Abstract
AMP-activated protein kinase (AMPK) serves as a fuel-sensing enzyme that is activated by binding of AMP and subsequent phophorylation by upstream kinases such as the tumor suppressor LKB1, when cells sense an increase in the ratio of AMP to ATP. Acute activation of AMPK stimulates fatty acid oxidation to generate more ATP and simultaneously inhibits ATP-consuming processes including fatty acid and protein syntheses, thereby preserving energy for acute cell surviving program, while the chronic activation leads to inhibition of cell growth. The goal of the present study is to explore the mechanisms by which AMPK regulates cell growth. Toward this end, we established stable cell lines by introducing a dominant negative mutant of AMPK α1 subunit or its shRNA into the prostate cancer C4-2 cells and other cells, or wild type LKB1 into the lung adenocarcinoma A549 and breast MB-MDA-231 cancer cells, both of which lack functional LKB1. Our results showed that the inhibition of AMPK accelerated cell proliferation and promoted malignant behavior such as increased cell migration and anchorage-independent growth. This was associated with decreased G1 population, downregulation of p53 and p21, and upregulation of S6K, IGF-1 and IGF1R. Conversely, treatment of the C4-2 cells with 5-aminoimidazole-4-carboxamide 1-Dribonucleoside (AICAR), a prototypical AMPK activator, caused opposite changes. In addition, our study using microarray and RT-PCR revealed that AMPK regulated gene expression involved in tumor cell growth and survival. Thus, our study provides novel insights into the mechanisms of AMPK action in cancer cells and presents AMPK as an ideal drug target for cancer therapy.
Keywords: AMPK, LKB1, cell proliferation, cell cycle, tumor suppressor, oncogenes
Introduction
AMP-activated protein kinase (AMPK) acts as a fuel-sensing enzyme that is highly conserved from yeast all through humans, consisting of three subunits, a catalytic subunit (α) and two regulatory subunits (β and γ) (Hardie, 2007). Each subunit in mammals contains two to three isoforms (α1, α2; β1, β2; γ1, γ2, γ3). AMPK is activated under such stress conditions as hypoxia, ischemia and exercise where the intracellular AMP level or the ratio of AMP to ATP is increased. As a result, AMP binds to the γ subunit, enabling phosphorylation of threonine 172 in the activation loop of the α catalytic subunit by upstream kinases such as LKB1 and CaMKK, yielding an active conformation. AMPK can also be activated by hormones and cytokines, such as two adipocytes-derived hormones, leptin and adiponectin, and IL6 and CNTF. In addition, AMPK can be activated by a variety of pharmacological agents. The prototypical activator is 5-aminoimidazole-4-carboxamide 1-D-ribonucleoside (AICAR), a cell permeable agent that is phosphorylated and converted to ZMP, an AMP analogue, after entering the cell. Importantly, two types of clinically used anti-diabetic drugs, metformin and thiazolidinediones (TZDs), have been known to activate AMPK (Hardie, 2007). Upon activation, AMPK phosphorylates a plethora of substrates. Therefore, the activation of AMPK promotes fatty acid oxidation to generate more ATP in coping with acute energy demand and inhibits ATP-consuming processes such as lipid and protein synthesis to preserve energy for cell surviving program (Hardie, 2007; Luoet al., 2005
Considerable amount of evidence has demonstrated that AMPK is implicated in the metabolic syndrome and cancer cell growth and metabolism (Hardie, 2007; Luo et al., 2005).Thus, decreases in AMPK activity are associated with insulin resistance, which can be improved by pharmacological activators of AMPK. Furthermore, many therapies that have proven useful in treating the metabolic syndrome in humans, including TZDs, metformin, caloric restriction and exercise, have been shown to activate AMPK. In addition to its association with the metabolic syndrome, AMPK is emerging as an important modulator of energy metabolism in cancer cells and thereby regulating their growth (Luo et al., 2005). First, it has been reported that obesity which often concurs with insulin resistance and decreases in AMPK activity has been shown to be associated with increased risk and/or mortality of many types of cancer such as those derived from breast, colon, colorectum, prostate, and ovary (Calle et al., 2003). Second, the metabolic syndrome manifesting hyperlipidemia, hyperglycemia, and hyperinsulinemia is a risk factor of several types of cancers (Chang and Ulrich, 2003; Luo et al., 2005). Thus, treatment of type 2 diabetes with metformin significantly reduces the incidence of cancers (Evans et al., 2005). Third, reduced levels of adiponectin have been found in plasma of patients with some cancers including breast and prostate cancers, and treatment of cancer cells with adiponectin attenuates their growth and even induces apoptosis (Barb et al., 2007; Grossmann et al., 2008). Finally, in animal studies, maneuvers that activate AMPK (e.g. treatment with AICAR, metformin and TZDs and exercise) can inhibit tumor development (Buzzai et al., 2007; Dowling et al., 2007; Huang et al., 2008; Isakovic et al., 2007; Lee, 2003; Rattan et al., 2005; Xiang et al., 2004).
At molecular levels, AMPK has been linked to oncogenes and tumor suppressors. Thus, the first physiological activator of AMPK is LKB1/Stk11(Carling, 2006). Loss-of-function mutations of LKB1account for an autosomal dominant genetic disease called Peutz-Jegher syndrome. The patients with this genetic disease develop benign tumors in their gastric-intestinal (GI) system and have an increased risk for GI adenocarcinomas. Most of the mutations impinge on the kinase domain of LKB1causing the loss of kinase activity. In addition, somatic mutations of the LKB1gene have been found in several other cancers, for example, in 34% of lung adenocarcinomas, 19% of squamous cell carcinomas (SCC) and other cancers (Ikediobi et al., 2006; Ji et al., 2007; Su et al., 1999). Although a complete ablation of LKB1 causes embryonic lethality in mouse, its heterozygous deletion increases the incidence of tumor in the intestine and stomach (Luo et al., 2005) and predisposes animals to carcinogenesis induced by 7,12 dimethylbenz(α) anthracene (DMBA), thus developing SCC of the skin and lung (Gurumurthy et al., 2008).
In addition, AMPK has been shown to phosphorylate and activate tuberous sclerosis complex protein 2 (TSC2) (Inoki et al., 2006). This protein together with TSC1 forms a GTPase activating protein for Rheb, an activator of mTOR. Loss-of-function mutations of TSC proteins cause another autosomal genetic disease, tuberous sclerosis characterized by harmatomas in multiple tissues. Recently, AMPK has been shown to phosphorylate and inhibit Raptor, another mTOR modulator (Gwinn et al., 2008). Thus, the net effect of AMPK activation is to inhibit mTOR, thereby impeding protein synthesis and cell cycle progression (Luo et al., 2005).
