Abstract
Rapid protein kinase D (PKD) activation and phosphorylation via protein kinase C (PKC) have been extensively documented in many cell types cells stimulated by multiple stimuli. In contrast, little is known about the role and mechanism(s) of a recently identified sustained phase of PKD activation in response to G protein-coupled receptor agonists. To elucidate the role of biphasic PKD activation, we used Swiss 3T3 cells because PKD expression in these cells potently enhanced duration of ERK activation and DNA synthesis in response to Gq-coupled receptor agonists. Cell treatment with the preferential PKC inhibitors GF109203X or Gö6983 profoundly inhibited PKD activation induced by bombesin stimulation for <15 min but did not prevent PKD catalytic activation induced by bombesin stimulation for longer times (>60 min). The existence of sequential PKC-dependent and PKC-independent PKD activation was demonstrated in 3T3 cells stimulated with various concentrations of bombesin (0.3–10 nm) or with vasopressin, a different Gq-coupled receptor agonist. To gain insight into the mechanisms involved, we determined the phosphorylation state of the activation loop residues Ser744 and Ser748. Transphosphorylation targeted Ser744, whereas autophosphorylation was the predominant mechanism for Ser748 in cells stimulated with Gq-coupled receptor agonists. We next determined which phase of PKD activation is responsible for promoting enhanced ERK activation and DNA synthesis in response to Gq-coupled receptor agonists. We show, for the first time, that the PKC-independent phase of PKD activation mediates prolonged ERK signaling and progression to DNA synthesis in response to bombesin or vasopressin through a pathway that requires epidermal growth factor receptor-tyrosine kinase activity. Thus, our results identify a novel mechanism of Gq-coupled receptor-induced mitogenesis mediated by sustained PKD activation through a PKC-independent pathway.
The understanding of the mechanisms that control cell proliferation requires the identification of the molecular pathways that govern the transition of quiescent cells into the S phase of the cell cycle. In this context the activation and phosphorylation of protein kinase D (PKD),4 the founding member of a new protein kinase family within the Ca2+/calmodulin-dependent protein kinase (CAMK) group and separate from the previously identified PKCs (for review, see Ref. 1), are attracting intense attention. In unstimulated cells, PKD is in a state of low catalytic (kinase) activity maintained by autoinhibition mediated by the N-terminal domain, a region containing a repeat of cysteinerich zinc finger-like motifs and a pleckstrin homology (PH) domain (1–4). Physiological activation of PKD within cells occurs via a phosphorylation-dependent mechanism first identified in our laboratory (5–7). In response to cellular stimuli (1), including phorbol esters, growth factors (e.g. PDGF), and G protein-coupled receptor (GPCR) agonists (6, 8–16) that signal through Gq, G12, Gi, and Rho (11, 15–19), PKD is converted into a form with high catalytic activity, as shown by in vitro kinase assays performed in the absence of lipid co-activators (5, 20).
During these studies multiple lines of evidence indicated that PKC activity is necessary for rapid PKD activation within intact cells. For example, rapid PKD activation was selectively and potently blocked by cell treatment with preferential PKC inhibitors (e.g. GF109203X or Gö6983) that do not directly inhibit PKD catalytic activity (5, 20), implying that PKD activation in intact cells is mediated directly or indirectly through PKCs. Many reports demonstrated the operation of a rapid PKC/PKD signaling cascade induced by multiple GPCR agonists and other receptor ligands in a range of cell types (for review, see Ref. 1). Our previous studies identified Ser744 and Ser748 in the PKD activation loop (also referred as activation segment or T-loop) as phosphorylation sites critical for PKC-mediated PKD activation (1, 4, 7, 17, 21). Collectively, these findings demonstrated the existence of a rapidly activated PKC-PKD protein kinase cascade(s). In a recent study we found that the rapid PKC-dependent PKD activation was followed by a late, PKC-independent phase of catalytic activation and phosphorylation induced by stimulation of the bombesin Gq-coupled receptor ectopically expressed in COS-7 cells (22). This study raised the possibility that PKD mediates rapid biological responses downstream of PKCs, whereas, in striking contrast, PKD could mediate long term responses through PKC-independent pathways. Despite its potential importance for defining the role of PKC and PKD in signal transduction, this hypothesis has not been tested in any cell type.
Accumulating evidence demonstrates that PKD plays an important role in several cellular processes and activities, including signal transduction (14, 23–25), chromatin organization (26), Golgi function (27, 28), gene expression (29–31), immune regulation (26), and cell survival, adhesion, motility, differentiation, DNA synthesis, and proliferation (for review, see Ref. 1). In Swiss 3T3 fibroblasts, a cell line used extensively as a model system to elucidate mechanisms of mitogenic signaling (32–34), PKD expression potently enhances ERK activation, DNA synthesis, and cell proliferation induced by Gq-coupled receptor agonists (8, 14). Here, we used this model system to elucidate the role and mechanism(s) of biphasic PKD activation. First, we show that the Gq-coupled receptor agonists bombesin and vasopressin, in contrast to phorbol esters, specifically induce PKD activation through early PKC-dependent and late PKC-independent mechanisms in Swiss 3T3 cells. Subsequently, we demonstrate for the first time that the PKC-independent phase of PKD activation is responsible for promoting ERK signaling and progression to DNA synthesis through an epidermal growth factor receptor (EGFR)-dependent pathway. Thus, our results identify a novel mechanism of Gq-coupled receptor-induced mitogenesis mediated by sustained PKD activation through a PKC-independent pathway.
EXPERIMENTAL PROCEDURES
Cell Culture
Stock cultures of Swiss 3T3-PKD.GFP cells, which overexpress wild type PKD, Swiss 3T3-PKDK618N.GFP cells, that overexpress a kinase-deficient PKD, and control Swiss 3T3-GFP cells were generated as previously described (8, 14). The cells were maintained at 37 °C in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal bovine serum in a humidified atmosphere containing 10% CO2 and 90% air. For experimental purposes, cells were plated in 100-mm dishes at 6 × 105 cells/dish or 35-mm dishes at 1 × 105 cells/dish and grown in DMEM containing 10% fetal bovine serum for 7–9 days until they became confluent and quiescent (35).
Immunoblotting and Detection of PKD, MARCKS, ERK, and RSK
Confluent, quiescent Swiss 3T3-GFP, Swiss 3T3-PKD.GFP, and Swiss 3T3-PKDK618N.GFP cells were lysed in 2× SDS-PAGE sample buffer (20 mm Tris/HCl, pH 6.8, 6% SDS, 2 mm EDTA, 4% 2-mercaptoethanol, 10% glycerol) and boiled for 10 min. After SDS-PAGE, proteins were transferred to Immobilon-P membranes. The transfer was carried out at 100 V, 0.4 A at 4 °C for 4 h using a Bio-Rad transfer apparatus. The transfer buffer consisted of 200 mm glycine, 25 mm Tris, 0.01% SDS, and 20% CH3OH. For detection of proteins, membranes were blocked using 5% nonfat dried milk in phosphate-buffered saline, pH 7.2, and then incubated for at least 2 h with the desired antibodies diluted in phosphate-buffered saline, pH 7.2, containing 3% nonfat dried milk. Primary antibodies bound to immunoreactive bands were visualized by enhanced chemiluminescence (ECL) detection with horseradish peroxidase-conjugated anti-mouse, anti-rabbit, or anti-goat antibodies. The phosphospecific antibodies used were as follows: the phospho PKD polyclonal antibodies Ser(P)916, Ser(P)744, and Ser(P)748 detect PKD only when it is phosphorylated on Ser916, Ser744, or Ser748; the phospho-ERK1/2 monoclonal antibody recognizes ERK1/2 only when they are phosphorylated on Thr202 and Tyr204 (pERK1/ERK2); the phospho-p90RSK polyclonal antibody is specific to p90RSK only when it is phosphorylated on Thr574; the phospho MARCKS polyclonal antibody is specific to MARCKS only when it is phosphorylated on Ser152 and Ser156 (pMARCKS). Autoluminograms were scanned using a GS-710 scanner (Bio-Rad), and the labeled bands were quantified using the Quantity One software program (Bio-Rad).
