Abstract
The recently characterized enzyme NEIL2 (Nei-like-2), one of the four oxidized base-specific DNA glycosylases (OGG1, NTH1, NEIL1, and NEIL2) in mammalian cells, has poor base excision activity from duplex DNA. To test the possibility that one or more proteins modulate its activity in vivo, we performed mass spectrometric analysis of the NEIL2 immunocomplex and identified Y box-binding (YB-1) protein as a stably interacting partner of NEIL2. We show here that YB-1 not only interacts physically with NEIL2, but it also cooperates functionally by stimulating its base excision activity by 7-fold. Moreover, YB-1 interacts with the other NEIL2-associated BER proteins, namely, DNA ligase IIIα and DNA polymerase β and thus could form a large multiprotein complex. YB-1, normally present in the cytoplasm, translocates to the nucleus during UVA-induced oxidative stress, concomitant with its increased association with and activation of NEIL2. NEIL2-initiated base excision activity is significantly reduced in YB-1-depleted cells. YB-1 thus appears to have a novel regulatory role in NEIL2-mediated repair under oxidative stress.
Reactive oxygen species (ROS)2 are believed to play an important role in inducing various disease pathologies including cancer, rheumatoid arthritis, cardiovascular disease, and aging (1, 2). ROS are formed endogenously as a byproduct of respiration and oxidative metabolism and exogenously by a variety of environmental agents including ultraviolet (UV) radiation (3–5). Of the total ultraviolet light spectrum (100–400 nm) present in the natural sunlight, only UVA (320–400 nm) and a small fraction of UVB (280–320 nm) reach the surface of the earth, as UVB is mostly absorbed by the atmosphere. Although UVA is the predominant genotoxic radiation in the natural environment, it does not react directly with DNA, but interacts with cellular chromophores like riboflavin, porphyrins, quinones, and reduced nicotinamide cofactors to produce singlet oxygen (6). UVA also has the ability to penetrate deeper into the skin to proliferating basal layers, and causes DNA damage leading to genomic instability.
Although cellular antioxidant defenses (e.g. catalase, peroxidase, and superoxide dismutase) effectively combat the effects of ROS, but oxidative DNA damage still occurs. Most of the DNA lesions, except double strand breaks, are repaired via the DNA base excision repair (BER) pathway, initiated with the excision of damaged base by a specific DNA glycosylase (7, 8). Four oxidized base-specific DNA glycosylases have been identified and characterized so far in mammalian cells. 8-Oxoguanine-DNA glycosylase (OGG1) and endonuclease III homolog 1 (NTH1) were characterized previously and preferentially excise oxidized purines and pyrimidines, respectively (9, 10), and were thought to be the two major oxidized base-specific DNA glycosylases in mammalian cells. However, a lack of phenotype or significant cancer propensity of OGG1- and NTH1-null mice suggested the contribution of other DNA glycosylases in the repair of oxidized bases (11–13).We and subsequently others have identified and characterized two more human DNA glycosylases, which are orthologs of Escherichia coli Nei, and thus named them NEIL (Nei-like)-1 and 2 (13–17). NEILs are distinct from NTH1 and OGG1 in both structural features and reaction mechanisms, but act on many of the same substrates (15, 16). We have shown recently that AP endonuclease 1, which is essential for OGG1/NTH1-initiated BER, is dispensable when BER is initiated by NEILs (18, 19). Unlike OGG1 and NTH1, which are active only with duplex DNA, NEIL1 and NEIL2 demonstrated an unusual activity in excising lesions from DNA bubble structures or single-stranded DNA. NEIL2 particularly showed a unique preference for excising lesions from a DNA bubble and has significantly lower activity for duplex DNA substrate (20). In contrast to OGG1 and NTH1 null cells that did not show increased sensitivity to ROS and radiation (13, 21) compared with wild type cells, NEIL1-depleted cells demonstrated enhanced radiation sensitivity (22). Furthermore, we have shown previously induction of NEIL1 after ROS exposure (23), however, NEIL2-mediated BER after oxidative stress has not yet been reported.
In the present study, we report that Y-box-binding protein-1 (YB-1) stably interacts with NEIL2 and other NEIL2-interacting partners such as DNA polymeraseβ (Polβ) and DNA ligase IIIα (Lig IIIα) that are components of the BER complex. Additionally, the interaction between YB-1 and NEIL2 is enhanced after oxidative stress, concomitant with enhanced activity of NEIL2, suggesting a critical role for YB-1 in oxidative DNA damage repair.
EXPERIMENTAL PROCEDURES
Cell Culture and Chemicals
Human colon carcinoma HCT116 cells (with wild-type p53), a gift of B. Vogelstein (The Johns Hopkins University), were cultured in McCoy’s 5A modified medium supplemented with 10% fetal bovine serum, 2 mm l-glutamine, penicillin (100 units/ml), and streptomycin (100µg/ml). At 70% confluence, the cells were treated with glucose oxidase (Roche Applied Science) at 100 ng/ml for 1 h, followed by washing with and incubation in fresh medium or otherwise as stated. This treatment did not affect cell viability, as judged by trypan blue staining and the rate of cell growth. Before UVA treatment, cells were incubated with riboflavin, a photosensitizer at a concentration of 0.25 mg/ml for 1 h. N-Acetyl l-cysteine (NAC) (from Sigma) was used as scavenger of ROS at an optimized concentration of 10 mm for 5 h prior to UVA or glucose oxidase treatment.
Purification of NEIL2
For biochemical studies, full-length C-terminal His6-tagged NEIL2 (residues 1–331) was expressed and purified from Sf9 insect cells (19). Two major non-overlapping domains of NEIL2 consisted of N-terminal (residues 1–198) and C-terminal (residues 199–331) fragments, both His tag at the C terminus, were purified on nickel-nitrilotriacetic acid-NTA-agarose, followed by SP-Sepharose chromatography (19).
Construction, Expression, and Purification of WT and Deletion Mutants of YB-1
The baculovirus containing N-terminal His-tagged YB-1 was a kind gift from Dr. G. W. Teebor (New York University School of Medicine). Wild type (WT) YB-1 was purified according to the protocol described earlier (24). The generation of plasmids containing full-length GST-YB-1 and five GST-YB-1 deletion mutants (GST-YB-1Δ1, -Δ2, -Δ3, -Δ4, and Δ5) have been described previously (25). Further deletion constructs Δ6, Δ7, Δ8, and Δ9 were generated by PCR amplification with appropriate primers, digested with BamHI/XhoI restriction sites, and cloned in corresponding BamHI/XhoI restriction sites into pGEX-4T. After transformation, bacterial clones producing fusion proteins were tested by Western blot using GST antibodies.
For purification of WT and mutant YB-1, colonies of freshly transformed E. coli BL21(DE3) RIPL (Stratagene) with expression plasmids for WT or different mutants were grown into Luria broth containing 150 µg/ml ampicillin and 100 µg/ml chloramphenicol at 37 °C. Isopropyl 1-thio-β-d-galactopyranoside (0.3 mm) was added when A590 reached ~0.4–0.5 and the incubation was continued overnight at 16 °C. Bacterial cell lysate was prepared after sonication with 50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 10% glycerol, 1 mm EDTA, 1 mm DTT, and 0.1% Nonidet P-40. Lysate was centrifuged and cleared supernatant was used in the equilibrated EZView™ red glutathione-agarose affinity beads (Sigma). After incubation at 4 °C for 2 h, the beads were washed three times with lysis buffer. GST-tagged (N terminus) proteins were eluted with 10 mm reduced glutathione in 50 mm Tris-HCl, pH 7.5. Purity of each deletion mutant was checked in SDS-PAGE and the protein concentration was measured using Bradford reagent.