Enzymes essential for free fatty acid synthesis, including fatty acid synthase (FASN) and acetyl CoA carboxylase (ACC), can be inhibited by AMPK by virtue of direct phosphorylation and/or regulation of transcription (Luo et al., 2005). FASN is regarded as a metabolic oncogene, as it is highly expressed in several types of cancers, such as breast, prostate and ovarian cancers (Baron et al., 2004; Kuhajda, 2000). Studies have shown that the inhibition of FASN activity by pharmacological agents or by siRNA attenuates cell proliferation of cancer cells and causes their apoptosis (De Schrijver et al., 2003; Thupari et al., 2001). Finally, AMPK has been shown to regulate the activity of the p53 tumor suppressor resulting in inhibition of cancer cell growth (Jones et al., 2005).
In light of the fact that cancer cells require high levels of protein and lipid synthesis for accelerated growth, we have tested the hypothesis that the activation of AMPK by pharmacological agents could lead to the inhibition of their growth and shown that it does so in prostate cancer cells via suppression of mTOR, FASN and ACC (Xiang et al., 2004). Thus, we attempt to extend these studies by examining if AMPK directly regulates the growth of prostate cancer cells. In this report, we demonstrate that loss of LKB1/AMPK activity leads to an increase in cell proliferation, migration and invasion of prostate cancer cells. Furthermore, our results reveal that AMPK exerts its tumor suppressive function by targeting multiple oncogenes and tumor suppressors.
Materials and Methods
Materials
AICAR was purchased from Toronto Research Chemicals Inc (North York, ON, Canada). Compound C were from EMD Chemicals, Inc, (Gibbstown, New Jersey). Antibodies for IGF1R were from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Antibodies for total and phospho-T172 of AMPK, S6K1 and phospho-S389 S6K1, and clyclin D1 were from Cell Signaling Technology, Inc. (Danvers, MA). Antibodies for p53 and p21 were from Millipore (Billerica, MA). Mission™ lentiviruses for the AMPK α1 subunit and scrambled shRNA were purchased from Sigma (Saint Louis, Missouri). Construction of dominant negative AMPK mutant cDNA for the dominant negative mutant of human AMPK α1 catalytic subunit (D139A) was kindly provided by Dr. Carling and cloned by PCR into a lentiviral vector where a flag epitope was added to the aminoterminus of the α 1 mutant (DNα1). Lentivirus was prepared as described previously (Wu et al., 2000)
Stable Cell Lines
Prostate cancer C4-2 cells in 10% FBS-RPMI1640 medium, prostate cancer PC3 cells and lung cancer A549 cells in 10% FBS-Ham’s F12K medium, and breast cancer MDA-MB-231 cells in 10% FBS-DMEM medium were cultured in 37°C cell culture incubator containing 5% CO2. NIH3T3 F442a cells were cultured in DMEM supplemented with 10% calf serum. The cells were infected with lentivirus expressing the dominant negative AMPK α1 mutant or parental empty viral vector (C4-2, PC3, and F442a cells) and selected with blasticidin for two weeks, or infected with lentivirus expressing LKB1 (A549 and MDA-MB-231 cells) or shRNA, and selected with puromycin.
Immunoblot
Immunoblots were carried out according to protocols provided by vendors of antibodies. Briefly, protein samples (25µg) were subjected to SDS-PAGE and electrophoretically transferred to PVDF membranes (Millipore). The membranes were sequentially blotted with the first and second antibodies, and developed by the enhanced chemiluminescence (ECL) method.
Fluorescence activated cell sorting (FACS) Analysis
Cells were allowed to grow to 70–80% confluence prior to trypsinization and resuspension in ice-cold buffer (65% phosphate-buffered saline, 35% ethanol) at a concentration of 1–5×106 cells/ml for at least 1 hr. The fixed cells were recovered by centrifugation (1,000×g, 5min), washed 3 times in PBS, and resuspended in 1 ml PBS containing 8 µg RNase A and 50µg propidium iodide (PI). The PI stained samples were then incubated in the dark at RT for 30 min and assayed by FACS on a Becton-Dickinson flow cytometer with Cellquest software.
Soft Agar Assay for Colony Formation
Anchorage independent assay was carried out on soft agar. Dividing cells were trypsinized and plated at a density of 20,000 cells/well in 0.6% agar-10% FBS-RPMI1640 onto solidified 1% bed agar in the same culture medium in 6 well plates. One milliliter of culture medium was added onto the top of solidified agar and changed every three days. After 10–14 days of culture in humidified tissue culture incubator, plates were stained with 0.5 ml MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a tetrazole) (10 mg/ml) for >1 h, and colonies photographed and counted under microscopy.
Cell Migration Assay
Cell migration was assayed using Transwell chambers with 8 µm pore size membranes (6.5 mm diameter inserted into 24 well plates), according to the protocol provided by manufacture (Corning, New York, USA). The lower chamber was filled with 600 µl DMEM containing 10% FBS. Cells (1 × 105) were suspended with 100 µl serum-free DMEM and evenly distributed onto the upper chamber. After 16h of plating, the cells remaining on the upper surface of the filters were removed with cotton swabs and those on the lower surface were fixed with 100% methanol and stained with 4',6-diamidino-2-phenylindole (DAPI) (Pierce, Rockford, IL, USA). The membranes with stained cells were cut off and mounted on a glass slide. Pictures were then taken under fluorescent microscope and cell number counted.
RNA preparation and Reversed Transcription (RT)
Total cellular RNA was isolated from 100-mm dish using RNeasy Mini Kit (Qiagen, Valencia, CA) and the reverse transcription reactions were carried out using a kit manufactured by Promega (Madison, WI).
Quantitative Real-time PCR Analysis
the expression of mRNA were examined by real-time PCR with the ABI 7300 Real-time PCR System (Applied Biosystems, Foster City, CA), using SYBRGREEN PCR Master Mix 2× reagent in 20 µl reaction volume. The primers for RT-PCR were designed and synthesized to give rise to 70 to 100 bp products, according to Invitrogen primer design program. The primer sequence is listed in Table 1. Each sample was amplified in triplicate and normalized to Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression. Results were evaluated by the comparative threshold cycle value method (2−ΔΔ Ct) for relative quantification of gene expression.
Table 1.
