Abstract
Conventional treatment of obesity reduces fat in mature adipocytes but leaves them with lipogenic enzymes capable of rapid resynthesis of fat, a likely factor in treatment failure. Adenovirus-induced hyperleptinemia in normal rats results in rapid nonketotic fat loss that persists after hyperleptinemia disappears, whereas pair-fed controls regain their weight in 2 weeks. We report here that the hyperleptinemia depletes adipocyte fat while profoundly down-regulating lipogenic enzymes and their transcription factor, peroxisome proliferator-activated receptor (PPAR)γ in epididymal fat; enzymes of fatty acid oxidation and their transcription factor, PPARα, normally low in adipocytes, are up-regulated, as are uncoupling proteins 1 and 2. This transformation of adipocytes from cells that store triglycerides to fatty acid-oxidizing cells is accompanied by loss of the adipocyte markers, adipocyte fatty acid-binding protein 2, tumor necrosis factor α, and leptin, and by the appearance of the preadipocyte marker Pref-1. These findings suggest a strategy for the treatment of obesity by alteration of the adipocyte phenotype.
Keywords: leptin, post-adipocyte, dedifferentiation, lipogenesis, oxidation
Conventional treatment of obesity by caloric restriction reduces fat in adipocytes through increased lipolysis and hepatic oxidation of fatty acids to ketones. However, the adipocytes retain their complement of lipogenic enzymes capable of resynthesis of fat, which probably explains the high rate of relapse after such treatment. We have previously reported that adenovirus-induced hyperleptinemia rapidly causes profound loss of body fat (1) without any increase in plasma free fatty acids (FFA) or ketones (2). Moreover, whereas pair-fed controls regained their body weight in approximately 2 weeks, the weight loss of the hyperleptinemic rats persisted for 2 months after leptin levels had returned to normal (G. Chen, K. Koyama, and R.H.U., unpublished observations). This study was designed to account for the striking clinical differences in hyperleptinemic weight loss. Specifically, we wished to determine whether the loss of fat without ketosis might signify in situ oxidation of FFA in fat tissue, and whether the lag in weight regain might reflect a reduction in lipogenic enzymes.
We report here that the ectopic hyperleptinemia induced by adenovirus transfer of the leptin gene transforms adipocytes into “post-adipocytes,” i.e., fat-depleted cells devoid of mRNA encoding lipogenic enzymes and adipocyte markers, but with increased expression of enzymes of fatty acid oxidation, uncoupling proteins, and a preadipocyte marker. This transformation of the adipocyte phenotype suggests molecular targets for pharmacologic treatment of morbid obesity.
METHODS
Animals.
Lean wild-type (+/+) male Zucker diabetic fatty (ZDF) rats were bred in our laboratory from [ZDF-Drt-fa (F10)] rats purchased from R. Peterson (University of Indiana School of Medicine, Indianapolis). Their genotype was confirmed by the method of Phillips et al. (3).
To induce hyperleptinemia in wild-type ZDF rats, we infused them for 30 min with 1012 plaque-forming units of recombinant adenovirus containing either the rat leptin cDNA (AdCMV-leptin) or the bacterial β-galactosidase gene (AdCMV-β-gal) under control of the cytomegalovirus (CMV) promoter. The 2-ml infusate was prepared as previously described (1) and delivered via polyethylene tubing (PE-50; Becton Dickinson) previously anchored in the left carotid artery of 9-week-old wild-type ZDF rats weighing 250–300 g. The catheter placement was carried out while the animals were under sodium pentobarbital anesthesia (50 mg/kg; Abbott) and exteriorized via a subcutaneous tunnel 3 days before virus infusion. Animals were studied in individual metabolic cages, and food intake and body weight were measured daily.
Tissue Preparation.
Animals were sacrificed under sodium pentobarbital anesthesia. Tissues were resected immediately, washed with sterilized ice-chilled 10 mM phosphate-buffered saline, and frozen in liquid nitrogen. Tissues were kept at −70°C until RNA extraction.
Extraction of Total RNA and Semiquantitation by Reverse Transcriptase–Polymerase Chain Reaction (RT-PCR).