Generation of Stable Transfectant of NEIL2
HCT116 cells were transfected with 1 µg each of NEIL2-FLAG (C terminus) or with the corresponding vector as described previously (26). After 24 h, the cells were trypsinized and plated onto fresh medium in the presence of zeocin to select for clones carrying stably integrated plasmid DNA. Zeocin sensitivity of HCT116 cells were determined to be ~200 µg/ml. Media was replaced each alternate day with 250, 500, and 1000 µg/ml zeocin. After 3 weeks, individual zeocin-resistant clones were transferred into 6-well plates. Surviving clones were expanded and the expressions of NEIL2-FLAG, as well as NEIL2, were tested. To maintain the stable cells, the zeocin concentration at 200 µg/ml was maintained throughout. The clone with stably expressing NEIL2-FLAG was named HCT116N.
Identification of NEIL2-associated Proteins
To identify NEIL2-associated proteins, HCT116N and its corresponding vector control cells were washed with phosphate-buffered saline (PBS) and then with Tris-buffered saline (TBS, 50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA). Cells were then lysed with cold lysis buffer (TBS containing 1% Triton X-100 and a protease inhibitor mixture (Roche)). Cell lysates were precleared by centrifugation, and the supernatants were immunoprecipitated with anti-FLAG M2 antibody (Sigma). Immunoprecipitates (IP) of both control and HCT116N cells were washed with TBS containing 0.5% Triton X-100, and complexes were eluted stepwise with 1 ml each of 50 mm Tris-HCl, pH 7.5, containing 250 mm, 500 mm, or 1 m NaCl. Each fraction was precipitated with acetone, separated by SDS-PAGE, and stained with Coomassie Blue. Bands appearing in the NEIL2-FLAG IP but not in the control IP were excised for in-gel trypsin digestion followed by MALDI-TOF mass spectrometry.
Generation of Anti-YB-1 Antibody
A synthetic peptide, CDGKETKAADPPAENS (27), corresponding to the deduced amino acid residues 299–313 of the human YB-1 was chemically coupled to keyhole limpet hemocyanin and used to raise polyclonal antibody in rabbits (Biomolecular Resource Facility Core, University of Texas Medical Branch (UTMB)). The antibody was affinity purified from crude antiserum by adsorption to and elution from the above synthetic peptide immobilized on CNBr-activated Sepharose 4B (Amersham Biosciences) and was used for Western blot analysis.
Co-immunoprecipitation
HCT116N cells were lysed in cold lysis buffer as described earlier (26). The IP from the lysates with anti-FLAG M2 antibody (Sigma) were washed extensively with cold TBS and tested for the presence of YB-1. Anti-FLAG antibody (Sigma) was used to detect NEIL2-FLAG after stripping the membrane using Restore™ Western blot stripping buffer (Pierce).
Subcellular Fractionation and Nuclear Extract Preparation
After UVA treatment, the cells were washed with ice-cold PBS and nuclear extract was prepared as described previously (28). Briefly, the cells were harvested by scraping from the culture dishes into ice-cold equilibration buffer (EB: 10 mm HEPES, pH 7.9, 10 mm KCl, 1.5 mm MgCl2, 2 mm DTT, 0.1 mm EDTA, 0.1 mm EGTA, and protease inhibitors) and collected by centrifugation at 12,000 rpm (18,000 × g) for 1 min at 4 °C. Cell pellets were resuspended in EB containing 0.1% Nonidet P-40 and incubated on ice for 15 min. The cytosolic fraction was then isolated by centrifugation at 14,000 rpm (25,000 × g) for 1 min, and the resulting pellet was washed once with EB, resuspended in nuclear extraction buffer (20 mm HEPES-KOH, pH 7.9, 0.42 m NaCl, 1 mm EDTA, 0.1 mm EGTA, 2 mm DTT, 20% glycerol and protease inhibitors) and incubated on ice for 1 h with occasional vortexing. The extracted nuclear proteins were isolated by centrifugation at 14,000 rpm (25,000 × g) for 30 min.
Incision Assay of DNA Glycosylases
Two 51-mer oligonucleotides containing either 5-OHU or 8-oxoG at position 26 from the 5′-end were purchased from Midland Certified Reagent. The complementary oligonucleotides that contained G or C opposite 5-OHU or 8-oxoG, respectively, or with sequences to generate bubble structures of 11 unpaired bases, and named B11 (Table 1) (20), were purchased from Invitrogen. The single-stranded oligonucleotides containing lesions were labeled at the 5′ terminus with [γ-32P]ATP and polynucleotide kinase before annealing to the complementary strand (20). These oligonucleotide substrates (5OHU·G, 5OHU·B11, or 8-oxoG·B11) were incubated with NEIL2 (50–100 fmol) alone or with different amounts of YB-1 (50–400 fmol) in a buffer containing 40 mm HEPES-KOH, pH 7.5, 50 mm KCl, 5% glycerol, and 100 µg/ml bovine serum albumin for 10 min. The reactions were stopped by adding 70% formamide and 30 mm NaOH, and the cleaved oligo products were then separated by denaturing gel electrophoresis in 20% polyacrylamide gel containing 7 m urea, 90 mm Tris borate, pH 8.3, and 2 mm EDTA. The rate of product formation was linear under these reaction conditions. The radioactivity in the DNA bands was quantified by analysis in PhosphorImager (Amersham Biosciences) using Image Quant software. The Km and kcat values of NEIL2 were determined after incubating 5-OHU·B11 oligonucleotide substrates (2.5– 80 nm) with NEIL2 (100 fmol) and YB-1 (200 fmol) for 3 min at 37 °C in the same reaction buffer as described above.
TABLE 1. Sequences of lesion-containing (L) and complementary (C) oligonucleotides.
C, complementary strand for generating duplex (G opposite 5-OHU) or bubbles of 11-nucleotide (B11) unpaired regions. The noncomplementary sequences to generate bubbles are underlined.
| Sequences |
|---|
| L: 5′-GCTTAGCTTGGAATCGTATCATGTAXACTCGTGTGCCGTGTAGACCGTGCC-3′ |
| X, 5-OHU or 8-oxoG |
| C: 5′-GGCACGGTCTACACGGCACACGAGTNTACATGATACGATTCCAAGCTAAGC-3′ |
| N, G |
| B11: 5′-GGCACGGTCTACACGGCACAAACAGCCCACGGATACGATTCCAAGCTAAGC-3′ |
Far Western Analysis
Purified proteins were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes (19, 29). After incubation in a denaturation buffer (6 m guanidine HCl in PBS) twice for 5 min at 4 °C, the membranes were incubated for 10 min each in serial dilutions of guanidine-HCl in PBS containing 1 mm DTT. The membranes were blocked with PBS containing 0.5% Tween 20 and 5% nonfat dry milk for 45 min at 20 °C. After two washes in PBS/Tween 20 (0.5%), nonfat dry milk, the blots were incubated with NEIL2 or with other proteins (10 pmol/ml) for 3 h at 4 °C in PBS/Tween 20 (0.5%), nonfat dry milk containing 1 mm DTT, 0.5 mm phenylmethylsulfonyl fluoride. The filters were washed (4 ×10 min) in PBS/Tween 20 (0.25%), nonfat dry milk and then incubated with affinity-purified anti-NEIL2 antibody (or with other antibodies as mentioned) prior to secondary goat anti-rabbit antibody incubation, followed by ECL (Amersham Biosciences).