Primers for Real-time PCR
| Primers | Forward | Reverse |
|---|---|---|
| EphA3 | TACTTCCGGGCAGACAAAGA | TTCTTGGTGAAGATGGAGGTC |
| FN | TCAGACAATGCAGTGGTCTTAAC | TCGTAGACACTGGAGACACTCAC |
| GAPDH | ACAGTCAGCCGCAT | GACAAGCTTCCCGT |
| IGF1 | TGTGGAGACAGGGGCTTTTA | ATCCACGATGCCTGTCTGA |
| IGF1R | AAAAACCTTCGCCTCATCC | TGGTTGTCGAGGACGTAGAA |
| LITAF | GGCATGAATCCTCC | AGCTCTGCAGTTGG |
| Magec2 | GTATATGCTGGGAGGGAGCA | GTCCCTGCACCCAAACTTTA |
| p53 | AGGCCTTGGAACTCAAGGAT | CCCTTTTTGGACTTCAGGTG |
| TNTSF15 | CAAGGGCACACCTGACAGT | CCTAGTTCATGTTCCCAGTGC |
| AMPKα1 | TGCGTGTACGAAGGAAGAATCC | TGTGACTTCCAGGTCTTGGAGTT |
| PCNA | GGCACTCAAGGACCTCATCAA | AATGCCTAAGATCCTTCTTCATCCT |
Gene Array experiments
All procedures were performed at Boston University Microarray Resource Facility exactly as described in Affymetrix GeneChip Expression Analysis Technical Manual (Affymetrix, Santa Clara, CA, current version available at www.affymetrix.com). Processing of all samples including cell culturing, RNA isolation and array hybridization steps was carried out in parallel, and scanning of hybridized microarrays was performed on the same scanner.
Data analysis
Transcript expression estimates were derived from probe-level hybridization intensities using the MAS5 algorithm (Affymetrix). Differentially expressed transcripts were identified using Cyber-T (Baldi and Long, 2001). Cyber-T uses a Bayesian approach to estimate the significance of expression differences between treatment conditions leveraging the observation that probesets with similar expression have similar levels of technical noise. Cyber-T p-values were then adjusted to account for multiple testing using the False Discovery Rate (FDR) method of Klipper-Aurbach et al (Klipper-Aurbach et al., 1995).
Results
Inhibition of cell proliferation by LKB1/AMPK
Previously, we have shown that AMPK activators, AICAR and rosiglitazone, inhibit the growth of prostate cancer cells, which is associated with suppression of mTOR/S6K, ACC and FASN (Xiang et al., 2004). In the present study we attempted to assess whether the effects of these pharmacological agents were mediated by AMPK. Toward this end, we used a lentiviral expression system to express LKB1 in two types of cancer cells, A549, a lung adenocarcinoma cell line, and MB-MDA-231, a breast cancer cell line, both of which do not have functional LKB1 because of mutations. In addition, we infected lentivirus coding for a dominant negative mutant of AMPK α1 subunit into two prostate cancer cell lines, PC3 and C4-2 that was derived from LNCaP cells, reflective of androgen-independent, more advanced stage of prostate cancer cells, and also into NIH3T3 F442a preadipocytes. Different cells (104 cells) were plated onto 6 well plates and the doubling time was calculated after 72 hours. As shown in table 2, the doubling time was markedly increased when wild type LKB1 was introduced back to A549 and MB-MDA-231 cells. In contrast, the rate of cell proliferation was accelerated when the dominant negative AMPK mutant was expressed in PC-3, C4-2, and F442a. This result clearly demonstrates that LKB1/AMPK exerts an inhibitory effect on cell proliferation.
Table 2.
Impact of LKB1/AMPK on Cell Doubling
| Cell Line | Doubling Time (H) |
|---|---|
| A549 | 21 |
| A549-LKB1 | 47 |
| MB-MDA-231 | 30 |
| MB-MDA-231-LKB1 | 41 |
| C4-2 empty | 28 |
| C4-2 DNα1 | 21 |
| PC3 empty | 20 |
| PC3 DNα1 | 16 |
| 3T3-F442a empty | 28 |
| 3T3-F442a DNα1 | 17 |
Wild type LKB1 or dominant negative mutant of AMPKα1 subunit was constructed in alentiviral vector, and virus was produced from HEK293FT cells and infected into the cells as indicated. The cells infected with empty virus were used as a control. Each pair of stable cells was plated in the same number and at 72 hours, viable cells were counted under hemacytometer after staining with trypan blue.
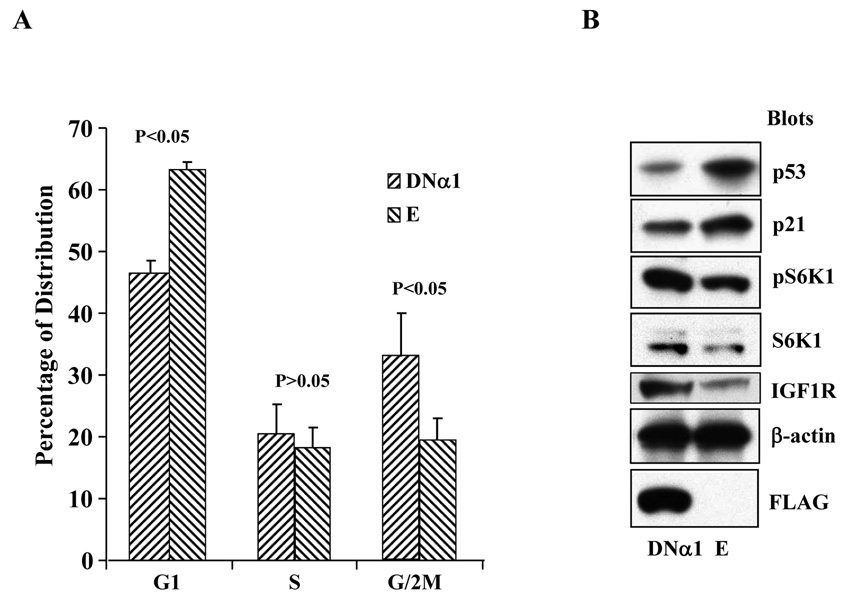
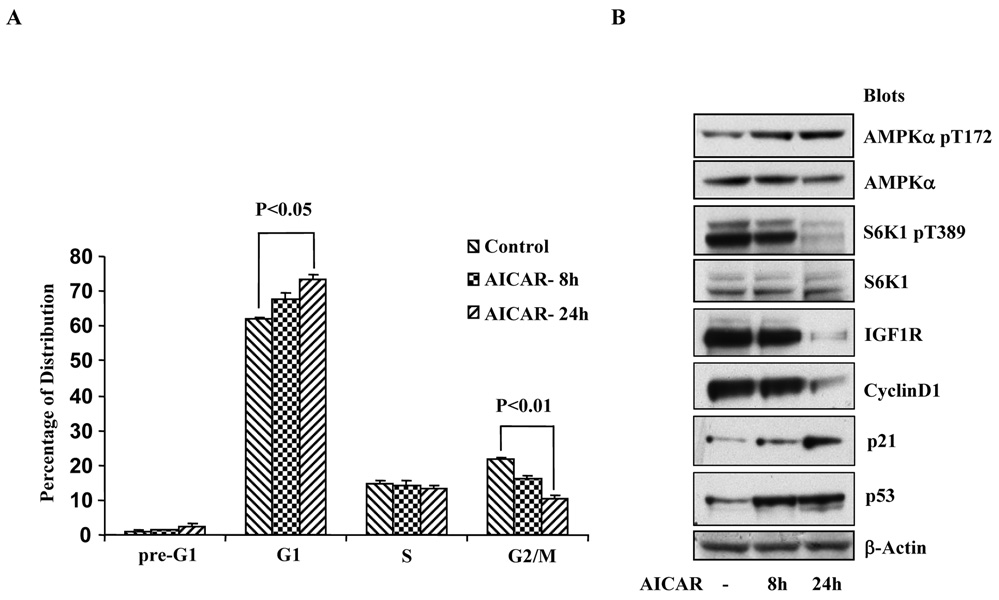
We then focused our attention to the prostate cancer C4-2 cells and first analyzed cell population at different stages of the cell cycle by FACS. As shown in Fig 1A, as compared with the cells containing the empty control viral vector, the G1 population of the cells expressing the α1 mutant (DNα1) was decreased (46% vs 63%, P<0.05), while G2/M population correspondingly increased (33% vs 20%). To unravel the molecular mechanisms underlying this difference, we carried out immunobloting and found that in the DNα1-containing cells, the abundance of p53 and p21 was reduced, whereas total S6K as well as its phosphorylated form, and expression of the IGF1 receptor increased (Fig 1B). In parallel, C4-2 cells were treated with AICAR and analyzed by FACS and Western blot. As shown in Fig 2A, treatment of the cells with AICAR from 8 to 24 hours progressively increased G1 population (P<0.05, 24h vs control), concomitant with a decrease in G2/M phase (P<0.01, 24h vs control). This was associated with upregulation of the tumor suppressors p53 and p21, and downregulation of oncogenic proteins including phosphorylation of S6K and expression of cyclin D1 and IGF1R (Fig 2B). Altogether, these results suggest that AMPK controls cell proliferation by modulating the activity and expression of a specific set of tumor suppressors and oncogenic molecules.