Total RNA from epididymal fat was extracted by the Trizol isolation method (Life Technologies, Gaithersburg, MD). RNA was treated with RNase-free DNase (Promega), and first-strand cDNA was generated from 1 μg of RNA in a 20-μl volume by using the oligo(dT) primer in the 1st-strand cDNA synthesis kit (CLONTECH). One microliter of the reverse transcription reaction mix was amplified with primers specific for rat acyl-CoA oxidase (ACO), carnitine palmitoyltransferase 1 (CPT-1), fatty acid synthetase (FAS), acetyl-CoA carboxylase (ACC), glycerol-3-phosphate acetyltransferase (GPAT), tumor necrosis factor-α (TNF-α), preadipocyte factor-1 (Pref-1), adipocyte fatty acid-binding protein (aP2), lipoprotein lipase (LPL), and uncoupling proteins (UCP) 1 and 2. All sequences are shown in Table 1. Linearity of the PCR was tested by amplification of 200 ng of total RNA per reaction from 15–50 cycles. The linear range was found to be between 15 and 40 cycles. In no case did the amount of RNA used for PCR exceed 200 ng per reaction. The samples were amplified for 25–35 cycles by using the following parameters: 92°C for 1 min, 55°C for 1 min, and 72°C for 1 min. β-Actin primers were used as a control. Levels of mRNA were expressed as the ratio of signal intensity for the target genes relative to that for β-actin. The PCR products were electrophoresed on an agarose gel and Southern blotting on a nylon membrane was carried out. Radiolabeled probes (Table 1) specific for each molecule were hybridized to the membrane and quantitated by a molecular imager (GS-363; Bio-Rad).
Table 1.
Sequences of PCR primers
| Gene | Sense primer (5′-3′) | Antisense primer (5′-3′) | Internal primers (5′-3′, 30-mers) | GenBank accession no. |
|---|---|---|---|---|
| β-Actin | TTGTAACCAACTGGGACGATATGG | GATCTTGATCTTCATGGTGCTAGG | GGTCAGGATCTTCATGAGGTAGTCTGTCAG | J00691 |
| ACO | GCCCTCAGCTATGGTATTAC | AGGAACTGCTCTCACAATGC | GCCTGCACTTTCTTCAGCCATCTTCAACGA | J02752 |
| CPT-1 | TATGTGAGGATGCTGCTTCC | CTCGGAGAGCTAAGCTTGTC | ACTCTGGTTGGAATCTGACTGGGTGGGATT | L07736 |
| ACC | ACTCCAGGACAGCACAGATC | TCTGCCAGTCCAATTCTAGC | ATGACATCTCGGCCATCTGGATATTCAGGG | M76767 |
| FAS | GTTTGATGGCTCACACACCT | TCAACTCACTCGAGGCTCAG | AAGAAGCATATGGCTTCAGCTTCAGCCTCA | M84761 |
| GPAT | TGATCAGCCAGGAGCAGCTG | AGACAGTATGTGGCACTCTC | CCAAGGAGCACCAGCAATTCATCACCTTTC | M77003 |
| UCP-1 | TGCAAGCACAAACGGTAGAC | CATCAGCTCTTTCTTCAGCT | TGGTGTACTTGGTCATTGCACAGCTGGGTA | M11814 |
| UCP-2 | AACAGTTCTACACCAAGGGC | AGCATGGTAAGGGCACAGTG | GTCATCTGTCATGAGGTTGGCTTTCAGGAG | U69135 |
| Pref-1 | ATTCGTCGACAAGACCTGCA | CCACCAGCCTGGTGAGCACG | TGTTGAGCTCTTTCATGGACACCTTCAGGA | U25680 |
| aP2 | GACCTGGAAACTCGTCTCCA | CATGACACATTCCACCACCA | TGGTCGACTTTCCATCCCACTTCTGCACAT | U75581 |
| TNF-α | CTCGAGTGACAAGCCCGTAG | TTGACCTCAGCGCTGAGCAG | ATAAGGTACAGCCCATCTGCTGGTACCACC | D00475 |
| LPL | CCTGAAGACTCGCTCTCAGA | TTGGTTTGTCCAGTGTCAGC | ATGTCCACCTCCGTGTAAATCAAGAAGGAG | L03294 |
Protein Extraction and Immunoblotting.