GST Pull-down Assay
Wild type YB-1 and its various deletion mutants with GST tag were coupled to glutathione-agarose affinity beads (EZView red glutathione-agarose affinity gel from Sigma). Purified NEIL2 (~400 ng) was allowed to interact with YB-1 and its different deletion mutants separately in a buffer containing 50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, 0.1% Triton X-100, and 1 mm DTT for 2 h. Beads were washed four times with 50 mm Tris-HCl, pH 7.5, 250 mm NaCl, 1 mm EDTA, 0.1% Triton X-100, and 1 mm DTT. The beads were then boiled with the sample buffer for Western analysis using anti-NEIL2 antibody.
Down-regulation of YB-1 by siRNA
For down-regulation of YB-1 in HCT116N cells, ON-Target Plus SMART pool (L-010213–00-0005 Human YBX1, NM_004559) was purchased from Dharmacon and the supplied protocol was followed. Briefly, 40 and 80 nm YB-1 siRNA was transfected with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. RNA and cell extract was prepared periodically to monitor the level of YB-1 expression by Western blot analysis and Real Time Quantitative RT-PCR.
UVA Treatment
For UVA treatment, cells were irradiated at 365 nm wavelength by a UV lamp (5 watt model UVLMS-38; Ultra-Violet Product, Ltd., Upland, CA). For NEIL2-YB-1 interaction, 40 J/m2 was used with an exposure time of 20 s. The dose was optimized in this study to avoid cell killing. The dosage was measured with the help of a UV dosimeter (UVX-36 from UVP Inc.). The UVX-36 sensor is designed and calibrated to accurately measure 365 nm radiation.
Measurements of Intracellular ROS Generation
The intracellular ROS level was determined by using 5-(and −6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA; Molecular Probes) as reported (30). When carboxy-H2DCFDA is oxidized by ROS it is irreversibly converted to the fluorescent form, DCF. Level of ROS was then measured after UVA treatment as a function of time. HCT116N cells were cultured in a 24-well plate and loaded with 50 µm carboxy-H2DCF-DA for 30 min at 37 °C. Fluorescence intensities of cells were determined with a FLx800 microplate reader (Bio-Tek Instruments, Winooski, VT) at 488 nm excitation and 530 nm emission (31). The -fold change in DCF fluorescence of UVA-treated cells compared with untreated cells was plotted.
RESULTS
Identification of YB-1 as a NEIL2-interacting Protein
All cellular processes are regulated and coordinated via various macromolecular interactions. To elucidate the NEIL2-initiated base excision repair pathway, we generated HCT116 cells stably expressing FLAG-tagged NEIL2 (HCT116N) for immunoaffinity purification to identify NEIL2-associated proteins. The NEIL2 immunocomplexes (IC) were isolated from extracts of HCT116N and corresponding vector control HCT116 cells using anti-FLAG antibody beads (Sigma). NEIL2-FLAG-associated proteins in the IC were eluted with increasing salt concentrations, and resolved on SDS-PAGE, followed by in-gel trypsin digestion and MALDI-TOF mass spectrometry. Among several NEIL2-interacting proteins eluted with 500 mm NaCl, we identified YB-1 (Fig. 1A, lane 2) and characterized its role in NEIL2-inititated BER.
FIGURE 1. Identification of YB-1 as NEIL2-associated protein.
A, SDS-PAGE of the 500 mm salt eluate of NEIL2-FLAG IC (lane 2) or empty vector (lane 1) isolated from HCT116N cell lysates. *, mass spectrometric analysis of the marked band. B, NEIL2-FLAG immunocomplex was tested for the presence of YB-1 by Western analysis (lane 3); lane 2, vector IP as control; lane 1, purified YB-1 as marker; lanes 4 and 5, corresponding cell lysates as input. Lower panel, Western analysis of IC and cell extracts used in the upper panel with anti-FLAG antibody. C, Far Western analysis showing direct interaction between YB-1 (lanes 5, 7, and 9) with WT, and the N-terminal (NTD) and C-terminal domains (CTD) of NEIL2. Lanes 1–3, Coomassie stain of marker, bovine serum albumin (BSA), and YB-1, respectively.
NEIL2 Stably Interacts with YB-1
Association of NEIL2 with YB-1 was further investigated by co-immunoprecipitation (co-IP) analysis. The presence of YB-1 in the NEIL2-FLAG IP (Fig. 1B, lane 3), but not in the control IP (Fig. 1B, lane 2), confirmed the association of YB-1 with NEIL2. We used equal amounts of lysates for IP (Fig. 1B, lanes 4 and 5).
To examine whether YB-1 interacts with NEIL2 directly or via association with other proteins, we examined in vitro association between NEIL2 and YB-1 by Far Western analysis. Fig. 1C shows that YB-1 (lane 5) interacted stably with NEIL2, but not with bovine serum albumin (lane 4).
We have shown previously that NEIL2 has two major domains: namely the N-terminal (residue 1–198) and the C-terminal (residue 199–331) domains (19). These domains were tested individually for their interaction with immobilized YB-1 by Far Western analysis. Fig. 1C shows that the N-terminal domain (lane 7) but not the C-terminal domain (lane 9) stably interacted with YB-1.
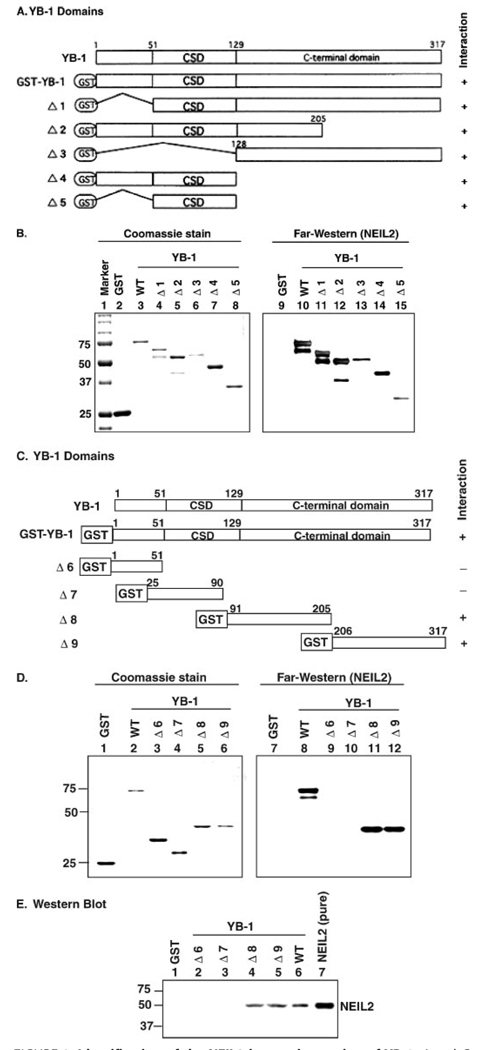
To identify its interacting region for NEIL2, we used various deletion mutants of YB-1 (Fig. 2A) (25). YB-1 has three major domains. Its N terminus is involved in transcriptional regulation, the middle (cold shock domain) is involved in nucleic acid binding, and the C-terminal region is involved in protein-protein interaction (32, 33). The interaction of these YB-1 mutants was again examined by Far Western analysis and it was found that NEIL2 interacted with all of these deletion mutants (Δ1– Δ5) of YB-1 (Fig. 2B, lanes 10–15) suggesting either a long stretch of YB-1 is involved or there are multiple sites of YB-1 that interact with NEIL2.