Figure 1. The effect of dominant negative mutant of AMPK α1 subunit on cell cycle profiles.
A. Stable C4-2 cells expressing the α1 subunit (DNα1) or empty vector (E) were established using lentiviral system and the cells at 70% confluence were subjected to FACS analysis, as described in Materials and Methods. The graph represents mean±STDV of a triplicate experiment (two-tailed t tests). B. In parallel, cell extracts were blotted with antibodies, as indicated.
Figure 2. The effect of AICAR on cell cycle profiles.
The same number of C4-2 cells (105 cells) was plated one day before treatment with or without AICAR for 8 and 24 hours and subjected to FACS analysis (A), and Western blot with antibodies, as indicated (B). The graph represents mean±STDV of a triplicate experiment (two-tailed t tests).
Regulation of Gene Expression by AMPK
Different behaviors of the C4-2 prostate cancer cells with or without the dominant negative mutant of AMPK α1 subunit prompted us to ask what downstream targets were regulated by AMPK. Thus, we performed a profiling analysis using Affymetrix whole-genome oligonucleotide microarrays. In doing so, total RNA was isolated from the C4-2 cells containing DNα1 or empty viral vector and reversely transcribed into cDNA, which was used as a template for in vitrotranscription. The obtained Biotin-labeled cRNA was purified, fragmented and hybridized to the microarray chip. The hybridized samples were stained with streptavidin-R-phycoerythrin and the signal was amplified using a biotinylated goat anti-streptavidin antibody. We derived transcript-level expression estimates from probe-level hybridization intensities for each sample using the MAS 5 algorithm as implemented in the GCOS software package (Affymetrix Inc.). Cyber-T analysis was performed in R 2.6.1 (for reference, see http://www.R-project.org) using the parameters recommended by Baldi and Long (Baldi and Long, 2001). For the initial Cyber-T analysis, all 54,675 probesets on the array were analyzed after first log transforming the expression estimates. All of the probesets were included in order to obtain robust estimates of the local variability in gene expression. We then restricted our subsequent search for differentially expressed transcripts to the 30,142 probesets that had sequence-specific signal in at least one sample (MAS 5 detection p-value below the Affymetrix recommended default of 0.04 in at least one sample). For these probesets, the Cyber-T p-value was adjusted to account for multiple testing using the False Discovery Rate (FDR) method of Klipper-Aurbach et al (Klipper-Aurbach et al., 1995). The 141 probesets with an FDR-adjusted p-value < 0.05 for the difference in expression between the two groups were considered to be differentially expressed. Upregulated or downregulated transcripts encoding protein products involved in tumorigenesis, proliferation and apoptosis were listed in Table 3 and 4.
Table 3.
Gene Expression Upregulated by Inhibition of AMPK
| Gene Title | Gene Symbol |
fdr | DN v E fold* |
Accession No. |
|---|---|---|---|---|
| AMP-activated protein kinase, alpha 1 catalytic subunit | PRKAA1 | 6.30E-06 | 3.35 | AF100763 |
| melanoma antigen family C 2 | MAGEC2 | 3.16E-03 | 3.07 | NM_016249 |
| insulin-like growth factor 1 | IGF1 | 7.85E-03 | 2.12 | AI972496 |
| inhibitor of DNA binding 2 | ID2 | 1.48E-02 | 1.81 | D13891 |
| insulin-like growth factor 1 receptor | IGF1R | 1.80E-02 | 1.72 | H05812 |
| ELL associated factor 2 | EAF2 | 1.37E-02 | 1.55 | NM_018456 |
| Integrin, beta 5 | ITGB5 | 4.78E-02 | 1.54 | BE138575 |
| clusterin | CLU | 1.13E-02 | 1.50 | M25915 |
| CDC42 effector protein (Rho GTPase binding) 3 | CDC42EP3 | 4.55E-02 | 1.43 | AL136842 |
| microtubule-associated protein 2 | MAP2 | 4.85E-02 | 1.41 | BF342661 |
| EPH receptor A3 | EPHA3 | 5.01E-03 | 1.40 | AF213459 |
| growth hormone receptor | GHR | 1.07E-02 | 1.34 | NM_000163 |
| FGFR1 oncogene partner 2 | FGFR1OP2 | 4.26E-02 | 1.25 | R91766 |
| transmembrane, prostate androgen induced RNA | TMEPAI | 4.90E-02 | 1.22 | NM_020182 |
| ras homolog gene family, member U | RHOU | 1.77E-02 | 1.16 | AL096776 |
| histone 2, H2be | HIST2H2BE | 2.85E-02 | 1.12 | NM_003528 |
| fibronectin 1 | FN1 | 2.63E-02 | 1.08 | AK026737 |
| centromere protein F, 350/400ka (mitosin) | CENPF | 4.59E-02 | 0.94 | NM_005196 |
| protein kinase, cAMP-dependent, catalytic, beta | PRKACB | 4.82E-02 | 0.92 | AA130247 |
| FK506 binding protein 5 | FKBP5 | 4.99E-02 | 0.85 | W86302 |
log2 fold-change
Table 4.
