Abstract
Our aim was to assess BMC of the hip over 8 yr in prepubertal children who participated in a 7-mo jumping intervention compared with controls who participated in a stretching program of equal duration. We hypothesized that jumpers would gain more BMC than control subjects. The data reported come from two cohorts of children who participated in separate, but identical, randomized, controlled, school-based impact exercise interventions and reflect those subjects who agreed to long-term follow-up (N = 57; jumpers = 33, controls = 24; 47% of the original participants). BMC was assessed by DXA at baseline, 7 and 19 mo after intervention, and annually thereafter for 5 yr (eight visits over 8 yr). Multilevel random effects models were constructed and used to predict change in BMC from baseline at each measurement occasion. After 7 mo, those children that completed high-impact jumping exercises had 3.6% more BMC at the hip than control subjects whom completed nonimpact stretching activities (p < 0.05) and 1.4% more BMC at the hip after nearly 8 yr (BMC adjusted for change in age, height, weight, and physical activity; p < 0.05). This provides the first evidence of a sustained effect on total hip BMC from short-term high-impact exercise undertaken in early childhood. If the benefits are sustained into young adulthood, effectively increasing peak bone mass, fracture risk in the later years could be reduced.
Key words: detraining, growth and development, exercise intervention, children, mechanical loading, peak bone mass
INTRODUCTION
The nature of bone to adapt to mechanical loading is well accepted. Bone responds to increased mechanical stress by getting stronger and responds to disuse by weakening and losing bone mass. Because bone loss and subsequent reductions in bone strength are largely age-related phenomena, the clinical implications of disuse are more pronounced among older adults. This may be caused in part by age-associated reductions in physical activity and also by the older skeleton’s reduced ability to respond to mechanical loading.(1) The result is an increased risk of fracture. Skeletal fragility is exacerbated by numerous mechanisms, including a failure to produce a skeleton of optimal mass and strength during growth.(2) Increasing evidence supports exercise during growth as having the greatest potential to reduce osteoporosis risk later in life,(3–5) and several researchers have reported positive effects of physical activity on the whole body,(6) lumbar spine,(7) and hip(8,9) in growing children. Whether these benefits persist into adulthood and contribute to the development of peak bone mass is unclear. Although a number of human studies have shown exercise-induced changes in the skeleton during growth may not be maintained long-term after exercise cessation,(10–12) we have previously shown skeletal benefits persist for 7 mo(8) to 3 yr after exercise cessation.(13) More recently, Warden et al.(14) found short-term exercise in growing rodents provided lifelong benefits to bone structure and strength. Whether short-term exercise in humans can provide similar lifelong benefits is unknown. The aim of this study was to investigate whether children who participated in a 7-mo targeted, impact exercise intervention exhibited skeletal benefits 7 yr after the intervention had ceased. We hypothesized that children who participated in the jumping intervention (100 jumps off a 24-in box 3 times/wk) would have greater bone mass accrual compared with children who were not exposed to the jumping exercises.
MATERIALS AND METHODS
Study design and participants
The subjects for this report were drawn from the BUGSY study (Building Growing Skeletons in Youth), an ongoing mixed longitudinal study of bone mineral accretion in growing children. The data reported come from two cohorts of children who participated in separate, but identical, randomized, controlled, school-based impact exercise interventions. Participants were recruited from local elementary schools in Corvallis, OR, to participate in the 7-mo jumping interventions. Studies were initiated in the fall of 1997 (pilot, n = 33) and fall of 1998 (n = 89). In each case, participants were randomly allocated (within classrooms) to either a jumping or stretching program and were assessed at baseline, postintervention (7 mo), and after 7 mo of detraining.(7,8) In 2002, funding was obtained to continue long-term surveillance on previous participants. The data presented here reflect only those subjects who agreed to long-term follow-up (N = 57; jumpers = 33, controls = 24; 47% of the original participants). Those subjects agreeing to long-term surveillance were reassessed at 31, 43, 55, 67, 79, and 91 mo from study entry. There were no differences in baseline age, height, weight, body composition, nutrient intakes, reported physical activity, maturity status, or race among the children who continued in the long-term follow-up compared with those who did not (p > 0.05; Table 1).
Table 1.