FIGURE 2. Identification of the NEIL2 interacting region of YB-1.
A and C, schematic representation of the GST-YB1 deletion mutants (Δ1–Δ5) and (Δ6–Δ9). B and D, Far Western analysis. Various GST-YB-1 deletion mutants were used for the interaction studies with NEIL2 (right panel). Left panel, Coomassie staining of the YB-1 deletion mutants. E, GST pull-down assay. NEIL2 incubated with glutathione-agarose affinity beads containing various deletion mutants of GST-tagged YB-1 (as indicated Δ6–Δ9), blotted with anti-NEIL2 antibody.
Further deletion mutants of YB-1 (Δ6–Δ9) were then generated to identify the NEIL2-interacting domain more precisely (Fig. 2C). Far Western analysis showed that NEIL2 interacted separately with YB-1 deletion mutants Δ8 (amino acid residues 91–205) and Δ9 (amino acid residues 206–317) (Fig. 2D, lanes 11 and 12), but not with Δ6 and Δ7 (Fig. 2D, lanes 9 and 10). This interaction pattern of YB-1 with NEIL2 was also confirmed by GST-pull down assay (Fig. 2E). These results thus indicate that a long C-terminal stretch of YB-1 (residues 91–317; including part of cold shock domain and whole C-terminal region) is involved in interaction with NEIL2. This fact is not unusual as previously it was reported that interaction between Smad3 and YB-1 involved cold shock domain and the whole C-terminal region (34).
YB-1 Stimulates NEIL2 Activity
The stable interaction between NEIL2 and YB-1 led us to investigate its effect on NEIL2 activity. A 5′ 32P-labeled 5-OHU-containing 51-mer oligonucleotide with an 11-nucleotide long bubble was used for the assay. Fig. 3A shows that YB-1 stimulated NEIL2 activity in a dose-dependent manner by ~7-fold. YB-1 also stimulated NEIL2 activity with 5OHU·G duplex oligonucleotide by ~3.5-fold (Fig. 3B).We used 5-OHU-containing bubble substrate (5-OHU·B11) in the remainder of our studies, as it was the preferred substrate for NEIL2. We have shown previously that NEIL2 removes 8-oxoG from bubble DNA (20), and here we have found that YB-1 also significantly increased the 8-oxoG excision activity of NEIL2 by ~5-fold (Fig. 3B).
FIGURE 3. Stimulation of NEIL2 activity by YB-1.
A, NEIL2 (50 fmol) was incubated for 10 min with the 5-OHU-containing 32P-labeled bubble substrate in the presence of increasing amounts of YB-1 (50 – 400 fmol), and the reaction products were analyzed in a 20% urea-polyacrylamide gel, and the cleaved products were quantitated (lower panel). B, NEIL2 incision activity on 5-OHU-containing 32P-labeled duplex substrate (lanes 1–5) or 8-oxoG containing 32P-labeled bubble substrate (lanes 6 –11) in the presence (+) or absence (−) of YB-1. Cleaved products were quantitated as shown in the lower panel. C, NEIL2 incision activity on 5-OHU-containing 32P-labeled bubble substrate in the presence of different GST-YB1 deletions as indicated in Fig. 2. Lower panel, quantification of the cleaved products. S, substrate; P, product. BSA, bovine serum albumin.
To investigate the mechanism of stimulation, we determined the kinetic parameters of NEIL2 in the presence or absence of YB-1. Table 2 showed that NEIL2 had comparable Km (~3.4–3.5 nm) in the presence or absence of YB-1; however, the catalytic efficiency (kcat/Km) of NEIL2 was nearly 3-fold higher in the presence of YB-1. This result indicates that NEIL2 turnover is significantly facilitated in the presence of YB-1.
TABLE 2.
Kinetic parameters of NEIL2 in the absence or presence of YB-1 with 5-OHU·B11 bubble DNA substrate
| NEIL2 | NEIL2 + YB-1 | |
|---|---|---|
| Vmax (nm/min) | 0.14 ± 0.01 | 0.38 ± 0.01 |
| Km (nm) | 3.53 ± 0.45 | 3.4 ± 0.32 |
| kcat (×10−3/min) | 112 ± 8 | 316 ± 7 |
| kcat/Km (×10−3 min−1 nm−1) | 31.8 ± 1.8 | 93.5 ± 6.7 |
Protein-Protein Interaction between YB-1 and NEIL2 Is Required for Stimulation of NEIL2 Activity
To investigate whether the interacting domain of YB-1 is sufficient to increase NEIL2 activity, we quantified NEIL2 activity in the presence of various deletion mutants of YB-1 (Fig. 3C).We observed that NEIL2 activity was increased with only the YB-1 mutants that interacted with NEIL2 (lanes 4–6). A non-interacting YB-1 mutant failed to stimulate NEIL2 activity (Fig. 3C, lane 7), indicating that protein-protein interaction was necessary for the stimulation.
Interaction of YB-1 with Other NEIL2-associated Proteins
We have shown previously that NEIL2-initiated repair involves PNK, Pol β, and Lig IIIα (19) and that NEIL2 stably interacts with Pol β and Lig IIIα. In view of the association between NEIL2 and YB-1, we investigated whether YB-1 interacted with any of these proteins that are involved in NEIL2-initiated PNK-dependent repair. Far Western analyses using purified proteins showed that YB-1, like NEIL2, interacted directly with Pol β and Lig IIIα (Fig. 4A, lanes 7 and 8), but not with PNK (Fig. 4A, lane 6).
FIGURE 4. Interaction of YB-1 with other NEIL2-associated proteins.
A, for Far Western analysis, recombinant PNK, Lig IIIα, Pol β, or bovine serum albumin (BSA) were run in SDS-PAGE, renatured, incubated with YB-1, and then blotted with anti-YB-1 antibody. Left panel, Coomassie staining of the corresponding proteins. B, Far Western analysis for the interaction studies between full-length or different GST-YB-1 deletion mutants (Δ6– Δ9) with Pol β (left panel) and Lig IIIα (right panel). C, Far Western analysis of the interaction between YB-1 and different C-terminal (16, 14, and 8 kDa) or N-terminal (31 and 22 kDa) deletion mutants of Pol β using anti-YB-1 antibody. Schematic representations of the Pol β domains are shown in the bottom panel.