Gene Expression Downregulated by Inhibition of AMPK
| Gene Title | Gene Symbol |
fdr | DN v E fold* |
Accession No. |
|---|---|---|---|---|
| oligophrenin 1 | OPHN1 | 4.77E-02 | −0.76 | NM_002547 |
| glutathione S-transferase A1 | GSTA1 | 2.57E-02 | −1.14 | NM_000846 |
| TP53TG3 protein | TP53TG3 | 4.71E-02 | −1.16 | NM_015369 |
| lipopolysaccharide-induced TNF factor | LITAF | 2.51E-02 | −1.18 | NM_004862 |
| B-cell translocation gene 1, anti-proliferative | BTG1 | 1.22E-03 | −1.21 | AL535380 |
| Rho family GTPase 3 | RND3 | 4.75E-03 | −1.29 | BG054844 |
| protein tyrosine phosphatase, receptor type, R | PTPRR | 4.80E-02 | −1.51 | U77917 |
| gap junction protein, beta 2, 26kDa (connexin 26) | GJB2 | 4.89E-02 | −1.54 | M86849 |
| baculoviral IAP repeat-containing 3 | BIRC3 | 3.22E-02 | −1.56 | U37546 |
| regulator of G-protein signalling 2, 24kDa | RGS2 | 4.83E-03 | −1.59 | NM_002923 |
| matrix metallopeptidase 16 (membrane-inserted) | MMP16 | 3.66E-02 | −1.66 | NM_022564 |
| egl nine homolog 3 (C. elegans) | EGLN3 | 2.73E-02 | −1.75 | AI378406 |
| matrilin 2 | MATN2 | 2.44E-02 | −1.89 | NM_002380 |
| LIM domain only 4 | LMO4 | 3.35E-03 | −1.92 | BC003600 |
| proteasome subunit, beta type 8 | PSMB8 | 1.83E-02 | −1.98 | U17496 |
| protein tyrosine phosphatase, receptor type R | PTPRR | 1.91E-02 | −2.00 | NM_002849 |
| CD24 molecule | CD24 | 1.67E-02 | −2.05 | BG327863 |
| phospholipase A2 | PLA2G2A | 4.75E-03 | −2.05 | NM_000300 |
| tumor necrosis factor (ligand) superfamily, member 15 | TNFSF15 | 2.92E-02 | −2.08 | NM_005118 |
| chemokine orphan receptor 1 | CMKOR1 | 2.88E-03 | −2.20 | AI817041 |
| epidermal growth factor | EGF | 4.29E-03 | −2.22 | NM_001963 |
| cadherin 3, type 1, P-cadherin (placental) | CDH3 | 4.98E-03 | −2.25 | NM_001793 |
| melanoma antigen family A 4 | MAGEA4 | 2.16E-03 | −2.30 | AW438674 |
log2 fold-change
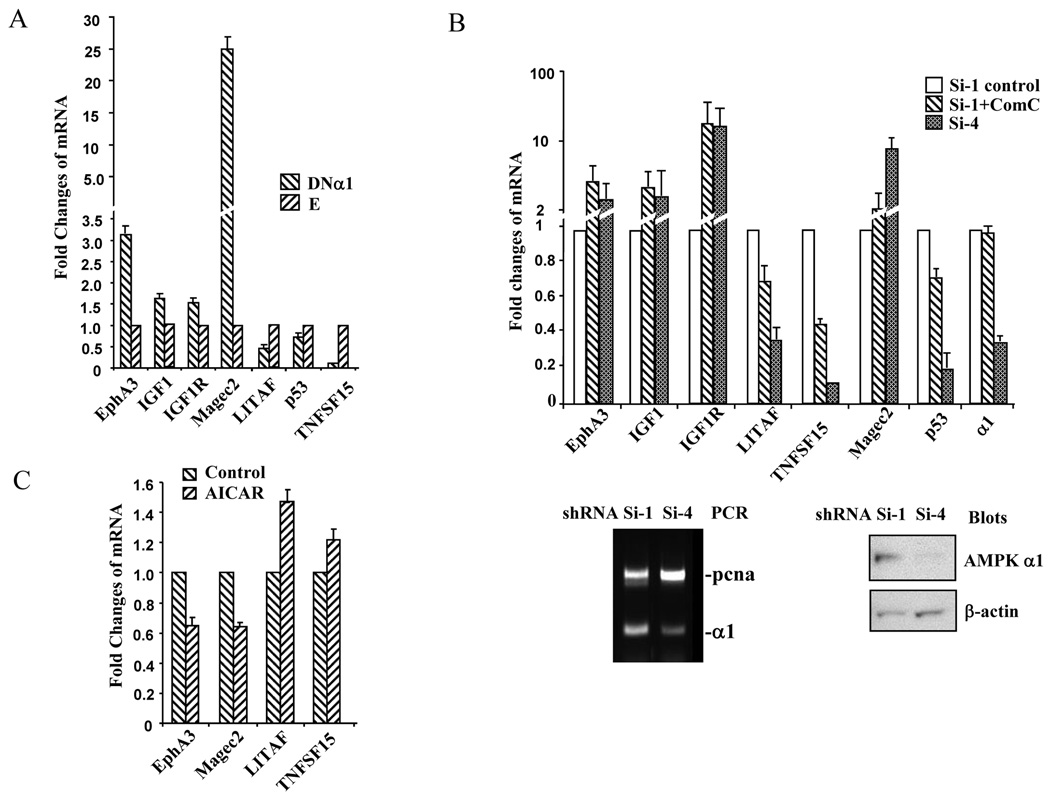
To validate the results of microarray analysis, we selected several genes that mostly interested us because of their potential roles in regulating the growth/survival of prostate cancer cells and carried out quantitative real time PCR. In doing so, we used different batches of C4-2 cells and prepared total RNA for RT-PCR. GAPDH was used as an internal control to normalize the signal for the transcripts of interests. The results of quantitative RT-PCR analysis were consistent with the microarray data, although fold changes were different to some extent attributable to different methods (Fig 3A). We found that cell proliferation-stimulating factors such as IGF1, IGF1R, and EphA3 were upregulated in the cells expressing the dominant negative AMPK α1 mutant, whereas the factors that might inhibit cell proliferation including p53, LITAF, and TNFSF15 were downregulated. To confirm the result obtained by expressing the DNα1 mutant, we made stable cell lines by infecting lentivirus containing shRNA for AMPK α 1 subunit, as opposed to scrambled shRNA control. Out of 4 α1 shRNA viruses, one displayed efficient silencing of the α 1 subunit (Fig 3B). The RT-PCR analysis revealed a similar trend of changes in gene expression when α 1 was silenced or when the cells containing scrambled shRNA were treated with compound C, a pharmacological AMPK inhibitor (Fig 3B). Finally, we assessed if activation of AMPK achieved the effects opposite to those observed in the DNα1 and α1 shRNA containing cells. Thus, the parental C4-2 cells were treated with AICAR for 24 hours and changes in expression of genes examined. Indeed, the changes caused by AICAR treatment were opposite to those in shRNA transfectants (Fig 3C). Of note, our microarray and/or real time PCR data also revealed that the expression of some potential oncogenes (e.g. EGF) was decreased in the DNα1 cells, whereas a few tumor suppressors upregulated (e.g. EAF2). The biological significance of such changes awaits further investigation.
