Abstract
Purpose
EphB4 receptors and their ephrinB2 ligands are essential for vascular development, but also play a role in pathological neovascularization (NV). We previously reported that soluble (s) forms of EphB4 and ephrinB2 significantly reduced retinal NV in a model of oxygen-induced retinopathy. This study investigates if these molecules suppress retinal NV by stimulation of endothelial cell (EC) apoptosis.
Methods
C57BL/6 mice at postnatal day 7 (P7) were exposed to 75% oxygen for 5 days (P12) and allowed to recover in room air to induce retinal NV. One eye was injected intravitreally with 150 ng in 1.5μls of sEphB4 or sEphrinB2 on P12 and P14, while contralateral eyes were injected with IgG antibody as control. Eyes were enucleated for histological analysis. At P16 TUNEL analysis and caspase-3 immunohistochemistry was performed on retinal sections to compare the apoptotic response between sEphB4 or sEphrinB2 injected eyes and controls. In vitro studies were performed with human retinal microvascular EC (HREC).
Results
Quantification of TUNEL positive vascular cells, located in areas of retinal NV, revealed approximately 2.5-fold increase in apoptosis in sEphrinB2 injected eyes compared to control eyes. Immunohistochemistry studies revealed co-localization of both TUNEL positive cells and caspase-3 positive cells with the endothelial marker, von Willebrand factor. Cultured HREC demonstrated significantly higher caspase-3 activity after a 3 hr stimulation with sEphrinB2 ± VEGF compared to IgG control ± VEGF (p<0.005). sEphB4 stimulation had no significant effect on caspase-3 activity in HREC cultures.
Conclusions
These data suggest that modulation of the endogenous ephrin signaling mechanism by sEphrinB2 may induce suppression of retinal NV via induction of apoptosis. Results of the in vitro studies suggest that sEphrinB2 may directly induce apoptosis of EC during pathological neovascularization.
Keywords: Angiogenesis, Apoptosis, EphrinB2, EphB4, Neovascularization, Retinopathy of Prematurity
Introduction
Eph receptor tyrosine kinases (RTKs) are recognized as the largest RTK family of receptors; 16 Eph receptors and 9 ephrin ligands, permit a number of different Eph-ephrin combinations, in a variety of cellular contexts, to achieve crucial cell positioning (Lackmann and Boyd 2008). Eph receptors bind to ephrin ligands on adjacent cells resulting in a unique bidirectional signaling that regulates cell migration, attraction, and repulsion as well as modulation of cell differentiation and proliferation (Pasquale 2008). Eph receptors and ephrin ligands have also emerged as critical regulators of cardiovascular development, as evidenced by studies in gene-deleted mice. Specifically, targeted mutations of ephrinB2 or EphB4 result in similar vascular defects of angiogenesis in the embryonic yolk sac, the developing head and trunk, and the developing heart (Wang et al. 1998; Adams et al. 1999; Gerety et al. 1999). EphrinB2 is typically expressed by arterial ECs (and vascular smooth muscle cells), while its receptor EphB4, is expressed by venous ECs, and these markers contribute to delineating developing arteries and veins through repulsive interactions (Gale et al. 2001; Shin et al. 2001). Recent studies have also revealed a role for ephrinB2 and EphB4 in physiologic, pathologic and tumor angiogenesis (Kuijper et al. 2007). EphrinB2 and its receptors have also been localized to neovascular membranes in patients with ocular angiogenic diseases (Umeda et al. 2004).