Characteristics at Baseline
|
Intervention groups |
||||||
|
Jumpers (n = 33) |
Controls (n = 24) |
|||||
| Male (n = 19) | Female (n = 14) | Total (n = 33) | Male (n = 16) | Female (n = 8) | Total (n = 24) | |
| Race (W/A/B/MR) | 18/1/0/0 | 13/1/0/0 | 31/2/0/0 | 16/0/0/0 | 8/0/0/0 | 24/0/0/0 |
| Age (yr) | 7.4 (1.0) | 7.9 (0.8) | 7.6 (1.0) | 7.9 (1.1) | 8.1 (0.8) | 7.9 (1.0) |
| Maturity (pre/post PHV) | 19/0 | 14/0 | 33/0 | 16/0 | 8/0 | 56/0 |
| Height (cm) | 126.7 (8.8) | 127.1 (8.8) | 126.8 (8.7) | 127.2 (7.2) | 129.8 (6.9) | 128.1 (7.0) |
| Weight (kg) | 27.8 (5.9) | 27.6 (6.1) | 27.7 (5.9) | 26.1 (3.8) | 28.4 (6.3) | 26.9 (4.7) |
| Relative fat mass (%)* | 16.8 (6.4) | 20.1 (3.1) | 18.1 (5.6) | 17.9 (6.3) | 20.9 (7.1) | 19.0 (6.6) |
| Bone variables | ||||||
| Total hip BMC | 11.64 (2.33) | 10.6 (2.96) | 11.22 (2.6) | 10.89 (2.53) | 10.1 (1.37) | 10.65 (2.24) |
| Femoral neck BMC | 1.89 (0.38) | 1.59 (0.37) | 1.77 (0.4) | 1.79 (0.4) | 1.5 (0.26) | 1.7 (0.4) |
| Trochanter BMC | 2.54 (0.68) | 2.47 (0.84) | 2.51 (0.73) | 2.24 (0.59) | 2.13 (0.54) | 2.21 (0.56) |
| Total calcium (mg) | 1265 (355) | 1220 (300) | 1248 (331) | 1257 (258) | 1263 (175) | 1259 (226) |
| Vitamin D (IU) | 420 (182) | 425 (66) | 423 (129) | 435 (85) | 453 (51) | 444 (66) |
| Team sports | ||||||
| [(n) %] reporting none | (5) 26.3 | (7) 50.0 | (12) 36.4 | (5) 31.2 | (3) 37.5 | (8) 33.3 |
| [(n) %] reporting ≥1 | (14) 73.7 | (7) 50.0 | (21) 63.6 | (11) 68.8 | (5) 62.5 | (16) 66.7 |
Values are mean (SD).
* Percent body fat estimated from triceps and subscapular skinfold.(22)
W, white; A, Asian; B, black; MR, mixed race.
Each child provided their assent to participate, and each child’s parent provided informed consent. Each parent and child pair completed health history, food frequency and physical activity questionnaires, and anthropometry assessments at each measurement interval. Bone scans of the hip were conducted at each measurement occasion.
Physical activity and nutritional assessment
Physical activity was assessed by parent and child using a modified self-reported physical activity questionnaire developed for children and adolescents.(15) Physical activity information was partitioned by general activity (such as physical education and general play) and participation in organized sports. Sporting activity was recorded as a dichotomous variable (no sport activities = 0; one or more sport activities = 1).
Dietary intake was assessed using the Harvard Medical School Youth Diet Survey developed for children and adolescents between the ages of 9 and 18 yr.(16) This food f
† Controls greater than jumpers, p < 0.05.
‡ Males greater than females, p < 0.05.
§ Males greater than females, p < 0.01.
¶ Females greater than males, p < 0.001. requency questionnaire is designed to be self-administered; however, to improve accuracy, the questionnaire was filled out by parent and child together.(17) A researcher familiar with the diet survey was available to answer questions regarding the classification of foods and serving sizes. Visual aids were also available to help participants and their parents to determine appropriate responses. Completed food surveys were sent to Harvard Medical School for analysis. Calcium was evaluated in the statistical models but was found to have no effect on the change in BMC parameters and was subsequently excluded. Calcium and vitamin D values at baseline and at the 91-mo follow-up are reported for descriptive purposes (Tables 1 and 2).
Table 2.
Characteristics at 91-mo Follow-Up
|
Intervention groups |
||||||
|
Jumpers (n = 29) |
Controls (n = 20) |
|||||
| Male (n = 18) | Female (n = 11) | Total (n = 29) | Male (n = 13) | Female (n = 7) | Total (n = 20) | |
| Race (W/A/B/MR) | 17/1/0/0 | 10/1/0/0 | 27/2/0/0 | 13/0/0/0 | 7/0/0/0 | 20/0/0/0 |
| Age (yr) | 15.0 (1.3) | 15.5 (1.4) | 15.2 (1.3) | 15.3 (1.4) | 15.8 (0.9) | 15.5 (1.2) |
| Maturity (pre/post PHV) | 4/14 | 0/11 | 4/25 | 2/11 | 0/7 | 2/18 |
| Height (cm) | 170.8 (8.9)† | 162.8 (5.4)† | 167.8 (8.6)‡ | 173.4 (8.4)† | 169.5 (3.0)† | 172.0 (7.1)‡ |
| Weight (kg) | 65.3 (15.1) | 58.2 (9.4) | 62.6 (13.5) | 63.1 (11.5) | 61.8 (16.8) | 62.7 (13.2) |
| Relative fat mass (%)* | 20.4 (9.2)§ | 29.8 (7.8)§ | 23.7 (9.7) | 16.5 (7.6)§ | 29.5 (8.5)§ | 21.0 (10.0) |
| Bone variables | ||||||
| Total hip BMC | 39.93 (9.56)¶ | 31.26 (5.40)¶ | 36.84 (9.22) | 39.31 (9.93)¶ | 30.15 (3.38)¶ | 36.1 (9.27) |
| Femoral neck BMC | 4.92 (0.88)¶ | 4.18 (0.53)¶ | 4.66 (0.81) | 4.73 (0.93)¶ | 4.21 (0.69)¶ | 4.55 (0.87) |
| Trochanter BMC | 11.11 (2.62)¶ | 8.31 (1.42)¶ | 10.1 (2.62) | 10.91 (2.54)¶ | 7.49 (1.05)¶ | 9.72 (2.69) |
| Total calcium (mg) | 1099 (510) | 989 (376) | 1057 (459) | 1329 (441) | 1194 (382) | 1282 (416) |
| Vitamin D (IU) | 254 (132) | 290 (215) | 267 (165) | 402 (197) | 276 (109) | 358 (179) |
| Team sports | ||||||
| [(n) %] reporting none | (5) 27.8 | (4) 36.4 | (9) 31.0 | (4) 30.8 | (2) 28.6 | (6) 30.0 |
| [(n) %] reporting ≥1 | (13) 72.2 | (7) 63.6 | (20) 69.0 | (9) 69.2 | (5) 71.4 | (14) 70.0 |
Values presented as mean (SD).