Epididymal fat pads were washed with ice-cold PBS (137 mM NaCl/1.5 mM KH2PO4/7 mM Na2HPO4/2.7 mM KCl) and homogenized with a Polytron homogenizer in a buffer (pH 7.5) containing 20 mM Tris⋅HCl, 1 mM EDTA, and 0.1 mM phenylmethanesulfonyl fluoride. The extract was centrifuged at 8,000 × g for 5 min at 4°C and then at 100,000 × g for 1 h at 4°C. The infranatant containing soluble protein was stored at −70°C until use. Protein concentration in the extract was measured by using the Bio-Rad protein assay with bovine serum albumin as a standard. Extracted protein (100 μg) from each pool was subjected to SDS/polyacrylamide gel electrophoresis (SDS/PAGE) and electrophoretically transferred to nitrocellulose. The blot was probed with goat polyclonal antibodies for rat leptin, peroxisome proliferator-activated receptor (PPAR)α, PPARγ (Santa Cruz Biotechnology), and rabbit antibody against UCP-2 (kindly provided by Barbara Kahn and Bradford Lowell of Beth Israel Hospital and Harvard Medical School, Boston). First antibody was detected by incubation with horseradish peroxidase-conjugated anti-goat or anti-rabbit antibody with enhanced chemiluminescence (Pierce) according to the manufacturer’s recommendation. Signal intensity was quantitated by a molecular imager with a chemiluminescence detector (Bio-Rad).
Plasma Measurements.
Fasting blood samples from the tail vein were collected between 1 and 3 p.m. in capillary tubes coated with EDTA. Plasma was stored at −20°C until the time of leptin assay with the Linco leptin assay kit (Linco Research, St. Charles, MO). Plasma insulin was assayed by radioimmunoassay (4). Plasma glucose was measured with a Glucose Analyzer II (Beckman).
Triglyceride (TG) Content of Fat Tissue.
Epididymal fat pads were resected, weighed, and immediately frozen in liquid nitrogen. About 100 mg of tissue was homogenized with a Polytron in 4 ml of buffer containing 18 mM Tris⋅HCl (pH 7.5), 300 mM d-mannitol, and 5 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA). Lipids were extracted by the method of Danno et al. (5).
Statistical Analysis.
All data are expressed as the mean ± SE of four samples. Statistical analysis was performed by unpaired Student’s t test or by one-way ANOVA.
RESULTS
Effect of Hyperleptinemia on Adipocyte TG Content.
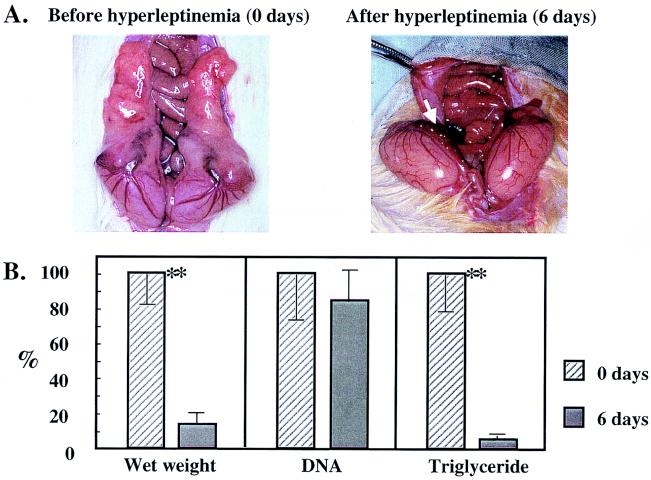
Hyperleptinemia was produced in 9-week-old lean, wild-type Zucker Diabetic Fatty rats (+/+) by the intravenous infusion of 1012 plaque-forming units of adenovirus containing the leptin cDNA (AdCMV-leptin). Plasma leptin levels exceeded 20 ng/ml in all of these rats, compared with less than 1 ng/ml in all control rats infused with adenovirus containing the β-galactosidase cDNA (AdCMV-β-gal). The hyperleptinemia was associated with a 30–50% reduction in food intake and a 32 ± 9 g weight loss during the 6 days of observation, at the end of which all grossly identifiable body fat had disappeared. Instead of normal epididymal fat pads, only strands of highly vascular tissue remained (Fig. 1A). The mean wet weight of the fat pads had declined by 87% and its total TG content by 95%, but the total DNA per fat pad had declined by only 16%, which was not significantly below uninfused controls (Fig. 1B). These findings suggested that the loss of TG was the result not of adipocyte death but rather of the 95% depletion of the adipocytes’ fat content. This interpretation is consistent with that of Sarmiento et al. (6), who demonstrated that in mice treated with recombinant leptin, necrosis and apoptosis of adipocytes are not the cause of the reduction in fat.
