Abstract
Hepassocin (HPS), is a liver-specific gene with mitogenic activity on isolated hepatocytes. It is up-regulated following partial hepatectomy and down-regulated frequently in heptocellular carcinoma (HCC). However, very little is known about the HPS transcription regulation mechanism. In this study, we identified HNF1α (hepatocyte nuclear factor-1α) as an important liver-specific cis-acting element for HPS using in vivo luciferase assays. Deletion of the HNF1 binding site not only led to a complete loss of HPS promoter activity in vivo but also abolished the induction of the HPS promoter by HNF1α. An electrophoretic mobility shift assay demonstrated that HNF1α interacted with the HPS gene promoter in vitro. Chromatin immunoprecipitation showed that HNF1α interacted with HMGB1 and CREB-binding protein, and all of them were recruited to the HPS promoter in vivo. Moreover, HNF1α expression was lower in HCC cell lines and tissues and correlated significantly with the down-regulation of HPS expression. Re-expression of HNF1α in human hepatoma HepG2 cells reinduced HPS expression. In contrast, knockdown of endogenous HNF1α expression by small interfering RNA resulted in a significant reduction of HPS expression. Furthermore, we found that partial hepatectomy and IL-6 significantly induced promoter activity of HPS, depending on STAT3 and HNF1 binding sites in the HPS promoter. These results demonstrate that the HNF1 binding site and HNF1α are critical to liver-specific expression of HPS, and down-regulation or loss of HNF1α causes, at least in part, the transcriptional down-regulation of HPS in HCC.
Hepassocin (HPS),3 also called HFREP-1 (hepatocyte-derived fibrinogen-related protein) and fibrinogen-like 1, is a liver-specific gene and belongs to the fibrinogen superfamily, the members of which share a common fibrinogen-like domain at their carboxyl termini (1). HPS is translated into a mature 312-amino acid protein after cleavage of a hydrophobic secretion signal. The position within the human genome is mapped to chromosome 8p22–21.3. It has been reported that the expression of HPS mRNA was detected mainly in murine livers. In situ hybridization studies revealed its presence in parenchymal hepatocytes but not in endothelial cells (1). Subsequent studies of human adult tissues demonstrated that HPS mRNA was strongly expressed in adult livers, fairly strongly in fetal livers, and weakly in pancreases but not in other tissues (2). HPS was induced 2 h after a 70% hepatectomy of mouse livers, and the second peak arrived 24 h later. The expression of HPS remained high until 72 h later and declined to the basal level thereafter (3), suggesting that HPS may function as a regulator of cell growth in liver regeneration. Functionally, HPS was initially described as generating mitogenic activity on isolated hepatocytes, whereas it did not promote DNA synthesis in non-liver cell lines in vitro. Further studies revealed expression patterns inconsistent with a tumor suppressor (4). Expression of the HPS/LFIRE-1 (liver fibrinogen-related gene-1) was frequently down-regulated or lost in HCC at both mRNA and protein levels, compared with their adjacent normal liver tissues, and the expression level was found to be strongly associated with the tumors' differentiation statuses (4). Exogenous HPS expression in human HCC cells inhibited their anchorage-dependent or -independent growth in vitro, and down-regulation of HPS by an antisense approach enhances cancer cell proliferation and colony formation in soft agar (4). Taken together, these results suggest that HPS plays an important role in the liver's development and physiological function and is associated with the progression of liver tumors. It was recently suggested that HPS in plasma almost completely binds to the fibrin matrix during clot formation and is strongly associated with fibrin and possibly fibrinogen (5). Additionally, IL-6 could increase HPS expression in HepG2 hepatoma cells in a dose-dependent manner, indicating that HPS may be an acute phase reactant (6).
Down-regulation of gene expression in HCC can occur via a variety of mechanisms. Previous studies have implicated allelic loss of HPS/LFIRE-1 on chromosome 8p22 in 57.1% (24 of 42) of HCC specimens through the loss of heterozygosity analysis (4). Because tissue-specific expression of genes is based on the presence of cis-acting sequences in their promoter and enhancer regions that interact with sequence-specific nuclear transcription factors that potentate or depress transcriptional initiation, we hypothesized that the down-regulation of HPS levels in HCC may occur at the transcriptional level. This reduction in HPS gene transcription could be caused by alterations in the quantity or function of regulatory nuclear transcription factors. In this study, we described identification and characterization of the regulatory elements that provided the liver-specific regulation of the HPS gene. Our results showed that liver-enriched HNF1α (hepatocyte nuclear factor-1α) was needed for appropriate expression of this gene, and down-regulation or loss of transcription factor HNF1α caused, at least in part, the transcriptional down-regulation of HPS in HCC. In addition, we have determined that HPS promoter activity was induced by pretreatment of cells with a variety of cellular mediators.
EXPERIMENTAL PROCEDURES
Cell Lines, Primary HCC, and Adjacent Tissues
Human hepatocarcinoma cell line HepG2 and human kidney cell line HEK293 were purchased from American Type Culture Collection (Manassas, VA). Human hepatocyte cell line L02, and hepatocarcinoma cell lines HL7702, SMMC-7721, and BEL-7402 were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). These were grown at 37 °C in 5% CO2 in Dulbecco's modified Eagle's medium (Invitrogen) and supplemented with penicillin (100 units/ml), streptomycin (10 μg/ml), and 10% fetal bovine serum (MDgenics Inc) and split 1:2 at confluences. Cells were detached via incubation with 0.05% trypsin and 0.04% EDTA in phosphate-buffered saline.
All HCC specimens were obtained from patients who underwent surgical resection. The primary tumor specimens were immediately frozen at –80 °C until RNA extraction. Both tumor and adjacent nontumor tissues were sampled at 1 cm3, respectively, and were confirmed by pathological examination.
5′-Rapid Amplification of cDNA Ends (5′-RACE)
5′-RACE was performed using human liver Marathon-ready cDNA (BD Biosciences Clontech) and gene-specific primers anchored in the 3′-untranslated region of HPS: 5′-TGCATATCTGCTGTACTGAAAGC-3′ and adapter-specific primers. Touchdown PCR cycling conditions were as follows: 2 min of initial denaturation at 95 °C PCR followed by five cycles of 95 °C for 30 s, 70 °C for 2.5 min, 5 cycles of 95 °C for 30 s, 70 °C for 2.5 min, 25 cycles of 95 °C for 30 s, 68 °C for 2.5 min, and a final extension at 72 °C for 10 min. The second round PCR was carried out with nested primers corresponding to positions 625–647 bp of HPS cDNA: 5′-GCAAGGGAATCTCCAGCTGTTCC-3′ and adapter-specific primers. The cycling conditions were as follows: 2 min of initial denaturation at 95 °C PCR followed by 25 cycles at 95 °C for 30 s, 65 °C for 30s, 72 °C for 2.5 min, and a final extension at 72 °C for 10 min. 5′-RACE products were cloned into pMD-18T simple vectors (TaKaRa Biotechnology Co., Ltd.) and sequenced. The TRANSFAC data base (available on the World Wide Web) was used to search for the potential cis-regulatory elements within HPS promoters. The threshold was set at 80.0.
Plasmid Constructions
The –2304 to +135 bp region relative to the transcription start site of HPS (accession number NM_004467) was amplified by PCR using primers P-2304 (5′-CCGCTCGAGGATCTGGAATTAAACCTTCCC-3′) and P+135 (5′-CCCAAGCTTAGTTTTGGTTACTATCATACC-3′). The PCR product was digested with XhoI and HindIII and inserted into a promoterless luciferase reporter vector pGL3/Basic, resulting in plasmid pGL3/–2304. The deletion mutants with various lengths of the promoter regions were prepared by PCR using P+135 as the 3′-end primer and the 5′-primers shown in Table 1. They were named pGL3/–1654, pGL3/–1058, pGL3/–784, pGL3/–614, pGL3/–554, pGL3/–454, pGL3/–64, pGL3/+57, and pGL3/+117, respectively. The numbers behind sprit represent the boundaries of the inserts relative to the transcription start sites.
TABLE 1.