We then used Far Western analysis to examine the interaction of Pol β and Lig IIIα with WT and different deletion mutants of YB-1. We observed that both Pol β and Lig IIIα interacted (Fig. 4B, lanes 5, 6 and 11, 12) within the C-terminal region of YB-1 (cf. Δ8 and Δ9 in Fig. 2). This region of YB-1 is also responsible for interaction with NEIL2 (Fig. 2). However, YB-1 did not have any effect on the DNA polymerase activity of Pol β or on Lig IIIα activity (data not shown).
To identify the region of Pol β involved in the interaction with YB-1, we performed Far Western analysis with the N-terminal fragments of Pol β having molecular masses of 8, 14, and 16 kDa, and to C-terminal fragments of 22 and 31 kDa. YB-1 interacted with the N-terminal 8-, 14-, and 16-kDa fragments of Pol β, along with the full-length protein (Fig. 4C, lanes 9 and 12–14), but not with the C-terminal fragments (Fig. 4C, lanes 10 and 11). These results indicate that the N-terminal 87-amino acid region of Pol β was sufficient for interaction with YB-1. In contrast, NEIL2 interacted with the 14- and 16-kDa fragments, but not with the 8-kDa domain of Pol β (19).
UVA-induced ROS Generation Increases Association of YB-1 with NEIL2
It is known that UVA radiation produces both singlet oxygen (1O2) and H2O2 in cells, which in turn induce oxidative damage to DNA (5, 35). We thus first tested the production of UVA-induced ROS in our system by determining the change in the fluorescence intensity of the oxidized carboxy-H2DCFDA after UVA treatment of HCT116N cells with riboflavin, used as a photosensitizer. The ROS level reached its peak within 2 h, which was about 12-fold higher compared with untreated cells, and by 24 h post-exposure, it continued to decrease (Fig. 5A). When cells were preincubated with NAC, a ROS scavenger, there was a significant decrease in the ROS level. Glucose oxidase was used as a positive control for ROS generation.
FIGURE 5. Interaction between NEIL2 and YB-1 under oxidative stress.
A, HCT116N cells were treated with UVA or glucose oxidase to induce oxidative stress for different times (as indicated in the figure) in the presence or absence of NAC, and ROS generation was evaluated by measuring the fluorescence of oxidized H2DCFDA. Riboflavin was used as a photosensitizer. B, nuclear extracts were prepared from HCT116N cells at different time points after UVA treatment (as indicated in the left panel) or glucose oxidase (GO) treatment (right panel), immunoprecipitated with anti-FLAG antibody-conjugated beads (Sigma), and analyzed by Western blot using anti-YB-1 or anti-FLAG antibody. Lower panel, quantification of the Western blot data (YB-1 bands, lanes 1–10) after normalization of NEIL2-FLAG bands. C, HCT116N cells were treated with UVA or glucose oxidase for the indicated times and the YB-1 levels were measured and quantified (lower panel) in nuclear extract or whole cell lysate on Western blots. Lamin B and α-tubulin were used as the loading control for nuclear extract and whole cell lysate respectively.
To investigate the association of YB-1 with NEIL2 after UVA treatment, nuclear extracts were prepared from HCT116N cells for immunoprecipitation with anti-FLAG affinity beads. Western analysis of NEIL2-FLAG IP showed increased association of YB-1 in UVA-treated cells after 2 h (Fig. 5B, lane 2); the highest level of interaction was found to occur at 15 h (Fig. 5B, lane 5). Pretreatment with NAC diminished the interaction (Fig. 5B, lanes 4 and 7) indicating the involvement of ROS generated after UVA treatment. Glucose oxidase treatment also showed elevated NEIL2-YB-1 interaction (Fig. 5B, lane 9) that falls off after NAC treatment (Fig. 5B, lane 10), as expected.
Increased Nuclear Translocation of YB-1 after Oxidative Stress
YB-1 is mostly a cytoplasmic protein. It translocates to the nucleus when cells are challenged with anticancer drugs, hyperthermia, or UV radiation (36, 37). However, its nuclear accumulation was never investigated under conditions of oxidative stress. As evident from immunoblot analysis of nuclear extracts, nuclear translocation of YB-1 started within 2 h of UVA treatment (Fig. 5C, lane 5) and reached its highest level around 15–24 h (Fig. 5C, lanes 8 and 9). NAC pretreatment of cells prevented the increased translocation of the YB-1 level after UVA irradiation (Fig. 5C, lanes 7 and 10), again suggesting a role of ROS in the nucleocytoplasmic transfer of YB-1. Glucose oxidase-generated ROS also increased accumulation of YB1 in the nucleus (Fig. 5C, lane 2). Western blot analysis using whole cell extract showed that the total YB-1 level remained the same after UVA treatment (Fig. 5C, lower panel).
Increased DNA Glycosylase/AP Lyase Activity of NEIL2 Immunocomplex after UVA Treatment
It is reasonable to postulate that UV-induced DNA damage signals an urgent need to repair cellular DNA. We hypothesized that one possible mechanism for such a cellular response is to enhance the efficiency of repair. The enhanced interaction between NEIL2 and YB-1 under oxidative stress thus prompted us to investigate the activity of the NEIL2-FLAG immunocomplex (IC) after UVA treatment. The NEIL2-FLAG IC obtained after UVA treatment showed significant increase (~3.5-fold in 2 h of treatment and 4.5-fold in 15 h of treatment) in NEIL2 activity with the 5-OHU·B11 substrate (Fig. 6, lanes 3 and 6). Interestingly, NAC pretreatment caused a dramatic decrease in activity (Fig. 6, lanes 5 and 8). Glucose oxidase treatment also increased the activity of NEIL2-IC (Fig. 6, lane 11) by ~3-fold. However, NAC pretreatment also caused a decrease in activity in glucose oxidase-treated samples (Fig. 6, lane 12). Similar levels of NEIL2 in NEIL2-FLAG IC in control versus UVA-treated cell extracts suggested that the enhanced interaction of YB-1 was responsible for the increase in NEIL2 activity.
FIGURE 6. Incision activity of NEIL2-FLAG immunocomplex after oxidative stress.
Incision activity of NEIL2-FLAG immunocomplex from UVA-treated cells (lanes 3–8) on 5-OHU containing 32P-labeled bubble substrate. NAC was used as a ROS scavenger (lanes 5, 8, and 12). Glucose oxidase (GO) was used as a positive control for ROS generation (lane 11). S, substrate; P, product.