Figure 1.
(A) The appearance of epididymal fat pads of a control rat infused with AdCMV-β-gal and a hyperleptinemic rat infused with AdCMV-leptin. Grossly visible fat is absent from the latter; arrow points to the stromal-vascular remnant. (B) Wet weight, DNA, and TG content of epididymal fat pads of rats before and 6 days after hyperleptinemia and expressed as percent of the prehyperleptinemic value. ∗∗, P < 0.01 versus day 6 of hyperleptinemia.
Effect of Hyperleptinemia on Expression of Enzymes of Fatty Acid Oxidation and Thermogenic Proteins.
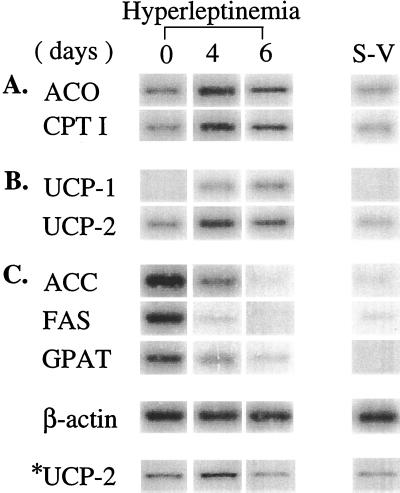
Fat loss in starvation and in insulin deficiency is associated with ketosis, whereas in hyperleptinemic fat loss plasma FFA and ketones remain normal (2). This lack of ketosis could signify that the fatty acids are oxidized in or near adipocytes, thus preventing them from reaching the liver, the site of ketogenesis. If so, one might expect increased expression of enzymes of fatty acid oxidation, such as CPT-1 and ACO, in adipose tissue during the disappearance of the fat. We observed an 8-fold increase in CPT-1 mRNA and a 6-fold increase in ACO mRNA in epididymal fat tissue of normal rats (Table 1 and Fig. 2A). That the energy generated by increased fatty oxidation was dissipated as heat can be inferred from the fact that the mRNA and the protein of the uncoupling proteins, UCP-1 and UCP-2, were also up-regulated (Table 2 and Fig. 2B), consistent with the many observations that leptin-induced weight loss exceeds that of pair-fed controls (7–9) and with our report of leptin-induced up-regulation of UCP-2 (10).
Figure 2.
Expression of genes involved in fatty acid metabolism in epididymal fat pads of normal rats before and after 4 or 6 days of hyperleptinemia. (A) mRNAs encoding enzymes of fatty acid oxidation, ACO and CPT-1. (B) mRNA of UCP-1 and UCP-2. (C) Enzymes of lipogenesis, ACC, FAS, and GPAT. S-V, stromal-vascular tissue from which adipocytes have been mechanically removed. ∗, Immunoblot.
Table 2.
