Abstract
Age-dependent bone loss has been well documented in both human and animal models. Although the underlying causal mechanisms are probably multifactorial, it has been hypothesized that alterations in progenitor cell number or function are important. Little is known regarding the properties of bone marrow stromal cells (BMSCs) or bone progenitor cells during the aging process, so the question of whether aging alters BMSC/progenitor osteogenic differentiation remains unanswered. In this study, we examined age-dependent changes in bone marrow progenitor cell number and differentiation potential between mature (3 and 6 mo old), middle-aged (12 and 18 mo old), and aged (24 mo old) C57BL/6 mice. BMSCs or progenitors were isolated from five age groups of C57BL/6 mice using negative immunodepletion and positive immunoselection approaches. The osteogenic differentiation potential of multipotent BMSCs was determined using standard osteogenic differentiation procedures. Our results show that both BMSC/progenitor number and differentiation potential increase between the ages of 3 and 18 mo and decrease rapidly thereafter with advancing age. These results are consistent with the changes of the mRNA levels of osteoblast lineage–associated genes. Our data suggest that the decline in BMSC number and osteogenic differentiation capacity are important factors contributing to age-related bone loss.
Key words: aging, mesenchymal stromal cells, osteoblast differentiation
INTRODUCTION
Aging is accompanied by a progressive loss of bone mass, decreased bone quality, and decreased bone strength, which together increase fracture risk.(1–4) These changes in bone structure and biomechanical properties result from a net imbalance between bone formation and bone breakdown. The decrease in the number and activity of bone-forming osteoblasts and increase in the number and activity of bone-resorbing osteoclasts are implicated in the progression of age-related bone loss.(5) It is also possible that, whereas the number of bone marrow stromal cells (BMSCs) is decreased,(6,7) the commitment of BMSCs to the osteogenic lineage is also decreased because of the alterations in the bone marrow microenvironment.(8–10) It is known that the levels of the stimulatory factors for bone formation such as TGFβ and interleukin-11 (IL-11) are decreased,(11,12) and the inhibitory factors for bone formation such as TNFα and IL-6 are increased in the bone marrow with age.(13,14) Clinical studies have shown that decreased bone mass is inversely correlated with an increase in marrow fat content (fatty marrow),(15,16) and increased marrow fat secretes large amounts of TNFα and IL-6,(17,18) which in turn inhibit osteoblastogenesis.(19) Thus, aging creates a bone marrow environment that is unfavorable for BMSC osteogenic differentiation and instead favors alternative differentiation pathways, for example, the adipogenic differentiation pathway.(16,20) To gain further insight into the mechanisms responsible for “aging bone,” we describe the isolation and characterization of bone marrow cells that are capable of multilineage differentiation from these aging mice and the impact of these changes on bone mass. Because only a fraction of these cells are actually the stem cells, we define our cells as enriched BMSCs.
MATERIALS AND METHODS
Reagents
Magnetic beads conjugated with anti-mouse CD11b (#558013) and CD45R/B220 (#551513) monoclonal antibodies and CD11b- (#557396) and CD45-FITC (#553079) monoclonal antibodies were purchased from BD Biosciences Pharmingen; Pan DC (#130-092-465) and stem cell antigen-1 (Sca-1) microbeads (130–092–529) were from Miltenyi Biotec. Pan DC is a mixture of CD11c and plasmacytoid dendritic cell antigen-1 (PDCA-1) antibodies. Myosin (#7784) and myogenin (#1853) monoclonal antibodies were from Abcam. Goat anti-mouse IgG-Cy3 conjugate (#81-6515) was from Zymed Laboratories. All other reagents were purchased from Sigma-Aldrich except where specified.
Animals
Male C57BL/6 mice were purchased from the aged rodent colony at the National Institute on Aging (Bethesda, MD, USA). Guinea pigs (Hartley) were purchased from Charles River Laboratories. All procedures were approved by the Institutional Committee for Animal Care and Use Committee (IACUC) at the Medical College of Georgia (MCG).
Preparation of bone marrow aspirates
Six mice per age group representing mature (3 and 6 mo old), middle-aged (12 and 18 mo old), and aged (24 mo old) C57BL/6 mice were used for the study. The mice were killed by CO2 overdose followed by thoracotomy. The femora and tibias were dissected free of soft tissues and kept in cold PBS on ice. The bones were cut open at both ends and flushed with complete isolation media (CIM) using a 22-gauge syringe followed by filtration through a 70-μm nylon mesh filter. CIM consisted of RPMI-1640 with 9% FBS, 9% horse serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 12 μM l-glutamine.(21) The bone marrow (combined from all six mice) was dispersed with a 25-gauge syringe to produce a single cell suspension. A small aliquot of cells was diluted in 3% acetic acid, and the nucleated cells were counted using a hemacytometer. This marrow aspirate was used for colony-forming unit (CFU) assays and BMSC isolation described below.
CFU assays
For the CFU-fibroblast (CFU-F) assay, 5 × 105 nucleated cells were seeded in 6-well plates in duplicate and incubated at 37°C in 5% CO2 atmosphere. Twenty-four hours after seeding, the media that contained nonadherent cells were removed and replaced with MesenCult media (Murine CFU-F Assay kit; StemCell Technologies). The cells were cultured continuously in the MesenCult media with fresh media replaced every third day. At day 14, the cells were washed with PBS, fixed in 4% paraformaldehyde (freshly prepared from its parapolymer) for 30 min, and stained with Giemsa stain. The cell density was preoptimized in a pilot experiment using the Murine CFU-F Assay kit (StemCell Technologies) according to the manufacturer's recommendations.
CFU-F assays with a guinea pig feeder cell layer were performed according to Kuznetsov and colleagues.(21,22) In brief, 3 × 106–10 × 106 nucleated mouse bone marrow cells were seeded in 25-cm2 plastic culture flasks. Three hours after seeding, unattached cells were removed by aspiration, and cultures were washed vigorously three times with DMEM; 1 × 107 nucleated feeder cells in 5 ml media were added to each 25-cm2 flask and cultured at 37°C in 5% CO2 atmosphere for 10 days with no further media replacement. Feeder cells were guinea pig bone marrow suspensions that were γ-irradiated with 6000 R just before adding to the flasks. Guinea pig cells were in αMEM (Hyclone) supplemented with 20% FBS (Atlanta Biologicals) and antibiotics. Colonies were stained with Giemsa as above.