Immunoprecipitation and Kinase Assay of PKD
Immunoprecipitations—Confluent Swiss 3T3-PKD.GFP cells were washed twice with DMEM and equilibrated in 5 ml of the same medium at 37 °C for 1–2 h. Some dishes were treated with various pharmacological agents during this equilibration period or with agonists for different times at the end of this period, as indicated in the corresponding figure legends. Cells were lysed in buffer A (50 mm Tris-HCl, pH 7.6, 2 mm EGTA, 2 mm EDTA, 1 mm dithiothreitol, 100 μg/ml leupeptin, 1 mm 4-(2-aminoethyl)-benzenesulfonyl fluoride, hydrochloride (Pefabloc), and 1% Triton X-100). PKD was immunoprecipitated with the PKD (PKD C-20) antiserum (1 μg/ml) raised against the C-terminal region of PKD (Santa Cruz Biotechnology). The immune complexes were recovered using protein-A coupled to agarose.
In Vitro Kinase Assays—Immune complexes were washed twice with lysis buffer, then twice with kinase buffer consisting of 30 mm Tris-HCl, pH 7.4, 10 mm MgCl2, 1 mm dithiothreitol. Autophosphorylation reactions were initiated by combining 20 μl of immune complexes with 5 μl of a phosphorylation mixture containing 100 μm [γ-32P]ATP in kinase buffer. After incubation at 30 °C for 10 min, the reactions were terminated by the addition of 1 ml of ice-cold kinase buffer and placed on ice. Immune complexes were recovered by centrifugation, and the proteins were extracted for SDS-PAGE analysis by the addition of 2× SDS-PAGE sample buffer (20 mm Tris/HCl, pH 6.8, 0.1 mm sodium orthovanadate, 1 mm EDTA, 6% SDS, 2 mm EDTA, 4% 2-mercaptoethanol, 10% glycerol). Dried SDS-PAGE gels were subjected to autoradiography to visualize autophosphorylated PKD. Autoradiograms were scanned using a Calibrated Densitometer GS800 (Bio-Rad), and the intensity of the detected bands was quantified using the Bio-Rad Quantity One 4.6 software.
For assays of exogenous substrate phosphorylation, immune complexes were processed as for autophosphorylation reactions, then substrate peptide (syntide-2 at a final concentration of 2.5 mg/ml) was added in the presence of [γ-32P]ATP (2 μCi/reaction diluted with cold ATP to give a final concentration of 100 μm) in kinase buffer (final reaction volume, 30 μl) and transferred to a water bath at 30 °C for 10 min. Reactions were terminated by adding 100 μl of 75 mm H3PO4, and 75 μl of the mixed supernatant was spotted to Whatman P-81 phosphocellulose paper. Papers were washed thoroughly in 75 μm H3PO4 and dried, and radioactivity incorporated into peptides was determined by detection of Cerenkov radiation in a scintillation counter.
For the measurements of in vitro PKD autophosphorylation, the following purified PKDs were used; either 15 ng/reaction recombinant purified PKD (purchased from Invitrogen (BioSource)) or PKD recovered from Swiss 3T3-PKD.GFP cell immune complexes by incubating the immune complexes with immunizing peptide (15 μg in ∼30 μl of kinase buffer) overnight on ice. The purified PKDs were then combined with phosphatidylserine and PDBu vesicles in kinase buffer (final volume, 50 μl) on ice (36). To initiate reactions, ATP at 200 μm was added, and the reactions were incubated at 30 °C for times indicated. Reactions were terminated by the addition of 2× SDS-PAGE sample buffer, resolved by SDS-PAGE on 8% gels, and analyzed by immunoblotting with the phosphospecific PKD antibodies Ser(P)744 and Ser(P)748.
siRNA Transfection
The SMART pool siRNA duplexes were purchased from Dharmacon (Lafayette, CO). PKD siRNA pool was designed to target against the mRNA of mouse PKD (GenBank™ accession number NM_008858) and consists of four selected siRNA oligonucleotides. The sequences wee as follows: oligo1, GAAGAGAUGUAGCUAUUAAUU; oligo2, GAAAGAGUGUUUGUUGUUAUU; oligo3, CAUAAGAGAUGUGCAUUUAUU; oligo4, CAGCGAAUGUAGUGUAUUAUU. For siRNA transfection the reverse transfection method was used; the siRNA pool was mixed with Trans IT-TKO Reagent (Mirus, Madison, WI) according to the manufacturer's protocol and added to 35-mm dishes. Swiss 3T3 cells were then plated on top of the siRNA/Trans IT-TKO complex at a density of 1 × 105 cells/35-mm dish. Control transfections were carried out with Dharmacon siCONTROL nontargeting siRNA four-oligo pool (Sc) (catalogue number D-001206-13). Six days after transfection, cells were used for experiments and subsequent Western blot analysis. Transfection efficacy was determined by transfecting fluorescein-labeled siRNA (Cell Signaling Technology, Beverly, MA) and counting fluorescence-positive cells by using a fluorescent microscope.
Assay of DNA Synthesis
Confluent and quiescent cultures of Swiss 3T3-PKD.GFP and Swiss 3T3-GFP cells were washed twice with DMEM and incubated with DMEM/Waymouth's medium (1:1, v/v) containing [3H]thymidine (0.2 μCi/ml, 1 μm) and various agonists as described in the figure legends. After 40 h of incubation at 37 °C, cultures were washed twice with PBS and incubated in 5% trichloroacetic acid at 4 °C for 20 min to remove acid-soluble radioactivity, washed with ethanol, and solubilized in 1 ml of 2% Na2CO3, 0.1 m NaOH. The acid-insoluble radioactivity was determined by scintillation counting in 6 ml of Beckman Readysafe.
Immunofluorescence
Cultures of Swiss 3T3-PKD.GFP cells were fixed in 4% paraformaldehyde for 20 min followed by permeabilization with 0.2% Triton X-100 in PBS for 5 min. The cultures were blocked in PBS supplemented with 5% goat serum and 2% bovine serum albumin (blocking solution) for 1 h at room temperature and incubated with the phospho-PKD antibody Ser(P)916 (1:1000) in blocking solution for another 24 h at room temperature. The cultures were then rinsed with PBS, and bound Ser(P)916 was detected by incubating with goat anti-rabbit secondary antibody conjugated to quantum dots (Qdot, Invitrogen) emitting at 655 nm (diluted 1:100 in PBS with 2% bovine serum albumin) for 1.5 h. Images were captured as uncompressed 12-bit TIFF files with a cooled (–12 °C) SPOT single color CCD digital camera (three pass method) driven by SPOT Version 2.1 software (Diagnostic Instruments, Inc., Sterling Heights, MI). Images were processed using Adobe Photo-shop CS.
Materials
[γ-32P]ATP (specific activity, 4500 Ci/mmol) was obtained from MP Biomedicals Inc., Solon, OH. Horseradish peroxidase-conjugated anti-rabbit IgG and enhanced chemiluminescence (ECL) reagents were from GE Healthcare. Purified PKD was obtained from BioSource (Invitrogen). Protein A-agarose and Pefabloc were from Roche Applied Science. Bombesin, vasopressin, EGF, PDGF, transforming growth factor-β, and Bisindolylmaleimide I (GF1) were obtained from Sigma. Gö6983 was from Calbiochem.