Expression of various genes and proteins in epididymal fat tissue before and after induction of hyperleptinemia in normal rats
| Function | Gene or protein | mRNA or protein level
|
||||
|---|---|---|---|---|---|---|
| Duration of hyperleptinemia
| ||||||
| 0 days | 4 days | 6 days | S–V | |||
| mRNA | Fatty acid oxidation | ACO | 0.24 ± 0.07d | 1.36 ± 0.36g,h,i | 0.64 ± 0.13a,i | 0.11 ± 0.03 |
| CPT-1 | 0.18 ± 0.06i | 1.25 ± 0.27g,h,i | 0.69 ± 0.22f,i | 0.09 ± 0.02 | ||
| Lipogenesis | ACC | 1.76 ± 0.56f,h,i | 0.21 ± 0.05c,d | 0.00 ± 0.00 | 0.03 ± 0.01 | |
| FAS | 1.48 ± 0.44f,h,i | 0.02 ± 0.01c,d | 0.00 ± 0.00 | 0.01 ± 0.00 | ||
| GPAT | 0.52 ± 0.21f,h,i | 0.16 ± 0.04c,d | 0.05 ± 0.02 | 0.00 ± 0.00 | ||
| Thermogenesis | UCP-1 | 0.00 ± 0.00 | 0.10 ± 0.03b,d | 0.28 ± 0.08a,f,i | 0.00 ± 0.00 | |
| UCP-2 | 0.23 ± 0.05d | 0.94 ± 0.21c,g,i | 0.41 ± 0.13b,i | 0.03 ± 0.01 | ||
| Preadipocyte marker | Pref-1 | 0.03 ± 0.01 | 0.63 ± 0.19b | 1.23 ± 0.34f,g,i | 0.48 ± 0.13b | |
| Adipocyte markers | aP2 | 1.00 ± 0.25f,h,i | 0.03 ± 0.01 | 0.04 ± 0.00 | 0.07 ± 0.02a,c | |
| TNF-α | 0.44 ± 0.10f,h,i | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.05 ± 0.01a,c | ||
| LPL | 0.78 ± 0.20c,i | 2.84 ± 0.58g,h,i | 0.27 ± 0.04i | 0.07 ± 0.02 | ||
| Protein | Thermogenesis | UCP-2 | 3.05 ± 0.77d | 9.43 ± 1.94g,h,i | 2.88 ± 0.43d | 2.13 ± 0.49 |
| Adipocyte marker | Leptin | 16.48 ± 3.46f,h,i | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.02 ± 0.01a,c | |
| Transcription factors | PPARγ | 17.18 ± 3.75f,h,i | 2.49 ± 0.48d,h | 0.01 ± 0.00 | 1.88 ± 0.34h | |
| PPARα | 2.17 ± 0.39 | 12.64 ± 2.66g,h,i | 6.48 ± 1.74d,g | 3.38 ± 0.68b | ||
Expression in stromal-vascular tissue (S–V) obtained from normal untreated rat appears on the right. Data are mean ± SE from four measurements of mRNA of various genes relative to β-actin mRNA or protein in arbitrary units.
P < 0.05 and
P < 0.01 versus 4th day of hyperleptinemia.
P < 0.05 and
P < 0.01 versus day 0.
P < 0.05 and
P < 0.01 versus 6th day of hyperleptinemia.
P < 0.05 and
P < 0.01 versus S–V.
Effect of Hyperleptinemia on Expression of Enzymes of Lipogenesis.
Because of the prolonged delay in the recovery of body weight after the hyperleptinemia had returned to normal, we suspected that down-regulation of lipogenic capacity of the adipocytes had occurred. We therefore semiquantified the expression of the lipogenic enzymes ACC, FAS, and GPAT. We observed complete disappearance of the mRNA of all 3 (Table 1 and Fig. 2C). In control rats injected with AdCMV-β-gal, no changes in expression of any of these genes could be detected (data not shown).
Effect of Hyperleptinemia on Expression of PPARα and PPARγ.
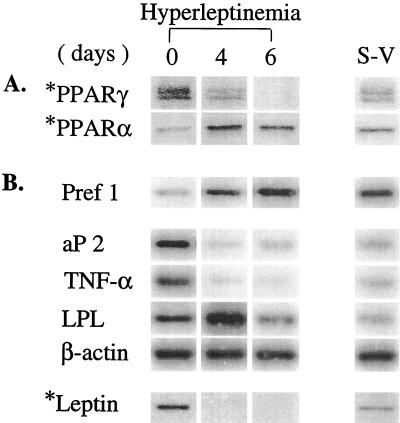
To gain further insight into the mechanism of the dramatic leptin-induced inversion of expression levels of enzymes of fatty acid oxidation and lipogenesis, we measured in epididymal tissue two of the transcription factors that regulate them, the α and γ PPAR isoforms. PPARγ2 is expressed exclusively in adipocytes and plays a role in their differentiation (11, 12) and in the expression of enzymes of lipogenesis (13). PPARα, by contrast, is most abundant in liver but is also expressed elsewhere in other tissues in which fatty acid oxidation is a normal function (14–16). By day 6, PPARγ protein was less than 1% of the control level. PPARα, by contrast, had increased 5.8-fold by day 4, when the enzymes of oxidation had peaked, and declined thereafter (Fig. 3A). These changes suggest that the increase in PPARα mediated the up-regulation of ACO and CPT-1 and that the reduced PPARγ played a role in down-regulating the lipogenic enzymes.
Figure 3.