Pathologic angiogenesis is controlled by the balance of pro-angiogenic factors (anti-apoptotic) and anti-angiogenic factors (pro-apoptotic). Several pro-apoptotic molecules have been identified that help regulate angiogenesis, including Fas-ligand (FasL), thrombospondin-1, and endostatin (Sakamaki 2004). The concept that the balance between pro- and anti-apoptotic factors plays a critical role in the regulation of angiogenesis and vascular regression has been validated in models of retinal neovascularization (NV) using mice deficient in both pro-apoptotic (i.e., Fas ligand) and anti-apoptotic (i.e., BCL-2) factors (Davies et al. 2003; Wang et al. 2005). Several Eph and ephrin molecules have both pro- and anti-angogenic properties, depending on the cellular context. EphB4 and ephrinB2 exhibit tumorogenic activities, including stimulation of new vessel formation within the tumor via reverse signaling of ephrinB2 (Pasquale 2008). However, Eph receptors and ephrin ligands also induce apoptosis in a variety of cell populations, including cancer cells, neural progenitors, and ECs (via EphB4) (Depaepe et al. 2005; Erber et al. 2006; Noren et al. 2006). We previously reported that soluble EphB4 or ephrinB2 significantly reduced retinal NV in a mouse model of oxygen-induced retinopathy (OIR) (Zamora et al. 2005). Therefore, the present study investigates whether these molecules suppress retinal NV by inducing apoptosis in retinal ECs.
Materials and methods
Animals
C57BL/6 mice were housed and bred in the Oregon Health & Science University animal care facility and treated in accordance with NIH guidelines and the guidelines of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. All animals were provided food and water ad libitum and were kept on a 12-hour light-dark schedule. To induce OIR, postnatal day (P) 7 mice, along with nursing females, were exposed to 75% oxygen for 5 days and then allowed to recover in room air on P12 (Smith et al. 1994). Room air litters were maintained under otherwise identical conditions as the hyperoxia-exposed mice.
Intravitreal Injections
Administration of anesthesia and injections were performed as previously described (Becker et al. 2003). Pups were deeply anaesthetized by isoflurane inhalation (0.5 L/min in oxygen). Approximately 1.5 μl of EphrinB2/Fc (n=8 mice) or EphB4/Fc (n=6 mice; 100 ng/μl; R&D Systems, Minneapolis, MN) was delivered intravitreally into the right eyes of oxygen-injured mice. Since these chimeric forms of ephrinB2/Fc and EphB4/Fc are monomers dimerized in their active form by the Fc portion of human IgG, 1.5μl of human whole IgG (100 ng/μl; Sigma; St. Louis, MO; n=12 mice) was delivered into the left eyes of oxygen-injured mice as a control protein injection. Intravitreal injections were performed with a Hamilton syringe connected to an ultrathin pulled borosilicate glass needle (outer diameter, ~50 μm). EphrinB2/Fc, EphB4/Fc, and human IgG proteins were diluted in sterile Dulbecco’s PBS (minus Ca++ and Mg++, pH ~7.4; Gibco/Invitrogen; Carlsbad, CA) prior to injection. Control and experimental injections were administered within 5 minutes of each other, and were given during the transition from hyperoxia to room air on P12 and repeated on P14. The mice were allowed to recover until P16 and were sacrificed by CO2 euthanasia. Both eyes were carefully enucleated from each mouse, placed in 10% neutral buffered formalin overnight, and then routinely processed for paraffin embedding. The eyes were then sectioned at 5 μm intervals, mounted on slides (SuperFrost Plus; Fisher Scientific, Pittsburgh, PA), and stored at room temperature until used for immunohistologic analysis.
Apoptosis Analysis
TUNEL analysis was performed on retinal cross sections from P16 eyes to compare the apoptotic response between soluble EphB4 (sEphB4), soluble ephrinB2 (sEphrinB2) and control injected eyes. Multiple sections (n= 10 sections per eye) on opposite sides of the optic nerve were randomly selected from each eye (n=6 soluble EphB4 or ephrinB2 injected eyes, n=12 control injected eyes). Sections were deparaffinized with xylene and hydrated in graded concentrations of ethanol and TBS. Apoptag Peroxidase In Situ Apoptosis Detection Kit (Intergen, Purchase, NY) was used to label exposed 3′-OH ends of DNA fragments in apoptotic cells, following the manufacturer’s instructions. Apoptotic cells were visualized with DAB substrate, and sections were counterstained with methyl green to aid in the morphological evaluation of the retinal cells. TUNEL positive cells that were located exclusively anterior to the ganglion cell layer were counted in a masked fashion and reported as a total number of pre-tuft positive apoptotic cells per section. All values are expressed as mean ± SD and statistical difference was determined using a Student t-test between experimental and control groups.