Primers used to synthesize 5′-truncated constructs of HPS
| Name | Forward (5′-3′) |
|---|---|
| P-1654 | 5′-CCGCTCGAGCCTGGCCATAATTTTGACCTT-3′ |
| P-1058 | 5′-CCGCTCGAGCAGTAGATAACAAAGAACAG-3′ |
| P-784 | 5′-CCGCTCGAGGAATAATCCCAATATTTTTGCT-3′ |
| P-614 | 5′-CCGCTCGAGTAATGGAGCTAACAAGTGCC-3′ |
| P-554 | 5′-CCGCTCGAGAAGGAACAACTTGTCCAGAG-3′ |
| P-454 | 5′-CCGCTCGAGCCTTGTTTTTTATAACTAGGA-3′ |
| P-64 | 5′-CCGCTCGAGTATTTCTAATCAAATAATGGA-3′ |
| P+57 | 5′-CCGCTCGAGAAGTTATTTAATGTCTCTGCA-3′ |
| P+117 | 5′-GTATGATAGTAACCAAAACT-3′ |
Vectors with a deletion in the HNF1-binding site (–463/–457 bp relative to the transcription start site) and STAT3-binding sites (–665/–657 and –750/–743 bp relative to the transcription start site) in HPS promoters were introduced using the Takara MutanBest kit (Takara Biotechnology Co., Ltd.) as described by the manufacturer. The primers pGL3/–1058-HNF1-Del-F (5′-TGCCTTGTTTTTTATAACTAGG-3′) and pGL3/–1058-HNF1-Del-R (5′-AATTGCCAAGGTGAAATGGTC-3′) were used to amplify the HNF1 deletion mutant from pGL3/–1058. The STAT3 deletion mutant (deletion of both STAT3-binding sites) was amplified by PCR using the following primers: pGL3/-1058-STAT3-Del(–750/–743)-F, 5′-TCTGAGTGAATAAATAGGGA-3′; pGL3/–1058-STAT3-Del(–750/–743)-R, 5′-TACCAAAGTTTTGTTTTCTT-3′; pGL3/–1058-STAT3-Del(–665/–657)-F, 5′-AGAAACTGTTACACAATACG-3′; pGL3/–1058-STAT3-Del(–665/–657)-R, 5′-TCTGGGTTCATCATTTCTTC-3′. The DNA sequences of mutated vectors were confirmed by sequence analyses.
The transcriptional factor HNF1α was generated by PCR using sense primer 5′-CCCAAGCTTATGGTTTCTAAACTGAGCCA-3′ and antisense primer 5′-GCTCTAGATACTGGGAGGAAGAGGCCA-3′ from the human liver cDNA library (BD Biosciences Clontech). The PCR products were digested with HindIII and XbaI and inserted into the pcDNA3.1/Myc-HisB vector (Invitrogen).
Transfection and Luciferase Assays
In Vitro Luciferase Assays—For in vitro luciferase assays, cells were seeded into 24-well plates and transiently transfected with different reporter plasmids as indicators. 24 h later, cells were collected and lysed in 100 μl of 1× passive lysis buffer. Luciferase assays were carried out with 50 μl of lysate using the dual luciferase reporter assay system in a chemiluminescence analyzer (FB12 luminometer; Berthold Detection Systems). Luciferase activities were expressed as -fold induction relative to values obtained from control cells. The results represent the mean of at least three independent transfection experiments, each carried out in duplicate. Renilla luciferase activity was used as an internal control for transfection efficiency.
In Vivo Luciferase Assays—For in vivo luciferase assays, mice were fed a normal diet with free access to food and water. Plasmids were transfected into the mouse liver using the TransIT In Vivo Gene Delivery System (Mirus, Madison, WI) by mouse tail vein injections according to the manufacturer's directions. In brief, 5.1-μl polymer solutions were incubated with 5–25 μgof plasmids in 200 μl of sterile water for 5 min at room temperature. The mixture was added to 1.9 ml of 1× delivery solution and incubated for 10–15 min, and the contents were delivered via syringe to the tail vein at a constant rate for 4–7 s. The mice were killed 24 h after injection. For luciferase determination, livers were removed and weighed (100 ± 3 mg) and homogenized in 1 ml of 1× passive lysis buffer at 4 °C; the lysate was centrifuged for 15 min at 13,000 rpm; 40 μl of supernatant was mixed with 40 μl of luciferase assay buffer (Promega, Madison, WI); and the chemiluminescence produced was measured in a luminometer (FB12 luminometer; Berthold Detection Systems). Renilla luciferase activity was used as an internal control for transfection efficiency. The results represent the mean of at least three independent transfection experiments; two individual mice were treated in every experiment, and three lobes of the liver, representing three different sites in the liver, were taken from each mouse.
Gel Mobility Shift Assays
Nuclear extracts were prepared from HepG2 and mouse liver, as previously described (7). The wild-type probe, 5′-CCTTGGCAATTATTAACCTGCC-3′, the underlined part of which corresponded to HNF1 recognition consensus sequenced in the promoter of HPS in the region –470/–457 bp, and the mutant probe, 5′-TCACCTTGGCAATTTGCCTT-3′, which deleted AA CC compared with the wild-type probe, were labeled with biotin at the 5′-end. Competition experiments were executed using a 100-fold excess of various unlabeled double-stranded DNA, which was added to the reaction mixture prior to the addition of the labeled probe. Supershifting was carried out using an anti-HNF1α antibody (catalog number sc-6547X; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). The LightShift chemiluminescent electrophoretic mobility shift assay kit (catalog number 20148; Pierce) was used to perform electrophoretic mobility shift assays. In brief, the reaction system was first prepared according to the manufacturer's protocol, and then DNA-protein complexes were electrophoresed on a 4% polyacrylamide gel in 0.5× Tris borate/EDTA electrophoresis buffer at 100 V and then transferred to a positive nylon membrane, UV-cross-linked, probed with streptavidin-horseradish peroxidase conjugate, and incubated with the substrate. The membrane was then exposed to x-ray film.
Chromatin Immunoprecipitation Assays
107 HepG2 cells were lysed with cell lysis buffer (10 mm Tris-HCl, pH 8.0, 10 mm NaCl, 0.2% Nonidet P-40) on ice for 10 min, centrifuged at 2500 rpm for 5 min at 4 °C, resuspended in nuclear lysis buffer (50 mm Tris-HCl, pH 8.0, 10 mm EDTA, 1% SDS), vortexed three times, sonicated six times to shear chromatin, and centrifuged at 12,000 rpm for 15 min at 4 °C. The supernatant was collected as the whole cell extract.
Protein-DNA immunocomplexes were immunoprecipitated with rabbit polyclonal antibodies against HNF1α (catalog number sc-8986; Santa Cruz Biotechnology), CBP (catalog number sc-22; Santa Cruz Biotechnology), HMGB1 (catalog number BC 003378; Protein Tech Group), or rabbit IgG (catalog number sc-2027; Santa Cruz Biotechnology). 100 μl of whole cell extract were incubated with antibodies in 900 μl of ChIP dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mm EDTA, 16.7 mm Tris-HCl, pH 8.0, 167 mm NaCl), with protein A/G-agrose beads as an adsorbent. Resins were washed with three kinds of buffers containing various detergents and NaCl in different concentrations. Following elution, DNA fragments were isolated and purified using phenol/chloroform. PCR was conducted using primers HPS-F(–503) (5′-CTTAGAGAGCAAATAAACTGACCAT-3′) and HPS-R(–309) (5′-TGAAGATTAAGTAAAAACGAAGTCC-3′) under the following conditions: 28 cycles, 52 °C, 5 units of Taq polymerase, and 25 pmol of each primer. Amplified products were analyzed on 1.5% Tris borate/EDTA-agrose gels.
HCC Tissue Microarray
The detection of HPS expression in human HCC tissues was performed using RNA in situ hybridization and a tissue microarray containing 142 formalin-fixed, paraffin-embedded human HCC tissues. The probe was 5′-TGTCTGT(T)TTAGCCGTGTAGGGGCC(G)CTGTAGTATACACCATTC(A)GGTTTGC-3′, labeled by digoxigenin at the 3′-end, and the sites modified by L-leucyl-β-naphthylamide are indicated in parentheses. Paraffin-embedded tissue sections were first treated with 0.3% Triton X-100 for 5 min and then with 0.2 n HCl for 20 min at room temperature. Next, they were treated with proteinase K in phosphate-buffered saline for 10 min at 37 °C, dehydrated in ethanol, and dried. Sections were hybridized at 42 °C for 19 h and then incubated with alkaline phosphatase-conjugated digoxigenin antibody at 37 °C for 1 h. Unbound conjugate was removed by washing in two changes of buffer 1 (0.1 m Tris-HC1, 0.15 m sodium chloride, pH 7.5) followed by one wash in buffer 3 (0.1 m Tris-HC1, 0.1 m sodium chloride, 0.05 m magnesium chloride hexahydrate, pH 9.5). Sections were counterstained with neutral red and mounted using glycerol aqueous mounting mediums.