(A) Expression of PPARα and PPARγ protein in epididymal fat pad of normal rats before and 4 and 6 days after induction of hyperleptinemia. (B) Expression of markers of mature adipocytes, aP2, TNF-α, leptin, and LPL, and a preadipocyte marker, Pref-1, in epididymal tissue of normal rats before and 4 and 6 days after induction of hyperleptinemia. S-V, stromal-vascular tissue from normal untreated rats. ∗, Immunoblot.
Effect of Hyperleptinemia on Markers of Adipocytes and Preadipocytes.
During the terminal phase of adipocyte differentiation, preadipocytes lose the marker Pref-1, an inhibitor of the differentiation process (17), and acquire new markers of mature adipocytes. PPARγ, a specific marker for adipocytes (11, 12), appears early in differentiation (18), and it has been proposed as a “master regulator” of this process (17, 19). As differentiation proceeds, adipocyte markers such as aP2, TNF-α, leptin, and LPL are expressed. The fact that PPARγ had disappeared from the lipid-depleted epididymal tissue of hyperleptinemic rats raised the possibility that the residual cells were not simply mature adipocytes emptied of fat, but rather were mature adipocytes that had lost their maturity markers and had acquired certain features of their progenitors (20, 21). We therefore semiquantified the mRNA and protein of preadipocyte and adipocyte markers at 0, 4, and 6 days after AdCMV-leptin infusion. As shown in Table 1 and Fig. 3B, the mRNA of adipocyte markers, aP2 and TNF-α and leptin protein, all declined to virtually undetectable levels on day 6 of the hyperleptinemia. mRNA of LPL, one of the first genes induced during differentiation (21), increased markedly on day 4, but on day 6 it declined to 34% of the control value (Table 2 and Fig. 3B). The loss or reduction in the expression of the four mature adipocyte markers examined was consistent with a regression of the phenotype of the differentiated cell toward that of the precursor. We therefore semiquantified the mRNA of the preadipocyte marker Pref-1 before and during hyperleptinemia. It was 21-fold higher than the control level after 4 days of hyperleptinemia and 41 times higher on day 6.
Since Pref-1 mRNA was present at low levels in the stromal-vascular cells of mature epididymal fat tissue (Fig. 3B), it seemed possible that the increase in Pref-1 observed after 6 days of hyperleptinemia might simply reflect an enrichment for preexisting preadipocytes as the result of the disappearance of “Pref-1-free” mature adipocytes. Consequently, we mechanically removed mature adipocytes from epididymal fat pads of normal rats and semiquantified Pref-1 mRNA in the residual stromal-vascular tissue. Pref-1 mRNA was 2.6 times greater in the epididymal tissue of the hyperleptinemic rats than in the control sample of normal rats from which adipocytes had been mechanically removed (Table 2 and Fig. 3B).
The mechanically obtained fat-free stromal-vascular tissue was too meager to weigh and its DNA content was less than 5% of that of fat-free epididymal tissue of hyperleptinemic rats, which, as mentioned, retained 81% of its original prehyperleptinemic total DNA. If the 41-fold increase in Pref-1 expression observed in the epididymal fat pad remnant of hyperleptinemic rats (Table 2) had occurred entirely in the stromal-vascular component, we calculate that a 640-fold increase in Pref-1 expression would have been required; if the changes had been localized to the “post-adipocytes,” a 38-fold increase would have been required. Therefore, it seems likely that this increase in Pref-1 represents induction rather than enrichment of the marker, and that the induction took place mostly, if not entirely, in adipocytes that had “dedifferentiated.”