To further characterize the apoptotic response, a subset of sections were immunolabeled with an antibody to activated caspase-3, as we have previously described (Davies et al. 2008). Briefly, following deparaffinization and rehydration, sections were boiled in 1mM EDTA for angiten retrieval. Following a blocking step, sections were incubated overnight at 4°C with cleaved caspase-3 antibody (Cell Signaling, Danvers, MA). Retinal sections were then incubated with biotinylated goat anti-rabbit IgG (Vector). ABC-HRP complex (Vector) was applied and visualized with DAB substrate and counterstained with methyl green.
Immunohistochemistry
Immunohistological analysis of proliferating cell nuclear antigen (PCNA) was performed on P16 eyes to examine the effect of sEphB4 and sEphrinB2 on retinal cell proliferation, using a rabbit polyclonal anti-PCNA antibody (Abcam, Cambridge, MA). To determine that ECs were a major retinal cell type undergoing apoptosis, retinal cross sections from P16 eyes were double-labeled combining either a rabbit polyclonal anti-von Willebrand Factor (vWF) (DAKO, Carpinteria, CA) or a rabbit polyclonal anti-GFAP antibody (DAKO) with the TUNEL assay. In addition, a subset of retinal sections were also double-labeled with antibodies to vWF and activated caspse-3, in order to confirm that ECs apoptosis was caspase-dependent. Following deparaffinization, antigen retrieval was accomplished by treating retinal tissue sections with 0.1% pepsin for 20 minutes at room temperature. The sections were rinsed with deionized water and then washed with Tris-buffered saline (50 mM Tris, 0.15 M NaCl (pH 7.5); TBS). Nonspecific binding sites were blocked with 2% normal goat serum (Vector Laboratories, Burlingame, CA), 0.1% BSA and 0.3% Triton X-100 in TBS for 60 minutes at room temperature. The sections were then incubated with experimental antibodies overnight at 4°C and followed with a TBS wash. Primary antibodies were then detected by incubation with goat, anti-rabbit IgG antibodies (1:200; Vector Labs), and the antibody-antigen complexes visualized using Fast Red as the substrate (Biogenex Laboratories, San Ramon, CA). For the PCNA analysis, multiple sections (n=6 sections per eye) were selected from each eye (n=4 sEphB4, n=6 sEphrinB2, n=7 control injected). PCNA positive cells located exclusively anterior to the ganglion cell layer were counted in a masked fashion and reported as a total number of PCNA positive cells per section. All values were expressed as mean ± SD and statistical difference was determined using a Student t-test between experimental and control groups.
Human Retinal Endothelial Cell Cultures
In vitro studies were performed with human retinal microvascular endothelial cells (ACBRI-181 Human Retinal Microvascular Endothelial Cells, Applied Cell Biology Research Institute, Kirkland, WA). These cells were isolated from a 17-year-old female and represent a mixture of venous and arterial cell populations. We have previously used them in unrelated functional assays (Smith et al. 2004). EC were cultured in MCDB-131 complete medium (Sigma), supplemented with 10% FBS, and endothelial growth factors (Clonetics, Cambrex Bioscience, Walkerville, MD; EGM-2 SingleQuot, but without addition of gentamicin, hydrocortisone, and FBS) and grown to 70–80% confluency. To examine the effect of sEphB4 and sEphrinB2 on apoptosis, HREC were serum starved overnight by substituting the complete medium with MCDB-131medium supplemented with only 0.1% FBS. HREC were then stimulated in triplicate with 2μg/mL sEphB4, sEphrinB2 (R&D Systems), or control IgG (Sigma) in the presence or absence of VEGF (10ng/mL, R&D Systems) for 3-hrs. EC culture conditions included co-stimulation with VEGF, which can alter EC responsiveness to ephrin molecules and may better represent the in vivo conditions that are associated with angiogenesis (Sturz et al, 2004). A caspase-3 activation assay (Molecular Probes, Eugene, OR) was performed according to the manufacturer’s protocol as a measure of apoptosis following the 3-hr stimulation. This assay has been previously characterized as a quantifiable indicator of apoptosis induced by a variety of apoptotic stimuli and in a variety of cell types, including ECs in vitro (Gurtu et al. 1997; Cutaia et al. 2005). Experimental variables were performed in triplicate with data presented from one of two experiments as mean ± SD. The statistical difference between all stimulations was determined using a one-way ANOVA, with a p value of < 0.05 considered statistically significant.