Reverse Transcription and Real Time PCR Analysis
Total RNA was extracted with RNAVzol reagent (Vigorous Biotechnology), and reverse transcription was applied using a Vigoscript first strand cDNA synthesis kit (Vigorous Biotechnology) according to the manufacturer's protocol. The cDNA was analyzed using real time PCR, according to the instructions from the kit. In brief, real time PCR was done using the Bio-Rad IQ™5 multicolor real time PCR detection system and SYBR Premix Ex Taq™ (2×) kit (TaKaRa Japan). The cycling conditions were as follows: 95 °C for 1 min, 40 cycles for 10 s at 95 °C, for 30 s at 55 °C, and for 30 s at 72 °C. SYBR Green fluorescence was measured after each elongation step. At the end of PCR, a melting curve analysis was performed by gradually increasing the temperature from 55 to 95 °C to determine purity. PCR was set up in triplicates. The relative quantification of HPS and HNF1α mRNA was analyzed using the comparative CT method and normalized to that of GAPDH. The following primers were used: HNF1 QP1, 5′-AGTGAGTCCGGGCTTCACAC-3′; HNF1QP2, 5′-TGAAGGTCTCGATGACGCTG-3′; HPSQP1, 5′-CTGGAGATTCCCTTGCGG-3′; HPSQP2, 5′-GTTTTAGCCGTGTAGGGG-3′; GAPDHQP1, 5′-TCAGTGGTGGACCTGACCTG-3′; GAPDHQP2, 5′-TGCTGTAGCCAAATTCGTTG-3′.
siRNA Transfection
HNF1α siRNAs were as follows: 5′-UGACAGCACUGCACAGCUUTT-3′ (sense strand) and 5′-AAGCUGUGCAGUGCUGUCATT-3′ (antisense strand). HepG2 cells were grown in 6-well plates to 50% confluence, and HNF1α siRNAs were transfected into HepG2 cells at 100 pmol/well with Vigofect reagent (Vigorous Biotechnology), according to the manufacturer's protocol. The nonspecific RNA duplexes (GenePharam, Shanghai, China) were used in control experiments. Cells were harvested after 48–72 h of incubation, and then real time PCR and Western blot were performed to detect interference effects.
Western Blot
For Western blots, 106 cells were lysed with 30 μl of TNT buffer (20 mm Tris-HCl (pH 7.5), 200 mm NaCl, 1% Triton X-100, 1 mm phenylmethylsulfonyl fluoride, and protease inhibitors leupeptin, aprotinin, pepstatin A, chymostatin, and antipain, each at a final concentration of 10 μg/ml). Then 20 μg of protein from each sample was loaded onto the gel. After separation by SDS-PAGE, proteins were transferred onto polyvinylidene difluoride membranes (Amersham Biosciences) and probed with various antibodies at the following concentrations: HNF1α (catalog number sc-8986; Santa Cruz Biotechnology), 1:1000; HPS (catalog number MAB1614; R&D Systems), 1:500; and β-actin (catalog number sc-47778; Santa Cruz Biotechnology), 1:1000. Chemiluminescent detection was conducted using Super-signal substrate (Pierce), according to the manufacturer's specifications.
Animal models
Male BALB/c mice (18–22 g body weight) were offered by the Beijing Institute of Radiation Medicine Animal Center (Beijing, China). All surgical procedures were approved by the Animal Care Committee of the Beijing Institute of Radiation Medicine. Animals received humane care according to the criteria outlined in Ref. 34. All of the mice were pentobarbital-anesthetized (50 mg/kg) before surgery. For partial hepatectomy (PHx), mice were subjected to midventral laparotomy with 70% liver resection, according to Higgins and Anderson (8). The bellies of control animals were cut open without PHx. For nephrectomy, the left kidney was surgically exposed, and then a silk suture was securely tied around the hilus renalis containing the artery, vein, and ureter before the kidney was removed (9). Sham animals underwent laparotomy without nephrectomy. For splenectomy, a small incision was made in the skin of the left flank. The peritoneum was opened, the splenic artery and vein were ligated with catgut, and the spleen was removed (10, 11). For sham-operated mice, the peritoneum was opened and surgically closed. Animals were sacrificed at the indicated time points after surgery.
Stimulation of Cells
L02 cells were treated with the following cytokines (R&D Systems, Inc. (Minneapolis, MN)): HGF (10 ng/ml), EGF (10 ng/ml), TNFα (10 ng/ml), IL-6 (10 ng/ml), vascular endothelial growth factor (10 ng/ml), or BSA (10 ng/ml) for 24 h. At the end of incubation, luciferase activity was determined as described under “Transfection and Luciferase Assays.”
For IL-6 stimulation, L02 cells were grown to 80% confluence, and then the medium was added with various concentrations of IL-6 with or without 1 μm dexamethasone. 24 h later, luciferase activity was measured to examine HPS promoter activity, real time PCR was carried out to explore the endogenous HPS mRNA levels with GAPDH as the internal control, and Western blot was performed to determine the endogenous HPS protein level with β-actin as the internal control.
Statistical Analyses
Statistical analyses were performed using SPSS version 12.0 (SPSS, Chicago, IL). Statistical values and results are expressed as mean ± S.D. A p value of <0.05 was considered statistically significant. Student's unpaired t test was used to analyze comparisons between groups. The Spearman test was used to analyze the correlation between parameters. Two-sided Fisher's exact tests were used to analyze the statistical association between clinical-pathological and RNA in situ hybridization variables.
RESULTS
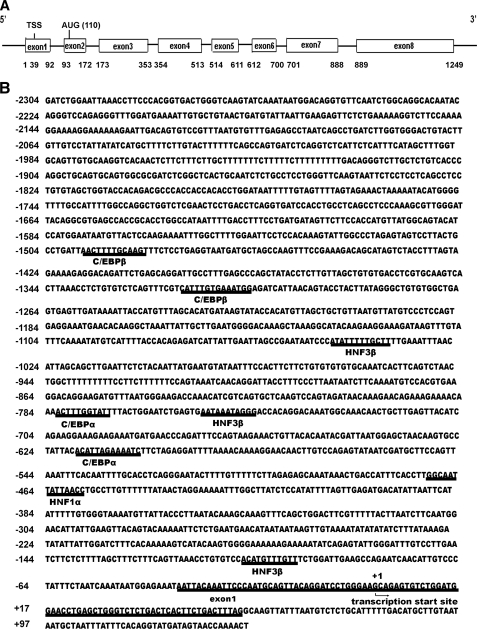
HNF1α Is Involved in Tissue-specific Regulation of HPS Gene Expression—The genomic structure for human HPS consists of eight exons separated by seven introns. The translational start site (AUG) is located within exon 2, which is 9891 bp downstream from exon 1. To identify the transcription initiation site(s), 5′-RACE was performed using an HPS-specific primer anchored in exon 6 to amplify total RNA from human liver tissue. Cloning and sequencing of these PCR products revealed two totally distinct DNA sequences. Both sequences extended exon 2 upstream and were located in different regions of exon 1 of the HPS gene. The longer sequence (718 bp; Fig. 1A), extended 54 base pairs into exon 1. We have designated this start site as +1 (Fig. 1B). This sequence was 38 bp shorter than the previously published cDNA HPS sequence in the GenBank™ (accession number NM_004467).
FIGURE 1.
A, genomic structure of HPS. The human HPS gene is located on chromosome 8p22–21.3, and the exon structure is indicated. B, transcriptional start sites and putative regulatory elements of the 5′-flanking region of the HPS gene. The 5′-RACE HPS products were cloned, sequenced, and compared with the known sequence for the HPS gene in GenBank™ (accession number NM_004467). The transcriptional start site was designated +1. The TRANSFAC data base (available on the World Wide Web) was used to identify potential cis-regulatory elements in the 5′-flanking sequence of the HPS gene (core sequence is underlined).
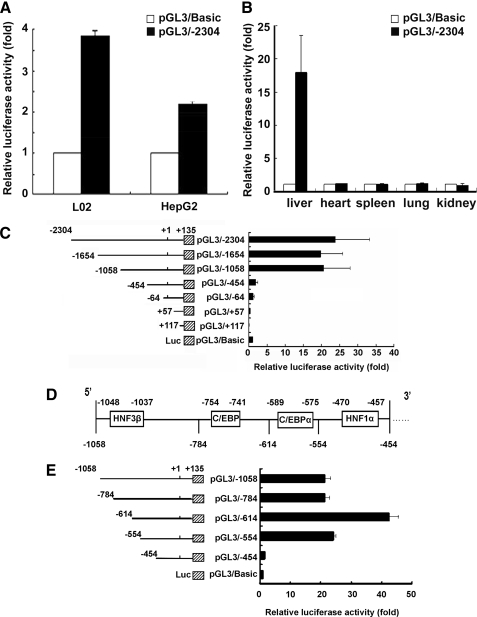
To functionally characterize the putative promoter, the 2.4-kb fragment, including 2304 bp of regions upstream of the transcriptional start site, exon 1, and a portion of intron 1, were cloned into a firefly luciferase-expressing vector (pGL3/–2304) (Fig. 1B). Transfection assays were carried out with the human hepatocyte cell line L02 cells and human hepatoma cell line HepG2 cells, and this promoter construct showed substantial transcriptional activity in both cells (Fig. 2A). Furthermore, promoter activities were measured following delivery of reporter vectors in mice by injection into tail veins. The use of this technology helped us study promoter activity in vivo (12–14). 24 h after the injection, promoter activity of pGL3/–2304 in the liver was about 18-fold higher than for those transfected with pGL3/Basic plasmid; however, no significant difference was detected in other organs, including the heart, kidney, lungs, and spleen (Fig. 2B).
FIGURE 2.