We used two different antibodies to detect the phosphorylated state of Ser744 and Ser748 in the PKD activation loop. One antibody (anti-Ser(P)744/Ser(P)748), obtained from Cell Signaling Technology, was raised against a peptide phosphorylated on serines equivalent to Ser744 and Ser748 of PKD but predominantly detects the phosphorylated state of Ser744, as shown originally in our laboratory (21). A second antibody, obtained from Abcam (ab17945), detects the phosphorylated state of Ser748. The specificity of this antibody was confirmed in our recent study using PKD with Ser744 and Ser748 mutated to unphosphorylatable alanines (22). All other reagents were from standard suppliers and were of the highest grade commercially available.
RESULTS
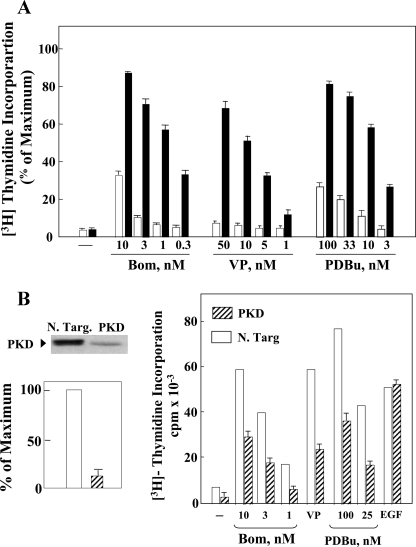
PKD Selectively Enhances DNA Synthesis in Response to Bombesin, Vasopressin, or PDBu—To determine whether PKD selectively facilitates DNA synthesis induced by Gq-coupled receptor agonists, Swiss 3T3 cells expressing PKD (termed Swiss 3T3 PKD.GFP cells) and parallel cultures of control cells (termed Swiss 3T3-GFP cells) all arrested in the G0 phase of the cell cycle were transferred to media containing [3H]thymidine and supplemented with bombesin (0.3–10 nm), vasopressin (1–50 nm), PDBu (1–100 nm), PDGF (1.25–10 ng/ml), transforming growth factor-β (0.6–10 ng/ml), EGF (0.3–2.5 ng/ml), or forskolin (10 μm). Reentry into S phase was determined by cumulative incorporation of radiolabeled precursor into DNA after 40 h of incubation.
Stimulation of Swiss 3T3 cells overexpressing PKD with bombesin, vasopressin, or PDBu induced a striking increase in [3H]thymidine incorporation into DNA, as compared with parallel cultures of Swiss 3T3-GFP cells (Fig. 1A). In contrast, the levels of DNA synthesis induced by increasing concentrations of PDGF, transforming growth factor-β, or EGF were virtually identical in Swiss 3T3-PKD.GFP and Swiss 3T3-GFP cells (supplemental Fig. S1). Moreover, PKD overexpression did not enhance DNA synthesis in response to forskolin, an agent that induces DNA synthesis via cAMP in Swiss 3T3 cells (37). These results show that PKD overexpression selectively enhanced DNA synthesis induced by Gq-coupled receptor agonists and PDBu in Swiss 3T3 cells.
FIGURE 1.
Panel A, PKD overexpression selectively potentiates DNA synthesis induced by bombesin, vasopressin, and PDBu in Swiss 3T3 cells. Confluent and quiescent cultures of Swiss 3T3 PKD.GFP cells (solid bars) and Swiss 3T3 GFP cells (open bars) were washed and incubated at 37 °C in 2 ml of DMEM/Waymouth's medium containing [3H]thymidine and the growth-promoting factors bombesin (Bom), vasopressin (VP), or PDBu, at the indicated concentrations. Results are expressed as a percentage mean ± S.E. (n = 3) of the maximal stimulation obtained with 10% fetal bovine serum (110 × 10–3 cpm/culture). Panel B, knockdown of endogenous PKD attenuates DNA synthesis in response to bombesin, vasopressin, or PDBu. Left, Swiss 3T3 cells were transfected with either non-targeting negative control (N. Targ.) or 75 nm PKD siRNA (PKD) as indicated. The cells were lysed, and PKD protein expression was assessed by Western blotting using the anti-PKD C-20 antibody. Shown here is are representative autoluminogram; similar results were obtained in four independent experiments. Autoluminograms were quantified by densitometric scanning. The results shown are the mean ± S.E. n = 4 and are expressed as percentage of the maximum level of PKD in non-targeting negative control cells (open bars). PKD expression was reduced by 88–92% (hatched bars). Right, Swiss 3T3 cells were transfected with either non-targeting negative control (open bars) or 75 nm PKD siRNA (hatched bars). After 6 days, when the cells were confluent and quiescent, the cultures were washed and incubated at 37 °C in 2 ml of DMEM/Waymouth's medium containing [3H]thymidine and the growth-promoting factors bombesin (Bom), 50 nm vasopressin (VP), and PDBu. Results are expressed as cpm/culture × 10–3; maximal stimulation, obtained in parallel cultures by stimulating with 10% fetal bovine serum, was 110 × 10–3 cpm/culture.
To determine the role of endogenous PKD in Gq-coupled receptor-induced mitogenesis in Swiss 3T3 cells, we depleted its expression using siRNAs that target specifically PKD. As shown in Fig. 1B, siRNAs targeting PKD produced striking knockdown of PKD protein (∼90%) and markedly attenuated the increase in DNA synthesis induced by bombesin, vasopressin, or PDBu in Swiss 3T3 cells. In contrast, DNA synthesis induced by EGF, a growth factor that does not activate PKD, was not affected by PKD knockdown. The results shown in Fig. 1 (and in supplemental Fig. S1) substantiate the notion that PKD plays a key and selective role in mediating Gq-coupled receptor-induced mitogenesis.
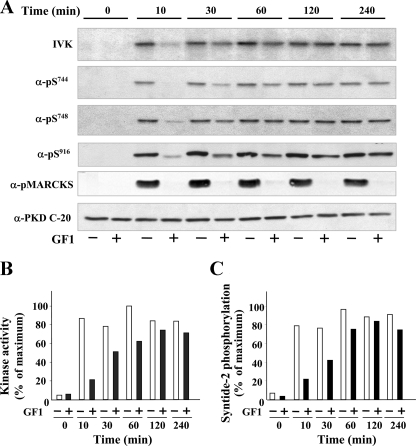
Bombesin Induces Early PKC-dependent and Late PKC-independent PKD Activation in Swiss 3T3 Cells—To elucidate the mechanism(s) of PKD activation that leads to enhanced DNA synthesis in response to Gq-coupled receptor agonists, we next determined whether in addition to the well characterized early, PKC-dependent mechanism of PKD phosphorylation and activation, bombesin also induces a second, PKC-independent phase of PKD activation in Swiss 3T3 cells. Quiescent cultures of these cells expressing PKD were pretreated with or without the preferential PKC inhibitor GF1, also known as bisindolylmaleimide I or GF109203X (38, 39), at 3.5 μm for 1 h (5, 6) and then stimulated with 10 nm bombesin for various times (10–240 min), as indicated in Fig. 2. Cell lysates were used to determine PKD phosphorylation at Ser744, Ser748, and Ser916 by SDS-PAGE followed by Western blotting using antibodies that specifically detect the phosphorylated state of each of these residues (4, 21, 40). Phosphorylation of PKD on Ser916 was also evaluated in single Swiss 3T3 cells by staining with PKD Ser(P)916 antibody and Qdot-labeled goat anti-rabbit antibody (supplemental Fig. S2).
FIGURE 2.