Results
TUNEL/Caspase-3/PCNA Analysis of Retinal Sections
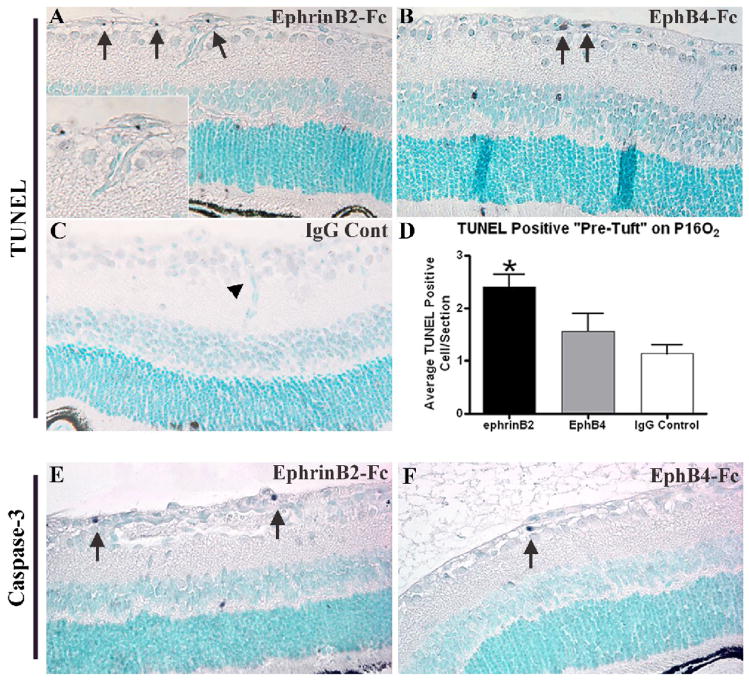
A TUNEL assay was performed on retinal sections from P16 eyes to determine if OIR eyes treated with sEphB4 or sEphrinB2 inhibited retinal NV through the induction of EC apoptosis. This time point was selected because of the marked tuft inhibition noted on P17 in the sEphB4 and sEphrinB2 treated eyes from our prior study using the mouse OIR model (Zamora et al. 2005). A significant increase of apoptosis in retinal vascular cells (pre-tufts) was observed in mice injected with sEphrinB2 (Fig. 1A, arrows, Fig 1D) compared to mice injected with control IgG (Fig. 1C, arrowhead). Treatment with sEphB4 did not result in a statistically significant increase in retinal vascular cell apoptosis in the pre-tufts (Fig. 1B, arrows, Fig. 1D). TUNEL positive cells were also noted in the inner and outer nuclear layers of the retina (Figs. 1A, 1B). We have previously quantified this finding, with the spatial and temporal pattern of neuronal apoptosis secondary to retinal ischemia in the OIR model (Davies et al. 2003). Activated caspase-3 was also localized in P16O2 sections. Qualitative assessment of the cleaved-caspase-3 immunostaining demonstrated positive cells localized to the neovascular “pre-tufts” in the ephrinB2-Fc injected mice (Fig. 1E, arrows), while the neovascular “pre-tufts” in the EphB4-Fc injected mice exhibited only an occasional caspase-3-positive cell (Fig. 1F, arrow).
Figure 1.
Increased apoptosis following treatment with sEphrinB2. TUNEL assays of representative sections depicting cells undergoing apoptosis (black, arrows) from P16 retinas following hyperoxia exposure and intravitreal injection with sEphrinB2 (A), sEphB4 (B) or IgG antibody as control (C). Quantification of TUNEL positive cells located exclusively anterior to the ganglion cell layer revealed a significant increase of apoptosis in sEphrinB2 treated retinas compared to controls (P<0.005). Treatment with sEphB4 did not reach statistical significance (P=0.211) (D). Apoptosis was confirmed by immunolabeling with a cleaved caspase-3 antibody in sEphrinB2 (E) and sEphB4 (F) treated eyes. Orig. Mag. 400x.
