Abstract
In animal cells, Akt (also called protein kinase B) is activated by stimuli that elevate the level of phosphatidylinositol 3,4,5-trisphosphate and is a major effector for eliciting responses that support cell growth and survival. We have shown previously that co-expression of Akt1 in budding yeast (Saccharomyces cerevisiae) along with hyperactive p110α, the catalytic subunit of mammalian phosphatidylinositol 3-kinase, results in Akt1 relocalization to cellular membranes and activation. In the present study, we show that activation of all three mammalian Akt isoforms by wild-type p110α causes deleterious effects on yeast cell growth. Toxicity of Akt in S. cerevisiae required its catalytic activity, its pleckstrin homology domain, and phosphorylation of its activation loop, but not phosphorylation of its hydrophobic motif. We demonstrate that expression in yeast of the only purported oncogenic allele, Akt1(E17K), leads to enhanced phenotypes. Ala-scanning mutagenesis of the VL1 region within the phosphatidylinositol 3,4,5-trisphosphate-interacting pocket of the Akt1 pleckstrin homology domain revealed that most residues in this region are essential for Akt1 activity. We found that active Akt leads to enhanced signaling through the yeast cell wall integrity pathway. This effect requires the upstream Rho1 activator Rom2 and involves both phosphorylation of the MAPK Slt2 and expression of its transcriptional targets, thus providing a quantitative reporter system for heterologous Akt activity in vivo. Collectively, our results disclose a heterologous yeast system that allows the functional assessment in vivo of both loss-of-function and tumorigenic Akt alleles.
Protein kinase B (PKB,3 also known as cellular Akt or c-Akt) belongs to the AGC (cAMP-dependent, cGMP-dependent, protein kinase C) family of protein kinases and is considered a central player in regulation of metabolism, cell survival, apoptosis, cellular proliferation, motility, transcription and cell-cycle progression (1, 2). The Akt subfamily comprises three mammalian isoforms, Akt1, Akt2, and Akt3 (PKBα, PKBβ, and PKBγ, respectively), whereas invertebrates, like flies and worms, have a single PKB/Akt protein. The relative expression of the three mammalian isoforms of Akt differs in different tissues: Akt1 is the predominant isoform in the majority of tissues, Akt2 is mainly present in insulin-responsive tissues, and Akt3 prevails in brain and testes. The three mammalian Akt members are products of different genes and share a common structure that consists of three conserved domains: an N-terminal pleckstrin homology (PH) domain, a kinase catalytic domain at the center, and a C-terminal regulatory domain containing the hydrophobic motif (HM) phosphorylation site (FXXF(S/T)Y) (3, 4). In the lower eukaryote Saccharomyces cerevisiae, an AGC protein kinase, Sch9, has been proposed as a functional ortholog of mammalian Akt, and is involved in nutrient sensing, ribosome biogenesis, lifespan, adaptation to osmotic stress, and cell-size control (5–10). In higher cells, Akt members transduce signals downstream the class I phosphatidylinositol 3-kinases (PI3Ks), which are also proto-oncogenic proteins. Class I PI3K are heterodimeric enzymes containing a catalytic subunit (p110α, -β, -δ, or -γ) and a regulatory subunit (p85α, p85β, p55γ, or p101). Activation of p110 catalytic subunits takes place by growth factor- and hormone-binding to tyrosine kinase- and G protein-coupled receptors, and involves the recruitment of the PI3K regulatory subunits to Tyr-phosphorylated receptors and scaffolding signaling proteins, as well as binding to Ras. The activated PI3K converts membrane bound phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol 3,4,5-trisphosphate (PIP3). The structure of the PH domain of Akt conforms a pocket that accommodates the polar head group of PIP3 (11). This interaction results in the translocation of the Akt kinase from the cytoplasm to the plasma membrane, where it undergoes activation. Mutational analyses have suggested that membrane localization is a primary requirement for Akt oncogenicity and mediation of PI3K-dependent signaling (12, 13). Akt activity is negatively regulated by phosphatidylinositol phosphatases that dephosphorylate PIP3. The major PI3K down-regulator is the PTEN phosphatase, which is considered a tumor suppressor because loss-of-function mutations are found in overt tumors (14) as well as in the germ line in hereditary diseases that predispose to cancer (15). PTEN dephosphorylates the 3′-phosphate of PIP3 and thereby negates the activity of PI3K (16). PIP3 is also metabolized to phosphatidylinositol 3,4-bisphosphate by 5-phosphoinositide phosphatases, like SHIP1 and SHIP2 (SH2-containing inositol phosphatase-1 and -2); but PIP3 and phosphatidyl inositol 3,4 bisphosphate mostly engage the same effectors. Once recruited to the plasma membrane, Akt is phosphorylated at two critical residues for its full activation. These residues include a Thr residue in the activation loop within the kinase catalytic domain (Thr-308, Thr-309, and Thr-305 for Akt1, Akt2, and Akt3, respectively) and a Ser residue on the hydrophobic motif (HM; Ser-473, Ser-474, and Ser-472 for Akt1, Akt2, and Akt3, respectively). The activation loop kinase for Akt is the phosphoinositide-dependent kinase 1 (PDK1). The HM kinase, also termed PDK2, has been identified recently as mTORC2 (17). The full activation of Akt permits a conformational change that results in substrate binding and a significant increase of the rate of catalysis.
The relevance of this pathway in clinical oncology has been evidenced by the frequency of mutations in its major down-regulator, the tumor suppressor PTEN (18) and, more recently, by the isolation of oncogenic mutations in both PI3K and Akt1 themselves (19–21). In previous reports, we described that heterologous expression of PI3K, PTEN, and Akt1 in S. cerevisiae reproduces several aspects of their function, including PIP3-dependent membrane translocation and phosphorylation of Akt1 at both activation residues by the yeast PDK1 orthologs and an unidentified endogenous kinase (22). We have also reported previously that this heterologous model system could be readily exploited to evaluate oncogenicity of both PI3K and PTEN mutants (23). Here, we show that catalytically active mammalian Akt inhibits yeast cell growth, thus extending the system to perform structure-function relationship studies on Akt in vivo.
EXPERIMENTAL PROCEDURES
Strains, Media, and Growth Conditions—The S. cerevisiae strains used in the present study were YPH499 (MATa ade 2–101 trp1–63 leu2–1 ura3–52 his3–200 lys2–801, P. Hieter), BY4741 (MATa his3Δ1; leu2Δ; met15Δ; ura3Δ, EUROSCARF, Germany), slt2Δ (BY4741 isogenic, slt2::KanMX4) bck1Δ (BY4741 isogenic, bck1::KanMX4), and rom2Δ (BY4741 isogenic, rom2::KanMX4). Escherichia coli DH5α F′[K12α (lacZYA-argF)U169 deoR supE44 thi-1 recA1 endA1 hsdR17 gyrA96 relA1 (α 80lacZ α M15)F′] was used for routine molecular biology techniques. YPD (1% (w/v) yeast extract, 2% (w/v) peptone, and 2% (w/v) dextrose/glucose) broth or agar was the general non-selective medium used for yeast cell growth. Synthetic minimal medium (SD) contained 0.17% yeast nitrogen base without amino acids, 0.5% ammonium sulfate, and 2% glucose and lacked appropriate amino acids and nucleic acid bases to maintain selection for plasmids. In SG (synthetic galactose) and SR (synthetic raffinose) media, glucose was replaced with 2% (w/v) galactose or 2% (w/v) raffinose, respectively. Galactose induction in liquid medium was performed by growing cells in SR to mid-exponential phase and then adding galactose to 2% for 4–6 h. Growth assays on plates were performed, typically, by growing transformants overnight in SD lacking leucine and uracil, or leucine and tryptophan, adjusting the culture to an absorbance A600 0.5, and spotting samples (5 μl) of the cell suspension and three serial 10-fold dilutions on to the surface of SD or SG plates lacking the appropriate nutrients to maintain selection for plasmids, followed by incubation at 30 °C for 2–3 days. The mTOR inhibitor rapamycin (Sigma-Aldrich) was added to SG plates lacking the appropriate nutrients at a final concentration of 0.2 μg/ml.