Time-course of bombesin-induced PKD phosphorylation and catalytic activation. Swiss 3T3 PKD.GFP cells were incubated in the absence (–, open bars) or in the presence (+, filled bars) of 3.5 μm GF1 for 1 h before stimulation of the cells with 10 nm bombesin for the indicated times. Panel A, the cultures were lysed with 2×SDS-PAGE sample buffer and analyzed by SDS-PAGE and immunoblotting with the following antibodies: phospho PKD Ser(P)916, Ser(P)744, Ser(P)748, and phospho-MARCKS. Equivalent loading of the gel was verified using Western blot analysis with an antibody directed against the C-terminal region of PKD (PKD-C20). Shown here are representative autoluminograms; similar results were obtained in five independent experiments. Quantification of 8 independent experiments carried out after 10 or 240 min of bombesin stimulation are shown in the supplemental data. Panel A, in vitro kinase. For IVK activity the cultures were lysed in ice-cold buffer, and PKD was immunoprecipitated from lysates with an anti-PKD (C-20) antibody bound to protein A-agarose and assayed for autophosphorylation, as described under “Experimental Procedures.” Shown here is a representative autoradiograph (IVK). Panel B, autoradiographs (IVK) were quantified by densitometric scanning. The results shown are the mean ± S.E. (n = 3) and are expressed as percentage of the maximum increase induced by treatment with bombesin. Panel C, PKD activity was measured by syntide-2 phosphorylation in immune complexes from lysates of cells that were incubated in the presence (filled bars) or in the absence (open bars) of 3.5 μm GF109203X for 1 h before stimulation with bombesin for the indicated times. The values shown (mean ± S.E. of at least two independent experiments) are expressed as percentage of the maximum increase induced by bombesin.
As shown in Fig. 2A, bombesin stimulation of Swiss 3T3 PKD.GFP cells induced striking PKD phosphorylation on the activation loop residues Ser744 and Ser748 and phosphorylation on Ser916, a well established autophosphorylation site (40). In agreement with previous results, cell treatment with GF1 prevented rapid PKD phosphorylation on Ser744, Ser748, and Ser916 induced by stimulation with bombesin (Fig. 2A). Because GF1 does not directly inhibit the catalytic activity of purified PKD in vitro kinase assays (5, 6, 11), including the activity of PKD isolated from 3T3 cells (shown in Fig. 6), these results imply that bombesin induced rapid multisite phosphorylation of PKD through PKCs.
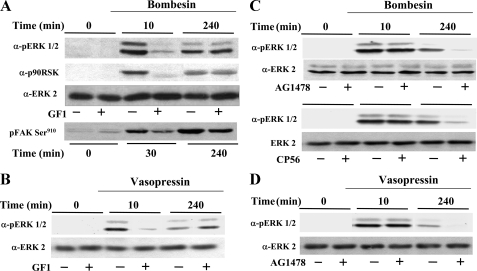
FIGURE 6.
PKD expression prolongs ERK signaling in response to bombesin or vasopressin through a PKC-independent but EGFR-dependent pathway. A, confluent and quiescent cultures of Swiss 3T3-PKD.GFP cells were washed and incubated at 37 °C in 2 ml DMEM in the presence (+) or in the absence (–) of 3.5 μm GF109203X (GF1) for 1 h before stimulation of the cells with 10 nm bombesin for the indicated times. The cultures were then lysed with 2× SDS-PAGE sample buffer and analyzed by SDS-PAGE and immunoblotting with phospho-ERK antibody (pERK1/2), phospho-p90rsk antibody (p90RSK), or phospho-focal adhesion kinase (FAK) Ser910. Shown here are representative autoluminograms. Similar results were obtained in four independent experiments. The membrane was further analyzed by Western blotting using ERK2 antibody (ERK2) to verify equal loading. B–D, confluent and quiescent cultures of Swiss 3T3-PKD.GFP cells were washed and incubated at 37 °C in 2 ml of DMEM in the absence (–) or presence (+) of 3.5 μm GF109203X (GF1), 500 nm AG1478, or 500 nm Compound 56 (CP56) as indicated for 1 h before stimulation of the cells with either 10 nm bombesin or 50 nm vasopressin for 10 or 240 min as indicated. The cultures were then lysed with 2× SDS-PAGE sample buffer and analyzed by SDS-PAGE and immunoblotting with phospho-ERK antibody (pERK1/2). Shown here are representative autoluminograms. Similar results were obtained in six independent experiments. The membrane was further analyzed by Western blotting using ERK2 antibody (ERK2) to verify equal loading.
The salient feature of the results illustrated in Fig. 2A was the shift from PKC-dependent to PKC-independent PKD phosphorylation on Ser916, Ser744, and Ser748 that was evident as early as 30 min after bombesin stimulation. Treatment with GF1 did not prevent PKD activation induced by bombesin stimulation for 120–240 min, as shown by Ser916 autophosphorylation monitored by Western blots (Fig. 2A; quantification of eight independent experiments is shown in supplemental Fig. S3) or by staining of individual cells (supplemental Fig. S2). Similarly, GF1 did not impede the phosphorylation of the activation loop residue Ser748 and only attenuated (but did not eliminate) the phosphorylation of Ser744 (see Western blots in Fig. 2A and quantification of eight independent experiments in supplemental Fig. S3). Western blotting with anti-PKD antibody (PKD C-20) confirmed that similar amounts of PKD were loaded in each lane (Fig. 2A).
To determine whether the catalytic activity of PKD (assayed by in vitro kinase assays) also displays biphasic activation, extracts of cells treated as indicated in Fig. 2 were immunoprecipitated with antibodies directed against the C-terminal region of PKD, and the resulting immune complexes were incubated with [γ32P]ATP. The reactants were analyzed by SDS-PAGE and autoradiography to determine the catalytic activity of PKD (in vitro kinase (IVK) in Fig. 2A and quantification of experiments in Fig. 2B). In addition, PKD kinase activity was also measured by phosphorylation of the peptide syntide-2, an excellent exogenous substrate for PKD (Fig. 2C). Bombesin stimulation of Swiss 3T3 PKD.GFP cells induced robust PKD catalytic activation in line with the assays of Ser916 autophosphorylation and phosphorylation at the activation loop residues Ser744 and Ser748. Treatment with the PKC inhibitor GF1 markedly inhibited PKD catalytic activation induced by bombesin stimulation for 10 min, but in striking contrast, GF1 did not prevent PKD activation induced by bombesin stimulation for longer times. Indeed, bombesin-induced PKD catalytic activation became insensitive to GF1 after 120–240 min of incubation (Fig. 2, B and C).
To verify that the inhibitory effect of GF1 on PKC-mediated phosphorylation of cellular substrates was maintained at all times of bombesin stimulation in Swiss 3T3 cells, we determined the phosphorylation state of MARCKS, a well established substrate of PKCs in these cells (41–43). MARCKS Ser152/156 phosphorylation was abolished by treatment with GF1 before bombesin stimulation at all times examined (Fig. 2A). Thus, sustained PKD activation in response to bombesin stimulation occurred in Swiss 3T3 cells with severely inhibited PKC activity.