Figure 3. Quantitative PCR analysis of gene expression.
Total RNA was isolated from C4-2 cells and reverse transcription performed, followed by quantitative PCR using primers listed in Table 1. Each sample was amplified in triplicate and normalized to GAPDH expression. Results were evaluated by the comparative threshold cycle value method for relative quantification of gene expression. Bars represent mean±STDV. A. The effects of DNα1 on expression of the genes selected. In each gene amplification, the value for DNα1 was expressed as fold of the empty control value (E). B. The effects of suppressing α1 subunit on gene expression by infecting lentivirus for α1 shRNA (Si-4), as compared with scrambled shRNA (Si-1) or treating the cells containing scrambled RNA with compound C (Si-1+ComC). The lower panel shows the silencing effect of shRNA on α1 expression by RT-PCR where PCNA was used as a control and by Western blot, where β-actin was a control. In each gene amplification, the value for Si-4 or Si-1+ComC was expressed as fold of the Si-1 value. C. Quantitative PCR analysis of gene expression was performed after treatment of C4-2 parental cells with or without AICAR. In each gene amplification, the value for AICAR treatment was expressed as fold of the value from untreated cells.
AMPK inhibits malignant properties of prostate cancer cells
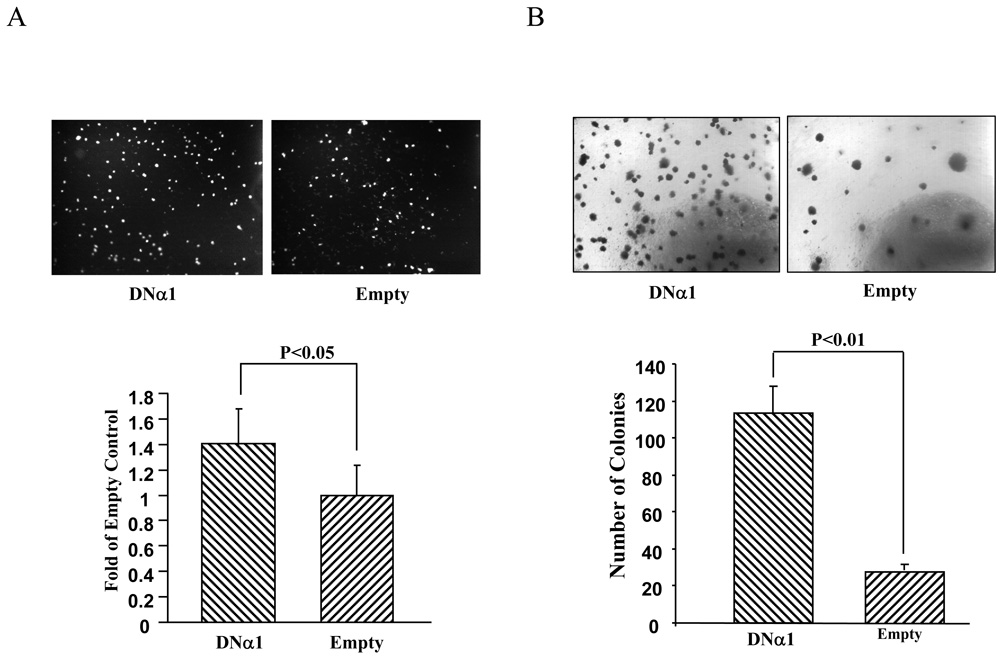
Next, we assessed if alteration of gene expression by AMPK is in line with changes in transformed phenotypes of C4-2 cells. First, cell migration was assayed using transwell chambers. Thus, the same number of the cells containing DNα1 or empty vector as a control was loaded on filter membranes and the cells were allowed to migrate through the membranes, stained with DAPI, photographed under fluorescent microscope and counted. Fig 4A shows that the expression of DNα1 significantly promoted the migration of C4-2 cells. Second, anchorage-independent growth of C4-2 cells was examined on soft agar. In this experiment, the same number of C4-2 cells was plated on soft agar and after two weeks, colonies were stained with MTT and counted. As shown in Fig 4B, the number of colonies of DNα1-C4-2 cells was remarkably greater than that of empty vector (approximately 4 times). Thus, our data demonstrate that the suppression of AMPK accelerates cell proliferation and augmentes malignancy of cancer cells.
Figure 4. Inactivation of AMPK enhances transformed behavior of C4-2 cells.
A. Cell migration assay. The DNα1- and empty-virus containing C4-2 cells were subjected to migration assay using Transwell chambers, as described in Materials and Methods. The migrated cells were stained with DAPI, photographed (one of triplicates was presented in upper panel) and counted. The results represent mean±STDV of three independent assays on the cells migrated through the membrane filter and are expressed as fold of the empty control cells (designated as 1). B. Assay of anchorage-independent growth on soft agar. 20,000 dividing cells containing DNα1 or empty vector were mixed in 0.6% agar-10% FBS-RPMI1640 and plated onto solidified 1% bed agar in the same culture medium in 6 well plates. After 14 days of culture, the colonies were stained with 0.5 ml MTT, photographed (the upper represents one of three individual wells) and counted (lower, one vision per well). The bar represents mean±STDV (n=3).
Discussion
Previously, we have shown that the AMPK activator AICAR inhibits the growth prostate cancer cells, which is associated with the inhibition of mTOR, FASN and, enzymes essential for protein and fatty acid synthesis and cell proliferation and/or of prostate cancer cells (Xiang et al., 2004). In those studies, we also observed same inhibitory effect of AICAR on the growth of the DU145 prostate cancer cells, LKB1 is inactivated by mutation such that AMPK is not able to be activated by (data not shown). Thus, the growth-inhibition of this cell line induced by may occur through a different mechanism, such as inhibition of DNA synthesis, IMP derived from AICAR/ZMP can disrupt the ratio of purine and pyramidine by increased synthesis of adenine and guanine-nucleotide, thus inhibiting synthesis independent of AMPK (Gong et al., 1993). To ascertain if AMPK per seexerts an inhibitory effect on prostate cancer cells, we established stable cell lines by the dominant negative AMPK α1 subunit or shRNA for α1. In keeping with previous data obtained with AICAR (Xiang et al., 2004), we found that the of AMPK in C4-2 cells accelerated cell proliferation and promoted phenotypes. These changes were correlated with increased activity of /S6K, and downregulation of p53 and p21. Previously studies from other labs shown that AMPK can phosphorylate S15 on p53 and thus regulates its function Jones et al., 2005). In our study, we did not observe changes in S15 phosphorylation (data not shown), but instead, we found that AMPK increased the abundance of p53, is consistent with a previous report (Rattan et al., 2005). Further, our data suggest this occurs at transcriptional level. Finally, our present study demonstrates novel that AMPK regulates a number of important modulators involved in cell, survival and tumorigenesis.