Plasmid Construction—Transformation of E. coli and yeast and other basic molecular biology methods were carried out using standard procedures. Plasmids pYES2-PTEN, pYES2-GFP-Akt1, pYES-GFP-AktK179M, pYES2-myr-Akt1, YCpLG-myc-p110α-CAAX, YCpLG-myc-p110α-wt, YCpLG-myc-p110α-E545K, YCpLG-myc-p110α-H1047R, and pYES3-GFP-Akt1 have been described (22, 23). pYES2-SHIP1 was constructed by subcloning human SHIP1 into pYES2 by PCR from pOTB7 SHIP1 (Mammalian Gene Collection, IMAGE ID 6304992, Geneservice, UK). To produce pYES2-GFP-Akt2, rat Akt2 coding sequence was amplified by PCR from the pEGFP-Akt2 plasmid (24) with primers bearing the appropriated restriction sites at their 5′ tails and cloned in-frame into the EcoRI/XbaI sites of pYES2-GFP. The latter plasmid had been constructed by amplifying the GFP sequence by PCR and cloning it into the HindIII/BamHI sites of pYES2. For pYES2-GFP-Akt3, the mouse Akt3 coding sequence was amplified by PCR from pSPORT1 Akt3 (Mammalian Gene Collection, IMAGE ID 30089997, Geneservice, UK) as above and cloned EcoRI-XbaI into pYES2-GFP. The primers used for amplification had EcoRI- or XbaI-containing tails in the upper and in the lower oligonucleotides, respectively, to allow directed cloning into the same sites of the pYES2-GFP. YCpLG-myc-p110γ and YCpLG-myc-p110δ were made by PCR from the plasmids pCMVSPORT6 p110γ (human sequence, Mammalian Gene Collection, IMAGE ID 5749986) and pCMVSPORT6 p110δ (mouse sequence, Mammalian Gene Collection, IMAGE ID 4192906), respectively. To produce plasmids pYES3-GFP-Akt1R25A, pYES3-GFP-Akt1E40K, pYES3-GFP-Akt1T308A, and pYES3-GFP-Akt1S473A site-directed mutagenesis was carried out using standard full plasmid amplification by DpnI-based PCR strategy with Turbo PfuI DNA polymerase (Stratagene) using pYES3-GFP-Akt1 as template. To obtain the plasmid pYES3-GFP-Akt1T308A,S473A we performed the same strategy using the pYES3-GFP-Akt1S473A plasmid as template. To obtain the plasmids pYES2-GFP-Akt1E17K, pYES2-GFP-Akt1T308D, pYES2-GFP-Akt1S473D, pYES2-GFP-Akt1F469A,F472A, pYES2-GFP-Akt1Y474A, pYES2-GFP-Akt11–467, and pYES2-GFP-Akt11–454 the same strategy was performed, but using pYES2-GFP-Akt1 as template. To obtain the plasmid pYES2-GFP-Akt1T308D,S473D we performed the same strategy using the pYES2-GFP-Akt1S473D plasmid as template. The Ala scanning collection of mutants (R15A, G16A, E17A, Y18A, I19A, K20A, T21A, W22A, R23A, and P24A), as well as the G16R and P24R mutations, were also obtained by PCR-based site-directed mutagenesis on pYES2-GFP-Akt1. The pYES2-GFP-Sch9 plasmid was constructed by PCR amplification from the Sch9 open reading frame from S. cerevisiae BY4741 genomic DNA. The primers used for amplification had BamHI- or EcoRI-containing tails in the upper and in the lower oligonucleotides, respectively, to allow directed cloning. PCR products were cleaved with BamHI-EcoRI to be inserted in the same sites of the pYES2-GFP vector. To generate the chimeras pYES2-Sch9-Akt1 and pYES2-Akt1-Sch9, standard sequential PCR-based method (25) was performed. The primers used for amplification of the different Sch9 or Akt1 regions had BamHI- or EcoRI-containing tails in the upper and lower oligonucleotides, respectively, to allow directed cloning. PCR products were cleaved with BamHI/EcoRI to be inserted in the same sites of the pYES2-GFP plasmid. The pMLP1-LACZ (URA3) plasmid (26) and its HIS3-based version were a gift of A. B. Sanz, R. García, and J. Arroyo. Oligonucleotide sequences used in this work are available upon request. In all cases, fidelity of the amplified DNA was verified by DNA sequencing.
Immunodetection by Western Blotting—The standard procedures were used for yeast cell growth, collection, and breakage, collection of proteins, fractionation by SDS-polyacrylamide gel electrophoresis, and transfer to nitrocellulose membranes. Rabbit anti-phospho-Akt1 (P-Thr-308) and rabbit anti-phospho-Akt1 (P-Ser-473) (Cell Signaling Technology) were used to detect the phosphorylation of the corresponding sites in PKB/Akt or the chimerical protein Sch9-Akt1. GFP fusion proteins were detected using a mouse anti-GFP antibody (JL-8, BD Biosciences, San Jose, CA). Yeast actin was detected by cross-reaction with a mouse anti-actin monoclonal antibody (Clone C4, MP Biomedicals, Irvine, CA). After washing, bound primary antibodies were revealed using horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibodies, as appropriate, and a chemiluminescence detection system (ECL, Amersham Biosciences).
Microscopy Techniques—For fluorescence microscopy on live cells (for the observation of GFP), cells from exponentially growing cultures were harvested by brief centrifugation, washed once with phosphate-buffered saline, and viewed directly. To monitor PI3K-dependent membrane localization of GFP-Akt, a time course was made to monitor membrane binding after induction of both p110 and GFP-Akt in galactose. Localization to membranes was significant at t = 4 h (>60%), and reached the maximum at t = 8 h (>90%). To allow detection of both positive and negative changes in GFP-Akt affinity for membranes, all data presented here were analyzed at 4 h after galactose induction. For statistics on cell populations, an average of >200 cells were counted for each experiment. Cells were examined with an Eclipse TE2000U microscope (Nikon) using the appropriate sets of filters. Digital images were acquired with Orca C4742-95-12ER charge-coupled device camera (Hamamatsu) and Aquacosmos Imaging Systems software.
β-Galactosidase Assays—Yeast cell extracts were prepared by harvesting cells by centrifugation from 10 ml of an exponentially growing culture after induction with galactose for 6 h. Then, cells were resuspended in 250 μl of breaking buffer (100 mm Tris-HCl, pH 8, 1 mm dithiothreitol, 20% glycerol), and glass beads (Glasperlen, ∼1 mm, Sartorius AG, Germany) were added to break cells in a Fast-Prep machine. Finally, extracts were clarified by centrifugation, and protein concentrations were measured using the Bradford method. β-Galactosidase assays were performed using the crude extracts obtained as described previously (27), scaling the protocol to a 96-well microtiter plate format. 10 μl of cell extract was mixed with 90 μl of Z buffer plus β-mercaptoethanol (0.03%) and 20 μl of o-nitrophenyl-β-d-galactopyranoside (4 mg/ml in Z buffer). The absorbance of the enzymatic reaction was measured at 415 nm on a microplate reader (Model 680, Bio-Rad) after at least 10 min of incubation at 30 °C and after the addition of 50 μl of 1 m Na2CO3 to stop the reaction. β-Galactosidase activity was expressed as nanomoles of o-nitrophenyl-β-d-galactopyranoside converted/min/mg of protein. Experiments were performed at least three times from independent yeast transformants.
RESULTS
In Vivo Activated Mammalian Akt1 Impairs Yeast Growth—In previous reports, we have shown that expression of membrane-targeted mammalian class I PI3K catalytic subunit (p110α-CAAX) driven by the galactose-inducible GAL1 promoter inhibited yeast growth, mainly by depletion of essential phosphatidylinositol-4,5-bisphosphate (PIP2) pools (22). However, WT p110α in the same expression system had no negative effects on growth (23) (Fig. 1A). Remarkably, co-expression of WT p110α with GFP-Akt1 led to poor growth, an effect that was more intense with the oncogenic p110α H1047R mutant protein, described to have an enhanced kinase activity (19, 28) (Fig. 1A). In contrast, the oncogenic mutation E545K, which owes its effect to abnormal interaction with the PI3K regulatory subunit p85 rather than to enhanced lipid kinase activity (29, 30), behaved like WT p110α regarding growth impairment in GFP-Akt1-expressing yeast cells (Fig. 1A). Thus, dose-dependent PIP3-driven Akt activation can be reproduced in yeast, and, furthermore, it could be monitored by analyzing its toxic effect. If it is true that Akt1 toxicity depends on intracellular PIP3 levels, it should be relieved by co-expression of the tumor suppressor PTEN, which operates as a PIP3 3-phosphatase. Indeed, as shown in Fig. 1B, co-expression of PTEN in cells expressing p110α and Akt1 rescued growth, while co-expression of SHIP1, a PIP3 5-phosphatase that generates phosphatidylinositol 3,4-bisphosphate instead of PIP2, was unable to revert the p110α-induced Akt1 toxicity. This result is in agreement with in vitro and structural data (11, 31) that support the idea that Akt1 is able to respond similarly to both PIP3 and phosphatidylinositol 3,4-bisphosphate.