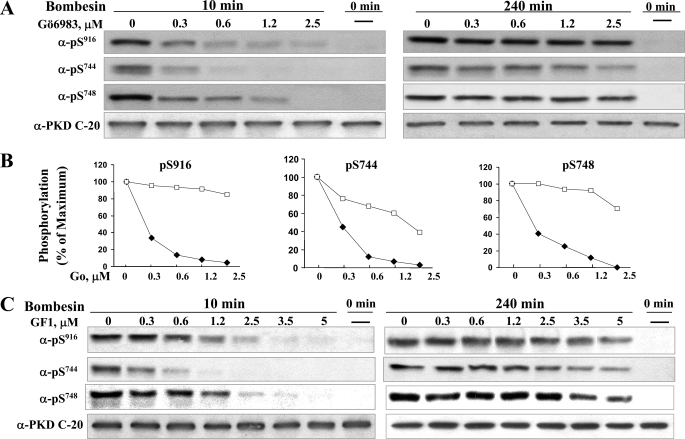
The results in Fig. 2 suggest that in addition to the well characterized early phase of PKD activation, bombesin stimulation of its endogenously expressed Gq-coupled receptor in Swiss 3T3 cells induces gradually a sustained phase of PKD phosphorylation and catalytic activation via a PKC-independent pathway. To substantiate the existence of biphasic PKD activation in response to bombesin receptor stimulation, we also tested the effect of Gö6983, a different PKC inhibitor that targets all isoforms of the PKC family but does not directly inhibit PKD activity (44). Specifically, we determined the effect of cell exposure to increasing concentrations of Gö6983 (0.3–2.5 μm) on PKD phosphorylation on Ser916, Ser744, and Ser748 induced by bombesin stimulation for either 10 or 240 min. As shown in Figs. 3, A and B, treatment with Gö6983 prevented PKD phosphorylation on Ser916, Ser744, and Ser748 produced by bombesin stimulation for 10 min. At 1.25 μm, Gö6983 inhibited PKD phosphorylation on these residues by 80–90%. In contrast, the inhibitory effect of Gö6983 on PKD multisite phosphorylation was strikingly diminished in cells stimulated with bombesin for 240 min. For example, treatment with 1.25 μm Gö6983 did not inhibit Ser916 autophosphorylation or phosphorylation of the activation loop residue Ser748 and only attenuated (by ∼40%) the phosphorylation of Ser744 (see Western blots in Fig. 3A and quantification in panels Fig. 3B). Similar dose-response results were obtained when Swiss 3T3 cells were treated with increasing concentrations of GF1 instead of Gö6983 (Fig. 3C).
FIGURE 3.
Effect of increasing concentrations of Gö6983 or GF1 on PKD phosphorylation on Ser916, Ser744, and Ser748 induced by bombesin stimulation for either 10 or 240 min. Swiss 3T3 PKD.GFP cells were incubated in the absence (–) or in the presence (+) of increasing concentrations of Gö6983 for 1 h before stimulation of the cells with 10 nm bombesin for either 10 min or 240 min as indicated and then lysed with 2×SDS-PAGE sample buffer. A, the samples were analyzed by SDS-PAGE and immunoblotting with the antibodies phospho-PKD Ser(P)916, Ser(P)744, Ser(P)748, and PKD-C20 to verify equal loading. Shown here are representative autoluminograms; similar results were obtained in three independent experiments. B, autoluminograms were quantified by densitometric scanning. The results shown are the mean ± S.E. (n = 3) and are expressed as the percentage of the maximum increase induced by treatment with bombesin in cells preincubated in the absence (open symbols) or the presence (closed symbols) of Gö6983. C, Swiss 3T3 PKD.GFP cells were incubated in the absence (–) or in the presence (+) of increasing concentrations of GF1 for 1 h before stimulation of the cells with 10 nm bombesin for 10 min or 240 min as indicated and then lysed with 2×SDS-PAGE sample buffer. The samples were analyzed by SDS-PAGE and immunoblotting with phospho PKD Ser(P)916, Ser(P)744, Ser(P)748 and PKD-C20 to verify equal loading. Shown here are representative autoluminograms; similar results were obtained in three independent experiments.
To determine whether bombesin induces PKC-dependent and PKC-independent phases of PKD activation at low concentrations, Swiss 3T3 PKD.GFP cells were treated with or without 3.5 μm GF1 and then stimulated with various concentrations of bombesin (0.1–10 nm) for either 10 or 240 min. Treatment with GF1 abrogated Ser744, Ser748, and Ser916 phosphorylation induced by cell stimulation with increasing concentrations of bombesin for 10 min. In contrast, stimulation of Swiss 3T3 PKD.GFP cells with bombesin for 240 min, even at a concentration as low as 0.3 nm, produced PKD multisite phosphorylation that was resistant to GF1 (supplemental Fig. 4). These results demonstrate biphasic PKD activation in response to physiological concentrations of the Gq-coupled receptor agonist bombesin (45, 46).
Using longer times of exposure, we also evaluated the effects of GF1 and bombesin on endogenous PKD in Swiss 3T3 cells, as shown in supplemental Fig. S5. The results indicate that bombesin, acting through its endogenously expressed receptor, induced PKC-dependent and PKC-independent phases of activation of endogenous PKD in Swiss 3T3 cells. Thus, biphasic PKD activation in response to Gq-coupled receptor agonist can be demonstrated in a system in which all the elements are expressed at physiological levels.
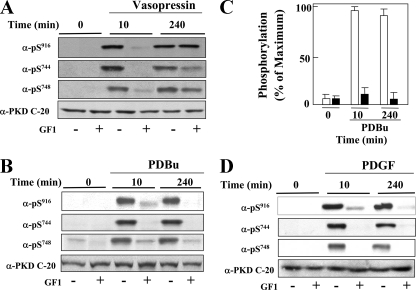
Role of PKC in Sustained PKD Activation Induced by 3T3 Cell Stimulation with Vasopressin, PDBu, or PDGF—To determine whether stimulation of a different mitogenic Gq-coupled receptor also induces biphasic PKD activation, we examined PKD phosphorylation on Ser744, Ser748, and Ser916 in cells treated with or without GF1 and stimulated with vasopressin. As shown in Fig. 4A, cell treatment with GF1 prevented PKD phosphorylation induced by vasopressin stimulation for 10 min. In contrast, GF1 did not prevent PKD phosphorylation induced by vasopressin stimulation for 240 min (Fig. 4A). A similar conclusion was drawn from experiments in which PKD phosphorylated on Ser916 was evaluated by staining individual Swiss 3T3 cells with PKD Ser(P)916 antibody and Qdot-labeled goat anti-rabbit antibody (supplemental Fig. S6).
FIGURE 4.
PKC-dependent and PKC-independent PKD activation in cells stimulated with vasopressin, PDBu, and PDGF. Swiss 3T3 PKD.GFP cells were incubated in the presence (+) or in the absence (–) of 3.5 μm GF1 for 1 h before stimulation with 50 nm vasopressin (panel A), 100 nm PDBu (panels B and C) or 10 ng/ml PDGF (panel D) for either 10 or 240 min as indicated. The cultures were then lysed with 2× SDS-PAGE sample buffer and analyzed by SDS-PAGE and immunoblotting with the antibodies phospho-PKD Ser(P)916, Ser(P)744, Ser(P)748, and PKD-C20 to verify equal loading. The results shown here are representative autoluminograms; similar results were obtained in at least three independent experiments. Panel C, quantification by densitometric scanning of Ser916 phosphorylation. The results shown are the means ± S.E. (n = 20) and are expressed as the percentage of the maximum increase induced by treatment with PDBu in cells treated in the absence (open bars) or the presence (closed bars) of 3.5 μm GF1 before PDBu stimulation.
Because PDBu bypasses receptor-mediated Gq/PLC activation, we also determined whether cell stimulation with PDBu induces biphasic PKD activation in Swiss 3T3 cells. In contrast to the results obtained with Gq-coupled receptor agonists, treatment with GF1 prevented PKD phosphorylation on Ser916, Ser744, and Ser748 induced by PDBu stimulation for either 10 or 240 min. A typical autoluminogram is presented in Fig. 4B and substantiated in multiple independent experiments monitoring Ser916 phosphorylation (Fig. 4C, bars; n = 20). The results obtained with PDBu implied that either Gq and/or PLC is required for eliciting the PKC-independent phase of PKD activation.