Increased plasma IGF-1 has been known to be a risk factor for many cancers including prostate cancer and thus the IGF-I pathway has been regarded as a potential target for cancer therapy (Sachdev and Yee, 2007). Previous studies have shown that a combination of low fat diet and exercise (DE) decreases plasma IGF-I and increases IGF-I binding protein 1 (IGFBP-1), an inhibitor of IGF-I (Ngo et al., 2003). Serum from men receiving DE intervention reduces the growth of LNCaP cells and induces their apoptosis in vitro, an event that can be reversed by adding IGF-I to the post-DE serum. In addition, the effect of post DE serum can be recapitulated by adding IGFBP-1 to the pre-DE serum. These studies clearly indicate that IGF-I plays an important role in control of LNCaP cell growth and survival. Although complicated mechanisms might be involved, the DE intervention can induce activation of AMPK in multiple tissues (Luo et al., 2005), which may contribute to the decrease of plasma levels of IGF-I. At posttranslational levels, it has been shown that AMPK specifically inhibits the IGF-I signaling pathway (He et al., 2006). Here for the first time, we show that that IGF-I and IGF-I receptor are downregulated by AMPK in a prostate cancer cell line.
Another interesting AMPK target is EphA3, which is among 14 members of the ephrin receptors making up the largest family of receptor tyrosine kinases in humans (Manning et al., 2002). These receptors are associated with 8 members of the ephrin ligands. The Eph/ephrin molecules are involved in various developmental processes. The EphA receptors are often upregulated in cancer cells (Pasquale, 2008) and could promote the growth of transformed cells in the absence of the ligands or inhibit their growth by binding to their corresponding ligands. A recent study of genome-wide mutations in human lung adenocarcinomas has demonstrated that the EphA3 gene is mutated at a high frequency, suggesting that the mutated EphA3 acts as an oncogene (Ding et al., 2008). EphA3 has also been found in prostate cancer cells, whose expression levels are associated with the degree of malignancy (Singh et al., 2008). Our study has shown that the inactivation of AMPK by expression of the dominant negative mutant or shRNA of AMPK α1 subunit or incubation of the cells with compound C leads to an upregulation of EphA3, in accord with accelerated cell growth, whereas the AMPK activator AICAR exerts an opposite effect. Thus, our data suggest a link between AMPK and oncogenic EphA3. In addition, we identified several other potential AMPK targets such as LITAF, TNFSF15, MAGEC2, and MAGEA4. Of note, our microarray data revealed that some growth-promoting factors (e.g. EGF and CD24) are downregulated and potential tumor suppressors upregulated (e.g. EAF2) when AMPK is inactivated. The significance of these findings is not clear at present. We will need to consider the global and integrated effects of AMPK on gene expression and growth suppression in the follow-up studies.
Jimenez et al (Jimenez et al., 2003) have performed genome-wide profiling in A549 cells after transfection of LKB1. By comparison, we could not find significant similarity between our results and theirs, except that TNFSF4 and TNFSF10 are increased by transfection of LKB1 in their study. It is not surprising that we have different findings, because of the following reasons: (1) different genetic background of these two cell lines, (2) multiple targets of LKB1 in addition to AMPK and (3), different approaches used in these two studies; while they employed transient expression, we made stable expression in the present study.
In the end, we should point out that the rationale behind this study is based on several observations. First, AMPK is often suppressed in obesity and metabolic syndrome (Luo et al., 2005). Second, epidemiological studies have indicated a positive association between the metabolic syndrome and prostate cancer (Lund Haheim et al., 2006). Third, in advanced stages of prostate cancer, the PTEN function is often found lost, leading to a constitutive activation of Akt, which in turn could cause an inhibition of AMPK (Horman et al., 2006; Majumder and Sellers, 2005). Altogether, these findings strongly suggest that AMPK plays a role in regulating the growth of malignant cells. In keeping with this notion, our present study shows that the inactivation of AMPK augments malignant behaviors of prostate cancer cells and its activation suppresses their growth. In addition, we demonstrate that many genes involved in cell growth and tumorigenesis are regulated by AMPK. Therefore, our findings offer us a new direction in elucidating the mechanism of AMPK action. And also, they provide us a good rationale to take AMPK as a candidate for cancer therapy.
Acknowledgements
Grants: This project is supported by NIH R01 grants, CA118918 and GM 057959 (to Z. Luo).
We thank Dr. David Carling for providing us the cDNA construct for the dominant negative mutant of AMPKα1 subunit.