FIGURE 1.
Akt1 inhibits growth of yeast cells when activated in vivo. A, growth of the yeast WT strain (YPH499) is impaired by expression of Akt1 in the presence of WT or tumor-related E545K and H1047R mutations of the catalytic subunit of PI3K, p110α. GFP-Akt1 was expressed from pYES2-GFP-Akt1 URA3-based plasmid under the control of the galactose-inducible GAL1 promoter, and all versions of p110α were expressed from LEU2-marked vectors of the YCpLG-myc-p110α series under the control of the same promoter. Serial 10-fold dilutions of cultures of representative transformants were spotted on synthetic medium lacking uracil and leucine under repressing (glucose; SD medium) or inducing (galactose; SG medium) conditions, as indicated. –, designates the corresponding YCpLG or pYES2-GFP empty vectors. B, Akt1-induced growth inhibition is counterbalanced by the 3-phosphatidylinositol phosphatase PTEN, but not by the 5-phosphatidylinositol phosphatase SHIP1. Akt1 was expressed from the TRP1-based pYES3-GFP-Akt1 plasmid, p110α from the YCpLG-myc-p110α plasmid, and PTEN or SHIP1 from pYES2-PTEN and pYES2-SHIP1, respectively, all under the control of the GAL1 promoter. Serial 10-fold dilutions were spotted as above on selective media lacking uracil, tryptophan, and leucine. “Vector” indicates the pYES3 empty plasmid.
Toxicity of Akt1 in Yeast Requires Its Kinase Activity and PIP3-dependent Membrane Recruitment—We used a K179M kinase-dead version of Akt1 to understand whether toxicity in yeast was due to its protein kinase activity. As shown in Fig. 2A, co-expression of p110α and Akt1K179M had no effect on yeast growth, bringing forward the conclusion that impairment of growth is a consequence of the phosphorylation of endogenous yeast protein targets by Akt1. Also, we expressed a myristoylatable version of Akt1, which is directed to membranes independently of PI3K-generated PIP3 (22). Peculiarly, myr-GFP-Akt1, extensively used as a constitutively active kinase in mammalian cells, was neither toxic in the absence nor in the presence of p110α (Fig. 2A), indicating that it cannot be used as a constitutively active Akt version in our model. This is consistent with our previous observation that myr-GFP-Akt1 is less efficiently phosphorylated than GFP-Akt1 in the yeast cell (22) and suggests that myr-GFP-Akt may be less available as a substrate for its activating endogenous kinases. Thus, the concentration of activated Akt1 at the particular spots where PIP3 is generated from endogenous PIP2 pools, rather than its indiscriminate attachment to membranes, seems a requirement for its toxicity in the yeast cell.
FIGURE 2.
Akt1 effects in yeast require an active kinase and are independent of rapamycin. A, mutation of the catalytic residue Lys-179 in Akt1 or its constitutive targeting to cellular membranes eliminates toxicity. GFP-Akt1, GFP-Akt1K179M, and myr-GFP-Akt1 were expressed from the corresponding pYES2-Akt1 vectors, and p110α from YCpLG-myc-p110α. Spotting of cell suspensions on agar media was performed as in Fig. 1A. B, Akt1 effects are not precluded by the presence of rapamycin. Akt1 and Akt1K179M were expressed from the corresponding pYES2-GFP-Akt1 plasmids, and p110α from YCpLG-myc-p110α. Serial 10-fold dilutions were spotted as above, except that the SG plates also contained rapamycin at a final concentration of 0.2 μg/ml, as indicated.
In mammalian cells, key effects of PI3K-dependent Akt activation related to control of cellular proliferation and survival rely on their downstream effector, the mammalian target of rapamycin (mTOR). Yeast Tor1 seems to have a significant degree of functional conservation with respect to its mammalian counterpart (32). However, inhibition of growth induced by PI3K and Akt1 in yeast was unaffected by the presence of rapamycin (Fig. 2B). Although we cannot discard that heterologous Akt might couple to Tor signaling in yeast, this result indicates that Akt1-induced toxicity is not mediated by the yeast rapamycin-dependent TORC1 complex.
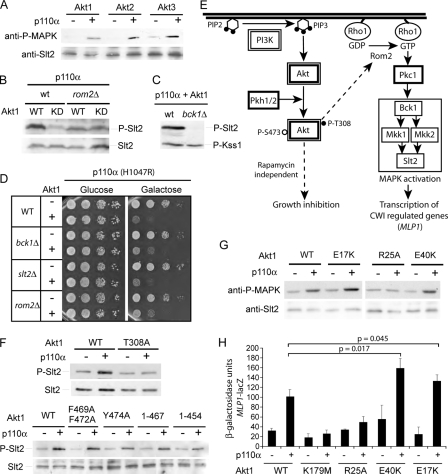
All Akt Isoforms Respond to PIP3 Production in Yeast Impairing Cell Growth—Next we tested in the yeast system other isoforms of PKB/Akt besides PKBα/Akt1, namely PKBβ/Akt2 and PKBγ/Akt3, by generating the corresponding fusions to GFP in the same expression vectors. All three Akt isoforms behaved equivalently in terms of p110α-dependent growth inhibition (Fig. 3A) and PIP3-dependent localization to the plasma membrane (data not shown). Upon p110α co-expression, Akt2 and Akt3 displayed enhanced phosphorylation at the activation sites equivalent to Thr-308 and Ser-473 in Akt1 (Fig. 3B). This indicates that toxicity of all Akt isoforms in yeast correlates to their PIP3-dependent activation in vivo. We also tested the four isoforms of WT p110 (α, β, δ, and γ). However, only p110α was capable of inducing toxicity when co-expressed with any Akt isoform (data not shown), suggesting that p110α is a more robust enzyme in vivo than the other isoforms.
FIGURE 3.
All three Akt isoforms are activated in vivo by p110α in yeast. A, growth of the yeast WT strain (YPH499) is equally impaired by expression of Akt1, Akt2, and Akt3 in the presence of the catalytic subunit of PI3K. The three Akt isoforms were expressed from the corresponding pYES2-GFP-Akt vectors, and p110α from the YCpLG-myc-p110α. Media and growth conditions were as in Fig. 1A. –, means empty YCpLG vector. B, in vivo activation of heterologous Akt isoforms in yeast. Lysates from the same transformants as in A co-expressing GFP-Akt1, -Akt2, and -Akt3 with or without p110α, as indicated, were analyzed by immunoblotting with the antibodies indicated: commercial phospho-specific anti-Akt1 antibodies directed against P-Thr-308 or P-Ser-473, anti-GFP antibodies, and anti-actin antibodies, as controls for equivalent protein loading.
Phosphorylation of Akt1 at Thr-308, but Not Ser-473, Is Essential for Toxicity in Yeast—To attest the contribution of the phosphorylation of Thr-308 and Ser-473 to activation of Akt in the yeast model, we mutated both residues to Ala in Akt1 by site-directed mutagenesis. These mutations did not affect PI3K-dependent re-localization of GFP-Akt1 to the yeast plasma membrane (data not shown). As expected, mutation of Thr-308 to Ala greatly eliminated toxicity of GFP-Akt1 (Fig. 4A), indicating that phosphorylation of this residue by yeast PDK1 orthologs is crucial for the activation of the Akt1 kinase in vivo in the yeast model. However, unexpectedly, the S473A mutation did not affect Akt1 toxicity, and a double T308A/S473A mutant behaved like the single T308A mutant (Fig. 4A). This implies that the observed phosphorylation of Akt1 at the HM is not linked to its effects on the yeast cell. Analyses of Akt1 P-Thr-308 and P-Ser-473 content in the T308A and S473A mutants verified that the corresponding epitopes were missing and revealed that phosphorylation of any of these two residues in yeast in the absence of the other was lower than in WT Akt1, but still responsive to p110α (Fig. 4B). We also generated and tested mutants that had each or both Thr-308 and Ser-473 sites in Akt1 mutated to Asp, a putative phospho-mimetic residue. We observed that the behavior of GFP-Akt1T308D and GFP-Akt1S473D was very similar to that of the mutants to Ala (data not shown), that is, the T308D mutation eliminated toxicity, whereas the S473D mutation did not add any effect. This might reflect that recognition by the endogenous PDK1-like kinases in situ is related to toxicity. Nevertheless, as shown in Fig. 4C, neither the T308D mutation interfered with Ser-473 phosphorylation, nor S473D had any effect on Thr-308 phosphorylation, whereas the equivalent changes to Ala negatively influenced each other phosphorylation. This might suggest that mutations to Asp lead to a more favorable conformation for the recognition of Akt1 by its heterologous activating kinases.