PDGF is known to increase diacylglycerol generation via tyrosine phosphorylation and activation of PLCγ rather than via Gq/PLCβ (47) and to stimulate PKD activation (6). To determine whether PDGF triggers biphasic PKD activation, cells were treated with or without 3.5 μm GF1 and then stimulated with 10 ng/ml PDGF for either 10 or 240 min. As shown in Fig. 4D, treatment with GF1 prevented PKD phosphorylation on Ser916, Ser744, and Ser748 induced by PDGF stimulation for either 10 or 240 min. Thus, in contrast to Gq-coupled receptor agonists, PDBu and PDGF induced PKD activation via PKC both at early and late times of stimulation. These results show for the first time that sustained PKD activation can be induced via PKC-dependent or PKC-independent mechanisms in the same cells.
Mechanism of PKD Catalytic Activation in Response to Bombesin in Swiss 3T3 Cells; Differential Regulation of Ser744 and Ser748 Phosphorylation—At least two mechanisms, autophosphorylation or transphosphorylation by a different upstream protein kinase, may contribute to PKD phosphorylation of activation loop Ser744 and Ser748 in response to stimulation with GPCR agonists. To distinguish between these mechanisms in Swiss 3T3 cells, we used different approaches to determine whether the catalytic activity of PKD is necessary for Ser744 and/or Ser748 phosphorylation during the early and late phases of GPCR-induced PKD activation in Swiss 3T3 cells.
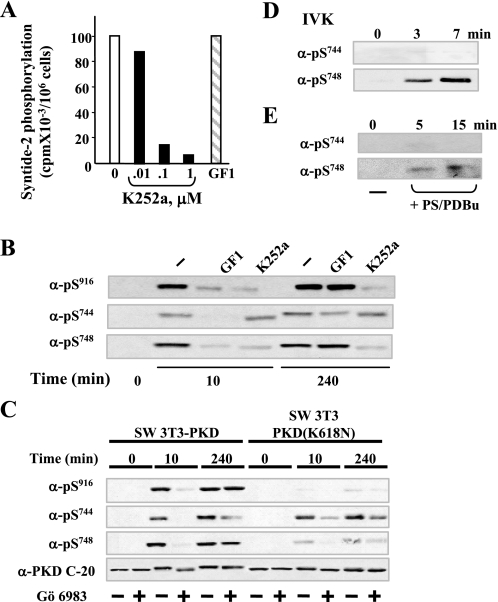
The furanosylated indolocarbazole K252a, a microbial alkaloid that inhibits various protein kinases, is a potent direct inhibitor of PKD catalytic activity (48) but a weak inhibitor of novel PKCs, including PKCδ and PKCε (49), the kinases implicated in mediating rapid Ser744 phosphorylation in Swiss 3T3 cells (50) and other cell types (1). As shown in Fig. 5A, the addition of K252a at 0.1–1 μm directly inhibited the catalytic activity of PKD isolated from bombesin-stimulated Swiss 3T3 cells, as measured by syntide-2 phosphorylation assays. In contrast, the addition of GF1 instead of K252a to the incubation mixture did not exert any inhibitory effect on PKD catalytic activity, even at the higher concentration (3.5 μm) tested in intact cells.
FIGURE 5.
Mechanism of PKD catalytic activation in response to bombesin in Swiss 3T3 cells; differential regulation of Ser744 and Ser748 phosphorylation. A, inhibition of in vitro PKD catalytic activity by K252a. Swiss 3T3 PKD.GFP cells were stimulated with bombesin for 10 min and then lysed in ice-cold buffer. PKD was immunoprecipitated from lysates with an anti-PKD C-20 antibody bound to protein A-agarose and assayed for syntide-2 phosphorylation activity in the presence of various concentrations of K252a (as indicated) or 3.5 μm GF1. B, Swiss 3T3-PKD.GFP were incubated in the absence (–) or in the presence 3.5 μm GF1 or 1 μm K252a as indicated for 1 h before stimulation with 10 nm bombesin for either 10 or 240 min. The cultures were then lysed with 2× SDS-PAGE sample buffer. C, Swiss 3T3-PKD.GFP or Swiss 3T3-PKDK618N.GFP were incubated in the absence (–) or in the presence (+) of 2.5 μm Gö 6983 for 1 h before stimulation of the cells with 10 nm bombesin for the indicated times. The cultures were then lysed with 2× SDS-PAGE sample buffer. All samples were analyzed by SDS-PAGE and immunoblotting with the antibodies phospho PKD Ser(P)916, Ser(P)744, Ser(P)748, and PKD-C20 to verify equal loading. The results shown here are representative autoluminograms; similar results were obtained in four independent experiments in panel B and three independent experiments in panel C. D, recombinant purified PKD was incubated with 100 μm ATP in kinase buffer for the times indicated. The reactions were terminated with 2× SDS-PAGE sample buffer. E, PKD eluted from Swiss 3T3 immunocomplexes was incubated with 200 μm ATP in kinase buffer for the times indicated. The reactions were terminated with 2× SDS-PAGE sample buffer. All samples were analyzed by SDS-PAGE and immunoblotting with the following antibodies phospho-PKD Ser(P)744 and Ser(P)748. All other details are as described under “Experimental Procedures.” The results shown here are representative autoluminograms; similar results were obtained in two independent experiments.
We next compared the effects of GF1 and K252a on bombesin-induced PKD phosphorylation on Ser916, Ser744, and Ser748 in intact Swiss 3T3 PKD.GFP cells. Cultures of these cells were incubated in the absence or presence of either 3.5 μm GF1 or 1 μm K252a for 1 h and then stimulated with 10 nm bombesin for either 10 or 240 min. Again, treatment with GF1 prevented PKD phosphorylation at Ser916, Ser744, and Ser748 induced by bombesin stimulation for 10 min, but it did not inhibit PKD phosphorylation on Ser916 and Ser748 and only attenuated PKD phosphorylation on Ser744 in response to bombesin stimulation for 240 min (Fig. 5B). The results obtained with K252a were strikingly different. Specifically, cell treatment with K252a abrogated early and late PKD phosphorylation at Ser748 induced by bombesin but did not prevent phosphorylation of PKD at Ser744 (Fig. 5B). We verified that treatment with K252a prevented PKD autophosphorylation on Ser916. These results suggested that PKD autophosphorylates at Ser748 but not on Ser744 in its activation loop.
To substantiate the interpretation of the results obtained with K252a, we also examined phosphorylation of Ser916, Ser744, and Ser748 in a PKD catalytic-deficient mutant of PKD (K618N) expressed in Swiss 3T3 cells (termed Swiss 3T3-PKDK618N.GFP cells (14)). As shown in Fig. 5C, the level of Ser744 phosphorylation of PKD (K618N) induced by bombesin stimulation for 10 or 240 min in cells pretreated with or without the PKC inhibitor Gö6983 was comparable with that of wild type PKD. These results indicate that agonist-induced PKD phosphorylation on Ser744 is mediated by transphosphorylation, initially via PKC but subsequently partly through a PKC-independent pathway that was evident in Swiss 3T3-PKDK618N.GFP cells.
In striking contrast to the results obtained with Ser744 phosphorylation, the level of Ser748 phosphorylation of PKD (K618N) induced by bombesin stimulation for either 10 or 240 min was markedly decreased as compared with wild type PKD, implying that the phosphorylation of this residue of the activation loop is mediated predominantly by autophosphorylation at both early and late times of GPCR stimulation (Fig. 5C). As expected, bombesin stimulation of Swiss 3T3-PKDK618N.GFP cells did not induce any detectable increase in PKD autophosphorylation on Ser916 (the faint bands seen correspond to endogenous PKD).
An additional line of evidence that supports the interpretation that Ser744 phosphorylation is mediated by transphosphorylation, whereas Ser748 phosphorylation of PKD results from autophosphorylation, is provided by studies in which PKD was activated in vitro by the addition of lipid activators (36), phosphatidylserine with either PDBu or diacylglycerol, and incubated for various times in the presence of ATP. The reactants were subjected to SDS-PAGE and immunoblotted with pSer744 or pSer748. Either purified recombinant PKD (Fig. 5D) or PKD isolated from Swiss 3T3 PKD.GFP cells by elution from immunoprecipitates (Fig. 5E) autophosphorylated on Ser748 rather than on Ser744.