For the CFU-osteoblast (CFU-Ob) assay, 5 × 105 nucleated cells were seeded in each well of 6-well plates in duplicate. Twenty-four hours later, the growth media were removed from the cells and replaced with osteogenic supplements (OSs, consisting of regular growth media DMEM plus 50 μM ascorbic acid-2-phosphates, 10 mM β-glycerophosphate, and 100 nM dexamethasone),(23) and cultured in OS continuously for 12 days with fresh media replaced every third day. The cells were washed, fixed, and stained with Fast Red Violet LB (Sigma-Aldrich).
For the CFU-adipocyte (CFU-Ad) assay, 1 × 106 nucleated cells were seeded in each well of 24-well plates in triplicate and treated with adipogenic induction media (DMEM containing 10% FBS, 1 μM Dex, 200 μM indomethacin, 10 μg/ml insulin, and 0.5 mM methylisobutylxanthine) for 2 days, followed by a 9-day incubation in maintenance media (growth medium plus 10 μg/ml insulin) with media replaced every other day. The cells were monitored daily using a microscope for the appearance of lipid droplets. For Oil-Red O staining, the cells were washed, fixed, and stained with Oil Red O solution as previously described.(24) Oil Red O solution was freshly prepared by diluting a stock solution (0.5 g of Oil Red O in 100 ml of isopropanol) with water (6:4) followed by filtration.
Isolation of BMSCs
The BMSCs were isolated using a protocol modified from Gimble et al.,(25) Peister et al,(26) and Tropel et al.(27) In brief, the single cell suspension described above (Preparation of bone marrow aspirates) was plated in 175-cm2 flasks at a density of 2 × 107 cells/flask. After a 3-h incubation at 37°C in 5% CO2, the media containing nonadherent cells were removed, and the adherent cells were washed two times gently with PBS to reduce the degree of hematopoietic lineage cell contamination. The cells were cultured in complete isolation media for 3–4 wk with media change every 3–4 days. At 70–80% confluence, the cells were lifted by incubation with trypsin/EDTA, washed, and resuspended at a density of 5 × 106 cells/ml in PBS containing 0.5% BSA and 2 mM EDTA. Fifty microliters each of magnetic nanoparticles conjugated to anti-mouse CD11b, CD45R/B220, and Pan DC monoclonal antibodies was added to every 1 × 107 total cells. The mixture of cells and antibody-conjugated microbeads was incubated at 12°C for 30 min and placed on the IMagnet (BD Biosciences Pharmingen) for 8 min at room temperature to allow magnetic beads to migrate and attach to one side of the tubes. The cells that were negative for these four antigens remained in the solution and were collected and subjected to a round of positive-selection using anti-Sca-1 microbeads. After several washes, the cells that attached to the microbeads (Sca-1–positive cells) were collected by removing the tube from the magnetic field, plated at a density of 50–100 cells/cm2, and amplified using regular growth media (DMEM with 10% FBS). These enriched BMSCs, which are “free” of monocytes, granulocytes, macrophages, myeloid-derived dendritic cells (DCs), natural killer cells, B-1 cells, B lymphocytes, T lymphocytes, classical DCs, plasmacytoid DCs, and macrophage progenitors, were cryopreserved or used for the experiments.
Fluorescence-activated cell sorting analysis of BMSCs to evaluate the efficiency of antibody depletion and selection.
Fluorescence-activated cell sorting (FACS) analyses were performed in the Flow Cytometry Core Laboratory at the Medical College of Georgia using a Becton Dickinson FACS Calibur flow cytometer. In brief, BMSCs were lifted by trypsin/EDTA, washed twice with PBS containing 5% heat-inactivated FBS and 0.16% sodium azide, and labeled with FITC-conjugated monoclonal antibodies against murine CD11b, CD45 (BD Pharmingen), and Sca-1 (Miltenyi Biotec) according to the manufacturer's instructions. This experiment was performed using cells that have been amplified for two passages after initial antibody selections. Ten thousand cells per sample were analyzed, and an equal amount of unlabeled cells was used for calibration. Experiments were performed in triplicate.
Retroviral transfection
Retroviral transfection was performed as previously described.(28) Briefly, the retroviral vector expressing green fluorescent protein (GFP) was constructed in a replication defective ΔU3nlsLacZ construct.(29) The LacZ sequence in the parental ΔU3nlsLacZ vector was excised by XbaI and BamHI and replaced with the full-length coding region for GFP cDNA. XbaI and BamHI restriction sites were incorporated into the 5`- and the 3`-end of the PCR products, respectively, when GFP cDNA was amplified by PCR. The retroviral particles (Ret-GFP) were produced by transfecting ΔU3-GILZ or ΔU3-GFP plasmid DNA into the retroviral packaging cell line 293GPG as described by Ory et al.(29)
For infection, 2 ml of viruses prepared above was added to the immuno-purified BMSCs prepared as above (seeded in a 60-mm dish the previous day at ∼70% confluence) and incubated at 37°C for 6 h in the presence of 8 mg/ml polybrene. The viruses were removed, and fresh media were added to the cells.
Differentiation
For induction of osteoblast differentiation, enriched BMSCs were plated at a density of 1 × 104 cells/cm2 in 96-well plates in triplicate. When the cells reached confluence, they were switched to osteogenic induction media (OS) and cultured continuously for 21 days with fresh media replaced every third day. For von Kossa staining, the cells were washed with calcium- and phosphate-free saline solution, fixed in 4% paraformaldehyde for 30 min, and stained with 5% silver nitrate solution for 30 min at room temperature in the dark. The cells were washed gently with double-distilled water, exposed to UV light for 30 min, and counterstained with 0.1% eosin. In some experiments, the cells were stained with 40 mM Alizarin Red S (ARS) solution (pH 4.2). Digital images were acquired by scanning plates using a CanoScan LiDE 80 flat-bed scanner (Canon).
For adipogenic induction, BMSCs were seeded in 24-well plates at a density of 30,000 cells/cm2. Two days after the cells reached confluence, they were treated with adipogenic induction media as described for the CFU-Ad assays.
For myogenic induction, BMSCs were plated at a density of 40–50% confluence and treated with 10 nmol/ml 5-azacytidine (5-aza) for 24 h. The 5-aza was washed away with PBS (two times) and replaced with regular growth media (DMEM) for 2–3 wk until myogenic markers were observed.(30) Cells in culture were immunolabeled with antibodies specific for indicated muscle proteins and detected by Cy3-conjugated secondary antibody as described below.