FIGURE 4.
Phosphorylation of Akt1 Thr-308, but not Ser-473 is required for toxicity in yeast. A, growth inhibition of the WT (YPH499) strain induced by WT Akt1 when co-expressed with p110α is attenuated by a T308A mutation, but not by S473A. The different Akt1 versions were expressed from the corresponding pYES3-GFP-Akt1 vectors, and p110α from the YCpLG-myc-p110α plasmid. Spotting on the appropriate selective media was performed as in Fig. 1A. B, substitution of the Thr-308 phosphorylation site for Ala diminishes but does not preclude phosphorylation at Ser-473 and vice versa. Lysates from WT (YPH499) cells expressing the indicated GFP-Akt1 versions in the presence and absence of p110α as in A were immunoblotted with the indicated phospho-specific anti-Akt1 antibodies, and with anti-GFP antibodies as a control for equivalent protein loading. C, phospho-mimetic mutations in either Thr-308 or Ser-473 do not influence phosphorylation of each other by yeast kinases. Experimental conditions were as in B. D, alignment of the C-terminal HM-containing extension of murine (m) isoforms of Akt and the human (h) alternatively spliced isoform of Akt3 (Akt3-γ1) showing the high degree of conservation at the hydrophobic motif (HM). Residues identical to the mAkt1 sequence are highlighted in bold. In lowercase, the residues that derive from alternative splicing in hAkt3-γ1. Multiple alignment was performed with ClustalW. The numbering corresponds to the mAkt1 sequence. Arrows indicate the hydrophobic residues targeted in this study. The asterisk marks the Ser-473 phosphorylatable site. E, mutational analyses on the C-terminal HM of Akt1. Lysates from WT (YPH499) cells expressing the indicated Akt1 WT or mutant versions from the corresponding pYES-GFP-Akt1 vectors in the presence ((+);YCpLG-myc-p110α) or absence ((–); YCpLG) of p110α were spotted on agar media as in Fig. 1A. F, Akt1 HM mutations or truncations do not affect phosphorylation of Thr-308. Lysates from the same transformants as in E were analyzed by immunoblotting with the indicated antibodies.
The observation that phosphorylation of the Ser-473 residue was dispensable for the toxicity of Akt1 in yeast prompted us to study the involvement of the whole C-terminal HM, a region that is highly conserved in the three isoforms of Akt (Fig. 4D). The Phe-469, Phe-472, and Tyr-474 residues (Fig. 4D, arrows) that surround Ser-473 have been described to be important for Akt structure and function (33). By site-directed mutagenesis, we generated two mutants with these hydrophobic residues mutated to Ala: GFP-Akt1F469A,F472A and GFP-Akt1Y474A. We observed that toxicity of these mutants in yeast was slightly reduced as compared with that of WT GFP-Akt1 (Fig. 4E), revealing a limited contribution of the HM to toxicity. We also generated a truncated version of Akt1 bearing a stop codon in position 468 (GFP-Akt11–467), thus lacking the whole HM. Moreover, based on the existence of an alternatively spliced form of Akt3, namely Akt3-γ1, that lacks a longer stretch at the C terminus of the Akt3 isoform (34) (Fig. 4D), we made a minimum length truncation of the Akt1 cDNA equivalent to the Akt3-γ1 splicing variant (GFP-Akt11–454). Like the HM point mutants, the truncated versions inhibited yeast growth in a p110α-dependent manner, although slightly less efficiently than WT GFP-Akt1 (Fig. 4E). For both HM point mutants and truncated versions, phosphorylation at the T-loop was still responsive to PI3K (Fig. 4F). In conclusion, the HM of Akt1 and the 14 residues preceding it, which are missing in a splicing variant of Akt3, contribute only partially to the effects of Akt1 in yeast.
The PH Domain of Akt1 Confers to the Kinase Domain of Sch9 Responsiveness to PIP3—Suggesting functional conservation between AGC kinase-controlled pathways from yeast to mammals, Sch9 has been reported to be a putative yeast ortholog of mammalian Akt (5). Overexpression of Sch9 led to a very mild reduction of yeast growth that was PI3K-independent (Fig. 5A). Actually, such mild growth inhibition was also observed in cells overexpressing Akt1 in the absence of p110α. We studied the influence of endogenous PIP3 production in the localization of GFP-Sch9. Overexpressed GFP-Sch9 was ubiquitous in the cytoplasm, excluded from vacuoles and frequently concentrated in cytoplasmic spots. Unlike GFP-Akt, re-localization of GFP-Sch9 to the plasma membrane did not occur when PIP3 was produced by p110α (Fig. 5B). Thus, Sch9 was not responsive to PIP3. Accordingly, the most striking difference between both AGC kinases is that the N-terminal PIP3-binding PH domain of Akt is replaced by a sphingosine long chain base-responsive sequence in yeast Sch9 (35, 36). We interchanged the N-terminal regulatory and C-terminal protein kinase domains of Sch9 and Akt1 producing the following chimeras: GFP-Akt11–148-Sch9411–824, bearing the Akt1 PH domain and the Sch9 kinase domain; and GFP-Sch91–410-Akt1149–480, bearing the Sch9 sphingoid-responsive region and the Akt1 kinase domain. Expression of the chimeras was equivalent to that of the wild-type fusions (Fig. 5C). Although the strong p110α-dependent effect of Akt1 on growth did not occur in either chimerical construct, the Akt1–148-Sch9411–824 had a greater inhibitory effect than Sch9 in the presence of p110α (Fig. 5A). Also, this chimera was efficiently re-located to the plasma membrane when p110α was present (Fig. 5B). In consequence, the kinase domain of Sch9 can reproduce the effects of heterologous Akt1 in yeast, albeit less efficiently. The GFP-Sch91–410-Akt1149–480 chimera behaved like GFP-Sch9 in terms of PI3K-independent toxicity and localization. Peculiarly, despite its apparent failure to re-locate to membranes, the GFP-Sch91–410-Akt1149–480 chimera displayed a p110α-dependent enhancement of phosphorylation at Thr-308 and Ser-473 (Fig. 5C). This suggests that the N-terminal domain of Sch9 may be sufficient to bring the chimera into proximity with yeast PDK1- and PDK2-like kinases, even though it may not retain the protein at the plasma membrane efficiently. Thus, stable recruitment at the plasma membrane seems to be required to induce full toxicity of Akt in yeast. The antibodies used proved to be specific for phosphorylated Akt1, failing to detect a putative phosphorylation on the conserved T-loop and HM residues of yeast Sch9 or the Akt1–148-Sch9411–824 chimera. Overall, these data support a very limited functional conservation between Sch9 and Akt1 and essentially underscore the importance of localization cues located in their N-terminal extensions for the function of these protein kinases.
FIGURE 5.
Comparative analyses on Sch9, Akt1, and chimerical constructs that interchange their regulatory and catalytic domains. A, growth of the WT (YPH499 strain) co-transformed with YCpLG (–) or YCpLG-myc-p110α (+), and pYES2 (Vector) or the same plasmid bearing GFP-Akt1, GFP-Sch9 or the chimerical GFP fusions indicated. Media and growth conditions were as in Fig. 1A. B, cellular localization of GFP-Sch9, GFP-Akt1, and the chimerical fusions. Transformants as in A were incubated in liquid synthetic medium with raffinose (2% w/v) as carbon source to mid-exponential phase, galactose was then added up to 2% (w/v) for induction and incubation proceeded for 4–6 h. Bars represent 5 μm. C, expression and analysis of the phosphorylation of Akt1, Sch9, and the chimerical proteins. Lysates from the same transformants as above were processed and immunoblotted with the same antibodies and techniques as in Fig. 4F.