Increased Activity of NEIL2 IC after Oxidative Stress Is Due to Specific Interaction between NEIL2 and YB-1
To provide further evidence that YB-1 is specifically involved in increasing NEIL2 activity after oxidative stress, we down-regulated YB-1 using siRNA. Real time RT-PCR analysis of the YB-1 mRNA level at 48 h after transfection of 80 nm YB-1 siRNA showed a significant decrease (~80%) in YB-1 mRNA levels (Fig. 7A, upper panel). The YB-1 protein level also showed a maximal decrease at 72 h using a similar amount of siRNA (Fig. 7A, lower panel). We also checked cell viability by trypan blue to confirm that there was no impaired cellular growth after siRNA treatment. We therefore down-regulated YB-1 using the optimized conditions, and then exposed the HCT116N cells to UVA and allowed them to grow for 15 h. The IC was isolated from the nuclear extracts of control and YB-1 siRNA-treated UVA-exposed cells. The marked decrease in NEIL2 activity (~5-fold) in the IC isolated from the YB-1 siRNA-treated cells (Fig. 7B, left panel, lane 3 versus lane 2) clearly indicated that the NEIL2-YB-1 interaction was responsible for enhanced NEIL2 activity. An equal amount of NEIL2-FLAG (judged by densitometric scanning) was used in the IC for incision assay (Fig. 7B, right panel, lanes 1 and 2). We also tested whether other NEIL2-interacting proteins (Pol β and Lig IIIα) have any effect on NEIL2 activity. Fig. 7C clearly shows that neither Pol β nor Lig IIIα has any effect on the activity of NEIL2.
FIGURE 7. Incision activity of NEIL2-FLAG immunocomplex after YB-1 down-regulation.
A, upper panel, real time RT-PCR for optimization of YB-1 down-regulation with different concentrations of YB-1 siRNA (20–80 nm) after 24 or 48 h treatment. Lower panel, Western analysis for YB-1 expression at the indicated time points and α tubulin as a loading control. B, incision activity of NEIL2-FLAG immunocomplex from YB-1 down-regulated, UVA-treated cells (left panel). Expression of NEIL2-FLAG and YB-1 in nuclear extract and their amount in NEIL2-FLAG IP from the nuclear extract (right panel). C, effect of Pol β (left panel) and Lig IIIα (right panel) on the incision activity of NEIL2.
DISCUSSION
The in vivo activity of DNA repair proteins appears to be significantly affected by interaction with partner proteins (38, 39). Whereas investigating NEIL2-associated proteins and their potential role in modulating NEIL2-initiated BER, we found that YB-1 physically associated with NEIL2 and enhanced NEIL2-initiated base excision repair. YB-1 was initially identified as the transcription factor that specifically binds to the Y-box element (inverted CCAAT box) of the major histocompatibility complex class II genes and modulates their activity (40). YB-1 was shown to have pleotropic roles in transcription, replication, and RNA metabolism (41–43). Several studies showed that YB-1 is associated in vitro directly or indirectly with several DNA replication and repair proteins, including MSH2, DNA polymerase δ, Ku80, and WRN (44, 45). However, the physiological significance of such association is unknown. One report documented stimulation of the DNA glycosylase activity of NTH1 because of stable interaction with YB-1 (24), suggesting its role in oxidative DNA base damage repair.
We have recently shown that NEIL2 stably interacts with downstream BER proteins, e.g. Pol β and Lig IIIα. Furthermore, the NEIL2 immunocomplex containing Pol β, PNK, and Lig IIIα efficiently repairs 5-OHU, supporting a model of repair coordination in which NEIL2 recruits these proteins to form a repair complex at the site of DNA damage (19). We have now shown that YB-1 could be a part of this complex, where it interacts with other NEIL2 partners such as Lig IIIα and Pol β. Interestingly, the interacting regions of Pol β for NEIL2 and YB-1 are different suggesting that their ternary complex could be formed without steric interference. However, YB-1 does not modulate the activity of Lig IIIα and Pol β; it only enhances NEIL2-initiated base excision, the first step in BER. UVA (320–400 nm), unlike UVB or UVC, produces singlet oxygen in vivo in the presence of cellular chromophores, e.g. riboflavin, porphyrins (6), and exogenous photosensitizers like quinones. UVA is not absorbed by the ozone layer, and constitutes more than 95% of solar UV radiation reaching the surface of the earth playing a causal role in the etiology of non-melanoma and melanoma skin cancers and photoaging (46, 47). As NEIL2-mediated oxidative damage repair is important for cellular defense mechanisms, we decided to test the role of UVA as a potential source of ROS. A significant increase in the level of ROS was observed in HCT116N cells after irradiation with 40 J/m2 UVA in the presence of riboflavin. Irradiation with UVA of 3 kJ/m2 is approximately equivalent to 1–2 min of exposure to midsummer sun at noon (48). Thus the dose used in our studies is physiologically relevant. Oxidative stress after UVA exposure induced oxidized DNA bases like 8-oxoG, and small amounts of DNA strand breaks and DNA-protein cross-links, which are mutagenic or toxic (49). A recent report showed that human OGG1, the major DNA glycosylase for repair of 8-oxoG in mammalian cells (21), is recruited to nuclear speckles in UVA-irradiated cells independent of the damage generation (50). Kozmin et al. (51) showed a major role of oxidative DNA damage, mostly 8-oxoG, in the genotoxicity of UVA radiation in yeast. Interestingly OGG1−/− mouse cells did not exhibit an increased frequency of UV light-induced mutations over that in WT(OGG1+/+) cells (52). This indicates that some other DNA glycosylase excises 8-oxoG from the DNA in mammalian cells. We have shown previously that NEIL2 removes 8-oxoG from bubble DNA substrates (20). In the present study we found that YB-1 significantly stimulates both the 8-oxoG and 5-OHU excision activities of NEIL2. Both 8-oxoG and5-OHU are abundant lesions in mammalian genomes, and are generated at comparable levels (53). Thus we propose that NEIL2 plays an important role in the repair of these mutagenic base lesions in mammalian cells.
Several laboratories have demonstrated direct involvement of YB-1 in the cellular response to genotoxic stress. YB-1 is primarily localized in the cytoplasm, but translocates to the nucleus after UV irradiation or treatment with anticancer drugs (36, 54). Nuclear localization of YB-1 appears to activate stress-inducible target genes (55, 56), suggesting that YB-1 is a stress-activated transacting factor. From our results, it appears that the nucleocytoplasmic trafficking of the YB-1 is also dependent on oxidative stress, which has not been previously reported. Here we demonstrated that the extent of NEIL2-YB-1 interaction was significantly increased after UVA- (or glucose oxidase-) induced nuclear translocation of YB-1. This increase is reflected in enhancement of the activity of NEIL2, and on the overall repair of the oxidized base in a reconstituted system (data not shown). Furthermore, the NEIL2 immunocomplex isolated from UVA-treated HCT116N cells previously treated with YB-1 siRNA had dramatically reduced repair activity compared with that in control cells. This strongly suggests that specific complex formation between NEIL2 and YB-1 is the limiting event in the repair reaction. Taken together, these results suggest that the repair proteins form complex(es) and act in concert for efficient repair. Whether NEIL2 forms a single multiprotein complex including YB-1 or multiple subcomplexes, warrants further investigation.
Acknowledgments
We are grateful to Sankar Mitra, Department of Biochemistry and Molecular Biology, for suggestions and critically reading the manuscript. We thank A. Kurosky, University of Texas Medical Branch Biomolecular Resources Facility, for MALDI-TOF MS and YB-1 antibody production and purification; George W. Teebor, New York University School of Medicine, for YB-1; Alan Tomkinson, University of Maryland, for Lig IIIα; and Michael Weinfeld, University of Alberta, for PNK expression plasmid; Sanat K. Mokkapati for suggestions regarding the kinetic parameter determination; and Muralidhar Hegde for DNA Lig IIIα protein purification. We also thank Wanda Smith for secretarial help with the manuscript and Dr. David Konkel for critically reading and editing the manuscript.