Functional analysis of the HPS promoter in HepG2 cells, L02 cells and mouse liver, and deletion mapping of the HPS promoter. A, nearly 2.4 kb of the 5′-flanking sequence upstream of the HPS promoter was cloned into pGL3/Basic vector (–2304 to +135). 500 ng of pGL3/–2304 or the empty pGL3/Basic vector alone were transfected into L02 cells or HepG2 cells. B, 5 μg of pGL3/–2304 were transfected into the mouse as described under “Experimental Procedures.” 24 h later, the kidney, liver, spleen, lung, and heart were excised, weighed, lysed, and assayed for luciferase activity. C, 5 μg of pGL3/–2304 and six progressive 5′ nested deletions, termed pGL3/–1654 (–1654 to +135), pGL3/–1058 (–1058 to +135), pGL3/–454 (–454 to +135), pGL3/–64 (–64 to +135), pGL3/+57 (+57 to +135), and pGL3/+117 (+117 to +135), were transfected into mouse liver, respectively. Left, schematic diagrams of the various HPS deletion constructs; right, graphical representation of relative luciferase activity. D, schematic representation of 5′-flanking sequence from –1058 to –454 bp. E, effects of deletions of HNF3β, CEBPα, and HNF1α elements in the HPS gene promoter on hepatocyte-specific expression. 5 μg of pGL3/–784 (–784 to +135), pGL3/–614 (–614 to +135), pGL3/–554 (–554 to +135), or pGL3/–454 (–454 to +135), respectively, were transfected into the mouse livers. All luciferase activity assays were performed 24 h post-transfection. The values were normalized to Renilla luciferase activity that was transfected concurrently in all of the assays to correct for transfection efficiency. Relative luciferase activity was expressed as means ± S.E. of triplicate measurements for the same assay (p < 0.05).
Then we investigated the minimal promoter region by constructing a series of 5′-flanking region deletion fragments, including exon 1 and a portion of intron 1, inserted into plasmids upstream of the luciferase reporter. As shown in Fig. 2C, pGL3/–2304, pGL3/–1654, and pGL3/–1058 constructs showed significant promoter activities to about 18-fold more than pGL3/Basic vector alone. Sequential deletions of the 5′ region up to –454 bp resulted in an obvious decrease in promoter activity, and deletion of an additional 390 bp or more, as found in pGL3/–454, led to an about 15-fold reduction in promoter activity. This finding suggested that there was a strong positive regulator of HPS expression within the –1058 to –454 bp region of the HPS promoter. We searched for potential cis-regulatory element(s) within this 604-bp promoter fragment (between nucleotides –1058 and –454) in the TRANSFAC data base, and this revealed multiple putative binding sites for liver-enriched transcription factors, including HNF3β, CEBPα, and HNF1α (Fig. 2D).
According to the search results, four fine deletion mutants (pGL3/–784, pGL3/–614, pGL3/–554, and pGL3/–454), which lacked the HNF3β, C/EBP, and HNF1α binding sites, respectively, were constructed, and reporter activity was analyzed in vivo as well. As shown in Fig. 2E, in contrast to pGL3/–1058, there was no significant difference in pGL3/–784 reporter activity. Deletion of 444 bp (resulting in –614 to +135 bp) substantially enhanced to 42.54-fold over the basal level and to 2-fold over pGL3/–1058, indicating that this region contained negative regulatory elements. A high level of activation was also maintained with the promoter fragment, including the region from –554 to +135 bp. However, deletion of a further 100 bp (resulting in –454 to +135 bp) substantially reduced ∼90% of activity compared with pGL3/–1058, indicating that the promoter region from –554 to –454 bp, possibly the HNF1α-binding sequence, was critical to the regulation of HPS promoter activity in vivo.
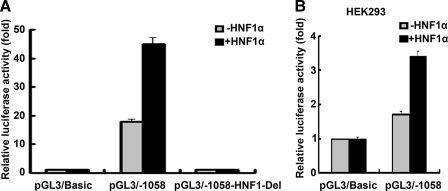
Sequence evaluation of the proximal HPS gene promoter revealed that the HNF1 recognition element was located between nucleotides –470 and –457, and to verify the contribution of the HNF1 binding sites to HPS promoter activity, pGL3/–1058 or deletion constructs termed pGL3/–1058-HNF1-Del (deletion of the region –470/–457bp) and HNF1α, were transiently co-transfected into mouse livers. In a typical experiment (Fig. 3A), the promoter activity of pGL3/–1058 reporter was activated to 18-fold over the basal level. Co-transfection of HNF1α with pGL3/–1058 led to a strong stimulation of reporter activity, up to 45-fold over the basal level and up to 2.5-fold over the maximum level obtained with pGL3/–1058 alone. However, analysis of the basal level of pGL3/–1058 promoter activity without the addition of exogenously expressed HNF1α showed a significant reduction upon deletion of the HNF1 binding site (pGL3/–1058-HNF1-Del). Moreover, deletion of the HNF1 binding site completely abolished the induction produced by overexpression of HNF1α. These data strongly supported the argument that HNF1α was important to the expression of HPS in vivo. Additionally, we also examined the HNF1α sensitivity of the promoter in nonliver cell lines. As shown in Fig. 3B, HNF1α enhanced pGL3/–1058 promoter activity up to ∼3.4-fold over the basal level and up to ∼1.7-fold over the level obtained with pGL3/–1058 alone in the HEK293 cells. Transfection of 293 cells with the HNF1α expression vector did not result in a strong induction of the activity of the HPS promoter. This may be explained by the previous report that other factors (15) interacting with HNF1 were also involved in the regulation of human HPS expression.
FIGURE 3.
Effects of HNF1α and deletions of HNF1 elements in the human HPS gene on hepatocyte-specific expression. A, 5 μg of the indicated promoter construct and 20 μg of HNF1α or pcDNA3.1 were co-transfected into the mouse liver. 24 h after transfection, the luciferase activity was measured. The values were normalized to Renilla luciferase activity that was transfected concurrently in all of the assays to correct for transfection efficiency. Results are presented as -fold inductions relative to the activity of the mice transfected with pGL3/Basic vector only, taken as 1.0 (first bar in the graph). B, HEK293 cells were co-transfected with 400 ng of HNF1α or pcDNA3.1 and 200 ng of the indicated promoter construct. 24 h after transfection, the luciferase activity was measured, and the results are presented as -fold inductions relative to the activity of cells co-transfected with pGL3/Basic only, taken as 1.0 (p < 0.05).
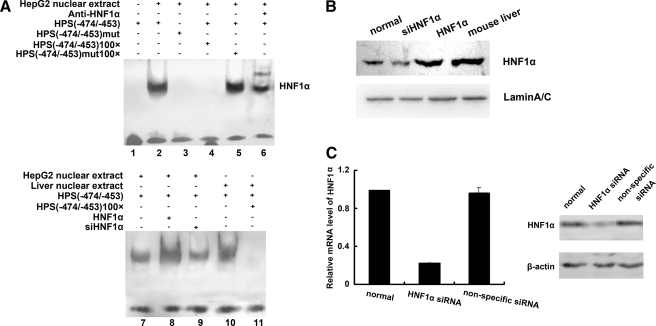
We further evaluated the binding of HNF1α to this element by an electrophoretic mobility shift assay using nuclear extracts from the human hepatocarcinoma cell line HepG2 (Fig. 4A). Significant amounts of a single DNA-protein complex were observed with nuclear extracts from HepG2 cells with the wild-type probe (Fig. 4A, lane 2) but not with the mutant HNF1 probe (Fig. 4A, lane 3). The nuclear extract binding was specific to HPS, having been effectively outcompeted by an excess of nonlabeled wild-type probe (Fig. 4A, lane 4) but not by an excess of nonlabeled mutant probe (Fig. 4A, lane 5). The DNA-protein complex from HepG2 nuclear extracts with the wild-type probe was identified by supershifting with specific HNF1α antibody (Fig. 4A, lane 6).
FIGURE 4.
Binding of HNF1α to the HPS promoter region. A, nuclear extracts, prepared from HepG2 cells or mouse livers, were prepared to analyze the binding to biotin-labeled oligonucleotides by an electrophoretic mobility shift assay. The oligonucleotides contained the sequence between –474 and –453 of the HPS promoter. The biotin-labeled oligonucleotides were incubated with biotin-labeled double-stranded probes with or without a mutation for the HNF1 binding site and resolved on a 4% nondenatured acrylamide gel. For competition assays, a 100-fold molar excess of unlabeled probe was added to the binding reaction mixture, and for the supershift assays, HNF1α antibody was added. Lane 1, binding reaction between water and biotin-labeled HNF1 probe. Lane 2, binding reaction between nuclear extracts and biotin-labeled HNF1 probe. Lane 3, binding reaction between nuclear extracts and biotin-labeled mutant HNF1 probe. Lane 4, competition binding between labeled and unlabeled HNF1 probe with HepG2 nuclear extract. Lane 5, competition binding between labeled HNF1 probe and unlabeled mutant HNF1 probe with HepG2 nuclear extract. Lane 6, supershift reaction between labeled HNF1 probe, nuclear extracts, and the HNF1α antibody. Lane 7, binding reaction between HepG2 nuclear extracts and biotin-labeled HNF1 probe. Lane 8, binding reaction between nuclear extracts derived from HepG2 cells that were transfected with 4 μg of HNF1α expression vector and biotin-labeled HNF1 probe. Lane 9, binding reaction between nuclear extracts derived from HepG2 cells that were transfected with HNF1α siRNA and biotin-labeled HNF1 probe. Lane 10, binding reaction between mouse liver nuclear extracts and biotin-labeled HNF1 probe. Lane 11, competition binding between labeled and unlabeled HNF1 probe with mouse liver nuclear extract. B, the amount of HNF1α in each reaction was examined by Western blot. C, HepG2 cells were transfected with HNF1α siRNA or nonspecific RNA duplex for 60 h. HNF1α expression was detected by real time PCR with GAPDH as the internal control and immunoblotting with β-actin as the internal control. -Fold induction represented the relative expression of HNF1α mRNA in HNF1α siRNA-treated HepG2 cells over that of normal controls.