PKD Expression Prolongs ERK Signaling through a PKC-independent but EGFR-dependent Pathway—The preceding results established that PKD is a critical element in mediating mitogenic signaling by Gq-coupled receptor agonists in Swiss 3T3 cells and that stimulation of endogenously expressed Gq-coupled receptors in these cells triggers PKD activation, not only via a well characterized early PKC-dependent phase but also through a novel late PKC-independent phase. We next determined whether the late phase of PKD activation plays a role in promoting long term mitogenic signaling in response to Gq-coupled receptor agonists.
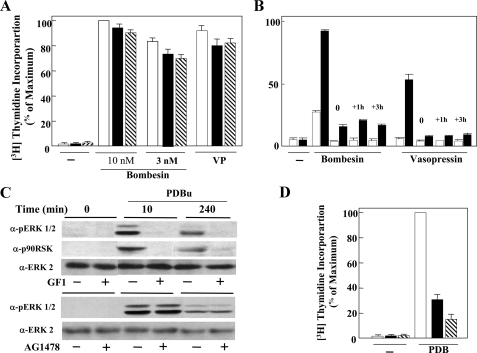
One of the mechanisms by which PKD facilitates GPCR-induced mitogenesis in Swiss 3T3 cells is by increasing the duration of the MEK/ERK/p90RSK activation (14), a pivotal pathway in mitogenic signaling (37). To examine whether PKD-mediated prolongation of ERK activation depends on PKC, lysates of Swiss 3T3-PKD.GFP cells incubated for 1 h in the absence or in the presence of 3.5 μm GF1 (5, 6) and subsequently challenged with or without bombesin for 10 or 240 min were analyzed by immunoblotting using a site-specific antibody that detects the activated form of ERK1/2 phosphorylated on Thr202 and Tyr204. In agreement with our previous results (14), bombesin induced prolonged ERK activation in Swiss 3T3-PKD.GFP cells, as it was noticeable even after 240 min of stimulation (Fig. 6A).
The salient feature of the results shown in Fig. 6A is that exposure to GF1 at a concentration that completely prevented rapid (10 min) ERK activation did not inhibit the prolonged ERK activation in response to bombesin stimulation. These results indicate that the increased duration of ERK activation induced by PKD signaling in bombesin-stimulated cells is mediated through the late, PKC-independent phase of PKD activation.
The 90-kDa ribosomal S6 kinases (RSK1–3) are serine/threonine protein kinases that are activated via ERK-mediated phosphorylation (51, 52). If PKD overexpression increases the duration of ERK catalytic activity within cells in response to GPCR agonists through a PKC-independent pathway, we would expect PKC-independent prolongation of the activation/phosphorylation of RSK, a downstream target of ERK. To test this possibility, we determined the effect of bombesin on RSK phosphorylation on Thr574, a site important for its activation (53). As shown in Fig. 6A, exposure to GF1 completely prevented rapid (10 min) RSK activation but did not inhibit the prolonged RSK activation in response to bombesin. Another downstream target of ERK is Ser910 of the nonreceptor-tyrosine kinase FAK (focal adhesion kinase) (54). Treatment with GF1 attenuated focal adhesion kinase phosphorylation on Ser910 induced by bombesin at early times of stimulation but did not block the prolonged phosphorylation of focal adhesion kinase on Ser910 in response to bombesin. Collectively, these results indicate that the increase in the duration of the ERK pathway induced by PKD signaling in bombesin-stimulated cells is mediated through the PKC-independent phase of PKD activation. A similar conclusion was drawn when the cells were stimulated with vasopressin instead of bombesin (Fig. 6B).
In a variety of cell types, GPCR agonists induce EGFR phosphorylation (55, 56) that leads to ERK signaling (37). Previously, we demonstrated that bombesin stimulation of Swiss 3T3 cells stimulates rapid ERK activation through an EGFR-independent pathway (57). Results shown in Fig. 6C confirmed these findings in Swiss 3T3-PKD.GFP cells, as treatment of these cells with the specific EGFR-tyrosine kinase inhibitor AG1478 (58) at a concentration (500 nm) that completely abolished EGF-induced ERK signaling in parallel cultures (results not shown) did not prevent ERK activation induced by stimulation with bombesin for 10 min. These results indicate that the rapid ERK activation induced by bombesin in PKD-overexpressing cells depends on PKC but not on EGFR tyrosine activity.
To obtain some indication of the steps linking the PKC-independent PKD activation with lengthened ERK signaling, we next determined whether EGFR-tyrosine kinase activity is necessary for the prolonged ERK activation induced by bombesin in Swiss 3T3-PKD.GFP cells. In striking contrast to the effects seen on rapid ERK activation, treatment with 500 nm AG1478 virtually abolished ERK activation induced by bombesin in Swiss 3T3-PKD.GFP cells during the second phase of PKD activation (Fig. 6C). Similar effects on prolonged ERK activation were obtained when Swiss 3T3-PKD.GFP cells were treated with compound 56 (59), a different selective inhibitor of EGFR-tyrosine kinase activity (Fig. 6C) or stimulated with vasopressin, a different Gq-coupled receptor agonist (Fig. 6D). Treatment with 500 nm AG1478 did not interfere with either the early or late phase of bombesin-induced PKD activation, as judged by Ser916 autophosphorylation (supplemental Fig. S7). These results show for the first time that the sustained phase of ERK activation induced by Gq-coupled receptor agonists in PKD-overexpressing 3T3 cells requires EGFR tyrosine kinase activity.
PKD Expression Potentiates DNA Synthesis through a PKC-independent but EGFR-dependent Pathway—Results shown above in Fig. 1 demonstrated that PKD overexpression selectively enhances DNA synthesis in response to the Gq-coupled receptor agonists bombesin and vasopressin in Swiss 3T3 fibroblasts. To determine whether PKD-mediated enhancement of DNA synthesis is mediated through the PKC-dependent and/or PKC-independent phases of PKD activation, cultures of Swiss 3T3-PKD.GFP cells, arrested in the G0 phase of the cell cycle, were transferred to media containing bombesin or vasopressin in the absence or in the presence of GF1. As shown in Fig. 7A, treatment with GF1 did not prevent the stimulation of DNA synthesis induced by bombesin or vasopressin in Swiss 3T3-PKD.GFP cells. Similar results were obtained when Gö6983 (1.25 μm) was added to the cultures instead of GF1. These results indicate that PKD enhances GPCR-induced DNA synthesis via a PKC-independent pathway.
FIGURE 7.