Immunocytochemistry
The cells grown in chamber slides were fixed with 4% paraformaldehyde solution for 30 min at room temperature. Slides were rinsed with PBS, blocked with 1% BSA in PBS for 1 h at room temperature, and incubated with FITC-conjugated anti-CD11b, CD45 (1:200 dilution), or Sca-1 (1:50 dilution) antibody for at least 1 h at room temperature. The slides were washed three times in PBS for 5 min each, covered with coverslips, and mounted with Vectashield mounting media (Vector Laboratories). Cultures were stained by immunocytochemistry for myogenic markers, myosin, or myogenin monoclonal antibody and detected by anti-mouse IgG-Cy3 conjugate. Images were visualized using a Nikon TE2000 fluorescence microscopy equipped with a COOLSNAP Monochrome Camera and processed with the Metamorph Imaging System.
Real-time RT-PCR analysis
Total cellular RNA was isolated from cells treated with or without OS using TRIZOL reagent (Invitrogen) as previously described.(24) Equal amounts of total RNA (2 μg) were reverse transcribed using TaqMan reverse transcription reagents (Applied Biosystems), and 1 μl of the cDNA was used as template for real-time PCR analysis using SYBR Green Master Mix (Applied Biosystems) and a Chromo-4 real-time PCR instrument (MJ Research) as previously described.(24) The PCR reactions were performed in triplicate, and the levels of mRNA expression were calculated by the ΔΔCt method using β-actin as an internal control.(31) The primer sequences used in the PCR reactions are listed in Table 1.
Table 1.
Primers Used in PCR Reactions
| Genes | GenBank no. | Forward (5′ to 3′) | Reverse (5′ to 3′) | Size (bp) |
| Runx2 | NM_009820 | CCACCACTCACTACCACACG | TCAGCGTCAACACCATCATT | 250 |
| Col la1 | U03419 | CACCCTCAAGAGCCTGAGTC | CGGGCTGATGTACCAGTTCT | 250 |
| Osteocalcin | U11542 | TTCTGCTCACTCTGCTGACC | TTTGTAGGCGGTCTTCAAGC | 250 |
| β-actin | NM_007393 | CTGGCACCACACCTTCTACA | GGTACGACCAGAGGCATACA | 190 |
Proliferation assay (MTT)
Cells were seeded in triplicate at a density of 1 × 104 cells/cm2 in 96-well plates (7 plates/experiment). One plate was used at each time point using a Cell Growth Determination kit (CGD-1; Sigma-Aldrich) according to the manufacturer's instructions.
Statistical analysis
Results are expressed as mean ± SE. Experiments were performed in triplicate except where noted. Data were analyzed using either ANOVA with Bonferroni posthoc testing or unpaired t-tests, using a commercial statistical package (Instat; Graphpad, San Diego, CA, USA); p < 0.05 was considered significant. Quantification of image data was done using NIH Image J software, version 1.38.
RESULTS
Changes in BMSC frequency with aging
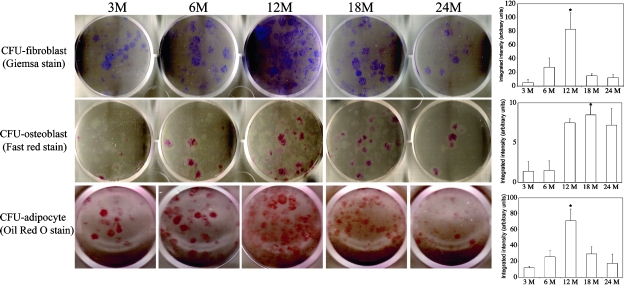
To determine whether the number or the differentiation potential of the BMSC/progenitor cells is changed during the aging process, we flushed bone marrow cells from 3-, 6-, 12-, 18-, and 24-mo-old C57BL/6 mice and performed CFU-F (fibroblast), CFU-Ob (osteoblast), and CFU-Ad (adipocyte) assays. We found that the number of bone marrow cells that are capable of forming colonies (CFU-F) increased between the ages of 3 and 12 mo and started to decrease after 12 mo of age (Fig. 1). A sharp decrease of CFU-F was observed at 24 mo of age. The ability of BMSCs to undergo adipogenic differentiation (CFU-Ad) showed a similar pattern as seen with CFU-F (i.e., CFU-Ad increased between ages of 3 and 12 mo and started to decrease after 12 mo, and a sharp decrease was seen at 24 mo of age). These results suggest that the number and differentiation potential of BMSCs continue to increase as the animals develop from mature (3 and 6 mo old) to middle age (12 mo old) and decrease rapidly as they age further (>18 mo). In contrast, peak osteogenic differentiation of an enriched BMSC population occurred at a slightly later time point (18 mo), consistent with data shown in Fig. 4. Results for these assays were quantified using NIH Image J software (version 1.38) and are presented as bar graphs (Fig. 1, right panels).
FIG. 1.
Assays for the number of colony-forming units for fibroblasts, osteoblasts, and adipocytes. Bone marrow cells from indicated ages of mice were cultured for 14 days in MesenCult media and stained with Giemsa (CFU-fibroblast), cultured for 12 days in osteogenic induction media and stained with Fast Red Violet LB (CFU-osteoblast), or cultured in adipogenic induction media for 2 days followed by a 9-day incubation in maintenance media and then stained with Oil Red O (CFU-adipocyte). Quantitative results for these assays are shown as bar graphs on right. CFU-fibroblast and CFU-osteoblast assays were performed in duplicate in 6-well plates with 5 × 105 nucleated cells/well. CFU-adipocyte assays were performed in triplicate in 24-well plates with 1 × 106 nucleated cells/well. Only one representative well is shown for each age group. These experiments were repeated in triplicate in two separate experiments (*p < 0.001 compared with 3 mo).
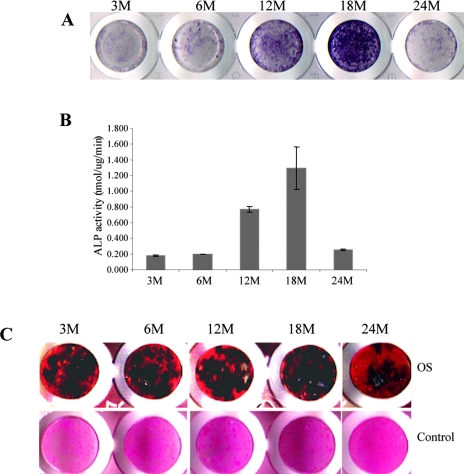
FIG. 4.
