Abstract
Borrelia burgdorferi sensu lato is the causative agent of Lyme disease (LD), an infectious disease occurring in North America, Europe, and Asia in different clinical stages. B. burgdorferi sensu lato encompasses at least 12 species, with B. burgdorferi sensu stricto, B. garinii, and B. afzelii being of highest clinical importance. Immunologic testing for LD as well as recent vaccination strategies exclusively refer to proteinaceous antigens. However, B. burgdorferi sensu stricto exhibits glycolipid antigens, including 6-O-acylated cholesteryl β-d-galactopyranoside (ACGal), and first the data indicated that this compound may act as an immunogen. Here we investigated whether B. garinii and B. afzelii also possess this antigen, and whether antibodies directed against these compounds are abundant among patients suffering from different stages of LD. Gas-liquid chromatography/mass spectroscopy and NMR spectroscopy showed that both B. garinii and B. afzelii exhibit ACGal in high quantities. In contrast, B. hermsii causing relapsing fever features 6-O-acylated cholesteryl β-d-glucopyranoside (ACGlc). Sera derived from patients diagnosed for LD contained antibodies against ACGal, with 80% of patients suffering from late stage disease exhibiting this feature. Antibodies reacted with ACGal from all three B. burgdorferi species tested, but not with ACGlc from B. hermsii. These data show that ACGal is present in all clinically important B. burgdorferi species, and that specific antibodies against this compound are frequently found during LD. ACGal may thus be an interesting tool for improving diagnostics as well as for novel vaccination strategies.
Lyme disease (LD)2 is caused by B. burgdorferi sensu lato (s.l.) and is transmitted by ticks of the genus Ixodes (1, 2). It is the most common tick-borne disease in the U.S. with an incidence of 6 per 100,000, with endemic areas such as Connecticut reaching 111 cases per 100,000 (3). LD is also frequent in Asia and Europe, particularly in Germany, Austria, Slovenia, and Sweden (2, 4). B. burgdorferi s.l. comprises at least 12 species with B. burgdorferi sensu stricto (Bbu), B. garinii (Bga), and B. afzelii (Baf) being of highest clinical importance (2). In the U.S., LD is exclusively caused by Bbu, whereas in Europe all human pathogenic species are found, with Bga and Baf being predominant (2, 5, 6). LD is an infectious disease occurring in different clinical stages: early localized infection is indicated by erythema migrans (EM) in ∼70–90% of the patients (7–9), and early disseminated infection often causes neurological manifestations, such as facial palsy, meningitis, meningoradiculitits, or meningoencephalitis (early neuroborreliosis (NB)) (2,8,9). The cardinal manifestation of late stage LD in the U.S. is Lyme arthritis (LA), with ∼70% of the untreated EM cases developing this syndrome (10, 11). In Europe, next to arthritis, acrodermatitis chronica atrophicans (ACA) is a frequent late manifestation, and has been associated with Baf (11).
Currently, diagnosis of LD is generally based on assessment of clinical features in combination with immunologic serum testing, where both ELISA and a confirming immunoblot are required (12, 13). However, because in Europe and Asia at least three species are causing LD, there is a substantial variation of immunodominant antigens, which requires the combination of various homologous antigens for effective serodiagnosis (14–16). Immunologic evaluation in these areas is therefore complicated, and no consensus has been established yet (12). In comparison to diagnostic procedures, vaccination strategies directed against LD so far have also been based on proteinaceous antigens: in the 1990s, recombinant vaccines based on OspA were found to be effective (17), but the production was discontinued, one reason being the high production costs in comparison to early treatment (2). Another concern raised against this approach was a potential triggering of autoimmune diseases by vaccination with Osps due to a similarity between an immunodominant epitope in OspA and human leukocyte function-associated antigen-1 (18).
In contrast to proteins, information on membrane glycolipids in Borrelia available today is rather scarce. In 1978, a preliminary compositional analysis of lipid extracts of B. hermsii causing relapsing fever (RF) indicated the presence of monoglucosyldiacylglycerol and acylated as well as non-acylated cholesteryl glucosides (19). Later, studies on Bbu indicated the presence of complex glycolipids as well, but no chemical analysis was performed (20, 21). A more recent study identified mono-α-d-galactosyldiacylglycerol (MGalD) in Bbu, and first data indicated that antibodies present in sera obtained from LD patients detected this antigen (22). We and others were recently able to show that Bbu furthermore exhibits cholesteryl 6-O-acyl-β-d-galactopyranoside (ACGal) as well as its non-acylated counterpart, cholesteryl β-d-galactopyranoside (βCGal) (23, 24). Patient sera reacted with ACGal more frequently as compared with MGalD (23), and antibodies could be raised in mice by intraperitoneal injection (24), indicating that this compound is a strong immunogen.
The aim of this study was to elucidate whether ACGal is a common structure present in the most relevant B. burgdorferi species of clinical importance, and whether it is a specific feature of Borrelia causing LD. Furthermore, we aimed at defining the frequency of the occurrence of antibodies against this antigen in patients suffering from LD. To this end, we performed a comparative structural analysis of glycolipid fractions of Bbu as well as the two other B. burgdorferi s.l. species of clinical importance, Baf and Bga, in comparison with B. hermsii (Bhe), the causative agent of relapsing fever. We found ACGal to be present in all B. burgdorferi species tested, whereas Bhe exhibited cholesteryl 6-O-acyl-β-d-glucopyranoside (ACGlc) instead. Antibodies against ACGal could be detected in the majority of patients diagnosed for arthritis or acrodermatitis, and these failed to cross-react with ACGlc. These data demonstrate that ACGal is an abundant, but still highly specific antigen in B. burgdorferi and thus a promising candidate for vaccine development and improvement of serologic methods.
EXPERIMENTAL PROCEDURES
Borrelia Strains—B. burgdorferi sensu stricto (B31, tick isolate, ATCC 35210) was kindly provided by B. Hammer, Institute for Microbiology and Hygiene, Charité, Berlin, Germany; B. garinii (A, tick isolate) was a gift by R. Ackermann, University Hospital of Cologne, Cologne, Germany. B. afzelii (PKo, human skin isolate) was provided by B.W., Munich, Germany. B. hermsii (HS1, tick isolate) was purchased from ATCC (Manassas, VA).
Cultivation of Borrelia—Glycerol stocks of Borrelia were stored at –80 °C. 100 μl was transferred to 5 ml of BSK-H complete medium containing 6% rabbit serum (Sigma-Aldrich, Taufkirchen, Germany). After 3–4 days of culture at 34 °C and microaerophilic conditions, viability of bacteria was ensured by darkfield microscopy, and cultures were transferred to 50 ml of medium. After culturing for another 4 days, bacteria were transferred to the final volume of 500 ml. Borrelia were harvested by centrifugation at 12,000 × g at 4 °C for 20 min followed by two washing steps with endotoxin-free water (Braun, Melsungen, Germany) under similar conditions. Bacteria were then subjected to analytical and preparative procedures. Sonicates were prepared by suspending dried Borrelia (5 mg) in 5 ml of 0.05 m sodium acetate followed by sonication four times for 2 min. The sonicate then was centrifuged for 3 min at 3,000 × g at 4 °C, and the supernatants were harvested and spun for 30 min at 12,000 × g at 4 °C. The resulting pellet was washed twice with phosphate-buffered saline (PBS, Invitrogen) and stored at –20 °C.