Footnotes
This work was supported in part by United States Public Health Service Grants CA102271 (to T. K. H.), P01 AG021830 (to I. B. and T. K. H.), P01 ES06676 (to T. K. H.), CA81063 (to R. C.), and E508457 (to K. K. B.), and the Intramural Research Program of the National Institutes of Health, NIEHS.
The abbreviations used are: ROS, reactive oxygen species; YB-1, Y box-binding protein 1; Ab, antibody; BER, base excision repair; IC, immunocomplex; IP, immunoprecipitate/immunoprecipitation; Lig IIIα, DNA ligase IIIα; NEIL, Nei-like; 5-OHU, 5-hydroxyuracil; 8-oxoG, 8-oxoguanine; NTH1, endonuclease III homolog 1; OGG1, 8-oxoguanine-DNA glycosylase 1; Pol β, DNA polymerase β; TBS, Tris-buffered saline; WT, wild type; NAC, N-acetyl l-cysteine; H2DCFDA, 5-(and –6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate; GST, glutathione S-transferase; DTT, dithiothreitol; PBS, phosphate-buffered saline; MALDI-TOF, matrix-assisted laser desorption ionization time-of-flight; RT, reverse transcriptase.
REFERENCES
- 1.Ames BN, Shigenaga MK, Hagen TM. Proc. Natl. Acad. Sci. U. S. A. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gotz ME, Kunig G, Riederer P, Youdim MB. Pharmacol. Ther. 1994;63:37–122. doi: 10.1016/0163-7258(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 3.Kasai H, Crain PF, Kuchino Y, Nishimura S, Ootsuyama A, Tanooka H. Carcinogenesis. 1986;7:1849–1851. doi: 10.1093/carcin/7.11.1849. [DOI] [PubMed] [Google Scholar]
- 4.Ogawa Y, Kobayashi T, Nishioka A, Kariya S, Hamasato S, Seguchi H, Yoshida S. Int. J. Mol. Med. 2003;11:149–152. [PubMed] [Google Scholar]
- 5.Pouget JP, Douki T, Richard MJ, Cadet J. Chem. Res. Toxicol. 2000;13:541–549. doi: 10.1021/tx000020e. [DOI] [PubMed] [Google Scholar]
- 6.Kvam E, Tyrrell RM. J. Investig. Dermatol. 1999;113:209–213. doi: 10.1046/j.1523-1747.1999.00653.x. [DOI] [PubMed] [Google Scholar]
- 7.Mitra S, Hazra TK, Roy R, Ikeda S, Biswas T, Lock J, Boldogh I, Izumi T. Mol. Cells. 1997:305–312. [PubMed] [Google Scholar]
- 8.Mitra S, Izumi T, Boldogh I, Bhakat KK, Hill JW, Hazra TK. Free Radic. Biol. Med. 2002;33:15–28. doi: 10.1016/s0891-5849(02)00819-5. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda S, Biswas T, Roy R, Izumi T, Boldogh I, Kurosky A, Sarker AH, Seki S, Mitra S. J. Biol. Chem. 1998;273:21585–21593. doi: 10.1074/jbc.273.34.21585. [DOI] [PubMed] [Google Scholar]
- 10.Lu R, Nash HM, Verdine GL. Curr. Biol. 1997;7:397–407. doi: 10.1016/s0960-9822(06)00187-4. [DOI] [PubMed] [Google Scholar]
- 11.Minowa O, Arai T, Hirano M, Monden Y, Nakai S, Fukuda M, Itoh M, Takano H, Hippou Y, Aburatani H, Masumura K, Nohmi T, Nishimura S, Noda T. Proc. Natl. Acad. Sci. U. S. A. 2000;97:4156–4161. doi: 10.1073/pnas.050404497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ocampo MT, Chaung W, Marenstein DR, Chan MK, Altamirano A, Basu AK, Boorstein RJ, Cunningham RP, Teebor GW. Mol. Cell. Biol. 2002;22:6111–6121. doi: 10.1128/MCB.22.17.6111-6121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takao M, Kanno S, Kobayashi K, Zhang QM, Yonei S, van der Horst GT, Yasui A. J. Biol. Chem. 2002;277:42205–42213. doi: 10.1074/jbc.M206884200. [DOI] [PubMed] [Google Scholar]
- 14.Bandaru V, Sunkara S, Wallace SS, Bond JP. DNA Repair. 2002;1:517–529. doi: 10.1016/s1568-7864(02)00036-8. [DOI] [PubMed] [Google Scholar]
- 15.Hazra TK, Kow YW, Hatahet Z, Imhoff B, Boldogh I, Mokkapati SK, Mitra S, Izumi T. J. Biol. Chem. 2002;277:30417–30420. doi: 10.1074/jbc.C200355200. [DOI] [PubMed] [Google Scholar]
- 16.Hazra TK, Izumi T, Boldogh I, Imhoff B, Kow YW, Jaruga P, Dizdaroglu M, Mitra S. Proc. Natl. Acad. Sci. U. S. A. 2002;99:3523–3528. doi: 10.1073/pnas.062053799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morland I, Rolseth V, Luna L, Rognes T, Bjoras M, Seeberg E. Nucleic Acids Res. 2002;30:4926–4936. doi: 10.1093/nar/gkf618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiederhold L, Leppard JB, Kedar P, Karimi-Busheri F, Rasouli-Nia A, Weinfeld M, Tomkinson AE, Izumi T, Prasad R, Wilson SH, Mitra S, Hazra TK. Mol. Cell. 2004;15:209–220. doi: 10.1016/j.molcel.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Das A, Wiederhold L, Leppard JB, Kedar P, Prasad R, Wang H, Boldogh I, Karimi-Busheri F, Weinfeld M, Tomkinson AE, Wilson SH, Mitra S, Hazra TK. DNA Repair. 2006;5:1439–1448. doi: 10.1016/j.dnarep.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dou H, Mitra S, Hazra TK. J. Biol. Chem. 2003;278:49679–49684. doi: 10.1074/jbc.M308658200. [DOI] [PubMed] [Google Scholar]
- 21.Klungland A, Rosewell I, Hollenbach S, Larsen E, Daly G, Epe B, Seeberg E, Lindahl T, Barnes DE. Proc. Natl. Acad. Sci. U. S. A. 1999;96:13300–13305. doi: 10.1073/pnas.96.23.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenquist TA, Zaika E, Fernandes AS, Zharkov DO, Miller H, Grollman AP. DNA Repair. 2003;2:581–591. doi: 10.1016/s1568-7864(03)00025-9. [DOI] [PubMed] [Google Scholar]
- 23.Das A, Hazra TK, Boldogh I, Mitra S, Bhakat KK. J. Biol. Chem. 2005;280:35272–35280. doi: 10.1074/jbc.M505526200. [DOI] [PubMed] [Google Scholar]