* Percent body fat determined from whole body DXA scans.
† Males greater than females, p < 0.05.
‡ Controls greater than jumpers, p < 0.05.
§ Females greater than males, p < 0.001.
¶ Males greater than females p < 0.01.
W, white; A, Asian; B, black; MR, mixed race.
Anthropometric measures
Standing height, sitting height, and weight were assessed at each visit. Standing and sitting height were measured to the nearest 0.1 cm using a wall-mounted stadiometer. A standard protocol outlined by Martin et al.(18) was used to assess sitting height. Weight was measured to the nearest 0.1 kg using an electronic weighing scale. Measurements were taken twice unless there was a discrepancy >0.4 cm (height and sitting height) or 0.4 kg (weight), and then a third measurement was taken. Recorded values were the average of two measurements or the median of three. Leg length was calculated by subtracting sitting height from standing height. For the analysis, a change in height from study entry (Δheight) was calculated.
Body fat was estimated using sex-specific prediction equations during the first 3 yr of data collection.(19) Two anatomical sites (triceps and subscapular) were measured on the participants right side using Lange (Cambridge Scientific Industries, Cambridge, MD, USA) skinfold calipers (precision error = 2% based on a subsample of 20 children randomly chosen from our population). Beginning with the 43-mo follow-up, body fat was determined from whole body DXA scans. Because of the differences in our methods of estimating body fat over time, these data are presented for descriptive purposes only. Trained and qualified technicians blinded to the group status of the participants conducted all measurements.
Bone mineral assessment
In this study, BMC (g) of the left proximal femur (total hip, femoral neck, trochanter) was evaluated using DXA (QDR 4500A; Hologic, Waltham, MA, USA). Bone measurements of the hip have an in-house precision error of 1–1.5%. Trained and qualified technicians conducted all measurements. Over the 8-yr period during which these data were collected, the Bone Research Laboratory acquired several upgrades to its DXA software. As a result, all scans from each of the nine measurement occasions were reanalyzed using the latest software version (Hologic QDR software, version 12.3, Delphi A) to ensure data accuracy. A single researcher was responsible for reanalyzing all of the scan data to improve reliability of the analyses. Spine and anthropometric phantoms were scanned daily and weekly, respectively, to maintain quality assurance of the QDR 4500A.
For each hip site a change in BMC (ΔBMC) accrual was calculated. For clarification, at each measurement occasion, we subtracted the study entry value from the values at subsequent visits. Therefore, for an individual measured at study entry, 7 mo, 19 mo, and then yearly for the following 7 yr, there are seven change (Δ) scores.
Biological maturity
Peak height velocity (PHV) is a commonly used biological parameter in growth studies that allows subjects to be aligned at comparable biological rather than chronological ages.(20) It is also the only sexual maturational landmark that occurs in both boys and girls and thus allows sex comparisons at the same maturational age to be performed.(21) In this study, bone measurements are considered in terms of time before and after PHV.(20,22) We were able to calculate actual height velocities for 13 boys and 8 girls who had gone through PHV. A further 25 were at or less than a year from PHV. Therefore, until the majority of the group go through PHV, a predicted value (years from PHV) has to be used in the analysis. Age of attainment of PHV was estimated by applying sex-specific anthropometric prediction equations.(23) These equations use markers of somatic growth to predict how far a child is, in years, from reaching PHV. The equations were developed using data from a longitudinal study of children’s growth and verified in two other similar longitudinal studies. The prediction equation was applied at each measurement occasion. As subjects approach PHV, the prediction increases in accuracy. For this analysis, the measurement occasion closest to PHV was used. Subjects were classified as either pre- or post-PHV at each measurement occasion (pre-PHV maturity = 0, post-PHV maturity = 1).