Cell Disintegration and Total Lipid Extraction—Lyophilized Borrelia cells were suspended in 15 ml of pyrogen-free water and subsequently disrupted by a sonifier (250 watts, Branson, Danbury, CT, using a 5-mm micro tip, output control 6, duty cycle 50%) 3 times for 5 min in ice-cooled water. 15 ml of n-butanol (LiChrosolv, Merck, Darmstadt, Germany) were added to the suspension, and the tube was shaken for 15 min. For phase separation it was centrifuged at 6200 × g for 1 h at 4 °C, and the butanol phase was taken off. The remaining phases (water, interphase, and pellet) were extracted again with 15 ml of n-butanol. Both butanol phases were united and re-extracted with 15 ml of pyrogen-free water. The total lipids were yielded after vacuum evaporation of the butanol phase as an oily solid.
Analytical TLC and Preparative TLC—To analyze the lipids, three solvent systems were used: CHCl3/MeOH (85:15, v/v) for glycolipids, CHCl3/MeOH/H2O (65:25:4, v/v) for phospholipids, and toluene/EtOH (85:15, v/v) for cholesteryl β-d-galactopyranoside (βCGal)/cholesteryl β-d-glucopyranoside (βCGlc) separation. The latter was applied 5 times (run and dried) for a clear distinction. The runs were performed on silica gel-coated aluminum plates (Kieselgel 60 F254, 0.2 mm, Merck) and stained with molybdenum stain solution (1 m H2SO4, 40 mm ammoniumheptamolybdat [(NH4)6Mo7O24·4H2O], 3 mm Cer(IV)-sulfate [Ce(SO4)2·4H2O]). For staining the plates were immersed into the molybdenum stain solution and heated to ∼250 °C using a heat gun. To purify βCGlc we employed an additional preparative step: The sample was loaded onto a 20-cm-length aluminum TLC plate (Kieselgel 60, 0.2 mm, Merck) and run in a saturated 20-cm-height chamber with CHCl3/MeOH (85:15, v/v). According to reference sample Rf values, the βCGlc was scraped off, eluted with MeOH (Chromasolv for high-performance liquid chromatography, Riedel de Haen, Seelze, Germany) on a suction filter (35 mm, porosity 4), and membrane-filtered (as above). Standard substances used as TLC references were purchased from Sigma-Aldrich for cholesterol, cholesteryl oleate, phosphatidyl choline, phosphatidyl glycerol, or from Research Plus (Manasquan, NJ) for cholesteryl β-d-glucopyranoside (βCGlc). Cholesteryl α-d-galactopyranoside (αCGal) was kindly provided by Dirk Warnecke, Biozentrum Klein Flottbek, Hamburg, Germany.
Flash Column Chromatography—A glass column (diameter, 2.2 cm) was filled with 125-cm3 silica gel (Kieselgel 60, 40–63 μm, Roth, Karlsruhe, Germany) and equilibrated with CHCl3 under N2 overpressure. The column was loaded with the extracted total lipids and eluted under N2 overpressure sequentially each with 2 column volumes of CHCl3, CHCl3/MeOH (49:1, v/v), CHCl3/MeOH (48:2, v/v), CHCl3/MeOH (47:3, v/v), CHCl3/MeOH (46:4, v/v), and finally 12 column volumes of CHCl3/MeOH/H2O (39:10:1, v/v). Fractions (10 ml) were collected, and every second one was checked by molybdenum stain staining for lipid content. The fractions comprising the same lipid were filtered through a PVDF membrane filter (TE 35, 0.2 μm, Schleicher & Schuell, Dassel, Germany), and were combined.
GLC and Combined GLC-MS—Compositional analysis employing GLC-MS was performed with 100 μg of each sample after mild methanolysis with 1.5 ml of 0.5 m HCl/MeOH for 1 h at 85 °C in sealed ampoules. The liquid was blown off by N2, and the sample was subsequently peracetylated with 1 ml of pyridine/acetanhydride (2:1, v/v) for 1 h at 80 °C and concentrated. The chromatography was run on a Hewlett Packard, model 5890 Series II (column: HP-5MS 30 m, Agilent, Böblingen, Germany) with a temperature gradient from 150 °C (3 min) to 320 °C at 5 °C/min. The mass spectra were detected and recorded by electron impact and chemical ionization (HP 5989A, Agilent).
NMR Spectroscopy—The glycolipids and phospholipids were dissolved in 0.5 ml of chloroform-d/methanol-d4 (9:1, v/v) and DMSO-d6, respectively (Cambridge Isotope Laboratories, Andover, MA), and the NMR spectra were recorded in 5-mm high precision NMR tubes (Promochem, Wesel, Germany) at 300 K. Proton (1H) and all proton-detected two-dimensional NMR spectra were run on a Bruker DRX-600 Avance spectrometer at 600 MHz. Carbon (13C) and distortionless enhancement by polarization transfer (DEPT 135) spectra were measured on a Bruker DPX-360 spectrometer at 90.6 MHz. The chemical shift values were referenced to internal tetramethylsilane (δH = 0.00 ppm) or CDCl3 (δc = 77.0 ppm). 1H/1H correlated spectroscopy (COSY), 1H/1H total correlated spectroscopy (TOCSY), 1H/13C heteronuclear multiple quantum coherence (HMQC), and 1H/13C heteronuclear multiple bond connectivity (HMBC) experiments were performed using standard Bruker software (XWinNMR 3.5).
Patient Sera—Sera derived from patients diagnosed for LD as well as sera derived from controls were provided by B.W. and V.F., Munich, Germany. Serodiagnosis was based on ELISA and subsequent confirmative immunoblotting. In total, 68 samples from patients (20 for EM, 19 for early NB, 14 for ACA, and 15 for LA), and 20 samples from healthy controls were tested.