PCNA was immunolocalized on P16 tissue sections (data not shown) and positive cells anterior to the ganglion cell layer were counted. The number of PCNA-positive cells from sEphB4 (12.9 ± 1.7), sEphrinB2 (9.4 ± 2.0), and control injected eyes (10.9 ± 1.8) were not significantly different.
Co-localization of von-Willebrand Factor and Apoptotic-positive cells
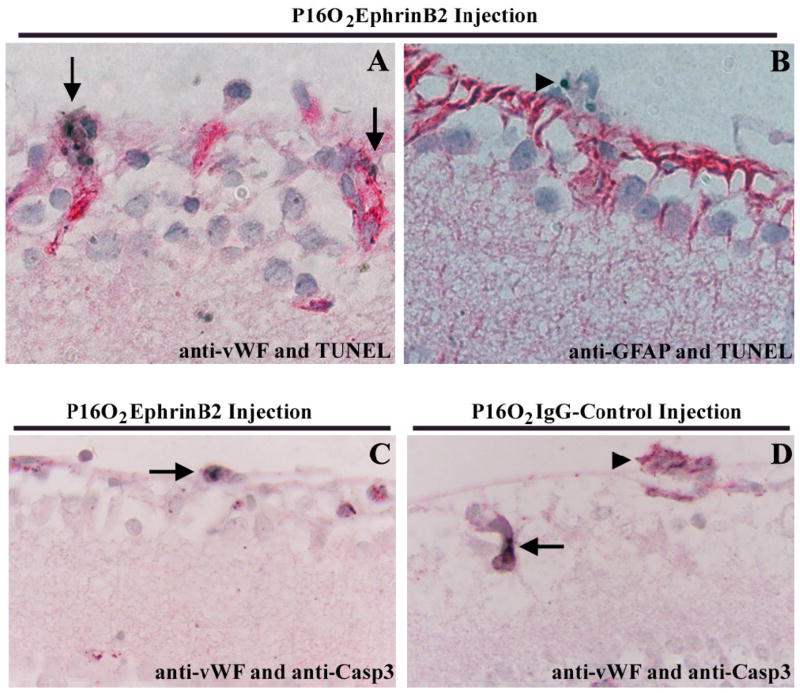
Double labeling for anti-vWF, an EC marker, and TUNEL, a method to detect apoptotic cells, showed co-localization within the neovascular tufts and along the inner limiting membrane in the “pre-tufts” in the ephrinB2-Fc injected eyes (Fig. 2A, arrows). On a rare occasion, in the GCL, an apoptotic cell was observed that did not co-localize with anti-vWF (data not shown). In addition, GFAP positive cells that were localized along the nerve fiber layer and inner nuclear layer did not co-localize with TUNEL positive cells (Fig. 2B). These results suggest that the majority of apoptotic cells along the inner retina are ECs. As additional evidence of this conclusion, cleaved caspase-3 was also co-localized with vWF positive cells in the “pre-tufts” (Fig. 2C, arrow). In contrast, Fig. 2D reveals a vWF positive vascular tuft (arrowhead) that does not exhibit cleaved caspase-3 cells in the IgG control injected eyes, though one positive cell (arrow) is seen in an intraretinal vessel, confirming the validity of the immunostaining procedure.
Figure 2.
Apoptotic endothelial cells on P16 retinas. A: Double labeling for vWF (red) and TUNEL (black, arrows) showed co-localization within the neovascular tuft and in “pre-tufts” along the inner limiting membrane. B: TUNEL positive cells (black, arrowhead) located within the neovascular tufts are not astrocytes as demonstrated by the absence of GFAP immunoreactivity (red). C: Double labeling for vWF (red) and cleaved caspase-3 (black, arrow) confirmed apoptotic ECs in neovascular tufts of sEphrinB2 treated eyes. D: In IgG control Injected eyes, a vWF positive vascular tuft (arrowhead) does not exhibit positive cleaved caspase-3 staining, though a positive cell (arrow) is observed within an intraretinal vessel. Orig. Mag. 1000x.