School-based exercise intervention
The specifics of the exercise intervention have been reported elsewhere.(7) Briefly, the exercise intervention was conducted from October to May during the 1997 and 1998 school years. Time was taken off for winter and spring breaks (∼1 and 3 wk, respectively). All children were involved in regularly scheduled physical education (PE) classes once a week for 30 min. The exercise intervention was incorporated into the regular school schedule. Children participated in either a jumping (intervention) or stretching (control) program 3 d/wk for 20 min on separate days from the regularly scheduled PE classes. The general format for each exercise session included a 5-min warm-up activity, 10 min of jumping or stretching, and a 5-min cool down. Children were introduced to the program, and jumpers were progressively trained to reach a maximum of 100 jumps per session by the fifth week of the program. The height of the box from which they jumped (24 in) did not change during the intervention. Average ground reaction forces for 100 jumps was ∼8 times body weight (using a single force plate) in a subsample of participants (n = 24).(24) The control groups had equivalent contact time with instructors and performed nonimpact stretching activities while their classmates were jumping. Compliance to the intervention as measured by attendance was ∼96% for both jumpers and stretchers in both cohorts (1997, 1998).
Statistical analysis
Descriptive results are expressed as mean ± SD (version 14.0; SPSS, Chicago, IL, USA). Intervention and control group comparisons were made with t-tests (p < 0.05), and Bonferroni adjustments were made for multiple comparisons. The hypotheses were tested using hierarchical (multilevel) linear modeling using random effects models (MlwiN version 1.0; Multilevel Models Project, Institute of Education, University of London, London, UK). This procedure has been described previously.(25)
To summarize the analyses, hierarchical models were developed for analyzing hierarchically structured data. In this example, indices of bone accrual were measured repeatedly in individuals (level 1 of the hierarchy) and between individuals (level 2 of the hierarchy). Analysis models that contain variables measured at different levels of the hierarchy are known as multilevel regression models. Additive, multilevel regression models were adopted to describe the ΔBMC from study entry.
where yij is the ΔBMC (g) on measurement occasion i in the jth individual; αj is the constant for the jth individual; βjxij is the slope of the ΔBMC (g) parameter with time from study entry (years from start; i.e., time from baseline visit) for the jth individual; and k1zij to knzij are the coefficients of explanatory variables (i.e., age at study start, sex, race, maturity, Δheight, Δweight, sport activity, and intervention group) at assessment at occasion i in the jth individual, and εij represents the level 1 residual (within individual variance) for the ith assessment of the ΔBMC (g) in the jth individual.
Modeling strategy
Models were built in a stepwise procedure; that is, predictor variables (κ, fixed effects) were added one at a time. Likelihood ratio statistics were used to judge the effects of including further variables. Predictor variables (κ) were accepted as significant if the estimated mean coefficient (E) was greater than twice the SE of the estimate (SEE); that is, p < 0.05. If the retention criteria were not met, the predictor variable was discarded. To allow for the nonlinearity of growth, years from start power functions were introduced into the linear models. Years from study start was centered on the middle time point of the study (38 mo), which allowed the intercept for all models to be in the middle of the data rather than at study entry (when years from study start would equal 0, a visit not being modeled directly). This factor (years from start centered) was added as both a fixed and random coefficient. Once confounders of growth, maturation, and physical activity were controlled, the effects of the intervention were evaluated by adding an intervention variable (intervention group; jumping = 1, controls = 0). These variables were retained if E was greater than twice the SEE; that is, p < 0.05. The models were used to predict ΔBMC at each visit occasion attributable to the factors in the models (Table 2).
RESULTS
After 8 yr of data collection, data from a total of 421 measurements from 57 individuals who were measured on three or more occasions were used. For all models, the significant variances at level 1 indicated that ΔBMC increased significantly within individuals over the 8-yr observation period (E > 2 × SEE; p < 0.05). At baseline, there were no differences in height, weight, hip BMC values, sports participation, maturation, calcium, or vitamin D intakes between jumpers and control subjects (Table 1). At the last follow-up visit (91 mo), 49 subjects (86% of those agreeing to long-term follow-up) returned for testing. There were no differences in weight, sports participation, maturation, or calcium and vitamin D intakes between jumpers and control subjects at 91 mo, but control subjects were taller than jumpers (p < 0.05; Table 2). When examining the overall growth trajectories (baseline through 91 mo), we found no differences between groups or sex for height or body mass development (p > 0.05).
Intervention effect
Sex and race did not have a significant independent effect on ΔBMC at any modeled bone site. The model for total hip ΔBMC (Table 3) indicates that, after controlling for age at study start, maturity, Δheight, Δweight, and sport activity, a significant independent intervention effect was found. This effect was significant at each measured time point after the intervention (p < 0.05). Subjects who participated in the jumping intervention gained an additional 0.55 g of BMC compared with control subjects (p < 0.05). An intervention effect was not observed at the trochanter or femoral neck (Table 3).
Table 3.