Immunoblotting of Glycolipids—MGalD derived from Bbu, ACGal from Bbu, Bga and Baf and ACGlc from Bhe (1 μg each), dissolved in tert-butanol/H2O (4:1), and Borrelia sonicate (30 μl, corresponding to 30 μg of dried bacteria) were pipetted directly on PVDF membranes (Immobilon P, Millipore, Bedford, MA) previously immersed in MeOH and PBS. Membranes were blocked with PBS/5% skim milk (Fluka, Buchs, Switzerland)/0.05% Tween 20 overnight at 4 °C. After washing with PBS/0.1% Tween 20, membranes were incubated with sera diluted 1,000-fold in PBS/5% skim milk/0.05% Tween 20 for 3 h at room temperature. After washing, a rabbit anti-human IgG-HRP conjugate (Santa Cruz Biotechnology, Santa Cruz, CA) diluted 10,000-fold in PBS/5% skim milk/0.05% Tween 20 was added and incubated for 1 h at room temperature. Blots were washed with PBS, and bands were detected with the ECL-system (Amersham Biosciences) as recommended by the manufacturer's protocol using Hyperfilm ECL-films (Amersham Biosciences). The same procedure was employed for detecting IgM using anti-human IgM-HRP conjugate (Santa Cruz Biotechnology). For TLC immunoblotting, Borrelia extracts were separated on TLC as described above. The run TLC plate was transferred to a membrane by application of heat employing a published protocol (25) with the following modifications: The dried TLC plate was slowly submersed into transfer buffer (25 mm Tris-HCl, pH 10.4, 20% MeOH) for 10 s. The plate was gently dried using a soft tissue leaving only a little wetness. The PVDF membrane (Millipore, Bedford, MA) with the same dimensions was put on top and was quickly straightened with a glass rod. Covered by a glass microfiber sheet (GF/A, Whatman, Brentford, UK), a 150 °C preheated electric iron was placed on the membrane and plate for 30 s. The membrane was peeled off and let dry. The development of the blots was similar as described above for immunoblotting of membranes.
ACGal Antibody Titer Determination—A 96-well plate (Nunc maxisorb, Nunc a/s, Roskilde, Denmark) was coated with 50 μl per well of a 30 μg/ml ACGal solution (in 0.1 m NaHCO3, pH 8.2) for 16 h at 4 °C. The plate was washed twice with distilled water, blocked with 200 μl per well blocking buffer (0.05 m Hepes, 0.15 m NaCl, 10 mg/ml bovine serum albumin, pH 7.4), and incubated for 1 h at room temperature. The plate was washed three times with 200 μl of washing buffer (0.05 m Hepes, 0.15 m NaCl, 1 mg/ml bovine serum albumin, pH 7.4) and incubated with 100 μl per well of sera diluted in washing buffer for 2 h. After washing (3×) the plate was incubated with 100 μl per well of a 1:1000 dilution of secondary antibody HRP conjugate (rabbit anti-human IgG-HRP, Santa Cruz Biotechnology) in washing buffer for an additional 2 h. Following a washing step (3×) the staining was started by addition of 100 μl per well tetramethylbenzidine solution (SeramunBlau slow (ELISA), Seramun Diagnostica, Heidese, Germany), incubated in the dark for a few minutes and stopped with 50 μl per well 2 m H2SO4. The optical density was measured at 450 nm in an ELISA reader (SPECTRA Fluor Plus, Tecan, Crailsheim, Germany). As a cut-off, the mean value of negative samples at the corresponding dilution plus the 2-fold standard deviation was used.
RESULTS
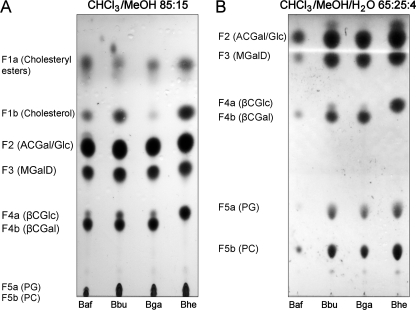
Comparative Analysis of Lipid Extracts from Different Borrelia burgdorferi Strains—Bbu, Bga, Baf, and Bhe were grown in BSK-H medium, harvested, and lyophilized as described under “Experimental Procedures.” Butanol extraction yielded 161 mg of total lipids for Bbu, 16.6 mg for Bga, 11.3 mg for Baf, as well as 25.0 mg for Bhe, respectively, corresponding to 26.7, 28.6, 24.7, and 28.8% of total dry weight (Table 1). The total lipids were subjected to TLC in CHCl3/MeOH 85:15 (v/v) to separate glycolipids and visualized with Mostain (Fig. 1A). In the lipid extract derived from Bbu, all previously described lipids (23) were detected except for two distinct patterns in the fraction formerly assigned as F4, F4a, and F4b (Fig. 1A). Furthermore, the phospholipid fraction F5 revealed two spots, which were subdivided into fractions F5a and F5b (Fig. 1, A and B).
TABLE 1.
Lipid proportions of total lipids and borrelial dry weight for the species B. burgdorferi s.s. (Bbu), B. afzelii (Baf), B. garinii (Bga), and B. hermsii (Bhe)
|
Fraction
|
Lipid
|
Proportion of total lipids
|
Proportion of cell dry weight
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bbu | Baf | Bga | Bhe | Bbu | Baf | Bga | Bhe | |||||||||
| % | ||||||||||||||||
| Total | 26.7 | 24.7 | 28.6 | 28.8 | ||||||||||||
| F1a | Cholesteryl esters | 2.1 | 2.3 | 3.4 | 2.5 | 0.6 | 0.6 | 1.0 | 0.7 | |||||||
| F1b | Cholesterol | 3.2 | 2.1 | 0 | 3.9 | 0.9 | 0.5 | 0 | 1.1 | |||||||
| F2 | ACGal/ACGlc | 22.5 | 22.8 | 18.5 | 23.4 | 6.0 | 5.6 | 5.3 | 6.7 | |||||||
| F3 | MGalD | 12.8 | 13.6 | 13.1 | 9.2 | 3.4 | 3.4 | 3.7 | 2.6 | |||||||
| F4a | βCGlc | 2.5 | 2.1 | 2.0 | 7.0 | 0.7 | 0.5 | 0.6 | 2.0 | |||||||
| F4b | βCGal | 9.9 | 11.6 | 11.1 | 0 | 2.6 | 2.9 | 3.2 | 0 | |||||||
| F5a&b | Phospholipids | 46.8 | 43.4 | 48.2 | 53.7 | 12.5 | 10.7 | 13.8 | 15.5 | |||||||
FIGURE 1.
TLC analysis of butanol extracted total lipids from the four Borrelia strains examined. 10 μl of the dissolved total lipids of B. afzelii (Baf), B. burgdorferi s.s. (Bbu), B. garinii (Bga), and B. hermsii (Bhe) were applied to silica gel thin-layer plates. The plates were run in CHCl3/MeOH 85:15 (v/v) (A) and CHCl3/MeOH/H2O 65:25:4 (v/v) (B), dried, stained with molybdenum stain, and heated. The visualized fractions were denoted from unpolar to polar as F1 to F5.
The lipid distribution in all three LD strains was homologous, with differences in proportions only (Table 1). Upon analytical TLC in CHCl3/MeOH/H2O (65:25:4, v/v), fraction F5 formerly identified as phosphatidylcholine (23) now revealed two distinct spots (Fig. 1B). Further analysis showed that lipid F5b co-migrates with the phosphatidylcholine standard, whereas F5a co-migrates with the phosphatidylglycerol standard. Both chemical structures were confirmed by NMR in DMSO-d6 and MALDI-TOF (data not shown) and are in line with previous reports (21, 26). Upon analytical TLC in different solvents (CHCl3/MeOH 85:15 (v/v), hexane/ethyl acetate 1:1 (v/v)) the fractions F1a and F1b, which are present only in minor portions of the total lipids (2–3%, Table 1), were compared with cholesterol and cholesteryl oleate standards and revealed that F1a co-migrated with cholesteryl oleate and F1b with cholesterol (not shown), which is part of the BSK-H medium (486 nm). Both were not further analyzed.