In Vivo Functional Studies of the PH Domain of Akt—To test the utility of the yeast model to study the function of the PIP3-binding PH domain of Akt in vivo, we generated three point mutations within this domain in Akt1: R25A, E40K, and E17K. The R25A and E40K mutations had been previously reported to respectively eliminate and enhance the affinity of the PH domain of Akt1 for PIP3 (12, 13). The E17K mutation was recently described as the first oncogenic mutation in PKB/Akt, and it also seems to enhance the affinity of the PH domain for lipids (20). Akt1R25A was not toxic for yeast cells, whereas the E40K and E17K mutations maintained toxicity when co-expressed with p110α (Fig. 6A). Because interaction with PIP3 is important for the activation of the kinase, we investigated Akt1 phosphorylation in these mutants. In agreement with the effect on yeast growth observed, basal phosphorylation of Thr-308 and Ser-473 was not enhanced in Akt1R25A by co-expression of p110α, whereas Akt1E17K and Akt1E40K, like WT Akt1, did show a PI3K-dependent enhancement of phosphorylation (Fig. 6B). Quantification of the proportion of yeast cells in the population that re-locates GFP-Akt1 to the plasma membrane provides a sensitive indicator of PIP3 levels at the yeast plasma membrane (23), so it should also serve as a readout of the affinity of the Akt1 PH domain to PIP3 in vivo. After 4 h of co-expression in galactose-based medium, 60% of p110α-expressing cells had their membrane decorated by Akt1, whereas Akt1E17K and Akt1E40K were present in the membranes of 80–90% of the cells (Fig. 6C). In contrast, Akt1R25A was not re-located to the plasma membrane in the same experimental conditions. These findings confirm in vivo that the R25A mutation precludes recognition of PIP3, whereas both the E17K and E40K mutations enhance the affinity of Akt1 for cellular membranes containing this lipid.
FIGURE 6.
Yeast as a tool to evaluate the oncogenicity of Akt1. A, function of the Akt1 PH domain is crucial for yeast growth inhibition. YPH499 transformants harboring the pYES3-GFP-Akt1 (upper panel; WT) or pYES2-GFP-Akt1 (lower panel; WT) plasmids, or the indicated mutants, and either YCpLG (–) or YCpLG-myc-p110α (+), were processed in the appropriated media as in Fig. 1A. B, functionality of the PH domain determines Akt1 activation in yeast. Lysates from the same transformants as in A, grown in the appropriated media were processed and immunoblotted with the same techniques and antibodies as in Fig. 4F. C, recruitment of GFP-Akt1 to yeast membranes reflects the affinity of its PH domain for PIP3. YPH499 transformants harboring YCpLG-p110α and pYES3-GFP-Akt1 or pYES2-GFP-Akt1 WT or mutant versions as above were grown in the appropriated liquid synthetic medium with raffinose (2% w/v) as carbon source to mid-exponential phase, and expression was induced by adding galactose up to 2% (w/v) and incubating for 4 h. Cultures were processed for fluorescence microscopy, and the percentage of cells with GFP-Akt1 decorating the plasma membrane over the total of fluorescent cells was counted. Data are the average from three different transformants processed in parallel. Error bars correspond to the standard deviation. p values were determined by Student's t test.
Activated Akt Stimulates the CWI MAPK Pathway Upstream the Rho-GTPase Exchange Factor Rom2—To check whether phosphorylation events elicited by active heterologous Akt might influence signaling in the yeast cell, the phosphorylation status of ERK-related yeast MAPKs was studied by using anti-phospho-ERK1/2 antibodies. We found that phosphorylation of the Slt2 MAPK, readily detectable with these antibodies (37), was enhanced in lysates co-expressing p110α and Akt isoforms (Fig. 7A). In contrast, overexpression of Sch9, either in the absence or in the presence of p110α, was unable to trigger Slt2 phosphorylation (data not shown). To prove that activation of the Slt2 MAPK pathway in these cells was indeed dependent on the catalytic activity of Akt and not on the presence of p110α, we comparatively examined lysates from cells expressing Akt1 or Akt1K179M in the presence of p110α. Slt2 phosphorylation was significantly higher in cells expressing catalytically active Akt1 than in cells expressing the kinase-dead version of the protein (Fig. 7B). To check whether Slt2 phosphorylation depended on the activation of the MAPK module we expressed p110α and Akt1 in a bck1Δ background, lacking the MAPKKK at the head of the cell integrity pathway (Fig. 7C). As expected, deletion of bck1 precluded Akt-dependent Slt2 phosphorylation without affecting the basal activation levels of other ERK-related kinases such as Kss1, also detectable by these anti-phospho-MAPK antibodies (Fig. 7C). This result indicates that activated Akt stimulates the pathway upstream the MAPK module. Recruitment of protein kinase C (Pkc1) by GTP-bound Rho1 is known to be the key for the activation of the Bck1-Mkk1/2-Slt2 MAPK module (see Fig. 7E). Rho1 function is positively regulated by GTPase exchange factors and, among them, Rom2 has a major involvement (38). We expressed p110α and Akt1 (or kinase-dead Akt1 as a negative control) both in a rom2Δ mutant and in an isogenic wt strain. We could not detect Akt-dependent activation of the pathway in the rom2Δ mutant, indicating that the Rom2 GTPase exchange factor is required for the activation of the yeast CWI pathway in response to heterologous Akt1 activation (Fig. 7B). However, deletion of different CWI genes, such as ROM2, BCK1, and SLT2, did not eliminate Akt-induced toxicity (Fig. 7D), proving that this effect cannot be exclusively ascribed to the activation of this pathway.
FIGURE 7.
Activation of the yeast CWI pathway provides a reporter for in vivo activation of heterologous Akt1. A, all three isoforms of Akt cause PIP3-dependent phosphorylation of the Slt2 MAPK. The same membranes as in Fig. 3B were re-hybridized with anti phospho-p44/p42 antibodies or anti-Slt2 antibodies, as a control for the amount of Slt2 in the lysates, as indicated. B, Slt2 activation requires an intact Akt1 kinase and the function of the ROM2 gene. Lysates from WT BY4741 S. cerevisiae strain or isogenic rom2Δ cells expressing Akt1 (WT) or Akt1K179M (KD, kinase-dead) from the corresponding pYES2-GFP-Akt1 plasmid and p110α from the YCpLG-myc-p110α plasmid, as indicated, were immunoblotted with anti-phospho-p44/p42 antibodies, and anti-Slt2 antibodies. C, MAPK phosphorylation in yeast cells expressing in vivo-activated Akt1 is specific for Slt2 and requires the upstream MAPKKK Bck1. Lysates from WT BY4741 and an isogenic bck1Δ mutant expressing Akt1 and p110α from the same vectors as above were analyzed by immunoblotting with anti-phospho-p44/p42 antibodies. D, deletions in essential components of the CWI MAPK pathway do not abrogate Akt-induced toxicity in yeast. BY4741 WT or isogenic rom2Δ, bck1Δ, and slt2Δ strains were co-transformed with YCpLG-p110α(H1047R) and pYES2-GFP (–) or pYES2-GFP-Akt1 (+) as indicated. Because the BY4741 strain is not as sensitive as YPH499, used in the rest of the experiments in this report, to Akt1-induced toxicity, hyperactive H1047R p110α mutant was used to enhance the phenotype. Experimental conditions were identical to those in Fig. 1. E, scheme of the effects of in vivo Akt activation in yeast: Conversion of cellular PIP2 pools in the yeast cell to PIP3 by PI3K p110 catalytic subunit artificially recruits Akt to the plasma membrane, where phosphorylation in both the T-loop (Thr-308) by PDK1 orthologs Pkh1 and Pkh2 (53) and the C-terminal HM (S473) by an unknown kinase (22) are enhanced. This leads to an activation of the Akt kinase that causes a rapamycin-independent negative effect on yeast growth as well as activation of the CWI MAPK signaling pathway that involves the function of the Rho1-GTPase exchange factor Rom2. Both effects require PIP3, T308 phosphorylation and Akt kinase activity, while phosphorylation of the HM is dispensable. F, Activation of the CWI pathway by different Akt1 mutants. Upper panel: Elimination of the Thr in the activation loop of Akt1 restrains its ability to activate the Slt2 MAPK. Lower panel: HM mutants or HM truncated versions of Akt1 are still able to induce PIP3-dependent Slt2 phosphorylation. The same membranes as in Fig. 4 (B and F) were hybridized with anti-P-p42/44 or anti-Slt2 antibodies as indicated. G, activation of the CWI MAPK Slt2 in the presence of Akt1 loss- and gain-of-function PH mutants. The same membranes as in Fig. 6B were re-hybridized with anti phospho-p44/p42 antibodies or anti-Slt2 antibodies, as indicated. H, quantification of Akt1 activity in vivo in the yeast cell using a pMLP1-lacZ CWI transcriptional reporter. YPH499 cells were co-transformed with YCpLG-p110α, and pYES2-GFP-Akt or pYES3-GFP-Akt WT or mutant versions as above, and the pMLP1-LACZ(URA3) or pMLP1-LACZ(HIS3) plasmids. Cells were grown in synthetic raffinose-based medium lacking uracil, leucine, and histidine or tryptophan, as required, induced by the addition of galactose for 6 h, and the expression of MLP1 promoter-driven lacZ was examined. β-Galactosidase activity is expressed in the y-axis as nanomoles of o-nitrophenyl-β-d-galactopyranoside converted/minute/mg of protein. Data are the average from triplicate experiments on independent yeast transformants (except WT, six replicas). p values were calculated by Student's t test. Error bars correspond to the ±S.D.