Direct binding of HNF1α to the probe dramatically increased in nuclear extracts from HepG2 cells transfected with HNF1α (lane 8) in contrast to the band of control cells (lane 7). Transfection with siRNA directed against HNF1α into HepG2 cells reduced the levels of HNF1α mRNA by 30% (p < 0.05) and of HNF1α protein by 40% (p < 0.05) (Fig. 4C). The formation of DNA-protein complexes between nuclear extracts from cells knocked down HNF1α, and wild-type probes become weaker as well (lane 9). These results indicated that the decreased HNF1α binding activity at the HNF1 site in the HPS promoter was due to the down-regulation of HNF1α. Similarly, nuclear extracts from the mouse liver formed a single major complex with the wild-type probe (lane 10); binding specificity was shown by competition for binding by an excess of nonlabeled wild-type probe (lane 11). Western blot analysis was presented to reflect the amounts of HNF1α in the reaction (Fig. 4B).
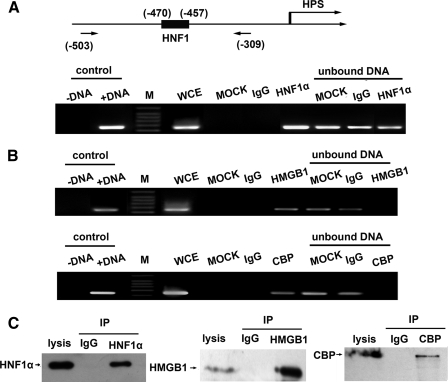
In vivo interaction of endogenously expressed HNF1α with the HPS proximal promoter was evaluated by ChIP analysis of anti-HNF1α antibody in HepG2 cells. As shown in Fig. 5A, PCR amplification of the region –503/–309 bp (with the HNF1 site at positions –470/–457 bp) using HNF1α immunoprecipitates as the template thus yielded a band of the expected size under all circumstances, which indicated the interaction of HNF1α with its recognition elements in vivo. Moreover, a trifling amplification band was yielded from rabbit IgG immunoprecipitates.
FIGURE 5.
HNF1α, HMGB1, and CBP are recruited to endogenous HPS promoters. A, schematic drawing of the HPS promoter region and the positions of PCR primers used for the ChIP assay are shown by arrows at the bottom (upper diagram). ChIP assays against HNF1α from extracts of HepG2 cells were performed. The following templates were used: DNA negative (just water as the negative control), DNA-positive (pGL3/–1058 plasmid as the positive PCR control), whole cell extract (a small aliquot of chromatin, which was saved before immunoprecipitations), mock (no antibody), IgG (rabbit total IgG), and HNF1α (HNF1α immunoprecipitate). Protein A/G-agrose-unbound DNA fractions were also used as control templates. B, ChIP assays with CBP immunoprecipitates and HMGB1 immunoprecipitates were performed using the same primers as in A. Positive and negative controls were equivalent to those in A. C, cleared whole cell extract was first incubated with control IgG or anti-HNF1α, anti-HMGB1, or anti-CBP antibody and then with protein A/G-agarose as an adsorbent. Bound proteins were detected by immunoblotting with antibodies to HNF1α, HMGB1, or CBP. IP, immunoprecipitation.
According to previous reports that coregulatory factors, such as CBP (15) and HMGB1 (7), interacted with HNF1α and were involved in liver-specific gene expression regulation, we carried out additional ChIP assays using the antibody against CBP or HMGB1. PCR amplifications using anti-CBP immunoprecipitates or anti-HMGB1 immunoprecipitates as templates both examined the HNF1 recognition region (amplicon –503/–309 bp) (Fig. 5B) and suggested that CBP and HMGB1 seemed to be recruited by HNF1α and participate in HPS transcription by interacting with HNF1α. Western blot was performed to examine the bound protein immunoprecipitated by HNF1α antibody, HMGB1 antibody, or CBP antibody (Fig. 5C).
HPS Expresses Down-regulation in Human HCC Tissues—We analyzed HPS mRNA expression in a series of 142 HCC patients using tissue microarray technology. Investigation of HPS mRNA expression was informative in 142 HCC tissue specimens. There were 113 males and 29 females with a median age of 50 years (range 18–98 years). The clinical-pathological features and RNA in situ hybridization results of the HCC tumor cohort are shown in Table 2. Among the 142 HCC tissue specimens, the proportion of specimens lacking HPS staining was 59.9% (85/142); the specimens with vague positive signals or less than 25% stained cells was 37 (26%); 16 specimens (11.3%) appeared with medium-positive signals, or 25–50% stained cells; and three specimens (2.1%) showed strong positive signals, or more than 50% stained cells. However, the decrease of HPS in HCC was not significantly correlated with sex, age, and histological grade (χ2 test).
TABLE 2.
HPS immunoreactivity in HCC tissue of 142 patients in relation to clinical-pathological characteristics
RNA in situ hybridization of HPS was performed in 142 HCCs. Minus and plus signs indicate the intensity of the staining. –, no positive signals; +, vague positive signals or less than 25% stained cells; ++, medium positive signals or 25–50% stained cells; +++, strong positive signals or more than 50% stained cells. The χ2 test was performed to test the significance of association.
| Clinical-pathological characteristics | n | - | + | ++ | +++ |
|---|---|---|---|---|---|
| Age at diagnosis | |||||
| <60 years | 110 | 70 | 24 | 14 | 1 |
| ≥60 years | 32 | 15 | 13 | 2 | 2 |
| Gender | |||||
| Female | 29 | 16 | 10 | 2 | 1 |
| Male | 113 | 69 | 27 | 14 | 2 |
| Histological grade | |||||
| All | 142 | 85 | 37 | 16 | 3 |
| G1 | 16 | 9 | 5 | 2 | |
| G2 | 84 | 52 | 21 | 8 | 2 |
| G3 | 42 | 24 | 11 | 6 | 1 |
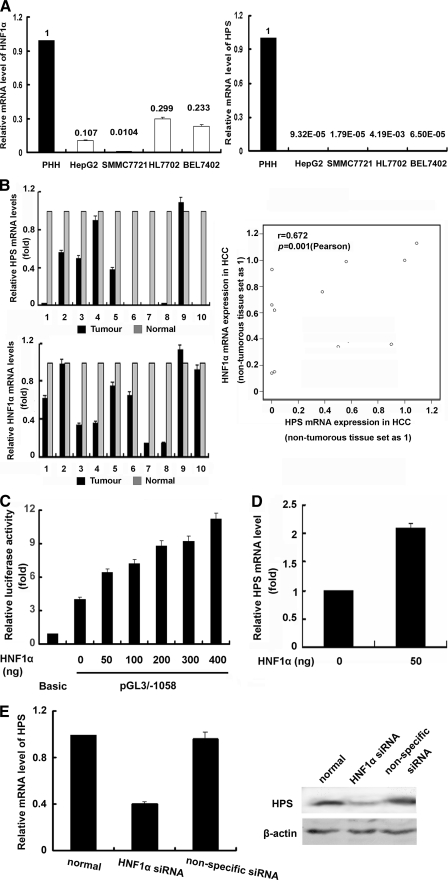
Reduced HNF1α Expression in HCC Causes Down-regulation of HPS—Since we have previously shown the regulation of HPS transcription by HNF1α, we wanted to investigate whether HNF1α was involved in the molecular mechanisms responsible for the down-regulation of HPS expression in human HCC tissues. To determine whether HNF1α and HPS mRNA showed coordinated expression patterns in different HCC cell lines and tissue samples, the expression levels of HPS and HNF1α were evaluated by real time PCR. As shown in Fig. 6A, strong reductions of HNF1α mRNA expression were found in all four HCC cell lines compared with primary human hepatocytes. Similarly, analysis of HNF1α mRNA expression in the tumor tissue of 10 HCC patients revealed a significant reduction of HNF1α expression in seven cases as compared with corresponding nonneoplastic liver tissue. Moreover, HPS was found down-regulated in all four HCC cell lines and nine HCC samples, and reduction of HNF1α expression correlated significantly with the down-regulation of HPS expression in HCC tissues (r = 0.672, p = 0.001) (Fig. 6B).
FIGURE 6.