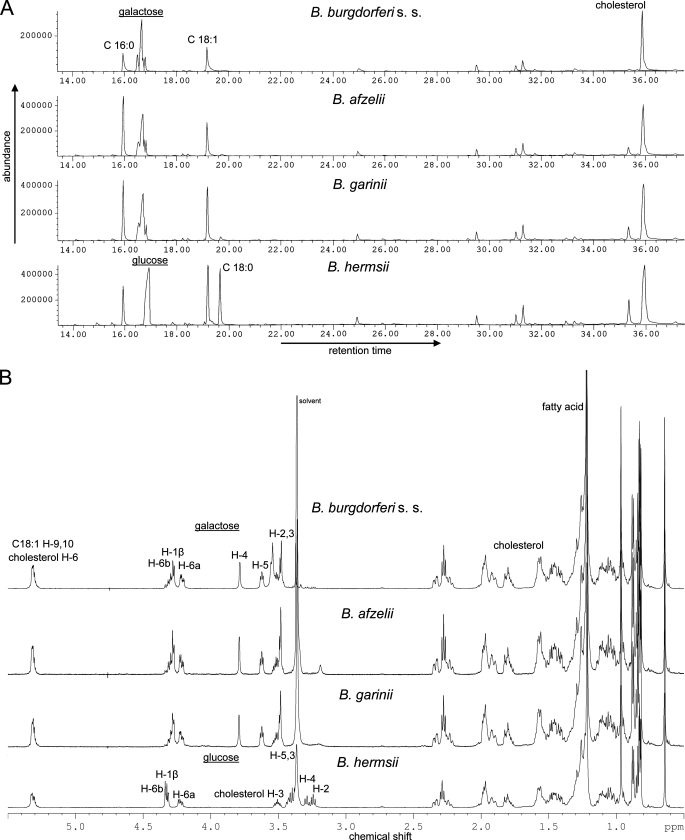
Presence of ACGal in B. garinii and B. afzelii—In Bbu, the fractions F2, F3, and F4b were analyzed by GLC-MS, NMR, and MALDI-TOF MS and gave identical results as published before (23), with F2 identified as cholesteryl 6-O-acyl-β-d-galactopyranoside (ACGal), F3 as mono-α-d-galactopyranosyldiacylglycerol (MGalD), and F4b as cholesteryl β-d-galactopyranoside (βCGal, data not shown), respectively. Bga and Baf exhibited glycolipids migrating at identical Rf values (Fig. 1). The major lipid in all three strains was fraction F2. It was identified in Baf and Bga by GLC-MS (Fig. 2A), NMR (Fig. 2B), and MALDI-TOF-MS (not shown) as cholesteryl 6-O-acyl-β-d-galactopyranoside, thus being identical with ACGal formerly identified in Bbu (Fig. 3). No significant differences in the fatty acid distribution could be observed, because in all three LD strains palmitic acid (16:0) and oleic acid (18:1) dominated.
FIGURE 2.
Comparative GLC-MS and 1H NMR analysis of acylated cholesteryl glycosides. A, fractions F2 (acylated cholesteryl glycosides) from B. burgdorferi s.s., B. afzelii, B. garinii, and B. hermsii were purified by flash chromatography and subjected to mild acid methanolysis and subsequent peracetylation. The samples were analyzed by GLC-MS as described under “Experimental Procedures.” The peaks were assigned according to retention time referenced by standards and mass spectra. B, purified fractions F2 of the four strains were dissolved in CHCl3/MeOD 9:1 (v/v), and 1H spectra were recorded in 600-MHz NMR spectrometer.
FIGURE 3.
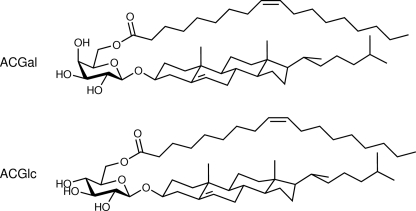
Chemical structures of identified acylated cholesteryl glycosides in LD and RF Borrelia. The structural analysis of fraction F2 in the three LD Borrelia revealed 6-O-acylated cholesteryl-β-d-galactopyranoside (ACGal), whereas the RF-causing B. hermsii contains 6-O-acylated cholesteryl-β-d-glucopyranoside (ACGlc) instead; depicted are the structures with oleic acid (18:1). The only difference in both structures is the configuration of the hydroxyl group of C-4 in the carbohydrate: in d-glucopyranoside equatorial and in d-galactopyranoside axial.
Identification of βCGlc in B. burgdorferi—As mentioned above, fraction F4a was not detected previously, potentially because it is present only in trace amounts (Table 1) and stains also faintly with sulfuric acid/EtOH, used in the first studies (23). Under conditions used for TLC, α-glycosides possess slightly higher Rf values as compared with β-glycosides; therefore we first considered F4a as cholesteryl α-d-galactopyranoside (αCGal). A comparison of chemically synthesized αCGal standard with F4a derived from Bbu applied to TLC in toluene/EtOH 85:15 (v/v) gave clearly distinct Rf values, ruling out αCGal. Next we compared F4a with cholesteryl β-d-glucopyranoside standard by the same procedure and found no differences in the retention of either. The column chromatography yielded a mixed pool of MGalD and F4a where the purity of F4a could be further enhanced by a preparative TLC step. A GLC-MS of isolated F4a revealed glucose and cholesterol but also galactose and glycerol, probably due to contaminations with MGalD. A 1H NMR spectrum of F4a was too weak to reveal clear evidence.
Presence of Cholesteryl 6-O-Acyl-β-d-glucopyranoside (ACGlc) in B. hermsii—Total lipids of Bhe were obtained, and the single lipids were isolated as described for the other strains. We focused on fraction F2, which was first analyzed by GLC-MS. The chromatogram (Fig. 2A) and corresponding mass spectra revealed glucose, cholesterol, and three different fatty acids as palmitic acid (16:0), oleic acid (18:1), and stearic acid (18:0), where the latter is present in the LD strains only in traces (Fig. 2A). The interpretation of the performed one-dimensional (1H (Fig. 2B), 13C, DEPT 135) and two-dimensional (COSY, TOCSY, HMQC, and HMBC) NMR measurements (Table 2) clarified the chemical structure of ACGlc in detail (Fig. 3). The chemical shift of the anomeric proton (H-1, δ4.29 ppm) and its coupling constant (J1,2 = 7.8 Hz) indicates β-configuration. The connectivity of the fatty acids, glucose, and cholesterol could be clearly deduced from the cross-signals in HMBC. Two cross-peaks at 4.3/4.2 ppm (Glc-H6a/6b) and 174.7 ppm (fatty acid-C1) revealed 6-O-acylated glucose, whereas cross-signals at 3.47 ppm (Cho-H3) and 101.7 ppm (Glc-C1) prove the presence of cholesteryl glucopyranoside (Table 2). The results of GLC-MS, NMR, and MS experiments unambiguously identified fraction F2 in Bhe as cholesteryl 6-O-acyl-β-d-glucopyranoside with a fatty acid distribution of 16:1 (2%), 16:0 (21%), 18:1 (44%), and 18:0 (33%).