CWI Activation Provides Biochemical and Transcriptional Reporters for Heterologous Akt Function—We next tested whether the phosphorylation levels of the Slt2 MAPK could be related to the ability of Akt to become activated in yeast. As shown in Fig. 7F, phosphorylation of Slt2 was dependent on the presence of Thr-308 at the activation loop of Akt1 but did not require its HM C-terminal extension. Thus, CWI MAPK activation by Akt1 matches its toxicity in yeast, as shown above in Fig. 4 (A and E). Also, Slt2 phosphorylation was slightly more prominent in lysates expressing GFP-Akt1E40K and GFP-Akt1E17K in the presence of p110α than in those expressing WT GFP-Akt1 in the same conditions (Fig. 7G), indicating that the CWI pathway is sensitive to the efficiency of GFP-Akt localization to the plasma membrane via its PH domain. The fact that in vivo activation of Akt modulates MAPK signaling in yeast opens the possibility of using transcriptional reporters to monitor Akt activity. We used a construct bearing the promoter of the MLP1 gene, a major transcriptional target of the CWI pathway (39) coupled to lacZ, so that β-galactosidase activity could be quantified (27). As shown in Fig. 7H, this system allowed drawing the same conclusions as qualitatively observed above. Importantly, mutations in the PH domain causing either loss or gain of function, such as the oncogenic E17K, could be traced by these means.
Ala Scanning of the VL1 Region at the PIP3-binding Pocket of Akt1 PH Domain—To further demonstrate the applicability of our model to gaining insight into Akt function and activation in vivo, we changed to Ala the amino acids in and around the VL1 loop of the PH domain of Akt1. This region configures a conserved structural element of the pocket that fits the polar head group of PIP3 (11) (residues Arg-15 to Pro-24; Fig. 8A). In agreement with a key role of this region in PIP3 recognition and Akt function, 9 out of the 10 Ala-scanning mutants led to important (G16A) or severe loss of function, as judged by both failure of p110α-triggered membrane recruitment and lack of toxicity in yeast (Fig. 8B). The exception was Pro-24 that, despite its vicinity to important residues, could be changed to Ala without influence in the activation or activity of the protein. This was not due to instability of the mutant protein, because all mutants were expressed in yeast to the same levels of as WT Akt1, as judged by immunoblot on yeast lysates (data not shown).
FIGURE 8.
Mutational and functional analysis of the VL1 region of Akt1. A, alignment of the 30 N-terminal residues of hAkt1 and mAkt1 with mAkt2 and mAkt3. Shaded boxes indicate identical residues taking mAkt1 as a reference. The image at the top shows the secondary structure in Akt1 associated to the sequences below. The region selected for the Ala-scanning mutational analysis is underlined. Asterisks mark residues previously mutated to alanine and reported to be essential (11). The black arrowhead indicates the residue Glu-17, in which the E17K oncogenic mutation maps. Gray arrowheads point the residues that have been mutated to Arg in this work. B, functional effects of Akt1 mutations at the VL1 region. Localization of GFP-Akt1 to membranes in the presence of PI3K was analyzed after 4 h of induction in galactose as in Fig. 6C. Data are the average from three different transformants processed in parallel. Error bars correspond to the standard deviation. p values were calculated by Student's t test (upper panel). Spotting on the appropriate selective media was performed as in Fig. 1A (lower panel; controls in glucose grew equivalently and are not shown).
The VL1 loop includes Glu-17, whose change to Lys yielded the above-mentioned gain-of-function oncogenic Akt1 allele (20). Other PH domains, such as that of Bruton's tyrosine kinase, include VL1 extensions rich in basic residues (40). Positively charged residues in this region provide a surface that could interact with negatively charged lipid membranes as well as facilitate interaction with the phosphate groups of phosphoinositides. From the DNA sequence of murine Akt1-coding DNA, we searched for feasible single point mutations that change any of the residues at the VL1 region to basic polar amino acids. Besides E17K (GAA to AAA), we found two other possible mutations in codons conserved in the human sequence at the DNA level: G16R (GGG to CGG) and P24R (CCA to CGA). Thus, we were prompted to study whether these mutations would also alter Akt1 PIP3 recognition and function. As shown in Fig. 8B, G16R maintained toxicity and led to a more efficient recruitment to PIP3-enriched membranes than G16A, although less than WT Akt1. Interestingly, the P24R mutant, a substitution that naturally exists in other PH domains, like that of the general receptor for phosphoinositides (GRP1 (41)), was slightly but reproducibly more responsive to PIP3 than WT Akt1 (Fig. 8B). These data underscore the sensitivity of the yeast system proposed here to study both loss- and gain-of-function mutants of Akt.
DISCUSSION
Humanized yeast systems are valuable tools to perform genetic and pharmacological screens on heterologous genes or proteins (42, 43). Such systems may take advantage on the conservation of pathways from yeast to mammals, allowing complementation of the function in yeast by the human gene; or, alternatively, expression of heterologous proteins may provide functions that do not naturally exist in yeast. Based on our former observations that mammalian Akt1 could be re-located to membranes and phosphorylated in a PI3K-dependent fashion in S. cerevisiae (22), we report here that in vivo activation of the different mammalian Akt isoforms in the yeast model leads to growth inhibition, an effect that relies on its catalytic activity. We propose this system as a valuable tool to evaluate in vivo the functional effects of Akt mutations, which may be relevant for studies on oncogenesis.
PIP3-dependent activation of Akt led to the activation of the CWI MAPK pathway that lies downstream the Rho1 GTPase and the protein kinase C yeast homolog Pkc1. Although hyperactivation of this pathway is known to negatively influence yeast growth (44), we show that Akt-induced Slt2 activation is not the cause of toxicity in yeast. We demonstrate that the Rho1 GAP Rom2 is required for activation of the pathway in yeast cells co-expressing PI3K and Akt. To know whether heterologous Akt directly phosphorylates Rom2 or operates on components upstream in the pathway will require further experimentation. We cannot discard that Akt phosphorylation is somehow altering the properties of the cell wall, and the CWI pathway is subsequently activated. Actually, electron microscopy on yeast cells expressing activated Akt show abnormal cell walls.4 Regardless of the mechanism involved, the activation of the Slt2 MAPK route provides the possibility of using a quantitative reporter of the function of Akt in an in vivo context.
Finding the heterologous targets of activated Akt in yeast might reveal novel conserved Akt substrates in the future. The fact that the Tor inhibitor rapamycin does not interfere with Akt toxicity indicates that TORC1 activation is not related to Akt1-induced toxicity in yeast. Accordingly, no regulation of yeast TORC1 by AGC kinases, such as the putative Akt ortholog Sch9, has been reported to our knowledge. Rather, it has been demonstrated that Sch9 acts downstream Tor1 (45), yielding it a more likely functional ortholog to the mTor target S6K than to Akt. In this regard, we show here that, in the presence of PI3K, Sch9 overexpression does not lead to the same growth inhibitory effects than Akt. Also, exchanging the N-terminal membrane-binding domains of Akt1 and Sch9 allowed mimicking the Akt phenotype only partially, even though PIP3-dependent membrane localization of the Sch9 C-terminal kinase domain mediated by the Akt1 N-terminal PH domain was as efficient as that of Akt1. We have observed, however, that overexpression of Akt isoforms by themselves, in the absence of PI3K, cause a very mild growth inhibition that is equivalent to the one observed when Sch9 is overexpressed, so we cannot discard that both kinases could share substrates.