Reduced HNF1α expression in HCC causes down-regulation of HPS. A, HNF1α and HPS mRNA expression was determined by quantitative reverse transcription-PCR in primary hepatocytes (PHH) and four HCC cell lines (HepG2, SMCC7721, HL7702, and BEL7402). The value represents the -fold change in mRNA level of HNF1α (left) or HPS (right) in relation to primary human hepatocytes (set as 1). B, correlation of HNF1α and HPS mRNA expression in 10 human HCC samples and corresponding nontumorous liver tissue samples. The Spearman test was used to analyze the correlation between parameters. C, various amounts of HNF1α and pGL3/–1058 were co-transfected into HepG2 cells. Control vector (pcDNA3.1) was added as necessary to keep the amount of transfected plasmids constant. 24 h after transfection, the luciferase activity was measured, and the results are shown as -fold inductions relative to the activity of cells co-transfected with pGL3/Basic only, taken as 1.0 (p < 0.05). D, HepG2 cells were transfected with 50 ng of HNF1α or control vector (pcDNA3.1) for 24 h. Total RNA were extracted and reverse transcribed. Real time PCR was performed in triplicates, and results were normalized to the endogenous control. -Fold induction represents the relative expression of HPS mRNA in HNF1α-treated HepG2 cells over that of control vector-treated. E, suppression of HNF1α decreased the expression of HPS. HepG2 cells were transfected with HNF1α siRNA or nonspecific RNA duplex for 60 h. HPS expression was detected by real time PCR with GAPDH as the internal control and immunoblotting with β-actin as the internal control. -Fold induction represented the relative expression of HPS mRNA in siHNF1-treated HepG2 cells over that of normal controls.
To verify whether changes of HNF1α expression might directly influence the transcriptional activity of the HPS promoter, increasing amounts of HNF1α were transfected with a Luc-reporter construct linked to the minimal HPS promoter (pGL3/–1058). As Fig. 6C shows, co-transfection of the 50-ng HNF1α expression vector with pGL3/–1058 reporter construct resulted in an about 6.4-fold increase of promoter activity over the basal level and in a more than 1.6-fold enhancement over the maximum level obtained with pGL3/–1058 alone. Further increases of the HNF1α expression vector led to a dose-dependent increase of the HPS promoter activity compared with the control.
To confirm that HNF1α may act as an activator of HPS gene transcription, real time PCR and RNA interference assays were performed. HepG2 cells were transfected with pcDNA3.1-HNF1α, and compared with cells transfected with pcDNA3.1, 50 ng of HNF1α vector promoted ∼2 fold up-regulation of HPS mRNA expression (Fig. 6D). When endogenous HNF1α expression was knocked down by siRNA, HPS mRNA was reduced 60% (p < 0.05), and protein was reduced 50% (p < 0.05). In addition, transfection with nonspecific siRNA only slightly decreased the level of HNF1α protein (Fig. 6E).
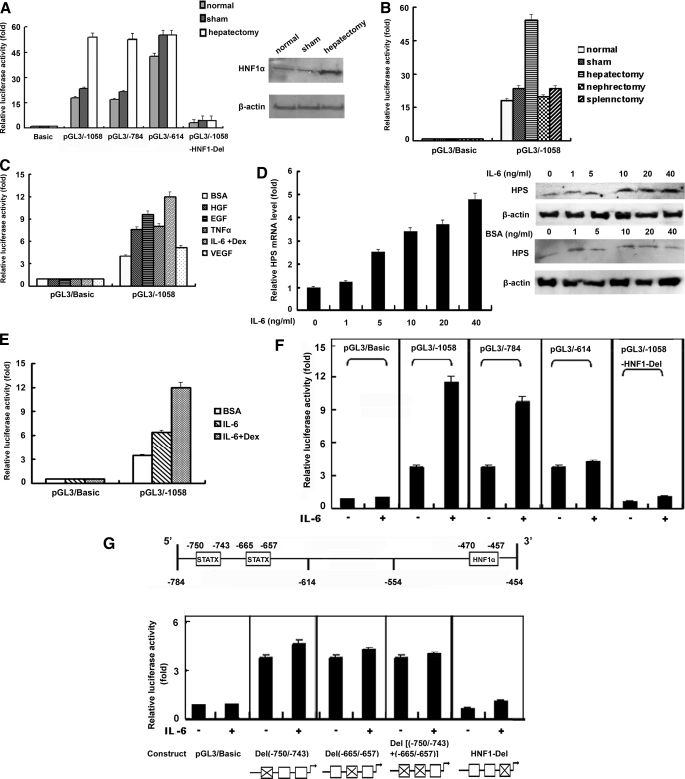
Effects of Hepatectomy and Cytokines on Promoter Activity of HPS Promoter Constructs—Previous studies have described how HPS may function as a regulator of cell growth in liver regeneration (3). To investigate the mechanism of up-regulation of expression in liver regeneration, several luciferase-HPS promoter constructs were injected into mouse tail veins, acute liver injury was induced by 70% liver resection, and luciferase activities were measured 24 h later. As shown in Fig. 7A, at 24 h after a 70% PHx treatment, no clear difference was found in pGL3/Basic reporter activity, whereas the promoter activity of pGL3/–1058 and pGL3/–784 increased about 3-fold over the sham control, up to about 50-fold over the basal level. In addition, no significant increase was observed in the sham control compared with the normal control. However, PHx did not increase the promoter activity of pGL3/–614 HPS promoter constructs compared with the sham control, indicating that the promoter region between –784 and –614 bp was critical for the up-regulation of HPS promoter activity in liver regeneration. In addition, PHx did not affect the promoter activity of reporter construct pGL3/–1058 with the deleted HNF1-binding site, suggesting that the HNF1-binding site was involved in increasing HPS by PHx. Moreover, we identified the enhancement of HNF1α expression following PHx (Fig. 7A, right), which may be involved in up-regulation of HPS expression in liver regeneration.
FIGURE 7.
Effects of hepatectomy and cytokines on promoter activity of HPS promoter constructs. A, 5 μg of the indicated promoter construct was transfected into each mouse liver, and acute liver injury was induced by administration of 70% liver resection. Sham group animals had their bellies cut open without surgery. Luciferase activity was measured 24 h later, and the results are shown as -fold induction relative to the activity of a normal mouse that was transfected with pGL3/Basic only and had not undergone laparotomy treatment, taken as 1.0 (p < 0.05). Western blot was performed to detect HNF1α expression with β-actin as the internal control. B, 5 μg of pGL3/Basic or pGL3/–1058 was transfected into each mouse liver, hepatectomy was performed by administration of 70% liver resection, nephrectomy was performed by the removal of the left kidney, and splenectomy was performed by excision of the spleen. Sham control animals had their bellies cut open without surgery. Luciferase activity was measured 24 h later, and the results are shown as described for A. C, L02 cells, following overnight serum starvation, were transfected with 500 ng of pGL3/Basic or pGL3/–1058 and were stimulated with the cytokine 10 ng/ml HGF, 10 ng/ml EGF, 10 ng/ml TNFα, or 10 ng/ml IL-6 with 1 μm dexamethasone or 10 ng/ml vascular endothelial growth factor. Cells treated with equivalent amounts of BSA were used as controls. 24 h later, luciferase activity was measured. The values were normalized to Renilla luciferase activity that was transfected concurrently to correct for transfection efficiency. Results were presented as -fold inductions relative to the activity of cells that were transfected with pGL3/Basic only and underwent BSA treatment, taken as 1.0 (p < 0.05). D, L02 cells were incubated with the indicated amounts of IL-6 in the presence of 1 μm dexamethasone. Cells treated with equivalent amounts of BSA were used as controls. -Fold changes in mRNA were determined by dividing the relative level of HPS mRNA in IL-6-treated cells by that of HPS mRNA in BSA-treated cells (left). HPS protein expression was detected by immunoblotting with β-actin as the internal control. E, L02 cells, following overnight serum starvation, were transfected with 500 ng of pGL3/Basic or pGL3/–1058, and 6 h after transfection, 10 ng/ml BSA, 10 ng/ml IL-6, or 10 ng/ml IL-6 with 1 μm dexamethasone were added in the growth medium. 24 h after transfection, luciferase activity was measured. Results are shown as described for C. F and G, L02 cells, following overnight serum starvation, were transfected with 500 ng of the indicated promoter construct, respectively, and 6 h later, cells were treated with 10 ng/ml BSA or 10 ng/ml IL-6 in the presence of 1 μm dexamethasone. Luciferase activity was measured 24 h later, and the results were shown as described for C. Below G are diagrams of the individual promoter constructs with the deleted elements indicated by X.
To explore the impact of nonhepatic tissue injury on hepatic HPS expression, nephrectomy and splenectomy were carried out, and the promoter activity of pGL3/–1058 in liver tissue was detected. As Fig. 7B shows, laparotomy did not induce pGL3/Basic reporter activity, and, after PHx, the promoter activity of pGL3/–1058 increased by about 3-fold over the sham control and by 52-fold over the basal level, whereas nephrectomy and splenectomy led to negligible increases in promoter activity over the normal control and the sham control. This suggested that the up-regulation of HPS expression in hepatic injury may be liver-specific.