Effect of aging on BMSC osteogenic differentiation. BMSCs from indicated ages of mice were cultured in osteogenic induction media for 10 or 21 days and assayed for ALP activity or mineralization. (A and B) After 10 days of treatment, cells were fixed with 3.7% formaldehyde, and stained with SIGMA FAST BCIP/NBT Buffered Substrate (A), or lysed in 0.05% Triton X-100 directly without fixing and assayed for ALP activity (B). ALP activity is given in Sigma units normalized to protein content, where 1 Sigma unit is equivalent to the enzyme activity needed to release 1 mol of p-nitrophenol per hour. The protein concentration was determined using Bradford reagent (*p < 0.01; + p < 0.001 compared with 3 mo). The experiment was done in triplicate in two separate experiments. (C) After 21 days of treatment, cells were fixed with formaldehyde and stained with silver nitrate for mineralized nodules (von Kossa staining). The cells were counterstained with eosin. These experiments were performed a minimum of three times in triplicate. Cells cultured in MDEM did not mineralize. Only one representative stained wells for each age group is shown.
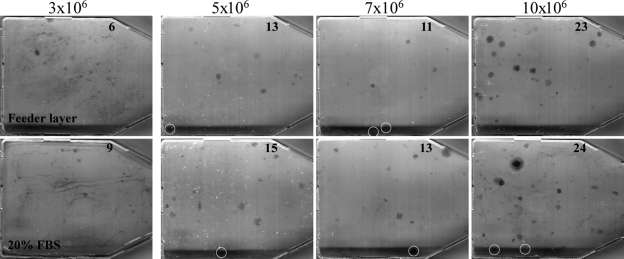
Earlier studies suggested that a feeder cell layer is needed to support marrow stromal fibroblast colony formation or CFU-F assays. The feeder cells are either γ-irradiated human or guinea pig bone marrow cells.(21) Evidence also showed that the bone marrow cells are capable of forming CFU-Fs without feeder cells.(26,32–34) To clarify whether feeder cells are necessary, we performed CFU-F assays using lethally irradiated guinea pig bone marrow cells as described by Kuznetsov and Gehron(21) or simply using regular growth media supplemented with 20% FBS, because both the murine CFU-F Assay Kit and feeder cell methods are supplemented with 20% serum in the media. Results showed that the cells cultured in DMEM with 20% FBS actually formed slightly more colonies (>50 cells in size) than those co-cultured with γ-irradiated guinea pig bone marrow cells at all seeding densities (Fig. 2), suggesting that the feeder cells are not an absolute requirement for the CFU-F assays. The numbers of colonies in each flask were counted visually, and colony number is indicated in the figure.
FIG. 2.
Effect of guinea pig feeder cells on CFU-fibroblast colony-forming efficiency; 3 × 106 to 10 × 106 nucleated mouse bone marrow cells were seeded in two sets of 25-cm2 plastic culture flasks. Top panels (Feeder layer): 3 h after seeding, unattached cells from one set of the flasks were removed by aspiration, washed vigorously three times with DMEM. 1 × 107 nucleated guinea pig bone marrow cells, freshly prepared and immediately γ-irradiated (6000 R), were added to each flask (5-ml volume). The cultures were incubated for 10 days and stained with Giemsa as in Fig. 1. Bottom panels (20% FBS): 24 h after seeding, the media in the other set of the flasks were removed, and the flasks were washed gently two times with DMEM. Five milliliters of DMEM supplemented with 20% FBS was added to each flask, incubated, and stained as above. Colonies (>50 cells in size) were counted visually, and the number of colonies in each flask is indicated. The white circles are used to highlight individual colonies.
Isolation and characterization of BMSCs from different aged mice
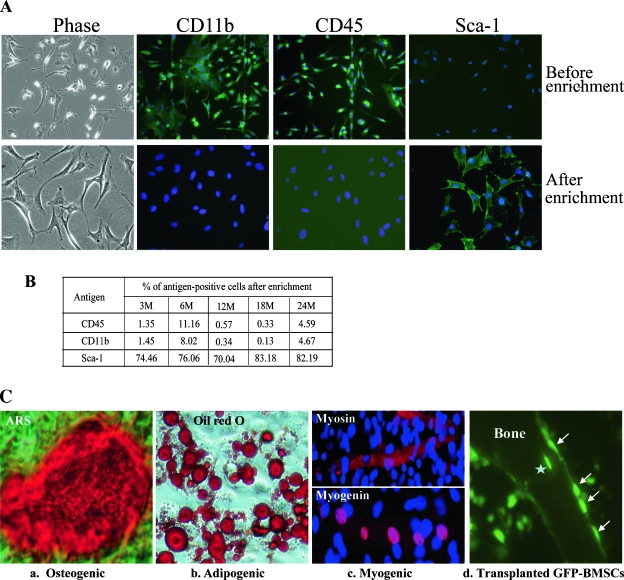
In addition to BMSCs, which can proliferate and form alkaline phosphatase (ALP)+ colonies in CFU-Ob assay, other cell populations in the bone marrow such as leukocytes also express ALP and form ALP+ colonies; therefore, CFU-Ob is not an accurate measure of true BMSC/progenitor cells. To determine whether the osteogenic differentiation potential of BMSCs is altered in aging, we isolated BMSCs from 3-, 6-, 12-, 18-, and 24-mo-old male C57BL/6 mice using negative immunodepletion and positive immunoselection approaches (see Materials and Methods section for detail). These BMSCs (Fig. 3A, phase) are negative for CD11b and CD45 and positive for Sca-1, as shown by immunolabeling of enriched cells from 18-mo-old mice using FITC-conjugated monoclonal antibodies (Fig. 3A, bottom panels). The bone marrow cell cultures that were not enriched by antibodies were mostly positive for CD11b and CD45 and were negative for Sca-1 (Fig. 3A, top panels). To confirm these results, FACS analyses were performed with BMSCs isolated from all five age groups of mice. The results showed that >88% of the cell populations are CD11b negative, >91% are CD45 negative, and >70% are Sca-1 positive (Fig. 3B). It was noted that the Sca-1–positive counts were lower in FACS analysis. This was because of the lower affinity of the Sca-1 antibody (see Materials and Methods section) and decreased Sca-1 expression in a fraction of the cells during amplification, because FACS analysis of Sca-1 antigen was performed after two rounds of amplification after initial antibody selections.
FIG. 3.