Multilevel regression models for change from study entry for total hip, trochanter and femoral neck aligned by years from study entry.
| Variables | ΔBMC |
|||||
| ΔTotal hip | ΔHip trochanter | ΔFemoral neck | ||||
| Random | Level 1 (within individuals) | Level 1 (within individuals) | Level 1 (within individuals) | |||
| Constant | 1.43 ± 0.14 | 0.20 ± 0.02 | 0.03 ± 0.003 | |||
| Level 2 (between individuals) | Level 2 (between individuals) | Level 2 (between individuals) | ||||
| Constant | Years from start centered | Constant | Years from start centered | Constant | Years from start centered | |
| Constant | 4.03 ± 0.88 | 1.62 ± 0.34 | 0.49 ± 0.11 | 0.16 ± 0.04 | 0.02 ± 0.006 | 0.01 ± 0.002 |
| Years from start centered | 1.62 ± 0.34 | 0.62 ± 0.13 | 0.16 ± 0.04 | 0.05 ± 0.01 | 0.01 ± 0.002 | 0.004 ± 0.001 |
| Fixed | Estimates | Estimates | Estimates | |||
| Constant | −7.37 ± 1.15 | −1.76 ± 0.05 | −0.53 ± 0.16 | |||
| Years from start centered | −0.55 ± 0.24 | −0.35 ± 0.09 | NS | |||
| Years from start centered2 | 0.21 ± 0.02 | 0.05 ± 0.01 | 0.02 ± 0.002 | |||
| Age at study start | 0.33 ± 0.11 | NS | 0.04 ± 0.02 | |||
| Sex | NS | NS | NS | |||
| Race | NS | NS | NS | |||
| Maturity | 1.23 ± 0.35 | 0.52 ± 0.13 | NS | |||
| ΔHeight | 0.51 ± 0.04 | 0.18 ± 0.02 | 0.04 ± 0.003 | |||
| ΔWeight | 0.13 ± 0.03 | 0.05 ± 0.01 | 0.02 ± 0.004 | |||
| Sport activity | 1.10 ± 0.21 | 0.23 ± 0.09 | 0.14 ± 0.3 | |||
| Intervention group | 0.55 ±0.22 | NS | NS | |||
Fixed effect values are presented as estimated mean coefficients ± SEE [ΔBMC g from study entry].
Random effects values presented as estimated mean variance ± SEE [ΔBMC g from study entry2].
Years from start centered is years from 38 mo.
Sex (0 = male, 1 = female); race (0 = white, 1 = nonwhite); maturity (0 = pre-PHV, 1 = post-PHV); Δheight (cm) from study entry; Δωeight (kg) from study entry; sport activity (0 = no sport activities, 1 = 1 or more sports activities); intervention group (0 = controls, 1 = jumpers).
Values are p < 0.05 (mean > 2 × SE estimate) unless noted as NS (NS, not significant.)
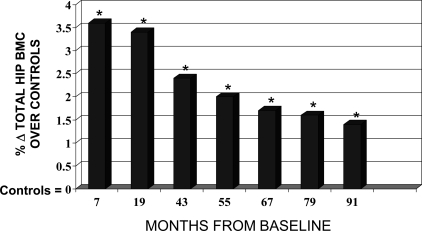
In terms of the percent contribution of the intervention to the percent change in BMC, we observed that, after controlling for growth, maturation, and sports participation, jumpers had 3.6% greater hip BMC than controls immediately after the intervention (7 mo). The relative contribution of the intervention subsided as the effects of growth and maturation assumed a greater contribution to the change in BMC over time (Fig. 1). This was most notable 2 yr after the exercise program ended (43 mo). The intervention effect leveled off in the last 3 yr, suggestive of a stable, persistent contribution of the intervention. At the last follow-up (91 mo), jumpers had 1.4% greater BMC at the hip compared with controls (p < 0.05; Fig. 1).
FIG. 1.
Jumping intervention effect on Δτotal hip BMC after 8 yr. Percent change in total hip BMC in jumpers above that of controls after 7 mo of exercise training, 1 yr of detraining (19 mo), and 4–8 yr of detraining (43–91 mo). The intervention participants had 3.6% greater bone mass than controls immediately after the intervention and 1.4% greater bone mass at the total hip than controls after 8 yr. *Results are adjusted for baseline age, ΔHt, ΔWt, maturity, and sports participation and are significant at each of the seven measurement intervals (p < 0.05).
DISCUSSION
Our aim was to study the long-term effects of a high-intensity jumping program on the growing skeleton. We report that, after 8 yr, children who participated in a 7-mo randomized, controlled jumping intervention had significantly greater hip BMC than controls. The intervention effect was significant at each measured time point after the intervention (Fig. 1). Although the relative contribution of the intervention effect decreased over time, it nevertheless persisted even after controlling for the effects of normal growth and maturation. We did not observe a sustained effect at the femoral neck, despite having previously reported increased FN BMC immediately after the intervention.(7) We attribute this to the greater variability in the femoral neck measure compared with the total hip and to the considerably smaller sample size in our long-term cohort.