Cytokines play an important role in liver mass restoration and repair after injury by acting on cell proliferation and apoptosis (16). To evaluate the effects of cytokines, including HGF, EGF, TNFα, vascular endothelial growth factor, and IL-6, known to be elevated during liver regeneration, and determine whether they regulate HPS expression, L02 cells, following overnight serum starvation, were transfected with the pGL3/–1058 vector and then treated with 10 ng/ml cytokines or BSA for 24 h, and reporter activity was analyzed. As shown in Fig. 7C, HPS promoter activity induced to varying degrees in L02 cells by stimulation of HGF, EGF, IL-6, and TNFα. The induction by cytokines was promoter-dependent, because no activation was detected with the promoterless pGL3/Basic vector. We noted that 10 ng/ml IL-6 induced a more than 3-fold promoter activity increase of pGL3/–1058 over BSA control and arrived at about 12-fold activity over the basal level. To further verify the induction of HPS expression by IL-6, endogenous HPS mRNA and protein levels in IL-6-treated L02 cells were detected. As shown in Fig. 7D, HPS mRNA expression was increased in a dose-dependent manner upon treatment with 1–40 ng/ml IL-6. Treatment with 1 ng/ml IL-6 resulted in a slight increase in HPS mRNA level; the levels were more profound at 10 ng/ml; 40 ng/ml IL-6 induced HPS mRNA expression that was ∼5-fold over the basal level. Not surprisingly, the enhancement of HPS transcription led to increased protein level with IL-6 stimulation, as shown in Western blot analysis.
Then we examined the achievement of the IL-6 receptor involved in regulating HPS expression. Because dexamethasone could stimulate the surface delivery of the hepatocyte IL-6 receptor, it is likely that dexamethasone augmented the effect of IL-6 (6). As shown in Fig. 7E, during treatment with 10 ng/ml IL-6 alone, the promoter activity of pGL3/–1058 was induced 1.8-fold compared with the cells treated with BSA, up to ∼7.2-fold over the basal level. Moreover, using both IL-6 and dexamethasone produced a more than 3-fold increase in HPS promoter activity compared with BSA control, up to ∼12-fold above pGL3/Basic, whereas treatment with dexamethasone singly led to a negligible increase of HPS promoter activity (data not shown). These results indicated that the induction of HPS upon IL-6 treatment also correlated with IL-6 receptor activity.
To identify the HPS gene promoter sequence that was responsible for IL-6-induced promoter activity, several of the luciferase-HPS promoter constructs were used in transient transfections in L02 cells in the absence or presence of IL-6 (Fig. 7F). The pGL3/Basic construct did not respond to IL-6 stimulation. The promoter activity of pGL3/–1058 and pGL3/–784 increased up to about 11-fold in cells treated with 10 ng/ml IL-6 over the basal level and to 2–3-fold over the cells treated with BSA, and the IL-6 response substantially decreased in pGL3/–614. Moreover, deletion of the HNF1 binding site resulted in the promoter construct pGL3/–1058-HNF1-Del blocking not only the IL-6 enhancement but also the basic promoter activity. These results suggested that –784 to –614 bp of the HPS promoter were responsible for IL-6-induced promoter activity of this gene and that the HNF1-binding site played an important role in the basal and IL-6-induced promoter activity of this gene. As Fig. 7G showed, two potential STAT3-binding sites were located in the region –750/–743 bp and –665/–657bp of the HPS promoter. Previous studies have revealed that IL-6-induced STAT3 amplifies HNF1-mediated transactivation of hepatic genes (17). Therefore, we examined whether the deletion of STAT3-binding sites affected the IL-6-induced promoter activity of the HPS gene and which STAT3-binding site was critical in the response to IL-6 stimulation. We transfected reporter construct pGL3/–1058 with mutated STAT3-binding sites in L02 cells and determined promoter activity in the presence or absence of IL-6. Results of these experiments showed that the promoter construct pGL3/–1058-STAT3-Del(–750/–743) with deletion of the first STAT3-binding site, the construct pGL3/–1058-STAT3-Del(–665/–657) deleted of the second STAT3-binding site, and combinations of these two mutants pGL3/–1058-STAT3-Del((–750/–743)+(–665/–657)) showed significant reductions in IL-6-induced promoter activities. However, the STAT3-binding site deletions did not affect the basic transcription activity of the reporter. The results of this experiment suggested that STAT3-binding sites play a crucial role in IL-6-induced promoter activity of HPS.
DISCUSSION
In this study, the promoter of the human HPS gene was cloned, and deletion constructs were created, mutated, and used to find important elements in the DNA sequence that could explain the liver-specific expression of this gene. We identified a binding site for HNF1 located at position –470 to –457 bp upstream of the transcription start site of human HPS and demonstrated that the HNF1 binding site was required for HPS promoter activity in vivo. Additionally, we confirmed the binding of endogenous HNF1α to the HPS proximal promoter in vitro by an electrophoretic mobility shift assay and in vivo by ChIP analysis strongly supported the argument that HNF1α was important to the in vivo expression of HPS. Overexpression of HNF1α in mouse livers could further enhance activity of the HPS promoter, and HPS promoter activity was augmented by expression of HNF1α in human embryonic kidney 293 cell line. Knocked down endogenous HNF1α expression by siRNA resulted in significant reductions of HPS expression. These results provided data to support the idea that HNF1 binding sites in the HPS promoter controlled hepatocyte-specific expression of HPS.
HNF1α protein is the most widely distributed regulator of liver-specific gene expression. Its potential binding sites have been found in regulatory regions of more than 100 genes, such as albumin, α-fetoprotein, α-fibrinogen, β-fibrinogen, α1-antitrypsin, transthyretin, aldolase B, and the hepatitis B virus large surface protein (18–23). Most often, these sites are localized in promoter regions and form clusters with binding sites of other transcription factors. However, it also has been shown that a highly efficient, liver-specific promoter can be obtained with only an HNF1 site (24). HNF1α expression was first regarded as a hepatocyte-specific transcriptional regulator, and later, its expression was also found in the kidney, intestine, and endocrine pancreas (25). Further studies revealed that HNF1α was also involved in regulating gene expression in these tissues (26–28). It has been reported that HNF1α occupied target gene promoters in diverse tissues but played an obligatory role in transcriptional activation only in cell- and promoter-specific contexts in which it was required to recruit histone acetylase activity (15). Because the HPS gene is not expressed in other organs (i.e. the kidney) with high levels of HNF1α expression, we propose that HNF1α binding to the HPS promoter is not only dependent on its requirement for transcription; the tissue-specific reliance on HNF1α for transcription of distinct genes is likely to be, at least in part, mediated by the role of HNF1α inducing localized hyperacetylation of chromatin, just as the phenylalanine hydroxylase promoter requires HNF1α for transcriptional activity and localized histone hyperacetylation only in liver tissue (15). CBP possessed intrinsic histone acetyltransferase activity, was identified as a partner for cAMP-response element-binding protein, and facilitated HNF1α to form preinitiation complexes at relevant promoters (29). In our ChIP analysis, we present evidence to show the binding of CBP to the HPS promoter in vivo. We also ascertain that another chromosomal protein, HMGB1, was recruited to the HPS promoter by HNF1α. Consequently, HNF1α can induce HPS promoter activity, consisting of the recruitment of histone acetylase activity to its target sites.
Our study revealed that there was a negative element in the HPS promoter located at position –784 to –614 bp upstream from the transcription start site of human HPS. Putative binding sites for several transcription factors were identified in this region, such as c-Ets, MyoD, VBP, and E4BP4. The regulatory roles of these transcription factor binding sites in HPS transcription are still not clear.
Previous studies have found that HPS was down-regulated or lost in HCC (4). Our finding of reduced HPS expression in HCC cell lines and tissues was in accordance with previous reports, and we also demonstrated that there was a strong correlation between the tissue-specific expression of HPS and the expression of HNF1α. The critical role of HNF1α and the HNF1 binding site in regulating HPS gene expression prompted us to postulate that decreased HPS gene transcription in HCC was mediated through HNF1α. This mechanism of HPS gene suppression is supported by our observation that HCC decreased the abundance and binding activity of HNF1α. To our knowledge, similar mechanisms may regulate the expression of other genes in HCC. For example, the tumor suppressor MIA2 (melanoma inhibitory activity 2), ALB (albumin), GST (glutathione S-transferase α), A1AT (α1-antitrypsin), and FGB (β-fibrinogen) are reduced in HCC, which correlate with reduced expression of HNF1α (30–32). A significant amount of HNF1α usually appears in differentiated hepatoma lines (33). Hepatoma dedifferentiation and suppression of hepatospecific gene expression is usually accompanied by a decrease in HNF1α expression (28). Moreover, a tumor suppressor function for HNF1α is supported by the phenotypes observed in two HNF1-deficient mouse strains (27). Furthermore, the growth suppressor activity of HPS in HCC also has been described. Therefore, our findings indicated a novel mechanism demonstrating the way in which HNF1α expression in HCC affected tumorigenicity through HPS regulation.