Characterization of BMSCs. (A) Marrow cell cultures (top) and antibody-enriched BMSCs from 18-mo-old mice (bottom) were immuno-labeled with FITC-conjugated monoclonal antibodies as indicated and detected using a fluorescence microscope. (B) FACS analysis of enriched BMSCs. BMSCs from indicated ages of mice were labeled with FITC-conjugated monoclonal antibodies (CD45, CD11b, and Sca-1, respectively) and analyzed. Percentages of cells positive for CD45, CD11b, and Sca-1 are shown. This experiment was performed in triplicate. (C) Multipotentiality of enriched BMSCs. BMSCs isolated from 18-mo-old mice were exposed to osteogenic, adipogenic, and myogenic differentiation media. (a) Cells were treated with osteogenic supplements for 21 days and stained with ARS for mineralized bone nodules. (b) Cells were treated with adipogenic induction media for 2 days, cultured in maintenance media for 14 days, and stained with Oil Red O. (c) Cells were treated with 5-azacytidine (5-Aza) for 24 h, cultured in regular growth media for 21 days, and immuno-labeled with indicated antibodies. These experiments were performed a minimum of three times in triplicate. (d) Bone tissue labeled with GFP antibody to show osteoblast-like lining cells (arrows) 6 wk after injection of GFP-BMSC into mouse tibias. Star indicates GFP-BMSC embedded in the bone. These experiments were repeated at least three times with similar results.
To characterize the enriched BMSCs, we treated cells from 18-mo-old mice with osteogenic, adipogenic, and myogenic induction media. Results showed that these BMSCs can differentiate into osteoblasts, adipocytes, and muscle-like cells as shown by Alizarin Red-S staining of mineralized bone matrix, Oil Red O staining of intracellular lipid vacuoles, and immunolabeling of muscle-specific proteins myosin and myogenin (Fig. 3C, a–c). Myosin and myogenin are muscle-specific markers expressed in cytosol and nucleus, respectively. Importantly, these cells have bone-forming capacity, as shown by an in vivo transplant experiment using GFP-expressing retrovirus-transduced BMSCs. In brief, GFP retrovirus–transduced BMSCs(35) from 18-mo-old mice were injected into the tibia of C57BL/6 mice. Four weeks after injection, the mice were killed, and bone tissues were sectioned and immunolabeled with GFP antibody. Results showed that the injected GFP-expressing cells not only proliferate actively in bone marrow but also differentiate into osteoblast-like lining cells or even incorporate into the trabecular bone (Fig. 3C, d). Together, these results showed that our antibody-enriched BMSCs contain a subset of multipotent stem cells.(27)
Osteogenic differentiation capacity of BMSCs from different-aged mice
To study whether aging alters BMSC ability for osteogenic differentiation, we first examined ALP activity. BMSCs isolated from different-aged mice (described above) were cultured in osteogenic induction medium (OS) for 10 days and stained with 1-Step NBT/BCIP buffered substrate to visualize the surface ALP+ cell populations. The results showed that the number of ALP+ cells increases in an age-dependent manner in BMSCs isolated from 3- to 18-mo-old mice and decreases sharply in cells from old mice (24 mo; Fig. 4A). In a parallel experiment, ALP activity was measured, and similar results were obtained (Fig. 4B). It is worth noting that the most intensive ALP staining or the highest ALP activity was observed in BMSCs of middle-aged mice (12 and 18 mo old) but not younger ones (3 and 6 mo old).
We next asked the question of whether ALP activity, an early osteoblast differentiation marker, can predict terminal differentiation state. The cells were cultured further for 21 days and stained with silver nitrate solution (von Kossa staining) to visualize mineralized bone nodules. Results showed that BMSCs from all age groups of mice can form mineralized nodules in a pattern similar to that seen in ALP staining, but the differences are less dramatic between mature (3 and 6 mo old) and middle-aged mice (12 and 18 mo old; Fig. 4C). The osteogenic potential of BMSCs from old mice (24 mo old), although still present, is significantly reduced, which is consistent with the ALP activity (Figs. 4A and 4B) and CFU-Ob assays (Fig. 1). Cells cultured in regular growth media (DMEM) did not mineralize regardless of ages. These data showed that BMSCs from all age groups of mice can undergo osteogenic differentiation and maturation, but the capacity or the potential for this biological process is significantly reduced with advancing age.
Real-time RT-PCR analysis of osteogenic gene expression
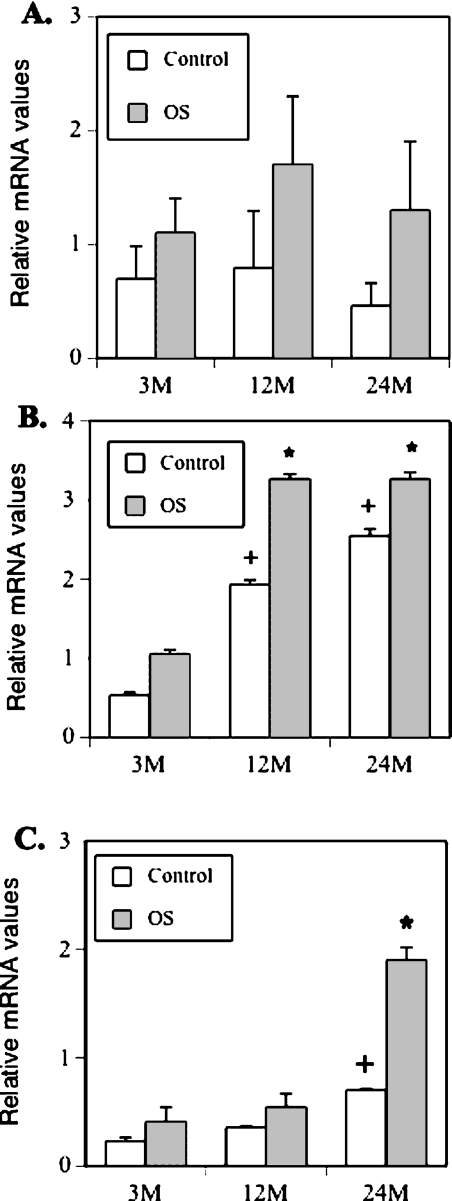
We examined the mRNA expression profile of the osteoblast lineage–associated genes. As representatives of mature, middle-aged, and aged groups, mRNA levels of BMSCs isolated from 3-, 12-, and 24-mo-old mice were analyzed. The cells were treated with or without OS for 14 days and harvested for total cellular RNA isolation. Equal amounts of total RNA were reverse transcribed, and the mRNA levels of Runx2/Cbfa1, type I collagen (Col1a1), and osteocalcin were analyzed by real-time PCR using CYBR Green Master Mix and the primers listed in Table 1. Results showed that basal levels of Runx2/Cbfa1 mRNA are higher in BMSCs from mature and middle-aged mice than that in old mice (Fig. 5A, open bars), although the levels did not significantly increase in response to osteogenic stimuli (OS, solid bars). In contrast, the levels of Col1a1 (Fig. 5B) and osteocalcin (Fig. 5C) mRNA increased in response to osteogenic stimulation. These data suggest that the BMSCs from all ages of mice have the intrinsic ability to differentiate into osteoblasts and form bone but that this ability is significantly reduced with aging.
