormezano I. Acquisition and extinction of the classically conditioning eyelid response in the albino rabbit. Science. 1962;136:650–652. doi: 10.1126/science.136.3516.650. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Alkon DL. Rabbit cerebellar slice analysis of long-term depression and its role in classical conditioning. Brain Research. 1993;631(2):235–240. doi: 10.1016/0006-8993(93)91540-9. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Gusev PA, Tomsic D, Alkon DL, Shi T. Intracellular correlates of acquisition and long-term memory of classical conditioning in purkinje cell dendrites in slices of rabbit cerebellar lobule HVI. The Journal of Neuroscience. 1998;18(14):5498–5507. doi: 10.1523/JNEUROSCI.18-14-05498.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs BG, Kehoe EJ. Cross-modal transfer as a function of initial training levels in classical conditioning with the rabbit. Learning & Behavior. 1987;15(1):47–54. [Google Scholar]
- Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit's classically conditioned nictitating membrane response. Behavioral Neuroscience. 1986;100(5):729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Lavond DG, Thompson RF. Classical conditioning in rabbits using pontine nucleus stimulation as a conditioned stimulus and inferior olive stimulation as an unconditioned stimulus. Synapse. 1989;3(3):225–233. doi: 10.1002/syn.890030308. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Logan CG, Rosen DJ, Thompson JK, Lavond DG, Thompson RF. Initial localization of the acoustic conditioned stimulus projection system to the cerebellum essential for classical eyelid conditioning. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(10):3531–3535. doi: 10.1073/pnas.84.10.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz JE, Rosen DJ, Chapman PF, Lavond DG, Thompson RF. Classical conditioning of the rabbit eyelid response with a mossy-fiber stimulation CS: I. pontine nuclei and middle cerebellar peduncle stimulation. Behavioral Neuroscience. 1986;100(6):878–887. doi: 10.1037//0735-7044.100.6.878. [DOI] [PubMed] [Google Scholar]
- Steinmentz JE, Sengelaub DR. Possible conditioned stimulus pathway for classical eyelid conditioning in rabbits. I. anatomical evidence for direct projections from the pontine nuclei to the cerebellar interpositus nucleus. Behavioral & Neural Biology. 1992;57(2):103–115. doi: 10.1016/0163-1047(92)90593-s. [DOI] [PubMed] [Google Scholar]
- Tracy JA, Britton GB, Steinmetz JE. Comparison of single unit responses to tone, light, and compound conditioned stimuli during rabbit classical eyeblink conditioning. Neurobiology of Learning & Memory. 2001;76(3):253–267. doi: 10.1006/nlme.2001.4024. [DOI] [PubMed] [Google Scholar]
- Watt CB, Mihailoff GA. The cerebellopontine system in the rat. I. autoradiographic studies. The Journal of Comparative Neurology. 1983;215(3):312–330. doi: 10.1002/cne.902150307. [DOI] [PubMed] [Google Scholar]
- Weible AP, McEchron MD, Disterhoft JF. Cortical involvement in acquisition and extinction of trace eyeblink conditioning. Behavioral Neuroscience. 2000;114(6):1058–1067. doi: 10.1037//0735-7044.114.6.1058. [DOI] [PubMed] [Google Scholar]
- Weiss C, Bouwmeester H, Power JM, Disterhoft JF. Hippocampal lesions prevent trace eyeblink conditioning in the freely moving rat. Behavioural Brain Research. 1999;99(2):123–132. doi: 10.1016/s0166-4328(98)00096-5. [DOI] [PubMed] [Google Scholar]
- Weisz DJ, Harden DG, Xiang Z. Effects of amygdala lesions on reflex facilitation and conditioned response acquisition during nictitating membrane response conditioning in rabbit. Behavioral Neuroscience. 1992;106(2):262–273. doi: 10.1037//0735-7044.106.2.262. [DOI] [PubMed] [Google Scholar]
- Welsh JP, Harvey JA. Pavlovian conditioning in the rabbit during inactivation of the interpositus nucleus. The Journal of Physiology. 1991;444:459–480. doi: 10.1113/jphysiol.1991.sp018888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo CH, Lobo DH, Baum A. Acquisition of a new-latency conditio ned nictitating membrane response-major, but not complete, dependence on the ipsilateral cerebellum. Learning & Memory. 1997;3(6):557–577. doi: 10.1101/lm.3.6.557. [DOI] [PubMed] [Google Scholar]