DISCUSSION
The results disclose that the profound loss of fat induced by sustained, chronic adenovirally induced hyperleptinemia in normal lean rats is associated with a major transformation of white fat tissue. This includes changes in the expression of the enzymes of fatty acid metabolism, of the transcription factors that influence their expression, and of molecular markers that distinguish mature adipocytes from preadipocytes. All of these dramatic changes occurred within 6 days of the infusion of AdCMV-leptin. The up-regulation in fat tissue of two major enzymes of fatty acid oxidation, CPT-1 and ACO, provides a likely explanation for the rapid disappearance of all visible fat without accompanying ketonemia (1, 2); it suggests that oxidation of an important fraction of fatty acids occurred in or very near the adipocytes—i.e., that fatty acids did not reach the liver in quantities sufficient to cause ketonemia. The increases in UCP-1 and -2 suggest that the energy generated by local oxidation was largely dissipated as heat, thus accounting for leptin’s ability to reduce weight in excess of its effect on food intake (1, 7–9). The virtual disappearance of the mRNA of three lipogenic enzymes, ACC, FAS, and GPAT, may signify a loss of lipogenic capability of adipocytes. The increase in PPARα protein, a known transcription factor for ACO and CPT-1 (22, 23), implies a role in the up-regulation of the expression of enzymes of fatty acid oxidation, while the reduction in PPARγ may signify a role in the reduction in expression of the lipogenic enzymes that it regulates. Finally, the markers of mature adipocytes, aP2 and TNF-α mRNA and leptin protein, disappeared, whereas LPL mRNA fell by 66% after a transient rise. At the same time, expression of Pref-1, a transmembrane protein expressed only in preadipocytes (17), increased dramatically to a peak on day 6. In other words, both the TGs and the markers of mature adipocytes disappeared from the fat pad with minimal reduction in the cell population, and the residual tissue bore a marker specific for preadipocytes. It seems reasonable, therefore, to conclude that these residual “post-adipocytes” were previously mature adipocytes that had lost their lipogenic enzymes and adipocyte markers and had acquired a new phenotype that is consistent with but does not prove uncoupled in situ oxidation of fatty acids.
These findings may have important pharmacologic implications in designing treatment strategies for obesity, a disorder that now affects almost half of the American population. Although therapy with recombinant leptin may be effective in some obese subjects (24), most such patients are expected to be resistant to the hormone (25). Although we produced no direct evidence that the effects of chronic hyperleptinemia on normal adipocytes are mediated by the disappearance of PPARγ and/or the increase in PPARα, our observation raises the possibility that fat content and phenotype of adipocytes can be manipulated by targeting PPAR ligands to adipocytes to enhance PPARα actions and/or reduce PPARγ actions. Such a strategy might bypass the hypothalamic targets of leptin that are thought to be resistant to leptin in obesity (25) and might therefore be more effective than leptin (24) in the management of this disorder (26). It also supports the greater efficacy of continuous leptin delivery by secreting cells over injections of recombinant leptin (27).
Acknowledgments
We thank Dr. Daniel Foster for critical examination of this manuscript. We thank Kay Mccorkle for technical support and Tess Perico for secretarial assistance. We acknowledge the grant support of the Department of Veterans Affairs, the National Institute of Diabetes, Digestive and Kidney Diseases, the Juvenile Diabetes Foundation International, Novo-Nordisk Corporation, and Sankyo, Inc.
ABBREVIATIONS
- PPAR
peroxisome proliferator-activated receptor
- ACO
acyl-CoA oxidase
- CPT-1
carnitine palmitoyl transferase 1
- TNF-α
tumor necrosis factor-α
- Pref-1
preadipocyte factor-1
- aP2
adipocyte fatty acid-binding protein 2
- UCP
uncoupling protein
- FFA
free fatty acids
- RT-PCR
reverse transcriptase–PCR
- TG
triglyceride
- ZDF
Zucker diabetic fatty
- FAS
fatty acid synthetase
- ACC
acetyl-CoA carboxylase