FIG. 5.
Real-time RT-PCR analysis of osteoblast-specific gene expression. BMSCs from 3-, 12-, and 24-mo-old mice were treated with or without osteogenic induction media for 14 days and harvested for total RNA isolation. The mRNA levels of Runx2/Cbfa1, type I collagen (Col), and osteocalcin were analyzed by real-time RT-PCR. The PCR reactions were performed in triplicate for each sample in two separate experiments (+ p < 0.001; *p < 0.01 compared with 3 mo).
BMSC proliferation
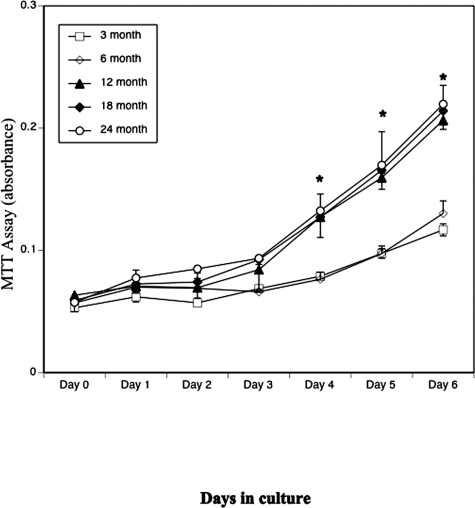
To determine whether the observed pattern of osteogenic differentiation was caused by altered rates of BMSC growth among different ages, we performed proliferation assays. BMSCs from all five age groups of mice were plated in triplicate in 96-well plates (1 × 104 cells/cm2), and cell proliferation was assayed every day for 7 consecutive days using a Cell Growth Determination kit (Sigma-Aldrich). As shown in Fig. 6, BMSCs from all age groups of mice started to proliferate 3–4 days after seeding, and growth continued until the end of the experiment (day 7). Thus, BMSCs from mice of all ages had proliferative potential, although the proliferative capacity was different among the groups. BMSCs from mice of 3 and 6 mo of age had lower proliferative rates than those of 12, 18, or 24 mo of age from day 4 onward (p < 0.001). These data suggest that the decrease of osteogenic differentiation observed in BMSC of aged mice was not caused by a decrease in BMSC proliferation.
FIG. 6.
Effect of aging on BMSC proliferation. BMSCs from 3-, 6-, 12-, 18-, and 24-mo-old mice were plated in triplicate in 96-well plates at a density of 1 × 104 cells/cm2, and cell proliferation was assayed using a Cell Growth Determination kit for 7 consecutive days. These experiments were repeated in triplicate in two separate experiments (*p < 0.01 compared with 3 mo).
DISCUSSION
We previously reported(36) that bone mass in C57Bl6 mice is relatively stable until 18 mo of age; however, between 18 and 24 mo, there was a rapid drop off in bone mass as assessed by bone densitometry and μCT. In this previous study, aging was associated with a decrease in osteoblast number, suggesting the possibility of decreased osteoblastic differentiation from bone progenitor cells. In this study, we described the osteogenic differentiation capacities of BMSCs derived from mice of five different age groups. It is known that decreased osteoblastic activity and increased osteoclastic activity contribute to age-related bone loss; however, little is known about the changes in BMSCs during the aging process. Because of technical limitations associated with the lack of specific surface markers, mouse BMSCs are difficult to isolate,(26) and the majority of the studies on aging BMSCs were conducted using whole marrow cells.(37,38) To gain insight into the role of BMSCs in the aging process, we developed a procedure based on several studies(25–27) and purified BMSCs from five age groups of C57BL/6 mice. The cells isolated using this procedure are capable of undergoing osteogenic, adipogenic, and myogenic differentiation (Fig. 3). These cells can proliferate and form bone in vivo as shown by transplant of GFP expression retrovirus–transduced cells (Fig. 3C, d). Thus, a subpopulation of these cells meet the criteria for the definition of pluripotent progenitor cells.(39,40) Comparative studies of osteogenic differentiation indicate that osteogenic differentiation capacity increases in an age-dependent manner in BMSCs isolated from mature to middle-aged mice (3–18 mo) but decreases rapidly thereafter. These results are consistent with the CFU assays, which showed that the numbers of colonies in CFU-F and CFU-Ob assays increase as animals grow from mature to middle age and decrease as they age further. Our results suggest that the BMSCs, irrespective of age, continue to retain proliferation and differentiation capacity and that the cells from younger mice are not necessarily more potent than that those from older ones in terms of proliferation or differentiation (i.e., the osteogenic and adipogenic differentiation capacity of BMSCs from 3- or 6-mo-old mice are not necessarily greater than the BMSCs from 12- or 18-mo-old mice). This conclusion is also supported by our results on mRNA expression of the key osteogenic gene Cbfa1/Runx2 (Fig. 5), where we found that there was no statistically significant difference in expression in Cbfa1 among BMSCs from mice of different ages. An alternative interpretation might be that, as the BMSCs age, a key subpopulation loses its “stem”-like characteristics and becomes more committed to differentiate along one pathway or the other (thus the increasing von Kossa and ALP activity in Fig. 4) until eventually these cells become exhausted, and osteoblast number decreases, as does BMD.(36) Data to support this possibility comes from studies by Ogawa et al.(41) In these studies on hematopoietic stem cells, the investigators reported that the more primitive multipotential “stem-like” cells tend to be in the dormant phase of the cell cycle. Thus, in their isolation procedure for hematopoietic stem cells, they placed isolated (or subcloned) cells into culture and selected those with less proliferative capacity (<20 cells) after 1 wk of culture. It is precisely this low proliferative potential that they use to identify “stem cells.” For proliferation data shown in Fig. 6, cells were cultured for <1 wk. Thus, it is possible that our results are caused by the fact that BMSCs cultures from the 3- and 6-mo-old mice have a greater percentage of multipotential progenitor cells (with lower proliferative capacity) and that this population decreases with increasing animal age.
A number of previous studies have examined the age dependency of the proliferative and differentiation capacity of progenitor cells from other species, and although most of these studies are consistent with our own, that progenitor cell number decreases with age, there are large variations in the animal species, sex, and ages used. A study by Stolzing and Scutt(42) used BMSCs from female Wistar rats between 3 and 56 wk of age and found that BMSCs from 56-wk-old rats, compared with 3-wk-old animals, had significantly decreased CFU-f number (57% lower), size (52% lower), and ALP expression (25% lower). Of note, in contrast to this study, the report by Stolzing and Scutt used adult (rather than aged) animals. A separate study by Chang et al.(43) studied human fetal versus adult BMSCs and also found that the adult cells showed decreased proliferation and differentiation capacity compared with fetal cells. However, they did not use an enriched bone marrow cell population, and the number of ages studied was limited.