How might the yeast model contribute to our understanding of the mechanisms that lead to Akt activation? Some speculations can be driven from our observations in yeast. First, it is intriguing that myr-Akt1, widely used as a constitutively activable version of Akt that bypasses the need for PI3K, is not toxic by itself in yeast. This suggested that PIP3 binding of Akt and not its sheer recruitment to membranes is critical for its activation, in agreement with previous structural studies (46) and in vivo Förster resonance energy transfer experiments (47). An alternative explanation to the failure of myr-Akt to behave as constitutively active in S. cerevisiae could be that recognition of endogenous Akt targets involved in the slow growth phenotype observed may rely on detachment of activated Akt from membranes, a process that myristoylation should prevent. Second, we can address the controversial question whether phosphorylation of Thr-308 conditions Ser-473 phosphorylation and vice versa. Our results with T308A and S473A mutants support the idea that phosphorylation of one of the residues is conformationally favorable, but not essential, for the phosphorylation of the other. On the other hand, the fact, that phosphorylation of either residue still occurs and is responsive to PIP3 when the other phosphorylatable residue is eliminated, is in agreement with reports that revealed that Thr-308 phosphorylation was unaffected in cells ablated for mTORC2 activity, the purported Ser-473 kinase (48), as well as with those showing that cells defective in PDK1 still phosphorylate Ser-473 (49). Third, Ser-473 phosphorylation, and even the whole HM, is largely dispensable for the growth inhibitory effects of Akt in yeast. This was surprising, considering the facts that Ser-473 phosphorylation is regarded as an important event for full activation of the kinase and that such residue is phosphorylated by yeast endogenous kinases. However, if the effects of Akt in yeast would mostly depend on the phosphorylation of local submembrane targets, these results are in agreement with the emerging view that Thr-308 phosphorylation is sufficient for membrane-bound Akt1 to efficiently phosphorylate local targets, whereas Ser-473 phosphorylation would be required for activity of the kinase on non-submembrane targets, such as nuclear substrates (50).
The best support for the value of our yeast model arises from functional analyses of Akt1 mutated at its PH domain. In our Ala scanning of the VL1 region and surrounding amino acids, which contributes to the structure of the phosphoinositide binding pocket, we prove that these residues are either essential (Arg-15, Glu-17, Tyr-18, Ile-19, Lys-20, Thr-21, Trp-22, and Arg-23) or very important (Gly-16) for the function of the PH domain. Mutations to Ala of Lys-14 within the β1 strand or Arg-25 within the β2 strand, the residues that flank the region targeted here, had also been reported to yield versions of Akt unable to bind PIP3, and thus non-activable (11). At least in the case of the R25A mutant, we also prove that yeast reproduces its loss of function. Remarkably, the only oncogenic mutation described to date in Akt1 maps to the Glu residue at position 17 (20), within the VL1 region, and involves its change into a basic residue. Expression in yeast of this allele in the presence of PI3K leads to an enhanced phenotype, as evidenced by a greater relocation of the mutant protein to cellular membranes. This is an important observation, because it proves that yeast is sensitive not only to loss-of-function but also to gain-of-function Akt mutants, allowing the use of this system to analyze putative oncogenic alleles. The E40K allele, which introduces a positive charge in the membrane-interacting interface of the protein outside the PIP3 binding pocket (13), also showed enhanced phenotypes in PIP3-producing yeast, although this mutation has not been related to tumorigenesis. We have also generated a novel point mutation leading to a P24R substitution that yielded Akt1 slightly more responsive to PIP3, as judged by membrane relocation of GFP-Akt1. This result suggests that the addition of positive charges at positions other than Glu-17 at the PIP3 binding pocket could enhance affinity for lipids. The P24R mutation, however, unlike Akt1E17K (20), did not lead to enhanced Thr-308 or Ser-473 Akt1 phosphorylation when expressed in mammalian cell lines.5 This indicates that it may not be relevant as an oncogenic allele, as suggested by the fact that no clinical reports are described involving this particular mutation.
In sum, our data illustrate the value of the humanized yeast model to readily study Akt structure-function relations. In this context, the development of qualitative or semi-quantitative visual readouts (such as yeast growth, GFP-Akt membrane relocation, or Slt2 MAPK phosphorylation), as well as reporter systems specific for Akt activation in yeast, such as the MLP1-lacZ described here, provide novel tools that could be further exploited in the future. For example, other mutations predicted to enhance the affinity for PIP3 of PH domains, such as those recently suggested by bioinformatic means by Park and co-workers (51), could be tested using this model. Besides genetic analyses, the system might also be utilized to test or screen for small molecules that display Akt inhibitory properties or, alternatively, to screen for mutations that confer resistance to known inhibitors. The latter strategy has recently been performed on PI3K quite successfully (52), proving that yeast-based systems can be a very helpful tool for the molecular analyses of key pharmacological targets, such as PKB/Akt.
Acknowledgments
We thank T. M. Badger, A. B. Sanz, R. García, and J. Arroyo for plasmids, J. Ortiz-Rincón for his help, and J. W. Thorner for critical comments on the manuscript.
This work was supported in part by Grant BIO2007-67299 from Ministerio Educación y Ciencia, Grant S-SAL-0246-2006 and the Programme for Universidad Complutense de Madrid Research Groups (920628) from Comunidad Autónoma de Madrid (to M. M.) and by Grant SAF2006-083139 from Ministerio de Educación y Ciencia, Grant ISCIII-RETIC RD06/0020 from Instituto de Salud Carlos III, Spain, and Fundación Mutua Madrileña (to R. P.).
Footnotes
The abbreviations used are: PKB, protein kinase B; PH, pleckstrin homology; HM, hydrophobic motif; PI3K, phosphatidylinositol 3-kinase; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol 3,4,5-trisphosphate; PTEN, phosphatase and tensin homolog; PDK1, phosphoinositide-dependent kinase 1; mTOR, mammalian target of rapamycin; GFP, green fluorescent protein; MAPK, mitogen-activated protein kinase; MAPKKK, MAPK kinase kinase; wt, wild type; CWI, cell wall integrity.
I. Rodríguez-Escudero, A. Andrés-Pons, R. Pulido, M. Molina, and V. J. Cid, unpublished data.
I. Rodríguez-Escudero, A. Andrés-Pons, R. Pulido, M. Molina, and V. J. Cid, unpublished observations.
References
- 1.Bellacosa, A., Kumar, C. C., Di Cristofano, A., and Testa, J. R. (2005) Adv. Cancer Res. 94 29–86 [DOI] [PubMed] [Google Scholar]
- 2.Manning, B. D., and Cantley, L. C. (2007) Cell 129 1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanada, M., Feng, J., and Hemmings, B. A. (2004) Biochim. Biophys. Acta 1697 3–16 [DOI] [PubMed] [Google Scholar]
- 4.Kumar, C. C., and Madison, V. (2005) Oncogene 24 7493–7501 [DOI] [PubMed] [Google Scholar]
- 5.Fabrizio, P., Pozza, F., Pletcher, S. D., Gendron, C. M., and Longo, V. D. (2001) Science 292 288–290 [DOI] [PubMed] [Google Scholar]