Strengths of the study include the randomized intervention, a highly specific exercise prescription, a rigorous analytical approach, and long-term subject retention. This study represents the first published report of the enduring effects (∼8 yr) of a randomized controlled exercise intervention on BMC gains in growing children. We attribute the lasting skeletal effects reported in this paper to a very specific exercise program (jumping) with an effective dose of 300 jumps per week off 24-in boxes. The statistical approach allowed us to observe the magnitude of the intervention effect after controlling for the effects of growth, maturation, and physical activity. Furthermore, the statistical design supported data retention, because a missed visit did not warrant subject exclusion from analyses (100% of subjects agreeing to long-term follow-up had at least three visits and were included in the analyses). However, subject retention was also high with 86% of the subjects whom agreed to long-term follow up measured at the last visit (91 mo).
Several limitations to the study must be acknowledged. First, we were unable to include lean mass in the models because our methods of estimating body composition varied between the two funding cycles, and we only had lean mass estimates beginning in 2002. Although lean mass has been shown to be a powerful predictor of the ΔBMC in growing children,(26,27) weight is highly related to lean mass in our data set (r = 0.82, p < 0.001) and thus was used in the models. This correlation was derived from data collected between 2002 and 2006 when lean mass was measured and included at least two measures from all 57 subjects. Second, we did not include minutes of weight-bearing activity as a variable in our models because of changes in our questionnaire methods. However, in a separate, long-term study of a similar intervention, we did not observe that minutes of weight-bearing activity contributed to changes in bone mass.(13) Thus, to control for physical activity, we used only sport participation and are confident that we are capturing the most important physical activity variable.
Finally, because of the 2D nature of the DXA assessment, we did not measure bone geometry. Understanding mineral distribution is important to understanding bone structural behavior. Given the diminishing effect of the intervention on BMC observed in our data, it is possible that the sustained benefits we observed may disappear before these children reach skeletal maturity. However, Warden et al.(28) found that structural and strength benefits from exercise-induced loading in rats persisted even as initial increases in bone mass diminished. It is plausible that our jumping intervention induced a structural change that we were not able to identify with DXA. Future studies should include measures of bone geometry to determine whether bone structure was affected by the intervention, and if so, whether those changes persist.
Approximately 50–80% of bone mass is genetically determined.(29,30) This leaves a substantial proportion of the variance in bone mass that may be modified by environmental stimuli such as exercise. The assertion that exercise undertaken in childhood may have substantial effects on the acquisition of peak bone mass and subsequent reductions in fracture risk in adulthood is often cited as the rationale for intervention studies. Whereas numerous studies have shown that exercise results in an immediate benefit,(7,31–35) there are few data from controlled longitudinal studies to suggest that exercise effects persist beyond a few years once training has ceased.(8,12)
Valdimarsson et al.(12) observed persistent effects from soccer training after 8 yr among active female athletes (18.3 ± 4.0 yr of age at baseline) and former female soccer players (40.0 ± 4.5 yr of age and retired for a mean of 9.7 yr at baseline). The authors report that soccer training was associated with greater BMD accrual during the playing years and steeper declines in bone mass in players compared with controls after retirement from soccer training. However, the higher BMD attributable to soccer training in youth among the former players was protective in that despite a greater rate of loss once training ceased, the players still had greater BMD compared with controls even 20 yr after retirement from soccer training.
Our study differed from Valdimarsson et al.(12) in two ways: (1) children in our study were prepubertal during exercise training and (2) our subjects participated in an acute 7-mo program of specifically defined impact exercise. However, despite the short-term exercise training, children in the jumping intervention had greater gains in BMC than nonjumpers 7 yr after the program ended. These findings are consistent with our recent report that a similar randomized, controlled intervention in growing children resulted in lasting effects on bone 3 yr after the intervention ended.(13) Although we cannot infer whether the effects of our intervention will persist into late adulthood, these data showed a long-lasting effect through childhood and adolescence not previously reported. Research suggests environmental stimuli during a critical window of time permanently affect subsequent structure, function, or developmental schedule of the organism.(36–38) This effect of environmental exposure is likely to be more marked in early life than at later ages, and the influence is more likely to exert a fundamental effect on the development of metabolic capacity.(36–38) If so, it is possible that exposure to sufficient mechanical stimuli during a “critical window” of pre- or early puberty may provoke a permanent change in bone metabolism that promotes enhanced accrual throughout growth. We hypothesize that the high forces delivered to the hip during the jumping intervention enhanced growth in our study cohort that resulted in significantly greater BMC values that persisted for 8 yr. Our research design allowed us to control lifestyle (e.g., dietary calcium, physical activity) and genetic (e.g., race, maturation timing) factors that may have persisting effects on bone mass and to observe the independent effects of our randomized, controlled, intervention on changes in bone mass in this cohort of growing children.
In conclusion, we report a sustained effect on BMC accrual from a simple, high impact jumping program. If this intervention became a regular activity within a mandatory physical education curriculum, children who choose not to engage in sport or physical activity outside of school would gain skeletal benefit. Furthermore, if the benefits are sustained into adulthood, effectively increasing peak bone mass, this could result in reductions in lifetime fracture risk.
ACKNOWLEDGMENTS
This study was supported by the National Institutes of Health (RO1 AR45655-08).