Because direct head-to-head comparison between our study and other similar studies is not possible, we cannot conclude that our observations are applicable to other animal strains or species. Particularly because results from other studies have shown that remarkable variations in growth and differentiation, potential exists even among commonly used strains of inbred mice.(26,44) Nevertheless, we speculate that a decrease in BMSC/progenitor number and hormonal and structural changes in the aging bone marrow environment may be key factors governing BMSC/progenitor cell lineage commitment. It is also possible, however, that the BMSCs lag behind the aging of other types of cells/tissues because the BMSCs are a special type of cell and they are needed for repair/regeneration.
A parabiotic study by Conboy et al.(45) found that the regenerative capacity of aging muscle satellite (progenitor) cells was substantially restored by the exposure of these older mice to the circulation of the younger mice in heterochronic experiments. These studies suggest that the environment plays an important role in maintaining the proliferative capacity of these progenitor cells.
In summary, our studies showed that BMSC/progenitor cell number and differentiation capacity decrease in an age-dependent manner. This study is consistent with our previous findings(36) showing a dramatic drop-off in the ability of mice between the ages of 18 and 24 mo to maintain a normal bone mass. We speculate, based on our findings, that changes in BMSC number and function, together with the changes in the bone marrow microenvironment (serum factors, etc.), may directly contribute to age-related bone loss.
ACKNOWLEDGMENTS
Funding for this research was provided by the Offices of the Dean of the School of Medicine and the Vice President for Research, and the Department of Orthopaedics, at the Medical College of Georgia. Funding was also provided by grants from the American Heart Association (to XS) and National Institutes of Health (AR049717 to MWH and DK058680 to CMI).
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Ferguson VL, Ayers RA, Bateman TA, Simske SJ. Bone development and age-related bone loss in male C57BL/6J mice. Bone. 2003;33:387–398. doi: 10.1016/s8756-3282(03)00199-6. [DOI] [PubMed] [Google Scholar]
- 2.Gudmundsdottir SL, Indridason OS, Franzson L, Sigurdsson G. Age-related decline in bone mass measured by dual-energy X-ray absorptiometry and quantitative ultrasound in a population-based sample of both sexes: Identification of useful ultrasound thresholds for osteoporosis screening. J Clin Densitom. 2005;8:80–86. doi: 10.1385/jcd:8:1:080. [DOI] [PubMed] [Google Scholar]
- 3.Hamrick MW, Shi X, Zhang W, Pennington C, Thakore H, Haque M, Kang B, Isales CM, Fulzele S, Wenger KH. Loss of myostatin (GDF8) function increases osteogenic differentiation of bone marrow-derived mesenchymal stem cells but the osteogenic effect is ablated with unloading. Bone. 2007;40:1544–1553. doi: 10.1016/j.bone.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rupprecht M, Pogoda P, Mumme M, Rueger JM, Puschel K, Amling M. Bone microarchitecture of the calcaneus and its changes in aging: A histomorphometric analysis of 60 human specimens. J Orthop Res. 2006;24:664–674. doi: 10.1002/jor.20099. [DOI] [PubMed] [Google Scholar]
- 5.Manolagas SC. Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 6.Bergman RJ, Gazit D, Kahn AJ, Gruber H, McDougall S, Hahn TJ. Age-related changes in osteogenic stem cells in mice. J Bone Miner Res. 1996;11:568–577. doi: 10.1002/jbmr.5650110504. [DOI] [PubMed] [Google Scholar]
- 7.Muschler GF, Nitto H, Boehm CA, Easley KA. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res. 2001;19:117–125. doi: 10.1016/S0736-0266(00)00010-3. [DOI] [PubMed] [Google Scholar]
- 8.Cao J, Venton L, Sakata T, Halloran BP. Expression of RANKL and OPG correlates with age-related bone loss in male C57BL/6 mice. J Bone Miner Res. 2003;18:270–277. doi: 10.1359/jbmr.2003.18.2.270. [DOI] [PubMed] [Google Scholar]
- 9.Haden ST, Glowacki J, Hurwitz S, Rosen C, LeBoff MS. Effects of age on serum dehydroepiandrosterone sulfate, IGF-I, and IL-6 levels in women. Calcif Tissue Int. 2000;66:414–418. doi: 10.1007/s002230010084. [DOI] [PubMed] [Google Scholar]
- 10.Kveiborg M, Flyvbjerg A, Rattan SI, Kassem M. Changes in the insulin-like growth factor-system may contribute to in vitro age-related impaired osteoblast functions. Exp Gerontol. 2000;35:1061–1074. doi: 10.1016/s0531-5565(00)00125-x. [DOI] [PubMed] [Google Scholar]
- 11.Pfeilschifter J, Diel I, Scheppach B, Bretz A, Krempien R, Erdmann J, Schmid G, Reske N, Bismar H, Seck T, Krempien B, Ziegler R. Concentration of transforming growth factor beta in human bone tissue: Relationship to age, menopause, bone turnover, and bone volume. J Bone Miner Res. 1998;13:716–730. doi: 10.1359/jbmr.1998.13.4.716. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi Y, Watanabe S, Ishii G, Takeda S, Nakayama K, Fukumoto S, Kaneta Y, Inoue D, Matsumoto T, Harigaya K, Fujita T. Interleukin-11 as a stimulatory factor for bone formation prevents bone loss with advancing age in mice. J Biol Chem. 2002;277:49011–49018. doi: 10.1074/jbc.M207804200. [DOI] [PubMed] [Google Scholar]
- 13.Ershler WB, Sun WH, Binkley N, Gravenstein S, Volk MJ, Kamoske G, Klopp RG, Roecker EB, Daynes RA, Weindruch R. Interleukin-6 and aging: Blood levels and mononuclear cell production increase with advancing age and in vitro production is modifiable by dietary restriction. Lymphokine Cytokine Res. 1993;12:225–230. [PubMed] [Google Scholar]
- 14.Wei J, Xu H, Davies JL, Hemmings GP. Increase of plasma IL-6 concentration with age in healthy subjects. Life Sci. 1992;51:1953–1956. doi: 10.1016/0024-3205(92)90112-3. [DOI] [PubMed] [Google Scholar]
- 15.Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop. 1971;80:147–154. doi: 10.1097/00003086-197110000-00021. [DOI] [PubMed] [Google Scholar]