References
- Baldi P, Long AD. A Bayesian framework for the analysis of microarray expression data: regularized t -test and statistical inferences of gene changes. Bioinformatics. 2001;17:509–519. doi: 10.1093/bioinformatics/17.6.509. [DOI] [PubMed] [Google Scholar]
- Barb D, Williams CJ, Neuwirth AK, Mantzoros CS. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr. 2007;86:s858–s866. doi: 10.1093/ajcn/86.3.858S. [DOI] [PubMed] [Google Scholar]
- Baron A, Migita T, Tang D, Loda M. Fatty acid synthase: a metabolic oncogene in prostate cancer? J Cell Biochem. 2004;91:47–53. doi: 10.1002/jcb.10708. [DOI] [PubMed] [Google Scholar]
- Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–6752. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- Carling D. LKB1: a sweet side to Peutz-Jeghers syndrome? Trends Mol Med. 2006;12:144–147. doi: 10.1016/j.molmed.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Chang CK, Ulrich CM. Hyperinsulinaemia and hyperglycaemia: possible risk factors of colorectal cancer among diabetic patients. Diabetologia. 2003;46:595–607. doi: 10.1007/s00125-003-1109-5. [DOI] [PubMed] [Google Scholar]
- De Schrijver E, Brusselmans K, Heyns W, Verhoeven G, Swinnen JV. RNA interference-mediated silencing of the fatty acid synthase gene attenuates growth and induces morphological changes and apoptosis of LNCaP prostate cancer cells. Cancer Res. 2003;63:3799–3804. [PubMed] [Google Scholar]
- Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67:10804–10812. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong YF, Srinivas RV, Fridland A. 5-Amino-4-idazolecarboxamide riboside potentiates the metabolism and anti-human immunodeficiency virus activity of 2',3'-dideoxyinosine. Mol Pharmacol. 1993;44:30–36. [PubMed] [Google Scholar]
- Grossmann ME, Nkhata KJ, Mizuno NK, Ray A, Cleary MP. Effects of adiponectin on breast cancer cell growth and signaling. Br J Cancer. 2008;98:370–379. doi: 10.1038/sj.bjc.6604166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurumurthy S, Hezel AF, Sahin E, Berger JH, Bosenberg MW, Bardeesy N. LKB1 deficiency sensitizes mice to carcinogen-induced tumorigenesis. Cancer Res. 2008;68:55–63. doi: 10.1158/0008-5472.CAN-07-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- He G, Sung YM, Digiovanni J, Fischer SM. Thiazolidinediones inhibit insulin-like growth factor-i-induced activation of p70S6 kinase and suppress insulin-like growth factor-I tumor-promoting activity. Cancer Res. 2006;66:1873–1878. doi: 10.1158/0008-5472.CAN-05-3111. [DOI] [PubMed] [Google Scholar]
- Horman S, Vertommen D, Heath R, Neumann D, Mouton V, Woods A, et al. Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase alpha-subunits in heart via hierarchical phosphorylation of Ser485/491. J Biol Chem. 2006;281:5335–5340. doi: 10.1074/jbc.M506850200. [DOI] [PubMed] [Google Scholar]
- Huang X, Wullschleger S, Shpiro N, McGuire VA, Sakamoto K, Woods YL, et al. Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem J. 2008;412:211–221. doi: 10.1042/BJ20080557. [DOI] [PubMed] [Google Scholar]
- Ikediobi ON, Davies H, Bignell G, Edkins S, Stevens C, O'Meara S, et al. Mutation analysis of 24 known cancer genes in the NCI-60 cell line set. Mol Cancer Ther. 2006;5:2606–2612. doi: 10.1158/1535-7163.MCT-06-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- Isakovic A, Harhaji L, Stevanovic D, Markovic Z, Sumarac-Dumanovic M, Starcevic V, et al. Dual antiglioma action of metformin: cell cycle arrest and mitochondria-dependent apoptosis. Cell Mol Life Sci. 2007;64:1290–1302. doi: 10.1007/s00018-007-7080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- Jimenez AI, Fernandez P, Dominguez O, Dopazo A, Sanchez-Cespedes M. Growth and molecular profile of lung cancer cells expressing ectopic LKB1: down-regulation of the phosphatidylinositol 3'-phosphate kinase/PTEN pathway. Cancer Res. 2003;63:1382–1388. [PubMed] [Google Scholar]
- Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Klipper-Aurbach Y, Wasserman M, Braunspiegel-Weintrob N, Borstein D, Peleg S, Assa S, et al. Mathematical formulae for the prediction of the residual beta cell function during the first two years of disease in children and adolescents with insulin-dependent diabetes mellitus. Med Hypotheses. 1995;45:486–490. doi: 10.1016/0306-9877(95)90228-7. [DOI] [PubMed] [Google Scholar]
- Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000;16:202–208. doi: 10.1016/s0899-9007(99)00266-x. [DOI] [PubMed] [Google Scholar]
- Lee IM. Physical activity and cancer prevention--data from epidemiologic studies. Med Sci Sports Exerc. 2003;35:1823–1827. doi: 10.1249/01.MSS.0000093620.27893.23. [DOI] [PubMed] [Google Scholar]
- Lund Haheim L, Wisloff TF, Holme I, Nafstad P. Metabolic syndrome predicts prostate cancer in a cohort of middle-aged Norwegian men followed for 27 years. Am J Epidemiol. 2006;164:769–774. doi: 10.1093/aje/kwj284. [DOI] [PubMed] [Google Scholar]
- Luo Z, Saha AK, Xiang X, Ruderman NB. AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci. 2005;26:69–76. doi: 10.1016/j.tips.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Majumder PK, Sellers WR. Akt-regulated pathways in prostate cancer. Oncogene. 2005;24:7465–7474. doi: 10.1038/sj.onc.1209096. [DOI] [PubMed] [Google Scholar]
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- Ngo TH, Barnard RJ, Leung PS, Cohen P, Aronson WJ. Insulin-like growth factor I (IGF-I) and IGF binding protein-1 modulate prostate cancer cell growth and apoptosis:possible mediators for the effects of diet and exercise on cancer cell survival. Endocrinology. 2003;144:2319–2324. doi: 10.1210/en.2003-221028. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Rattan R, Giri S, Singh AK, Singh I. 5-Aminoimidazole-4-carboxamide-1-beta-Dribofuranoside inhibits cancer cell proliferation in vitro and in vivo via AMP-activated protein kinase. J Biol Chem. 2005;280:39582–39593. doi: 10.1074/jbc.M507443200. [DOI] [PubMed] [Google Scholar]
- Sachdev D, Yee D. Disrupting insulin-like growth factor signaling as a potential cancer therapy. Mol Cancer Ther. 2007;6:1–12. doi: 10.1158/1535-7163.MCT-06-0080. [DOI] [PubMed] [Google Scholar]
- Singh AP, Bafna S, Chaudhary K, Venkatraman G, Smith L, Eudy JD, et al. Genome-wide expression profiling reveals transcriptomic variation and perturbed gene networks in androgen-dependent and androgen-independent prostate cancer cells. Cancer Lett. 2008;259:28–38. doi: 10.1016/j.canlet.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su GH, Hruban RH, Bansal RK, Bova GS, Tang DJ, Shekher MC, et al. Germline and somatic mutations of the STK11/LKB1 Peutz-Jeghers gene in pancreatic and biliary cancers. Am J Pathol. 1999;154:1835–1840. doi: 10.1016/S0002-9440(10)65440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thupari JN, Pinn ML, Kuhajda FP. Fatty acid synthase inhibition in human breast cancer cells leads to malonyl-CoA-induced inhibition of fatty acid oxidation and cytotoxicity. Biochem Biophys Res Commun. 2001;285:217–223. doi: 10.1006/bbrc.2001.5146. [DOI] [PubMed] [Google Scholar]
- Wu X, Wakefield JK, Liu H, Xiao H, Kralovics R, Prchal JT, et al. Development of a novel trans-lentiviral vector that affords predictable safety. Mol Ther. 2000;2:47–55. doi: 10.1006/mthe.2000.0095. [DOI] [PubMed] [Google Scholar]
- Xiang X, Saha AK, Wen R, Ruderman NB, Luo Z. AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms. Biochem Biophys Res Commun. 2004;321:161–167. doi: 10.1016/j.bbrc.2004.06.133. [DOI] [PubMed] [Google Scholar]