TABLE 2.
Chemical shifts and carbohydrate coupling constants of cholesteryl 6-O-acyl-β-d-glucopyranoside from B. hermsii in CDCl3/MeOD 9:1 (600 MHz for 1H (δH = 0.00 TMS), 90.6 MHz for 13C (δC = 77.0, internal CDCl3)], 300 K
| Proton | δ 1H | Carbon | δ 13C | |||
|---|---|---|---|---|---|---|
| [ppm] | [ppm] | |||||
| Glucose | H-1β | 4.29 | J1,2 = 7.8 Hz | C-1 | 101.7 | |
| H-2 | 3.20 | J2,3 = 8.4 Hz | C-2 | 73.8 | ||
| H-3 | 3.36 | C-3 | 76.8 | |||
| H-4 | 3.25 | J4,5 = 9.2 Hz | C-4 | 70.7 | ||
| H-5 | 3.38 | J5,6a = 6.6 Hz | C-5 | 74.1 | ||
| H-6a | 4.19 | J6a,6b = 12.0 Hz | C-6 | 64.0 | ||
| H-6b | 4.28 | J6b,5 = 2.4 Hz | ||||
| Cholesterol | H-1a | 0.98 | C-1 | 37.6 | ||
| H-1b | 1.77 | |||||
| H-2a | 1.86 | C-2 | 30.1 | |||
| H-2b | 1.53 | |||||
| H-3 | 3.47 | C-3 | 80.0 | |||
| H-4a | 2.30 | C-4 | 39.1 | |||
| H-4b | 2.20 | |||||
| - | - | C-5 | 140.8 | |||
| H-6 | 5.27 | C-6 | 122.4 | |||
| H-7a | 1.45 | C-7 | 32.2 | |||
| H-7b | 1.90 | |||||
| H-8 | 1.38 | C-8 | 32.2 | |||
| H-9 | 0.84 | C-9 | 50.5 | |||
| - | - | C-10 | 37.0 | |||
| H-11a | 1.41 | C-11 | 21.4 | |||
| H-11b | 1.36 | |||||
| H-12a | 1.10 | C-12 | 40.1 | |||
| H-12b | 1.94 | |||||
| - | - | C-13 | 42.7 | |||
| H-14 | 0.90 | C-14 | 57.1 | |||
| H-15a | 1.46 | C-15 | 24.6 | |||
| H-15b | 0.99 | |||||
| H-16a | 1.75 | C-16 | 28.5 | |||
| H-16b | 1.17 | |||||
| H-17 | 1.01 | C-17 | 56.5 | |||
| H-18 | 0.61 | C-18 | 12.1 | |||
| H-19 | 0.93 | C-19 | 19.6 | |||
| H-20 | 1.29 | C-20 | 36.1 | |||
| H-21 | 0.83 | C-21 | 19.0 | |||
| H-22R | 1.26 | C-22 | 36.5 | |||
| H-22S | 0.89 | |||||
| H-23R | 1.26 | C-23 | 24.2 | |||
| H-23S | 1.07 | |||||
| H-24 | 1.06 | C-24 | 39.8 | |||
| H-24 | 0.97 | |||||
| H-25 | 1.43 | C-25 | 28.3 | |||
| H-26 | 0.77 | C-26 | 22.8 | |||
| H-27 | 0.73 | C-27 | 23.0 | |||
| Fatty acid 16:0 | - | - | C-1 | 174.7 | ||
| H-2 | 2.25 | C-2 | 34.6 | |||
| H-3 | 1.52 | C-3 | 25.3 | |||
| H-4... | 1.18 | C-4 | 29.6 | |||
| H-15 | 1.22 | C-15 | 23.1 | |||
| H-16 | 0.79 | C-16 | 14.4 | |||
| Fatty acid 18:1 | H-8, H-11 | 1.93 | C-8, C-11 | 27.5 | ||
| H-9, H-10 | 5.28 | C-9, C-10 | 130.35 | 130.03 | ||
| H-17 | 1.22 | C-17 | 23.1 | |||
| H-18 | 0.79 | C-18 | 14.4 |
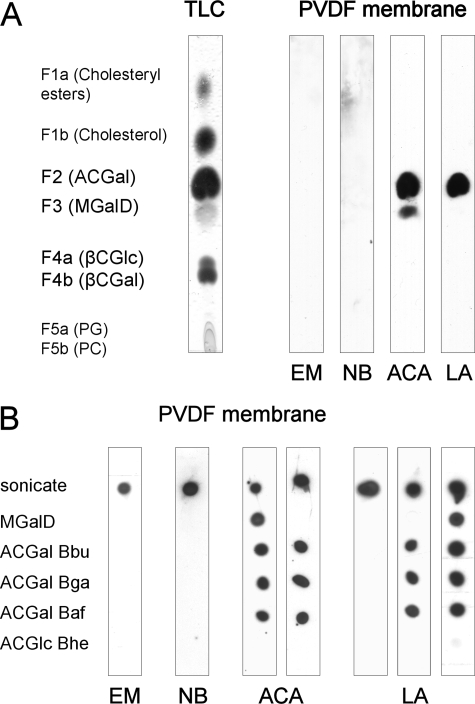
ACGal and MGalD Are the Only Glycolipid-Antigens Recognized by Sera from Patients—One of the major aims of this study was to determine the frequency by which antibodies against Borrelia glycolipids are encountered in patients during LD. Previous studies investigated low numbers of sera, focusing on MGalD (22) or MGalD, ACGal, and CGal (23), whereas βCGlc was not investigated before. Therefore, we aimed at elucidating which glycolipids are immunogenic by testing pooled sera. We applied the butanol extracts, which were separated on TLC plates and blotted onto PVDF membranes as described under “Experimental Procedures.” Membranes were incubated with diluted patient sera, which had been pooled for each clinical stage (EM, NB, ACA, and LA, respectively). We did not observe specific recognition of any glycolipid when pooled sera from patients diagnosed with EM or NB were tested (see Fig. 4A). In contrast, pooled sera from patients suffering from ACA exhibited antibodies directed against ACGal and MGalD, whereas sera from patients with LA interacted exclusively with ACGal. No antibodies against βCGal or βCGlc were detected. Based on this finding we focused our further analysis on ACGal and MGalD.
FIGURE 4.