Caspase-3 Activation in Human Retinal Endothelial Cells
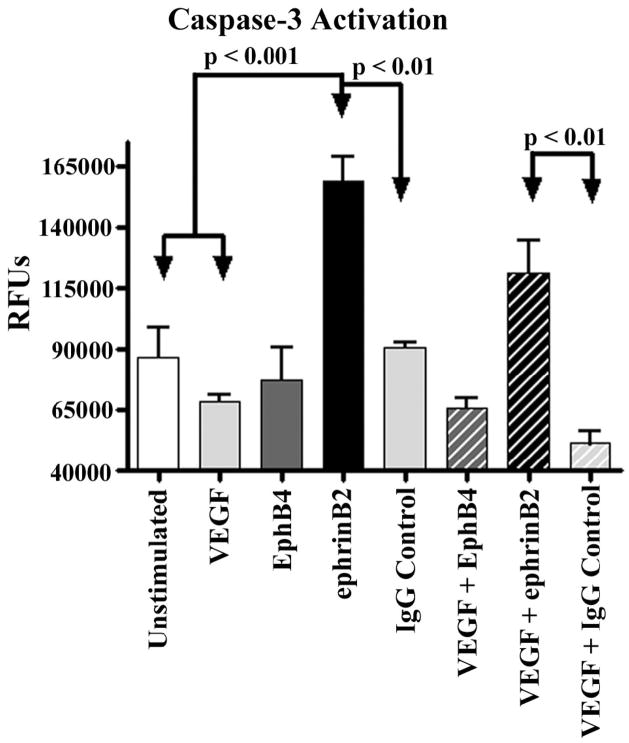
To identify the mechanisms by which sEphB4 and sEphrinB2 inhibit retinal NV we stimulated primary HREC cultures with VEGF in the presence or absence of sEphB4, sEphrinB2 or IgG control and measured caspase-3 activity (Fig. 3). Stimulation with sEphrinB2 significantly increased caspase-3 activity above unstimulated, VEGF recovered and IgG stimulated HREC. Furthermore, co-stimulation with sEphrinB2 and VEGF demonstrated increased caspase-3 activity compared to co-stimulation with VEGF and IgG control. Stimulation with sEphB4 alone or in combination with VEGF showed no difference in caspase-3 activity compared to all controls.
Figure 3.
Primary HREC cultures were stimulated with sEphB4, sEphrinB2 or IgG antibody as control in the presence or absence of VEGF and then assayed for caspase-3 activity as a measure of apoptosis. HREC were serum starved overnight, prior to stimulation. As expected treatment with VEGF alone significantly reduced caspase-3 activity. Stimulation with sEphrinB2 significantly increased caspase-3 activity above unstimulated even in the presence of VEGF, compared to control stimulations. Treatment with sEphB4 alone or in combination with VEGF was not associated with any alteration in caspase-3 activity compared to all controls (N=3).
Discussion
In addition to their role in artery and vein demarcation, Eph receptors and ephrin ligands exhibit angiogenic properties and have been localized to new capillaries in models of wound healing and tumor angiogenesis (Adams et al. 1999; Shin et al. 2001). EphrinB2 has also been localized to fibroproliferative membranes from patients with ischemic retinopathies, including retinopathy of prematurity (Umeda et al. 2004). In contrast, other studies have shown that Eph/ephrin signaling can attenuate EC growth induced by angiogenic growth factors (Martiny-Baron et al. 2004). Soluble forms of Ephs and ephrins are also known to activate both positive and negative regulators of intracellular signaling pathways, consistent with their pro- and anti-angiogenic properties (Tong et al. 2003).
It has been previously demonstrated that various forms of soluble ephrin ligands and receptors can profoundly reduce retinal and choroidal NV (He et al. 2005; Zamora et al. 2005; Chen et al. 2006). The in vivo mouse and in vitro human studies presented here suggest that reduction of preretinal NV by treatment with sEphrinB2 may be attributed to the induction of EC apoptosis. This reduction may be due to activation of endogenous EphB4 or blocking reverse signaling of endogenous ephrinB2. Ephrin signaling has been shown to control brain size by regulating apoptosis of neural progenitors (Depaepe et al. 2005). In addition, EphB4 activation by sEphrinB2 can directly induce apoptosis in breast cancer cells in culture and in vivo in a breast cancer mouse model (Noren et al. 2006). Similar to our results, induction of EC apoptosis and altered cell survival has been observed in studies of tumor angiogenesis by modulation of EphB4/ephrinB2 signaling (Noren et al. 2004; Huang et al. 2007).