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Turner CH, Takano Y, Owan I. Aging changes mechanical loading thresholds for bone formation in rats. J Bone Miner Res. 1995;10:1544–1549. doi: 10.1002/jbmr.5650101016. [DOI] [PubMed] [Google Scholar]
- 2.Raisz LG. Pathogenesis of osteoporosis: Concepts, conflicts, and prospects. J Clin Invest. 2005;115:3318–3325. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NIH Consensus. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–795. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- 4.Karlsson M. Has exercise an antifracture efficacy in women. Scand J Med Sci Sports. 2004;14:2–15. doi: 10.1111/j.1600-0838.2003.00322.x. [DOI] [PubMed] [Google Scholar]
- 5.Borer KT. Physical activity in the prevention and amelioration of osteoporosis in women: Interaction of mechanical, hormonal and dietary factors. Sports Med. 2005;35:779–830. doi: 10.2165/00007256-200535090-00004. [DOI] [PubMed] [Google Scholar]
- 6.Bailey DA, McKay HA, Mirwald RL, Crocker PR, Faulkner RA. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: The university of Saskatchewan bone mineral accrual study. J Bone Miner Res. 1999;14:1672–1679. doi: 10.1359/jbmr.1999.14.10.1672. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs RK, Bauer JJ, Snow CM. Jumping improves hip and lumbar spine bone mass in prepubescent children: A randomized controlled trial. J Bone Miner Res. 2001;16:148–156. doi: 10.1359/jbmr.2001.16.1.148. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs RK, Snow CM. Gains in hip bone mass from high-impact training are maintained: A randomized controlled trial in children. J Pediatr. 2002;141:357–362. doi: 10.1067/mpd.2002.127275. [DOI] [PubMed] [Google Scholar]
- 9.Petit MA, McKay HA, MacKelvie KJ, Heinonen A, Khan KM, Beck TJ. A randomized school-based jumping intervention confers site and maturity-specific benefits on bone structural properties in girls: A hip structural analysis study. J Bone Miner Res. 2002;17:363–372. doi: 10.1359/jbmr.2002.17.3.363. [DOI] [PubMed] [Google Scholar]
- 10.Gustavsson A, Olsson T, Nordstrom P. Rapid loss of bone mineral density of the femoral neck after cessation of ice hockey training: A 6-year longitudinal study in males. J Bone Miner Res. 2003;18:1964–1969. doi: 10.1359/jbmr.2003.18.11.1964. [DOI] [PubMed] [Google Scholar]
- 11.Nordstrom A, Olsson T, Nordstrom P. Bone gained from physical activity and lost through detraining: A longitudinal study in young males. Osteoporos Int. 2005;16:835–841. doi: 10.1007/s00198-004-1749-4. [DOI] [PubMed] [Google Scholar]
- 12.Valdimarsson O, Alborg HG, Duppe H, Nyquist F, Karlsson M. Reduced training is associated with increased loss of BMD. J Bone Miner Res. 2005;20:906–912. doi: 10.1359/JBMR.050107. [DOI] [PubMed] [Google Scholar]
- 13.Gunter K, Baxter-Jones A, Mirwald R, Almstedt H, Fuller A, Durski S, Snow CM. Jump starting skeletal health: A 4-year longitudinal study assessing the effects of jumping on skeletal development in pre and circum pubertal children. Med Sci Sports Exer. 2007;39:S42. doi: 10.1016/j.bone.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Warden SJ, Fuchs RK, Castillo AB, Nelson IR, Turner CH. Exercise When Young Provides Lifelong Benefits to Bone Structure and Strength. J Bone Miner Res. 2007;22:251–259. doi: 10.1359/jbmr.061107. [DOI] [PubMed] [Google Scholar]
- 15.Slemenda CW, Miller JZ, Hui SL, Reister TK, Johnston CC., Jr Role of physical activity in the development of skeletal mass in children. J Bone Miner Res. 1991;6:1227–1233. doi: 10.1002/jbmr.5650061113. [DOI] [PubMed] [Google Scholar]
- 16.Rockett HR, Wolf AM, Colditz GA. Development and reproducibility of a food frequency questionnaire to assess diets of older children and adolescents. J Am Diet Assoc. 1995;95:336–340. doi: 10.1016/S0002-8223(95)00086-0. [DOI] [PubMed] [Google Scholar]
- 17.Baranowski T, Domel SB. A cognitive model of children's reporting of food intake. Am J Clin Nutr. 1994;59(1 Suppl):212S–217S. doi: 10.1093/ajcn/59.1.212S. [DOI] [PubMed] [Google Scholar]
- 18.Martin A, Carter JEL, Hendy KC, Malina RM. Segment lengths. In: Lohman T, Roche AF, Martorell R, editors. Anthropometric Standardization Reference Manual. 2nd ed. Champaign, IL, USA: Human Kinetics; 1991. pp. 9–44. [Google Scholar]