Immunoblots of LD patient sera with separated Borrelia lipids. A, a TLC plate spotted with the total lipids of B. burgdorferi s.s. was run in CHCl3/MeOH 85:15 (v/v) and transferred to a PVDF membrane using hot iron. Although the TLC was stained with Mostain, the membrane was first incubated with pooled sera from patients diagnosed for erythema migrans (EM), neuroborreliosis (NB), acrodermatitis chronica atrophicans (ACA), or Lyme arthritis (LA), then with anti-human IgG-HRP and eventually with ECL solution. B, 1 μg of sonicate of B. burgdorferi s.s. as positive control, MGalD purified from the same and the acylated cholesteryl glycosides (ACGal and ACGlc) from B. burgdorferi s.s. (Bbu), B. afzelii (Baf), B. garinii (Bga), and B. hermsii (Bhe) were spotted on a PVDF membrane strip. The stripes were incubated with the individual LD patient sera, secondary antibody, and developed with the ECL/film system. Depicted are samples from different LD stages and different recognition patterns.
ACGal Is the Dominant Antigen in Late Stage LD—To assess the frequency of antibodies against ACGal and MGalD in patients at different stages of LD, both ACGal and MGalD were tested by dot blots with the individual sera. To investigate the specificity of antibodies against ACGal, ACGlc from Bhe was included in the analysis. PVDF membranes were spotted with purified ACGal of all three LD strains investigated, as well as MGalD from Bbu and ACGlc from Bhe. As a positive control, total Borrelia sonicates were employed. Blots were incubated with patient sera, and antigen-specific IgG was detected as described under “Experimental Procedures.” Antibodies directed against MGalD were detected in two sera from EM patients (10% of samples investigated) and six sera from patients suffering from late disease (21.1%), whereas none of the sera derived from NB patients displayed anti-MGalD antibodies (Fig. 4B and Table 3).
TABLE 3.
Occurrences of antibodies directed against MGalD and ACGal in LD sera from EM, NB, ACA, and LA determined by dot blots
|
LD
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Localized
|
Early diss.
|
Late
|
Controls | ||||||||
| EM | NB | Combined | ACA | LA | |||||||
| n (%) | |||||||||||
| 20 (100) | 19 (100) | 29 (100) | 14 (100) | 15 (100) | 20 (100) | ||||||
| Sonicate | 9 (45.0) | 19 (100) | 29 (100) | 14 (100) | 15 (100) | 8 (40) | |||||
| MGalD | 2 (10.0) | 0 (0) | 6 (21.1) | 3 (21.4) | 3 (20) | 0 (0) | |||||
| ACGal Bbu | 1 (5.0) | 4 (21.1) | 24 (82.8) | 11 (78.6) | 13 (86.7) | 0 (0) | |||||
| ACGal Bga | 1 (5.0) | 4 (21.1) | 24 (82.8) | 11 (78.6) | 13 (86.7) | 0 (0) | |||||
| ACGal Baf | 1 (5.0) | 4 (21.1) | 24 (82.8) | 11 (78.6) | 13 (86.7) | 0 (0) | |||||
| ACGlc Bhe | 0 (0) | 0 (0) | 1 (3.5) | 1 (7.1) | 0 (0) | 0 (0) | |||||
In contrast, IgG directed against ACGal was detected in patients from all groups, including EM and NB (1 out of 20 and 5 out of 19, respectively). Strikingly, 24 out of 29 (82.8%) patients diagnosed for late disease exhibited antibodies against ACGal, with no apparent differences between patients diagnosed for ACA or LA, respectively (Table 3). In every case, antibodies were found against ACGal from all species tested. One of the sera derived from a patient diagnosed for ACA exhibited antibodies against ACGlc from Bhe, whereas antibodies against ACGal were absent. We exclusively detected anti-glycolipid IgG antibodies, whereas no antibodies of the IgM subtype were found (data not shown). We determined anti-ACGal antibody titers in sera tested positive for ACGal by dot blot employing an ELISA technique based on adsorption of ACGal to microtiter plates as described under “Experimental Procedures.” Here, the majority of sera derived from patients diagnosed for ACA and LA exhibited antibody titers greater than 1:1000, with two LA patients exhibiting titers of 1:32,000 (Table 4).
TABLE 4.
Anti-ACGal titers in sera from dot blot-positive LD patients as determined by ELISA
|
LD
|
|||||
|---|---|---|---|---|---|
|
Localized
|
Early diss.
|
Late
|
|||
| EM | NB | Combined | ACA | LA | |
| n (%) | |||||
| 1 (100) | 4 (100) | 24 (100) | 11 (100) | 13 (100) | |
| <1,000 | 1 (100) | 1 (25) | 8 (33) | 4 (36) | 4 (31) |
| ≥1,000 | 0 (0) | 3 (75) | 16 (67) | 7 (64) | 9 (69) |
| 1,000 | 0 (0) | 2 (50) | 6 (25) | 2 (18) | 4 (31) |
| 2,000 | 0 (0) | 0 (0) | 1 (4) | 0 (0) | 1 (8) |
| 4,000 | 0 (0) | 1 (25) | 6 (25) | 4 (36) | 2 (15) |
| 8,000 | 0 (0) | 0 (0) | 1 (4) | 1 (9) | 0 (0) |
| 16,000 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 32,000 | 0 (0) | 0 (0) | 2 (8) | 0 (0) | 2 (15) |
DISCUSSION
In this study we performed a comparative analysis of glycolipids derived from Borrelia strains causing LD as well as from Bhe causing RF to elucidate the potential of these compounds as candidates for vaccine development or for improvement of diagnosis of LD. We found a strikingly similar pattern of glycolipids within Bbu, Bga, and Baf. In particular, ACGal turned out to be the most abundant glycolipid among all strains tested, because it comprises ∼45% of glycolipids, 22% of total lipids, and 6% of the dry weight. βCGal and MGalD were also found in all strains at comparable levels, and additionally we found that these strains contained βCGlc. However, this glycolipid was found in significantly lower levels when compared with ACGal and βCGal. Because glycolipids isolated from Bhe also predominantly contained cholesterol, we can conclude that the majority of glycolipids present in Borrelia spp., with the exception of MGalD based on diacylglycerol, contain cholesterol. However, in contrast to B. burgdorferi strains, Bhe contained ACGlc and βCGlc, thus exclusively exhibiting cholesterol-containing glycolipids based on glucose. The presence of non-acylated and acylated cholesteryl glucosides in Bhe was postulated before (19), but data were limited to a compositional analysis lacking connectivity and anomeric configuration.