Although sEphB4 caused suppression of retinal NV in our prior study (Zamora et al. 2005), it did not significantly increase apoptosis following injection into the vitreous, nor did it increase caspase-3 activity following stimulation of HREC cultures. Anti-angiogenic properties of Eph/ephrin molecules are dependent on specific culture conditions or local microenvironments and sEphB4 may require additional factors to induce apoptosis. Alternatively, our current results suggest that sEphB4 is not affecting apoptosis directly, but perhaps inhibiting retinal NV by other means. There is evidence in the literature demonstrating that perturbations of EphB4/ephrinB2 interactions, including the use of soluble forms of EphB4, results in altered EC migration/adhesion, cell-cell contacts, and subsequent tube formation (Martiny-Baron et al. 2004; Foo et al. 2006; Kertesz et al. 2006). Future studies will be required to determine if modulation of EphB4/ephrinB2 interactions by sEphB4 impacts retinal EC in the OIR model, resulting in altered EC migration and tube formation.
Acknowledgments
This project was supported by NEI EY011548 (MRP), NEI EY014909 (JRS), NEI EY10572 (core grant), Fight for Sight (MRP), Collins Medical Trust (MRP), and Research to Prevent Blindness (Career Development Award (JRS) and an unrestricted grant to Casey Eye Institute).
Footnotes
Commercial Relationships Disclosure: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams RH, et al. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13(3):295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker MD, et al. Immunohistology of antigen-presenting cells in vivo: a novel method for serial observation of fluorescently labeled cells. Invest Ophthalmol Vis Sci. 2003;44(5):2004–9. doi: 10.1167/iovs.02-0560. [DOI] [PubMed] [Google Scholar]
- Chen J, et al. Inhibition of retinal neovascularization by soluble EphA2 receptor. Exp Eye Res. 2006;82(4):664–73. doi: 10.1016/j.exer.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Cutaia M, et al. Alkaline stress-induced apoptosis in human pulmonary artery endothelial cells. Apoptosis. 2005;10(6):1457–67. doi: 10.1007/s10495-005-1402-5. [DOI] [PubMed] [Google Scholar]
- Davies MH, Eubanks JP, Powers MR. Increased retinal neovascularization in Fas ligand-deficient mice. Invest Ophthalmol Vis Sci. 2003;44(7):3202–10. doi: 10.1167/iovs.03-0050. [DOI] [PubMed] [Google Scholar]
- Davies MH, Stempel AJ, Powers MR. MCP-1 deficiency delays regression of pathologic retinal neovascularization in a model of ischemic retinopathy. Invest Ophthalmol Vis Sci. 2008;49(9):4195–202. doi: 10.1167/iovs.07-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depaepe V, et al. Ephrin signalling controls brain size by regulating apoptosis of neural progenitors. Nature. 2005;435(7046):1244–50. doi: 10.1038/nature03651. [DOI] [PubMed] [Google Scholar]
- Erber R, et al. EphB4 controls blood vascular morphogenesis during postnatal angiogenesis. Embo J. 2006;25(3):628–41. doi: 10.1038/sj.emboj.7600949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo SS, et al. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell. 2006;124(1):161–73. doi: 10.1016/j.cell.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Gale NW, et al. Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Dev Biol. 2001;230(2):151–60. doi: 10.1006/dbio.2000.0112. [DOI] [PubMed] [Google Scholar]
- Gerety SS, et al. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol Cell. 1999;4(3):403–14. doi: 10.1016/s1097-2765(00)80342-1. [DOI] [PubMed] [Google Scholar]
- Gurtu V, Kain SR, Zhang G. Fluorometric and colorimetric detection of caspase activity associated with apoptosis. Anal Biochem. 1997;251(1):98–102. doi: 10.1006/abio.1997.2220. [DOI] [PubMed] [Google Scholar]