Some liver-specific genes, which are known to be regulated by HNF1α, including hepatic metabolic genes, like those encoding G6Pase and PEPCK, and hepatic acute-phase response genes, like those encoding fibrinogen, α1-antitrypsin, and C-reactive protein, have been reported to show transcriptional up-regulation by IL-6 during the acute phase inflammatory response. This is to help restore homeostasis and restrict proteolytic and/or fibrogenic activity and tissue damage (6, 17). In our series of experiments, we found that partial hepatectomy and IL-6 significantly induced HPS promoter activity, and the induction was dependent on IL-6 receptor activation, largely for the augmentation effect on the induction, by combining IL-6 with dexamethasone. Sequence examination of the HPS promoter revealed two potential STAT3-binding sites. Our results showed that both of these STAT3-binding sites were involved in induced promoter activity of HPS by IL-6 as well. Still, no response was examined in the promoter construct with the deletion of the HNF1 binding site in either the presence or absence of IL-6. It is known that a mechanism whereby a tissue-specific transcription factor and DNA binding element are present in the promoter of many liver-specific genes may interact with induced transcription factors in response to specific external signals (17). Therefore, we presume that HNF1α may coordinate with the IL-6/IL-6R/STAT3 pathway, leading to synergistic transcriptional up-regulation of the HPS promoter to maintain metabolic homeostasis during times of growth and repair, like the IGFBP-1, G6Pase, and α-fibrinogen promoters (17).
In conclusion, we provide evidence that the transcription factor HNF1α plays an important role in HPS promoter activity in vivo and demonstrate that down-regulation or loss of HNF1α causes, at least in part, the transcriptional down-regulation of HPS in HCC. During liver regeneration, induction of HPS mRNA expression occurs at the transcriptional level, consequent to specific up-regulation of critical regulators of HPS gene expression as well.
This work was partially supported by the Special Funds for Major State Basic Research of China (2006CB910802) and the National Program on Key Basic Research Projects (2004CB518903).
Footnotes
The abbreviations used are: HPS, hepassocin; HCC, heptocellular carcinoma; HGF, hepatocyte growth factor; EGF, epidermal growth factor; TNF, tumor necrosis factor; BSA, bovine serum albumin; RACE, rapid amplification of cDNA ends; siRNA, small interfering RNA; PHx, partial hepatectomy; TNF, tumor necrosis factor; BSA, bovine serum albumin; IL, interleukin; CBP, CREB-binding protein; ChIP, chromatin immunoprecipitation; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
References
- 1.Hara, H., Uchida, S., Yoshimura, H., Aoki, M., Toyoda, Y., Sakai, Y., Morimoto, S., Fukamachi, H., Shiokawa, K., and Hanada, K. (2000) Biochim. Biophys. Acta 1492 31–44 [DOI] [PubMed] [Google Scholar]
- 2.Hara, H., Yoshimura, H., Uchida, S., Toyoda, Y., Aoki, M., Sakai, Y., Morimoto, S., and Shiokawa, K. (2001) Biochim. Biophys. Acta 1520 45–53 [DOI] [PubMed] [Google Scholar]
- 3.Yan, J., Ying, H., Gu, F., He, J., Li, Y. L., Liu, H. M., and Xu, Y. H. (2002) Cell Res. 12 353–361 [DOI] [PubMed] [Google Scholar]
- 4.Yan, J., Yu, Y., Wang, N., Chang, Y., Ying, H., Liu, W., He, J., Li, S., Jiang, W., Li, Y., Liu, H., Wang, H., and Xu, Y. (2004) Oncogene 23 1939–1949 [DOI] [PubMed] [Google Scholar]
- 5.Rijken, D. C., Dirkx, S. P., Luider, T. M., and Leebeek, F. W. (2006) Biochem. Biophys. Res. Commun. 350 191–194 [DOI] [PubMed] [Google Scholar]
- 6.Liu, Z., and Ukomadu, C. (2008) Biochem. Biophys. Res. Commun. 365 729–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu, M., Wang, J., Li, W., Yuan, Y. Z., Li, C. Y., Qian, X. H., Xu, W. X., Zhan, Y. Q., and Yang, X. M. (2008) Nucleic Acids Res. 36 1209–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higgins, G. M., and Anderson, R. M. (1931) Arch. Pathol. 12 186–202 [Google Scholar]
- 9.Zheng, F., Plati, A. R., Potier, M., and Striker, G. E. (2003) Am. J. Pathol. 162 1339–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huston, J. M., Ochani, M., Ballina, M. R., and Ulloa, L. (2006) J. Exp. Med. 203 1623–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wardemann, H., Boehm, T., Dear, N., and Carsetti, R. (2002) J. Exp. Med. 195 771–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu, F., Song, Y. K., and Liu, D. (1999) Gene Ther. 6 1258–1266 [DOI] [PubMed] [Google Scholar]
- 13.Andre, F. M., Cournil-Henrionnet, C., Vernerey, D., Opolon, P., and Min, L. M. (2006) Gene Ther. 13 1619–1627 [DOI] [PubMed] [Google Scholar]
- 14.Simone Wattiaux De Coninck, M. L., Wattiaux, R., and Jadot, M. (2004) J. Gene Med. 6 877–883 [DOI] [PubMed] [Google Scholar]
- 15.Parrizas, M., Maestro, M. A., Boj, S. F., Paniagua, A., Casamitjana, R., Gomis, R., Rivera, F., and Ferrer, J. (2001) Mol. Cell. Biol. 21 3234–3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diehl, A. M., and Rai R. M. (1996) FASEB J. 10 215–227 [DOI] [PubMed] [Google Scholar]
- 17.Leu, J. I., Crissey, M. A. S., Leu, J. P., Ciliberto, G., and Taub, R. (2001) Mol. Cell. Biol. 21 414–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lichtsteiner, S., Wuarin, J., and Schibler, U. (1987) Cell 5 963–973 [DOI] [PubMed] [Google Scholar]
- 19.Cereghini, S., Blumenfeld, M., and Yaniv, M. (1988) Genes Dev. 2 957–974 [DOI] [PubMed] [Google Scholar]
- 20.Courtois, G., Morgan, J. G., and Campbell, L. A. (1987) Science 238 688–692 [DOI] [PubMed] [Google Scholar]
- 21.Costa, R. H., Lai, E., and Grayson, D. R. (1988) Mol. Cell. Biol. 8 81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsutsumi K., Ito K., and Ishikawa K. (1989) Mol. Cell. Biol. 9 4923–4931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang, H. K., Wang, B. Y., and Yuh, C. H. (1989) Mol. Cell. Biol. 9 5189–5197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blumenfeld, M., Maury, M., Chouard, T., Yaniv, M., and Condamine, H. (1991) Development 113 589–599 [DOI] [PubMed] [Google Scholar]
- 25.Cereghini, S. (1996) FASEB J. 10 267–282 [PubMed] [Google Scholar]
- 26.Yang, Q., Yamagata, K., Fukuim, K., Cao, Y., Nammo, T., Iwahashi, H., Wang, H., Matsumura, I., and Hanafusa, T. (2002) Diabetes 5 1785–1792 [DOI] [PubMed] [Google Scholar]
- 27.Pontoglio, M., Barra, J., Hadchouel, M., Doyen, A., Kress, C., Bach, J. P., Babinet, C., and Yaniv, M. (1996) Cell 84 575–585 [DOI] [PubMed] [Google Scholar]
- 28.van Wering, H. M., Huibregtse, I. L., van der Zwan, S. M., de Bie, M. S., Dowling, L. N., Boudreau, F., Rings, E. H., Grand, R. J., and Krasinski, S. D. (2002) J. Biol. Chem. 277 27,659–27,667 [DOI] [PubMed] [Google Scholar]
- 29.Dohda, T., Kaneoka, H., Inayoshi, Y., Kamihira, M., Miyake, K., and Iijima, S. (2004) J. Biochem. (Tokyo) 136 313–319 [DOI] [PubMed] [Google Scholar]
- 30.Bosserhoff, A. K., Moser, M., Scholmerich, J., Buettner, R., and Hellerbrand, C. (2003) J. Biol. Chem. 278 15225–15231 [DOI] [PubMed] [Google Scholar]
- 31.Clairmont, A., Ebert, T., Weber, H., Zoidl, C., Eickelmann, P., Schulz, W. A., Sies, H., and Ryffel, G. H. (1994) Cancer Res. 54 1319–1323 [PubMed] [Google Scholar]
- 32.Courtois, G., Baumhueter, S., and Crabtree, G. R. (1988) Proc. Natl. Acad. Sci. U. S. A. 85 7937–7941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayashi, Y., Wang, W., Ninomiya, T., Nagano, H., Ohta, K., and Itoh, H. (1999) J. Clin. Pathol. Mol. Pathol. 52 19–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. (1996) Guide for the Care and Use of Laboratory Animals, pp. 21–55, National Academy Press, Washington, DC