- 24.Marenstein DR, Ocampo MT, Chan MK, Altamirano A, Basu AK, Boorstein RJ, Cunningham RP, Teebor GW. J. Biol. Chem. 2001;276:21242–21249. doi: 10.1074/jbc.M101594200. [DOI] [PubMed] [Google Scholar]
- 25.Ise T, Nagatani G, Imamura T, Kato K, Takano H, Nomoto M, Izumi H, Ohmori H, Okamoto T, Ohga T, Uchiumi T, Kuwano M, Kohno K. Cancer Res. 1999;59:342–346. [PubMed] [Google Scholar]
- 26.Bhakat KK, Mokkapati SK, Boldogh I, Hazra TK, Mitra S. Mol. Cell. Biol. 2006;26:1654–1665. doi: 10.1128/MCB.26.5.1654-1665.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohga T, Koike K, Ono M, Makino Y, Itagaki Y, Tanimoto M, Kuwano M, Kohno K. Cancer Res. 1996;56:4224–4228. [PubMed] [Google Scholar]
- 28.Schreiber E, Matthias P, Muller MM, Schaffner W. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jayaraman L, Moorthy NC, Murthy KG, Manley JL, Bustin M, Prives C. Genes Dev. 1998;12:462–472. doi: 10.1101/gad.12.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, Joseph JA. Free Radic. Biol. Med. 1999;27:612–616. doi: 10.1016/s0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 31.Boldogh I, Bacsi A, Choudhury BK, Dharajiya N, Alam R, Hazra TK, Mitra S, Goldblum RM, Sur S. J. Clin. Investig. 2005;115:2169–2179. doi: 10.1172/JCI24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graumann PL, Marahiel MA. Trends Biochem. Sci. 1998;23:286–290. doi: 10.1016/s0968-0004(98)01255-9. [DOI] [PubMed] [Google Scholar]
- 33.Wolffe AP. Bioessays. 1994;16:245–251. doi: 10.1002/bies.950160407. [DOI] [PubMed] [Google Scholar]
- 34.Higashi K, Inagaki Y, Fujimori K, Nakao A, Kaneko H, Nakatsuka I. J. Biol. Chem. 2003;278:43470–43479. doi: 10.1074/jbc.M302339200. [DOI] [PubMed] [Google Scholar]
- 35.Petersen AB, Gniadecki R, Vicanova J, Thorn T, Wulf HC. J. Photochem. Photobiol. B Biol. 2000;59:123–131. doi: 10.1016/s1011-1344(00)00149-4. [DOI] [PubMed] [Google Scholar]
- 36.Koike K, Uchiumi T, Ohga T, Toh S, Wada M, Kohno K, Kuwano M. FEBS Lett. 1997;417:390–394. doi: 10.1016/s0014-5793(97)01296-9. [DOI] [PubMed] [Google Scholar]
- 37.Stein U, Jurchott K, Walther W, Bergmann S, Schlag PM, Royer HD. J. Biol. Chem. 2001;276:28562–28569. doi: 10.1074/jbc.M100311200. [DOI] [PubMed] [Google Scholar]
- 38.Dianova II, Bohr VA, Dianov GL. Biochemistry. 2001;40:12639–12644. doi: 10.1021/bi011117i. [DOI] [PubMed] [Google Scholar]
- 39.Vidal AE, Boiteux S, Hickson ID, Radicella JP. EMBO J. 2001;20:6530–6539. doi: 10.1093/emboj/20.22.6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Didier DK, Schiffenbauer J, Woulfe SL, Zacheis M, Schwartz BD. Proc. Natl. Acad. Sci. U. S. A. 1988;85:7322–7326. doi: 10.1073/pnas.85.19.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kohno K, Izumi H, Uchiumi T, Ashizuka M, Kuwano M. Bioessays. 2003;25:691–698. doi: 10.1002/bies.10300. [DOI] [PubMed] [Google Scholar]
- 42.Sommerville J, Ladomery M. Chromosoma (Berl.) 1996;104:469–478. doi: 10.1007/BF00352111. [DOI] [PubMed] [Google Scholar]
- 43.Sommerville J, Ladomery M. FASEB J. 1996;10:435–443. doi: 10.1096/fasebj.10.4.8647342. [DOI] [PubMed] [Google Scholar]
- 44.Hasegawa SL, Doetsch PW, Hamilton KK, Martin AM, Okenquist SA, Lenz J, Boss JM. Nucleic Acids Res. 1991;19:4915–4920. doi: 10.1093/nar/19.18.4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaudreault I, Guay D, Lebel M. Nucleic Acids Res. 2004;32:316–327. doi: 10.1093/nar/gkh170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lowe NJ, Meyers DP, Wieder JM, Luftman D, Borget T, Lehman MD, Johnson AW, Scott IR. J. Investig. Dermatol. 1995;105:739–743. doi: 10.1111/1523-1747.ep12325517. [DOI] [PubMed] [Google Scholar]
- 47.Wang SQ, Setlow R, Berwick M, Polsky D, Marghoob AA, Kopf AW, Bart RS. J. Am. Acad. Dermatol. 2001;44:837–846. doi: 10.1067/mjd.2001.114594. [DOI] [PubMed] [Google Scholar]
- 48.Parrish JA, Jaenicke KF, Anderson RR. Photochem. Photobiol. 1982;36:187–191. doi: 10.1111/j.1751-1097.1982.tb04362.x. [DOI] [PubMed] [Google Scholar]
- 49.Sarasin A. Mutat. Res. 1999;428:5–10. doi: 10.1016/s1383-5742(99)00025-3. [DOI] [PubMed] [Google Scholar]
- 50.Campalans A, Amouroux R, Bravard A, Epe B, Radicella JP. J. Cell Sci. 2007;120:23–32. doi: 10.1242/jcs.03312. [DOI] [PubMed] [Google Scholar]
- 51.Kozmin S, Slezak G, Reynaud-Angelin A, Elie C, de Rycke Y, Boiteux S, Sage E. Proc. Natl. Acad. Sci. U. S. A. 2005;102:13538–13543. doi: 10.1073/pnas.0504497102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kappes UP, Runger TM. Radiat. Res. 2005;164:440–445. doi: 10.1667/rr3434.1. [DOI] [PubMed] [Google Scholar]
- 53.Wagner JR, Hu CC, Ames BN. Proc. Natl. Acad. Sci. U. S. A. 1992;89:3380–3384. doi: 10.1073/pnas.89.8.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayakawa H, Uchiumi T, Fukuda T, Ashizuka M, Kohno K, Kuwano M, Sekiguchi M. Biochemistry. 2002;41:12739–12744. doi: 10.1021/bi0201872. [DOI] [PubMed] [Google Scholar]
- 55.Bargou RC, Jurchott K, Wagener C, Bergmann S, Metzner S, Bommert K, Mapara MY, Winzer KJ, Dietel M, Dorken B, Royer HD. Nat. Med. 1997;3:447–450. doi: 10.1038/nm0497-447. [DOI] [PubMed] [Google Scholar]
- 56.Oda Y, Sakamoto A, Shinohara N, Ohga T, Uchiumi T, Kohno K, Tsuneyoshi M, Kuwano M, Iwamoto Y. Clin. Cancer Res. 1998;4:2273–2277. [PubMed] [Google Scholar]