- 19.Williams DP, Going SB, Lohman TG, Harsha DW, Srinivasan SR, Webber LS, Berensen GS. Body fatness and risk for elevated blood pressure, total cholesterol, and serum lipoprotein ratios in children and adolescents. Am J Public Health. 1992;82:358–364. doi: 10.2105/ajph.82.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baxter-Jones AD, Mirwald RL, McKay HA, Bailey DA. A longitudinal analysis of sex differences in bone mineral accrual in healthy 8-19-year-old boys and girls. Ann Hum Biol. 2003;30:160–175. doi: 10.1080/0301446021000034642. [DOI] [PubMed] [Google Scholar]
- 21.Baxter-Jones ADG, Eisenmann JC, Sherar LB. Controlling for maturation in pediatric exercise science. Pediatr Exer Sci. 2005;17:18–30. [Google Scholar]
- 22.Malina RM. Secular changes in growth, maturation, and physical performance. Exerc Sport Sci Rev. 1978;6:203–255. [PubMed] [Google Scholar]
- 23.Mirwald RL, Baxter-Jones AD, Bailey DA, Beunen GP. An assessment of maturity from anthropometric measurements. Med Sci Sports Exerc. 2002;34:689–694. doi: 10.1097/00005768-200204000-00020. [DOI] [PubMed] [Google Scholar]
- 24.Bauer J, Fuchs R, Smith G, Costello M, Snow CM. Force characteristics of children participating in exercise that increases bone mass. J Appl Biomechanics. 2001;17:142–152. [Google Scholar]
- 25.Goldstein H. Multilevel Statistical Models. 2nd ed. London, UK: Arnold; 1995. [Google Scholar]
- 26.Wang MC, Bachrach LK, Van Loan M, Hudes M, Flegal KM, Crawford PB. The relative contributions of lean tissue mass and fat mass to bone density in young women. Bone. 2005;37:474–481. doi: 10.1016/j.bone.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 27.Witzke KA, Snow CM. Lean body mass and leg power best predict bone mineral density in adolescent girls. Med Sci Sports Exerc. 1999;31:1558–1563. doi: 10.1097/00005768-199911000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Warden SJ, Fuchs RK, Castillo AB, Nelson IR, Turner CH. Exercise when young provides lifelong benefits to bone structure and strength. J Bone Miner Res. 2007;22:251–257. doi: 10.1359/jbmr.061107. [DOI] [PubMed] [Google Scholar]
- 29.Pocock NA, Eisman JA, Hopper JL, Yeates MG, Sambrook PN, Eberl S. Genetic determinants of bone mass in adults. A twin study. J Clin Invest. 1987;80:706–710. doi: 10.1172/JCI113125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slemenda CW, Christian JC, Williams CJ, Norton JA, Johnston CC., Jr Genetic determinants of bone mass in adult women: A reevaluation of the twin model and the potential importance of gene interaction on heritability estimates. J Bone Miner Res. 1991;6:561–567. doi: 10.1002/jbmr.5650060606. [DOI] [PubMed] [Google Scholar]
- 31.Bass SL, Saxon L, Daly RM, Turner CH, Robling AG, Seeman E, Stuckey S. The effect of mechanical loading on the size and shape of bone in pre-, peri-, and postpubertal girls: A study in tennis players. J Bone Miner Res. 2002;17:2274–2280. doi: 10.1359/jbmr.2002.17.12.2274. [DOI] [PubMed] [Google Scholar]
- 32.Blimkie CJ, Rice S, Webber CE, Martin J, Levy D, Gordon CL. Effects of resistance training on bone mineral content and density in adolescent females. Can J Physiol Pharmacol. 1996;74:1025–1033. [PubMed] [Google Scholar]
- 33.Heinonen A, Sievanen H, Kannus P, Oja P, Pasanen M, Vuori I. High-impact exercise and bones of growing girls: A 9-month controlled trial. Osteoporos Int. 2000;11:1010–1017. doi: 10.1007/s001980070021. [DOI] [PubMed] [Google Scholar]
- 34.Mackelvie KJ, McKay HA, Khan KM, Crocker PR. A school-based exercise intervention augments bone mineral accrual in early pubertal girls. J Pediatr. 2001;139:501–508. doi: 10.1067/mpd.2001.118190. [DOI] [PubMed] [Google Scholar]
- 35.McKay HA, MacLean L, Petit M, MacKelvie-O'Brien K, Janssen P, Beck T, Khan KM. “Bounce at the Bell”: A novel program of short bouts of exercise improves proximal femur bone mass in early pubertal children. Br J Sports Med. 2005;39:521–526. doi: 10.1136/bjsm.2004.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mericq V. Low birth weight and endocrine dysfunction in postnatal life. Pediatr Endocrinol Rev. 2006;4:3–14. [PubMed] [Google Scholar]
- 37.Lucas A. Programming by early nutrition in man. Ciba Found Symp. 1991;156:38–50. [PubMed] [Google Scholar]
- 38.Fewtrell MS, Prentice A, Jones SC, Bishop NJ, Stirling D, Buffenstein R, Lunt M, Cole TJ, Lucas A. Bone mineralization and turnover in preterm infants at 8-12 years of age: The effect of early diet. J Bone Miner Res. 1999;14:810–820. doi: 10.1359/jbmr.1999.14.5.810. [DOI] [PubMed] [Google Scholar]