- He S, et al. Soluble EphB4 regulates choroidal endothelial cell function and inhibits laser-induced choroidal neovascularization. Invest Ophthalmol Vis Sci. 2005;46(12):4772–9. doi: 10.1167/iovs.05-0502. [DOI] [PubMed] [Google Scholar]
- Huang X, et al. EphB4 overexpression in B16 melanoma cells affects arterial-venous patterning in tumor angiogenesis. Cancer Res. 2007;67(20):9800–8. doi: 10.1158/0008-5472.CAN-07-0531. [DOI] [PubMed] [Google Scholar]
- Kertesz N, et al. The soluble extracellular domain of EphB4 (sEphB4) antagonizes EphB4-EphrinB2 interaction, modulates angiogenesis, and inhibits tumor growth. Blood. 2006;107(6):2330–8. doi: 10.1182/blood-2005-04-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijper S, Turner CJ, Adams RH. Regulation of angiogenesis by Eph-ephrin interactions. Trends Cardiovasc Med. 2007;17(5):145–51. doi: 10.1016/j.tcm.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Lackmann M, Boyd AW. Eph, a protein family coming of age: more confusion, insight, or complexity? Sci Signal. 2008;1(15):re2. doi: 10.1126/stke.115re2. [DOI] [PubMed] [Google Scholar]
- Martiny-Baron G, et al. Inhibition of tumor growth and angiogenesis by soluble EphB4. Neoplasia. 2004;6(3):248–57. doi: 10.1593/neo.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren NK, et al. The EphB4 receptor suppresses breast cancer cell tumorigenicity through an Abl-Crk pathway. Nat Cell Biol. 2006;8(8):815–25. doi: 10.1038/ncb1438. [DOI] [PubMed] [Google Scholar]
- Noren NK, et al. Interplay between EphB4 on tumor cells and vascular ephrin-B2 regulates tumor growth. Proc Natl Acad Sci U S A. 2004;101(15):5583–8. doi: 10.1073/pnas.0401381101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133(1):38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Sakamaki K. Regulation of endothelial cell death and its role in angiogenesis and vascular regression. Curr Neurovasc Res. 2004;1(4):305–15. doi: 10.2174/1567202043362072. [DOI] [PubMed] [Google Scholar]
- Shin D, et al. Expression of ephrinB2 identifies a stable genetic difference between arterial and venous vascular smooth muscle as well as endothelial cells, and marks subsets of microvessels at sites of adult neovascularization. Dev Biol. 2001;230(2):139–50. doi: 10.1006/dbio.2000.9957. [DOI] [PubMed] [Google Scholar]
- Smith JR, et al. Susceptibility of retinal vascular endothelium to infection with Toxoplasma gondii tachyzoites. Invest Ophthalmol Vis Sci. 2004;45(4):1157–61. doi: 10.1167/iovs.03-1105. [DOI] [PubMed] [Google Scholar]
- Smith LE, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35(1):101–11. [PubMed] [Google Scholar]
- Tong J, et al. Manipulation of EphB2 regulatory motifs and SH2 binding sites switches MAPK signaling and biological activity. J Biol Chem. 2003;278(8):6111–9. doi: 10.1074/jbc.M208972200. [DOI] [PubMed] [Google Scholar]
- Umeda N, et al. Expression of ephrinB2 and its receptors on fibroproliferative membranes in ocular angiogenic diseases. Am J Ophthalmol. 2004;138(2):270–9. doi: 10.1016/j.ajo.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93(5):741–53. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- Wang S, Sorenson CM, Sheibani N. Attenuation of retinal vascular development and neovascularization during oxygen-induced ischemic retinopathy in Bcl-2−/− mice. Dev Biol. 2005;279(1):205–19. doi: 10.1016/j.ydbio.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Zamora DO, et al. Soluble Forms of EphrinB2 and EphB4 Reduce Retinal Neovascularization in a Model of Proliferative Retinopathy. Invest Ophthalmol Vis Sci. 2005;46(6):2175–82. doi: 10.1167/iovs.04-0983. [DOI] [PubMed] [Google Scholar]