- 16.Moore SG, Dawson KL. Red and yellow marrow in the femur: Age-related changes in appearance at MR imaging. Radiology. 1990;175:219–223. doi: 10.1148/radiology.175.1.2315484. [DOI] [PubMed] [Google Scholar]
- 17.Moller DE. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2000;11:212–217. doi: 10.1016/s1043-2760(00)00272-1. [DOI] [PubMed] [Google Scholar]
- 18.Skolnik EY, Marcusohn J. Inhibition of insulin receptor signaling by TNF: Potential role in obesity and non-insulin-dependent diabetes mellitus. Cytokine Growth Factor Rev. 1996;7:161–173. doi: 10.1016/1359-6101(96)00021-4. [DOI] [PubMed] [Google Scholar]
- 19.Maurin AC, Chavassieux PM, Frappart L, Delmas PD, Serre CM, Meunier PJ. Influence of mature adipocytes on osteoblast proliferation in human primary cocultures. Bone. 2000;26:485–489. doi: 10.1016/S8756-3282(00)00252-0. [DOI] [PubMed] [Google Scholar]
- 20.Beresford JN, Bennett JH, Devlin C, Leboy PS, Owen ME. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci. 1992;102:341–351. doi: 10.1242/jcs.102.2.341. [DOI] [PubMed] [Google Scholar]
- 21.Kuznetsov S, Gehron RP. Species differences in growth requirements for bone marrow stromal fibroblast colony formation In vitro. Calcif Tissue Int. 1996;59:265–270. doi: 10.1007/s002239900121. [DOI] [PubMed] [Google Scholar]
- 22.Kuznetsov SA, Friedenstein AJ, Robey PG. Factors required for bone marrow stromal fibroblast colony formation in vitro. Br J Haematol. 1997;97:561–570. doi: 10.1046/j.1365-2141.1997.902904.x. [DOI] [PubMed] [Google Scholar]
- 23.Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. [PubMed] [Google Scholar]
- 24.Shi X, Shi W, Li Q, Song B, Wan M, Bai S, Cao X. A glucocorticoid-induced leucine-zipper protein, GILZ, inhibits adipogenesis of mesenchymal cells. EMBO Rep. 2003;4:374–380. doi: 10.1038/sj.embor.embor805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gimble JM, Robinson CE, Wu X, Kelly KA, Rodriguez BR, Kliewer SA, Lehmann JM, Morris DC. Peroxisome proliferator-activated receptor-gamma activation by thiazolidinediones induces adipogenesis in bone marrow stromal cells. Mol Pharmacol. 1996;50:1087–1094. [PubMed] [Google Scholar]
- 26.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 27.Tropel P, Noel D, Platet N, Legrand P, Benabid AL, Berger F. Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res. 2004;295:395–406. doi: 10.1016/j.yexcr.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 28.Zhang W, Yang N, Shi XM. Regulation of MSC osteogenic differentiation by glucocorticoid-induced leucine zipper (GILZ) J Biol Chem. 2008;283:4723–4729. doi: 10.1074/jbc.M704147200. [DOI] [PubMed] [Google Scholar]
- 29.Ory DS, Neugeboren BA, Mulligan RC. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc Natl Acad Sci USA. 1996;93:11400–11406. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18:1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- 31.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holmes C, Khan TS, Owen C, Ciliberti N, Grynpas MD, Stanford WL. Longitudinal analysis of mesenchymal progenitors and bone quality in the stem cell antigen-1null osteoporotic mouse. J Bone Miner Res. 2007;22:1373–1386. doi: 10.1359/jbmr.070604. [DOI] [PubMed] [Google Scholar]
- 33.Novitzky N, Makgoka SJ, Mohamed R. Basic fibroblast growth factor corrects proliferative derangement of bone marrow stroma and CD34+ population following allogeneic stem cell transplantation. Exper Hematol. 2001;29:1432–1438. doi: 10.1016/s0301-472x(01)00744-5. [DOI] [PubMed] [Google Scholar]
- 34.Yang M, Li K, Ng PC, Chuen CKY, Lau TK, Cheng YS, Liu YS, Li CK, Man Pan Yuen P, Edward James A, Lee SM, Fok TF. Promoting effects of serotonin on hematopoiesis: Ex vivo expansion of cord blood CD34+ stem/progenitor cells, proliferation of bone marrow stromal cells, and antiapoptosis. Stem Cells. 2007;25:1800–1806. doi: 10.1634/stemcells.2007-0048. [DOI] [PubMed] [Google Scholar]
- 35.Yang N, Zhang W, Shi XM. Glucocorticoid-induced leucine zipper (GILZ) mediates glucocorticoid action and inhibits inflammatory cytokine-induced COX-2 expression. J Cell Biochem. 2008;103:1760–1771. doi: 10.1002/jcb.21562. [DOI] [PubMed] [Google Scholar]
- 36.Hamrick MW, Ding KH, Pennington C, Chao YJ, Wu YD, Howard B, Immel D, Borlongan C, McNeil PL, Bollag WB, Curl WW, Yu J, Isales CM. Age-related loss of muscle mass and bone strength in mice is associated with a decline in physical activity and serum leptin. Bone. 2006;39:845–853. doi: 10.1016/j.bone.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 37.D'Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115–1122. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- 38.Kahn A, Gibbons R, Perkins S, Gazit D. Age-related bone loss. A hypothesis and initial assessment in mice. Clin Orthop. 1995;313:69–75. [PubMed] [Google Scholar]
- 39.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 40.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 41.Ogawa M, LaRue AC, Drake CJ. Hematopoietic origin of fibroblasts/myofibroblasts: Its pathophysiologic implications. Blood. 2006;108:2893–2896. doi: 10.1182/blood-2006-04-016600. [DOI] [PubMed] [Google Scholar]
- 42.Stolzing A, Scutt A. Age-related impairment of mesenchymal progenitor cell function. Aging Cell. 2006;5:213–224. doi: 10.1111/j.1474-9726.2006.00213.x. [DOI] [PubMed] [Google Scholar]
- 43.Chang PL, Blair HC, Zhao X, Chien YW, Chen D, Tilden AB, Chang Z, Cao X, Faye-Petersen OM, Hicks P. Comparison of fetal and adult marrow stromal cells in osteogenesis with and without glucocorticoids. Connect Tissue Res. 2006;47:67–76. doi: 10.1080/03008200600584074. [DOI] [PubMed] [Google Scholar]
- 44.Phinney DG, Kopen G, Isaacson RL, Prockop DJ. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: Variations in yield, growth, and differentiation. J Cell Biochem. 1999;72:570–585. [PubMed] [Google Scholar]
- 45.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]