Sequencing of the Bbu strain B31 genome (27) identified genes encoding for a galactosyltransferase (mgs, bb0454 (28)) indicating that these bacteria glycosylate cholesterol from the host (or culture medium) and incorporate it into their membranes. This incorporation of cholesterol obtained from their hosts into glycolipids appears to be a special feature of Borrelia sp., shared only with a few other pathogenic bacteria like Mycoplasma sp. (29, 30) or Helicobacter sp. (31). However, it is important that the anomeric configuration β distinguishes ACGlc isolated from Bhe from the cholesteryl 6-O-acyl-α-d-glucopyranoside found in Helicobacter pylori (32). At this point, we can only speculate why Borrelia exhibit the consistent feature of incorporating cholesterol derivates. It was recently shown that β-glucosylation of host-derived cholesterol by H. pylori protects the bacterium from the immune system of the host (33). Thus, the abundance of glycosylated cholesterol derivatives among Borrelia sp. may be indicative of a similar mechanism of immune evasion.
We tested sera derived from patients diagnosed for LD at different stages for the presence of antibodies against Borrelia glycolipids focusing on MGalD, ACGal, and, for reasons of testing specificity, ACGlc from Bhe. IgG antibodies directed against ACGal were found in sera of all LD stages and none of the controls, however, the most striking finding was that during late stages of disease over 80% of patients developed antibodies against ACGal reaching titers of up to 1:32,000. These findings also show that ACGal acts as a stronger antigen as compared with MGalD, because only about 20% of late stage sera exhibited antibodies against MGalD. This is in excellent agreement with our earlier work (23). These results furthermore demonstrate that the development of anti-glycolipid antibodies is a feature of late stage LD. In addition, our data furthermore indicate that antibodies against ACGal are highly specific, because the structurally closely related glycolipid ACGlc from Bhe was only detected by one serum, which did not contain anti-ACGal antibodies. Taken together, this indicates that ACGal is a strong antigen, which is abundant among Borrelia, yet specific for Borrelia-causing LD. In contrast, MGalD is not unique to B. burgdorferi s.l., because it is present in Bhe, (19), other spirochetes (34), and even numerous vertebrates (35). Regarding the antigenic epitope in ACGal, the lack of recognition of βCGal and ACGlc by the sera tested indicates that at least the Gal and the fatty acid moiety, either partially or entirely, are important for immunization. This is in agreement with the consideration that the carbohydrate could form the polar head of the lipid jutting out of the membrane while cholesterol and fatty acid form the hydrophilic tail, which is concealed within lipid bilayer.
Our results indicate that ACGal may be a candidate for improving diagnosis of LD as well as vaccination strategies. Regarding the value of testing for ACGal antibodies in serodiagnosis of LD, the results point to a promising sensitivity particularly in late stages of disease combined with an outstanding specificity. Thus, including ACGal into an antigen panel for serodiagnosis of LD may increase test reliability considerably, in particular, because ACGal, as we show here, is abundant among the three clinically most relevant B. burgdorferi s.l. species. Presence of ACGal in the recently described species B. spielmanii, remains to be investigated; however, so far this agent has been detected in a low number of cases with EM but not in disseminated disease (5). Furthermore, our observations that ACGal is 1) a strong antigen, 2) abundant among B. burgdorferi substrains, and 3) induces highly specific antibodies, together with the previous observation that administration of ACGal induces antibodies (24), make it a highly promising vaccine candidate. In theory, immunization strategies based on ACGal, as compared with approaches based on proteinaceous antigens, would have the advantage that ACGal as a major constituent of the membrane of the bacteria is unlikely to be differentially expressed by the bacteria during different stages of disease, as has been shown for several other antigens, such as Osps. Also, as of yet there are no reports on host molecules bearing significant homologies to ACGal. In the case of LYMErix™, the potential development of autoantibodies against leukocyte function-associated antigen-1 due to homologies with OspA was one of the reasons that led to the withdrawal of this vaccine from the market (18, 36). However, because glycosylated cholesterol derivates so far only have been described in bacteria, fungi, plants, and slime molds (30, 31, 37), the development of antibodies during ACGal vaccination cross-reacting with host tissues is unlikely. Finally, we have shown previously that ACGal, in contrast to MGalD (38), does not induce pro-inflammatory cytokines (23) indicating that adverse acute events during administration of this compound would also be highly unlikely.
Taken together, our data define acylated cholesterol glycosides as a major constituent of Borrelia lipid membranes, with B. burgdorferi sensu lato exhibiting ACGal exclusively. Because we detected antibodies against ACGal in the majority of patients suffering from late stage LD we propose that this compound is a useful tool for improving LD diagnostics as well as a promising vaccine candidate.
Acknowledgments
We thank Hermann Moll, as well as Heiko Kässner (both of the Research Center Borstel, Germany) for skilful help with GLC-MS and NMR analysis, respectively. The introduction to TLC blotting by Sabine Gronow and Irina von Cube (both of the Research Center Borstel, Germany) is gratefully acknowledged. Furthermore, we are thankful for the technical assistance of Fränzi Creutzburg and Diana Woellner (Institute for Microbiology and Hygiene, Charité Medical Center, Berlin, Germany).
Footnotes
The abbreviations used are: LD, Lyme disease; ACA, acrodermatitis chronica atrophicans; ACGal, cholesteryl 6-O-acyl-β-d-galactopyranoside; ACGlc, cholesteryl 6-O-acyl-β-d-glucopyranoside; Baf, B. afzelii; Bbu, B. burgdorferi sensu stricto; Bga, B. garinii; Bhe, B. hermsii; βCGal, cholesteryl β-d-galactopyranoside; βCGlc, cholesteryl β-d-glucopyranoside; ELISA, enzyme-linked immunosorbent assay; EM, erythema migrans; GLC-MS, gas-liquid chromatography-mass spectrometry; LA, Lyme arthritis; MALDI-TOF, matrix-assisted laser desorption ionization/time-of-flight; MGalD, mono-α-d-galactopyranosyldiacylglycerol; MS, mass spectrometry; NB, neuroborreliosis; Osp, outer-surface protein; OspA, outer-surface protein A; PBS, phosphate-buffered saline; PVDF, polyvinylidene difluoride; RF, relapsing fever; s.l., sensu lato; s.s., sensu stricto; HRP, horseradish peroxidase.