- 6.Pedruzzi, I., Dubouloz, F., Cameroni, E., Wanke, V., Roosen, J., Winderickx, J., and De Virgilio, C. (2003) Mol. Cell. 12 1607–1613 [DOI] [PubMed] [Google Scholar]
- 7.Jorgensen, P., Rupes, I., Sharom, J. R., Schneper, L., Broach, J. R., and Tyers, M. (2004) Genes Dev. 18 2491–2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roosen, J., Engelen, K., Marchal, K., Mathys, J., Griffioen, G., Cameroni, E., Thevelein, J. M., De Virgilio, C., De Moor, B., and Winderickx, J. (2005) Mol. Microbiol. 55 862–880 [DOI] [PubMed] [Google Scholar]
- 9.Kaeberlein, M., Powers, R. W., III, Steffen, K. K., Westman, E. A., Hu, D., Dang, N., Kerr, E. O., Kirkland, K. T., Fields, S., and Kennedy, B. K. (2005) Science 310 1193–1196 [DOI] [PubMed] [Google Scholar]
- 10.Pascual-Ahuir, A., and Proft, M. (2007) Cell Cycle 6 2445–2447 [DOI] [PubMed] [Google Scholar]
- 11.Thomas, C. C., Deak, M., Alessi, D. R., and van Aalten, D. M. (2002) Curr. Biol. 12 1256–1262 [DOI] [PubMed] [Google Scholar]
- 12.Bellacosa, A., Chan, T. O., Ahmed, N. N., Datta, K., Malstrom, S., Stokoe, D., McCormick, F., Feng, J., and Tsichlis, P. (1998) Oncogene 17 313–325 [DOI] [PubMed] [Google Scholar]
- 13.Aoki, M., Batista, O., Bellacosa, A., Tsichlis, P., and Vogt, P. K. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 14950–14955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sansal, I., and Sellers, W. R. (2004) J. Clin. Oncol. 22 2954–2963 [DOI] [PubMed] [Google Scholar]
- 15.Eng, C. (2003) Hum. Mutat. 22 183–198 [DOI] [PubMed] [Google Scholar]
- 16.Sulis, M. L., and Parsons, R. (2003) Trends Cell Biol. 13 478–483 [DOI] [PubMed] [Google Scholar]
- 17.Sarbassov, d. D., Guertin, D. A., Ali, S. M., and Sabatini, D. M. (2005) Science 307 1098–1101 [DOI] [PubMed] [Google Scholar]
- 18.Altomare, D. A., and Testa, J. R. (2005) Oncogene 24 7455–7464 [DOI] [PubMed] [Google Scholar]
- 19.Samuels, Y., and Ericson, K. (2006) Curr. Opin. Oncol. 18 77–82 [DOI] [PubMed] [Google Scholar]
- 20.Carpten, J. D., Faber, A. L., Horn, C., Donoho, G. P., Briggs, S. L., Robbins, C. M., Hostetter, G., Boguslawski, S., Moses, T. Y., Savage, S., Uhlik, M., Lin, A., Du, J., Qian, Y. W., Zeckner, D. J., Tucker-Kellogg, G., Touchman, J., Patel, K., Mousses, S., Bittner, M., Schevitz, R., Lai, M. H., Blanchard, K. L., and Thomas, J. E. (2007) Nature 448 439–444 [DOI] [PubMed] [Google Scholar]
- 21.Bleeker, F. E., Felicioni, L., Buttitta, F., Lamba, S., Cardone, L., Rodolfo, M., Scarpa, A., Leenstra, S., Frattini, M., Barbareschi, M., Grammastro, M. D., Sciarrotta, M. G., Zanon, C., Marchetti, A., and Bardelli, A. (2008) Oncogene 27 5648–5650 [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez-Escudero, I., Roelants, F. M., Thorner, J., Nombela, C., Molina, M., and Cid, V. J. (2005) Biochem. J. 390 613–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andres-Pons, A., Rodriguez-Escudero, I., Gil, A., Blanco, A., Vega, A., Molina, M., Pulido, R., and Cid, V. J. (2007) Cancer Res. 67 9731–9739 [DOI] [PubMed] [Google Scholar]
- 24.He, L., Simmen, F. A., Mehendale, H. M., Ronis, M. J., and Badger, T. M. (2006) J. Biol. Chem. 281 11126–11134 [DOI] [PubMed] [Google Scholar]
- 25.Yon, J., and Fried, M. (1989) Nucleic Acids Res. 17 4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Pena, J. M., Diez-Muniz, S., Nombela, C., and Arroyo, J. (2008) J. Biotechnol. 133 311–317 [DOI] [PubMed] [Google Scholar]
- 27.Bermejo, C., Rodriguez, E., Garcia, R., Rodriguez-Pena, J. M., Rodriguez de la Concepcion, M. L., Rivas, C., Arias, P., Nombela, C., Posas, F., and Arroyo, J. (2008) Mol. Biol. Cell 19 1113–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, Z., and Roberts, T. M. (2006) Cell Cycle 5 675–677 [DOI] [PubMed] [Google Scholar]
- 29.Carson, J. D., Van Aller, G., Lehr, R., Sinnamon, R. H., Kirkpatrick, R. B., Auger, K. R., Dhanak, D., Copeland, R. A., Gontarek, R. R., Tummino, P. J., and Luo, L. (2008) Biochem. J. 409 519–524 [DOI] [PubMed] [Google Scholar]
- 30.Miled, N., Yan, Y., Hon, W. C., Perisic, O., Zvelebil, M., Inbar, Y., Schneidman-Duhovny, D., Wolfson, H. J., Backer, J. M., and Williams, R. L. (2007) Science 317 239–242 [DOI] [PubMed] [Google Scholar]
- 31.Frech, M., Andjelkovic, M., Ingley, E., Reddy, K. K., Falck, J. R., and Hemmings, B. A. (1997) J. Biol. Chem. 272 8474–8481 [DOI] [PubMed] [Google Scholar]
- 32.Powers, T., Dilova, I., Chen, C. Y., and Wedaman, K. (2004) Curr. Top. Microbiol. Immunol. 279 39–51 [DOI] [PubMed] [Google Scholar]
- 33.Yang, J., Cron, P., Good, V. M., Thompson, V., Hemmings, B. A., and Barford, D. (2002) Nat. Struct. Biol. 9 940–944 [DOI] [PubMed] [Google Scholar]
- 34.Brodbeck, D., Hill, M. M., and Hemmings, B. A. (2001) J. Biol. Chem. 276 29550–29558 [DOI] [PubMed] [Google Scholar]
- 35.Liu, K., Zhang, X., Lester, R. L., and Dickson, R. C. (2005) J. Biol. Chem. 280 22679–22687 [DOI] [PubMed] [Google Scholar]
- 36.Roelants, F. M., Torrance, P. D., and Thorner, J. (2004) Microbiology 150 3289–3304 [DOI] [PubMed] [Google Scholar]
- 37.Martin, H., Rodriguez-Pachon, J. M., Ruiz, C., Nombela, C., and Molina, M. (2000) J. Biol. Chem. 275 1511–1519 [DOI] [PubMed] [Google Scholar]
- 38.Bickle, M., Delley, P. A., Schmidt, A., and Hall, M. N. (1998) EMBO J. 17 2235–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia, R., Bermejo, C., Grau, C., Perez, R., Rodriguez-Pena, J. M., Francois, J., Nombela, C., and Arroyo, J. (2004) J. Biol. Chem. 279 15183–15195 [DOI] [PubMed] [Google Scholar]
- 40.Baraldi, E., Djinovic, C. K., Hyvonen, M., Surdo, P. L., Riley, A. M., Potter, B. V., O'Brien, R., Ladbury, J. E., and Saraste, M. (1999) Structure 7 449–460 [DOI] [PubMed] [Google Scholar]
- 41.Lietzke, S. E., Bose, S., Cronin, T., Klarlund, J., Chawla, A., Czech, M. P., and Lambright, D. G. (2000) Mol. Cell. 6 385–394 [DOI] [PubMed] [Google Scholar]
- 42.Mager, W. H., and Winderickx, J. (2005) Trends Pharmacol. Sci. 26 265–273 [DOI] [PubMed] [Google Scholar]
- 43.Simon, J. A., and Bedalov, A. (2004) Nat. Rev. Cancer. 4 481–492 [DOI] [PubMed] [Google Scholar]
- 44.Watanabe, Y., Irie, K., and Matsumoto, K. (1995) Mol. Cell. Biol. 15 5740–5749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Urban, J., Soulard, A., Huber, A., Lippman, S., Mukhopadhyay, D., Deloche, O., Wanke, V., Anrather, D., Ammerer, G., Riezman, H., Broach, J. R., De Virgilio, C., Hall, M. N., and Loewith, R. (2007) Mol. Cell. 26 663–674 [DOI] [PubMed] [Google Scholar]
- 46.Milburn, C. C., Deak, M., Kelly, S. M., Price, N. C., Alessi, D. R., and van Aalten, D. M. (2003) Biochem. J. 375 531–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calleja, V., Alcor, D., Laguerre, M., Park, J., Vojnovic, B., Hemmings, B. A., Downward, J., Parker, P. J., and Larijani, B. (2007) PLoS Biol. 5 e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacinto, E., Facchinetti, V., Liu, D., Soto, N., Wei, S., Jung, S. Y., Huang, Q., Qin, J., and Su, B. (2006) Cell 127 125–137 [DOI] [PubMed] [Google Scholar]
- 49.Williams, M. R., Arthur, J. S., Balendran, A., Van der, K. J., Poli, V., Cohen, P., and Alessi, D. R. (2000) Curr. Biol. 10 439–448 [DOI] [PubMed] [Google Scholar]
- 50.Bhaskar, P. T., and Hay, N. (2007) Dev. Cell. 12 487–502 [DOI] [PubMed] [Google Scholar]
- 51.Park, W. S., Heo, W. D., Whalen, J. H., O'Rourke, N. A., Bryan, H. M., Meyer, T., and Teruel, M. N. (2008) Mol. Cell. 30 381–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zunder, E. R., Knight, Z. A., Houseman, B. T., Apsel, B., and Shokat, K. M. (2008) Cancer Cell. 14 180–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Casamayor, A., Torrance, P. D., Kobayashi, T., Thorner, J., and Alessi, D. R. (1999) Curr. Biol. 9 186–197 [DOI] [PubMed] [Google Scholar]