- GPAT
glycerol-3-phosphate acyltransferase
- LPL
lipoprotein lipase
- CMV
cytomegalovirus
- AdCMV-leptin
recombinant adenovirus containing the rat leptin cDNA under control of the CMV promoter
- AdCMV-β-gal
recombinant adenovirus containing the bacterial β-galactosidase gene under control of the CMV promoter
References
- 1.Chen G, Koyama K, Yuan X, Lee Y, Zhou Y T, O’Doherty R, Newgard C B, Unger R H. Proc Natl Acad Sci USA. 1996;93:14795–14799. doi: 10.1073/pnas.93.25.14795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimabukuro M, Koyama K, Chen G, Wang M Y, Trieu F, Lee Y, Newgard C B, Unger R H. Proc Natl Acad Sci USA. 1997;94:4637–4641. doi: 10.1073/pnas.94.9.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips M S, Liu Q, Hammond H A, Dugan V, Hey P J, Caskey C J, Hess J F. Nat Genet. 1996;13:18–19. doi: 10.1038/ng0596-18. [DOI] [PubMed] [Google Scholar]
- 4.Yalow R S, Berson S A. J Clin Invest. 1960;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danno H, Jincho Y, Budiyanto S, Furukawa Y, Kimura S. J Nutr Sci Vitaminol. 1992;38:517–521. doi: 10.3177/jnsv.38.517. [DOI] [PubMed] [Google Scholar]
- 6.Sarmiento U, Benson B, Kaufman S, Ross L, Qi M, Scully S, DiPalma C. Lab Invest. 1997;77:243–256. [PubMed] [Google Scholar]
- 7.Campfield L A, Smith F J, Guisez Y, Devos R, Burn P. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 8.Halaas J L, Gajiwala K S, Maffei M, Cohen S L, Chait B T, Rabinowitz D, Lallone R L, Burley S K, Friedman J M. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 9.Pelleymounter M A, Cullen M J, Baker M B, Hecht R, Winters D, Boone T, Collins F. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Y T, Shimabukuro M, Koyama K, Lee Y, Wang M Y, Trieu F, Newgard C B, Unger R H. Proc Natl Acad Sci USA. 1997;94:6386–6390. doi: 10.1073/pnas.94.12.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tontonoz P, Hu E, Spiegelman B M. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 12.Tontonoz P, Hu E, Graves R A, Budavari A I, Speigelman B M. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 13.Schoonjans K, Staels B, Auwerx J. Biochim Biophys Acta. 1996;1302:93–109. doi: 10.1016/0005-2760(96)00066-5. [DOI] [PubMed] [Google Scholar]
- 14.Gottlicher M, Widmark E, Li Q, Gustafsson J A. Proc Natl Acad Sci USA. 1992;89:4653–4657. doi: 10.1073/pnas.89.10.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Issemann I, Green S. Nature (London) 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 16.Keller H, Dreyer C, Medin J, Mahfoudi A, Ozato K, Wahli W. Proc Natl Acad Sci USA. 1993;90:2160–2164. doi: 10.1073/pnas.90.6.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smas C M, Sul H S. Cell. 1992;73:725–734. doi: 10.1016/0092-8674(93)90252-l. [DOI] [PubMed] [Google Scholar]
- 18.Chawla A, Schwarz E J, Dimaculangan D D, Lazar M A. Endocrinology. 1994;135:798–800. doi: 10.1210/endo.135.2.8033830. [DOI] [PubMed] [Google Scholar]
- 19.MacDougald O A, Lane M D. Curr Biol. 1995;5:618–621. doi: 10.1016/s0960-9822(95)00125-4. [DOI] [PubMed] [Google Scholar]
- 20.Yeh W C, McKnight S. Curr Opin Cell Biol. 1995;7:885–890. doi: 10.1016/0955-0674(95)80074-3. [DOI] [PubMed] [Google Scholar]
- 21.Dani C, Amri E Z, Bertrand B, Enerback S, Bjursell G, Grimaldi P, Ailhaud G. J Cell Biochem. 1990;43:103–110. doi: 10.1002/jcb.240430202. [DOI] [PubMed] [Google Scholar]
- 22.Osumi T, Wen J K, Hashimoto T. Biochem Biophys Res Commun. 1991;175:866–871. doi: 10.1016/0006-291x(91)91645-s. [DOI] [PubMed] [Google Scholar]
- 23.Mascaro C, Acosta E, Ortiz J A, Marrero P F, Hegardt F G, Haro D. J Biol Chem. 1998;273:8560–8563. doi: 10.1074/jbc.273.15.8560. [DOI] [PubMed] [Google Scholar]
- 24.Phillips D F. J Am Med Assoc. 1998;280:869–870. [Google Scholar]
- 25.Caro J F, Kolaczynski J W, Nyce M R, Ohannesian J P, Opentanova I, Goldman W H, Lynn R B, Zhang P L, Sinha M K, Considine R V. Lancet. 1996;348:159–161. doi: 10.1016/s0140-6736(96)03173-x. [DOI] [PubMed] [Google Scholar]
- 26.Friedman J M, Halaas J L. Nature (London) 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 27.Morsy M A, Gu M C, Zhao J Z, Holder D J, Rogers I T, Pouch W J, Motzel S I, Klein H J, Gupta S K, Liang X, et al. Gene Ther. 1998;5:8–16. doi: 10.1038/sj.gt.3300565. [DOI] [PubMed] [Google Scholar]