References
- 1.Burgdorfer, W., Barbour, A. G., Hayes, S. F., Benach, J. L., Grunwaldt, E., and Davis, J. P. (1982) Science 216 1317–1319 [DOI] [PubMed] [Google Scholar]
- 2.Steere, A. C., Coburn, J., and Glickstein, L. (2004) J. Clin. Invest. 113 1093–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (2007) MMWR Morb. Mortal Wkly Rep. 56 573–57617568368 [Google Scholar]
- 4.Dennis, D. T., and Hayes, E. B. (2002) in Lyme borreliosis: Biology, Epidemiology and Control (Stanek, G., ed) pp. 251–280, CABI publishing, Oxford, United Kingdom
- 5.Fingerle, V., Schulte-Spechtel, U. C., Ruzic-Sabljic, E., Leonhard, S., Hofmann, H., Weber, K., Pfister, K., Strle, F., and Wilske, B. (2008) Int. J. Med. Microbiol. 298 279–290 [DOI] [PubMed] [Google Scholar]
- 6.Hubalek, Z., and Halouzka, J. (1997) Eur. J. Epidemiol. 13 951–957 [DOI] [PubMed] [Google Scholar]
- 7.Smith, R. P., Schoen, R. T., Rahn, D. W., Sikand, V. K., Nowakowski, J., Parenti, D. L., Holman, M. S., Persing, D. H., and Steere, A. C. (2002) Ann. Intern. Med. 136 421–428 [DOI] [PubMed] [Google Scholar]
- 8.Steere, A. C., and Sikand, V. K. (2003) N. Engl. J. Med. 348 2472–2474 [DOI] [PubMed] [Google Scholar]
- 9.Huppertz, H. I., Böhme, M., Standaert, S. M., Karch, H., and Plotkin, S. A. (1999) Eur. J. Clin. Microbiol. Infect. Dis. 18 697–703 [DOI] [PubMed] [Google Scholar]
- 10.Steere, A. C., Schoen, R. T., and Taylor, E. (1987) Ann. Intern. Med. 107 725–731 [DOI] [PubMed] [Google Scholar]
- 11.Stanek, G., and Strle, F. (2003) Lancet 362 1639–1647 [DOI] [PubMed] [Google Scholar]
- 12.Reed, K. D. (2002) J. Clin. Microbiol. 40 319–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilske, B., Fingerle, V., and Schulte-Spechtel, U. (2007) FEMS Immunol. Med. Microbiol. 49 13–21 [DOI] [PubMed] [Google Scholar]
- 14.Wilske, B., Busch, U., Fingerle, V., Jauris-Heipke, S., Preac Mursic, V., Rossler, D., and Will, G. (1996) Infection 24 208–212 [DOI] [PubMed] [Google Scholar]
- 15.Hauser, U., Krahl, H., Peters, H., Fingerle, V., and Wilske, B. (1998) J. Clin. Microbiol. 36 427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goettner, G., Schulte-Spechtel, U., Hillermann, R., Liegl, G., Wilske, B., and Fingerle, V. (2005) J. Clin. Microbiol. 43 3602–3609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steere, A. C., Sikand, V. K., Meurice, F., Parenti, D. L., Fikrig, E., Schoen, R. T., Nowakowski, J., Schmid, C. H., Laukamp, S., Buscarino, C., and Krause, D. S. (1998) N. Engl. J. Med. 339 209–215 [DOI] [PubMed] [Google Scholar]
- 18.Gross, D. M., Forsthuber, T., Tary-Lehmann, M., Etling, C., Ito, K., Nagy, Z. A., Field, J. A., Steere, A. C., and Huber, B. T. (1998) Science 281 703–706 [DOI] [PubMed] [Google Scholar]
- 19.Livermore, B. P., Bey, R. F., and Johnson, R. C. (1978) Infect. Immun. 20 215–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honarvar, N., Schaible, U. E., Galanos, C., Wallich, R., and Simon, M. M. (1994) Immunology 82 389–396 [PMC free article] [PubMed] [Google Scholar]
- 21.Radolf, J. D., Goldberg, M. S., Bourell, K., Baker, S. I., Jones, J. D., and Norgard, M. V. (1995) Infect. Immun. 63 2154–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hossain, H., Wellensiek, H. J., Geyer, R., and Lochnit, G. (2001) Biochimie. (Paris) 83 683–692 [DOI] [PubMed] [Google Scholar]
- 23.Schröder, N. W., Schombel, U., Heine, H., Göbel, U. B., Zähringer, U., and Schumann, R. R. (2003) J. Biol. Chem. 278 33645–33653 [DOI] [PubMed] [Google Scholar]
- 24.Ben-Menachem, G., Kubler-Kielb, J., Coxon, B., Yergey, A., and Schneerson, R. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 7913–7918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taki, T., Handa, S., and Ishikawa, D. (1994) Anal. Biochem. 221 312–316 [DOI] [PubMed] [Google Scholar]
- 26.Belisle, J. T., Brandt, M. E., Radolf, J. D., and Norgard, M. V. (1994) J. Bacteriol. 176 2151–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fraser, C. M., Casjens, S., Huang, W. M., Sutton, G. G., Clayton, R., Lathigra, R., White, O., Ketchum, K. A., Dodson, R., Hickey, E. K., Gwinn, M., Dougherty, B., Tomb, J. F., Fleischmann, R. D., Richardson, D., Peterson, J., Kerlavage, A. R., Quackenbush, J., Salzberg, S., Hanson, M., van Vugt, R., Palmer, N., Adams, M. D., Gocayne, J., Weidman, J., Utterback, T., Watthey, L., McDonald, L., Artiach, P., Bowman, C., Garland, S., Fuji, C., Cotton, M. D., Horst, K., Roberts, K., Hatch, B., Smith, H. O., and Venter, J. C. (1997) Nature 390 580–586 [DOI] [PubMed] [Google Scholar]
- 28.Ostberg, Y., Berg, S., Comstedt, P., Wieslander, A., and Bergström, S. (2007) FEMS Microbiol. Lett. 272 22–29 [DOI] [PubMed] [Google Scholar]
- 29.Rottem, S. (2002) Biochem. Biophys. Res. Commun. 292 1289–1292 [DOI] [PubMed] [Google Scholar]
- 30.Smith, P. F. (1971) J. Bacteriol. 108 986–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haque, M., Hirai, Y., Yokota, K., Mori, N., Jahan, I., Ito, H., Hotta, H., Yano, I., Kanemasa, Y., and Oguma, K. (1996) J. Bacteriol. 178 2065–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirai, Y., Haque, M., Yoshida, T., Yokota, K., Yasuda, T., and Oguma, K. (1995) J. Bacteriol. 177 5327–5333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wunder, C., Churin, Y., Winau, F., Warnecke, D., Vieth, M., Lindner, B., Zähringer, U., Mollenkopf, H. J., Heinz, E., and Meyer, T. F. (2006) Nat. Med. 12 1030–1038 [DOI] [PubMed] [Google Scholar]
- 34.Livermore, B. P., and Johnson, R. C. (1974) J. Bacteriol. 120 1268–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steim, J. M. (1967) Biochim. Biophys. Acta 144 118–126 [DOI] [PubMed] [Google Scholar]
- 36.Nigrovic, L. E., and Thompson, K. M. (2007) Epidemiol. Infect. 135 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warnecke, D., Erdmann, R., Fahl, A., Hube, B., Müller, F., Zank, T., Zähringer, U., and Heinz, E. (1999) J. Biol. Chem. 274 13048–13059 [DOI] [PubMed] [Google Scholar]
- 38.Kinjo, Y., Tupin, E., Wu, D., Fujio, M., Garcia-Navarro, R., Benhnia, M. R., Zajonc, D. M., Ben-Menachem, G., Ainge, G. D., Painter, G. F., Khurana, A., Hoebe, K., Behar, S. M., Beutler, B., Wilson, I. A., Tsuji, M., Sellati, T. J., Wong, C. H., and Kronenberg, M. (2006) Nat. Immunol. 7 978–986 [DOI] [PubMed] [Google Scholar]