PKD expression potentiates DNA synthesis in response to bombesin or vasopressin through a PKC-independent but EGFR-dependent pathway. A, confluent and quiescent cultures of Swiss 3T3 PKD.GFP cells were washed and incubated at 37 °C in 2 ml of DMEM/Waymouth's medium containing [3H]thymidine and either 10 and 3 nm bombesin (Bom) or 50 nm vasopressin (VP) either in the absence (open bars) or presence of either 3.5 μm GF109203X (closed bars) or Go6978 (hatched bars). After 40 h, DNA synthesis was assessed by measuring the [3H]thymidine incorporated into acid-precipitable material. Results are expressed as a percentage mean ± S.E. (n = 3) of maximum stimulation obtained with 10% fetal bovine serum (105 × 10–3 cpm). B, confluent and quiescent cultures of Swiss 3T3 PKD.GFP cells were washed and incubated at 37 °C in 2 ml of DMEM/Waymouth's medium containing [3H]thymidine and either 10 nm bombesin (Bom) or 50 nm vasopressin (VP). Some of these cultures were incubated in the absence (open bars) or presence (closed bars) of 500 nm AG1478, added at the same time as bombesin or vasopressin (0 h) or 1 h or 3 h after the addition of bombesin or vasopressin (+1 h, +3 h). DNA synthesis was assessed by measuring the [3H]thymidine incorporated into acid-precipitable material. Results are expressed as a percentage mean ± S.E. (n = 3) of maximum stimulation obtained with 10% fetal bovine serum (98 × 10–3 cpm) C, confluent and quiescent cultures of Swiss 3T3-PKD.GFP cells were washed and incubated at 37 °C in 2 ml of DMEM in the absence (–) or presence (+) of 3.5 μm GF1 or 500 nm AG1478 for 1 h before stimulation of the cells with 100 nm PDBu for 10 or 240 min as indicated. The cultures were then lysed with 2× SDS-PAGE sample buffer, then analyzed by SDS-PAGE and immunoblotting with phospho-ERK antibody (pERK1/2) or phospho-p90RSK antibody (p90RSK) as indicated. Shown here are representative autoluminograms. Similar results were obtained in four independent experiments. The membrane was further analyzed by Western blotting using ERK2 antibody (ERK2) to verify equal loading. D, confluent and quiescent cultures of Swiss 3T3 PKD.GFP cells were washed and incubated at 37 °C in 2 ml of DMEM/Waymouth's medium containing [3H]thymidine and 100 nm PDBu either in the absence (open bars) or presence of 3.5 μm GF109203X (closed bars) or Go6978 (hatched bars). After 40 h DNA synthesis was assessed by measuring the [3H]thymidine incorporated into acid-precipitable material. Results are expressed as a percentage mean ± S.E. (n = 3) of maximum stimulation obtained with 10% fetal bovine serum (105 × 10–3 cpm).
Because PKD facilitates GPCR-induced mitogenesis in Swiss 3T3 cells at least in part by increasing the duration of the MEK/ERK/p90RSK activation (14) and the results in Fig. 6 indicated that PKD induces prolonged ERK activation through EGFR, we determined whether treatment with AG1478 prevents the enhancement of DNA synthesis induced by Gq-coupled receptor agonist in Swiss 3T3-PKD.GFP cells. As shown in Fig. 7B, treatment of the cells with 500 nm AG1478 strikingly attenuated DNA synthesis induced by bombesin in PKD-overexpressing 3T3 cells. Interestingly, the addition of the EGFR-tyrosine kinase inhibitor 1 or 3 h after bombesin also prevented bombesin-induced DNA synthesis, implying that EGFR-tyrosine kinase activity is required during the sustained phase of PKD activation (Fig. 7B).
As shown above, PKD also enhanced PDBu-elicited DNA synthesis and increased the duration of ERK activation. However, in contrast to GPCR agonists, PDBu stimulated early and late PKD activation via PKC (Fig. 4). Therefore, we predicted that treatment with either GF1 or Gö6983 should inhibit PDBu-induced DNA synthesis in Swiss 3T3-PKD.GFP cells. As shown in Fig. 7, cell treatment with GF1 (3.5 μm) markedly inhibited prolonged ERK and p90RSK activation (Fig. 7C) as well as stimulation of DNA synthesis (Fig. 7D) induced by PDBu in Swiss 3T3-PKD.GFP cells. These results imply that the enhancement of DNA synthesis by PKD in PDBu-stimulated cells is mediated through a PKC-dependent pathway. Furthermore, PDBu-induced early or late ERK signaling did not depend on EGFR-tyrosine kinase activity, as shown by the results obtained with AG1478 (Fig. 7C). Collectively, these results support the notion that Gq-coupled receptor activation induces prolonged ERK signaling and enhanced DNA synthesis through a PKD-dependent but PKC-independent pathway that requires EGFR-tyrosine kinase activity, whereas PDBu stimulated these responses via a PKC/PKD-dependent cascade in the same cell type.
DISCUSSION
The experiments presented here were designed to elucidate the biological significance and underlying mechanism(s) of a recently identified sustained phase of PKD activation using a well defined cellular system where PKD expression potently and selectively enhances mitogenic signaling induced by Gq-coupled receptor agonists. In this context we continued to exploit Swiss 3T3 fibroblasts, a cell line used extensively as a model system to elucidate mechanisms of mitogenic signaling induced by activation of endogenously expressed GPCRs (32–34). Previously, we demonstrated that PKD overexpression in these cells enhances DNA synthesis and cell division in response to the GPCR agonists bombesin and vasopressin (8, 14). Here, we extended these findings, demonstrating that PKD strikingly potentiated DNA synthesis induced by the Gq-coupled receptor agonists in a selective manner in Swiss 3T3 cells (see supplemental Fig. S1). Furthermore, we showed that siRNA-mediated knockdown of endogenous PKD in these cells markedly attenuated DNA synthesis in response to bombesin, vasopressin, or PDBu. Consequently, subsequent experiments were designed to dissect PKC-dependent and PKC-independent phases of PKD activation in Swiss 3T3 cells and to clarify the contribution of these phases to PKD-mediated mitogenic signaling in response to Gq-coupled receptor agonists in these cells.
Our studies produced several lines of evidence indicating that stimulation of the endogenously expressed Gq-coupled receptors in Swiss 3T3 cells leads to rapid PKC-dependent followed by sustained PKC-independent PKD activation. Specifically, treatment with preferential PKC inhibitors profoundly inhibited PKD activation induced by bombesin stimulation for <15 min but did not prevent PKD catalytic activation induced by bombesin stimulation for longer times (>60 min). Indeed, bombesin-induced PKD activation became virtually PKC-independent after 120–240 min of bombesin stimulation. The existence of sequential PKC-dependent and PKC-independent PKD activation was demonstrated in Swiss 3T3 cells treated with different PKC inhibitors and stimulated with increasing concentrations of bombesin. Biphasic PKD activation was also elicited by stimulation with vasopressin, an agonist that induces mitogenic signaling in Swiss 3T3 cells via a different Gq-coupled receptor. In contrast, cell stimulation with PDBu, a direct activator of PKC/PKD that bypasses receptor-mediated Gq/PLC pathways, induced PKC-dependent PKD activation both at early and late times of stimulation. Similarly, stimulation of Swiss 3T3 cells with PDGF, a polypeptide growth factor that increases diacylglycerol generation via tyrosine phosphorylation and activation of PLCγ (47), also induced PKC-dependent PKD activation at early and late times of cell stimulation. Collectively, our results show for the first time that PKC-independent sustained PKD activation is induced specifically by Gq-coupled receptor agonists, implying that either Gq and/or PLC is required for eliciting the PKC-independent phase of PKD activation. The results also show that PKC-dependent and PKC-independent pathways leading to sustained PKD activation are operative within the same cells.
To elucidate the mechanism by which Gq-coupled receptor agonists induce biphasic PKD activation, we examined the phosphorylation of each of the key residues of the activation loop of PKD, namely Ser744 and Ser748. In theory, the phosphorylation of these residues in response to cell stimulation could involve PKD-mediated autophosphorylation and/or transphosphorylation by upstream kinases. Several lines of evidence implicated both mechanisms in the phosphorylation of the activation loop of PKD in response to Gq-coupled receptor agonists. Specifically, we found that cell treatment with the direct PKD inhibitor K252a abolished bombesin-stimulated PKD phosphorylation on Ser748, but in striking contrast, K252a did not prevent PKD phosphorylation on Ser744. These results suggest that GPCR-induced activation loop Ser748 phosphorylation is mediated predominantly by PKD autophosphorylation, whereas activation loop Ser744 phosphorylation is apparently regulated by transphosphorylation. This interpretation was substantiated by results obtained with Swiss 3T3 cells expressing a kinase-deficient PKD mutant. In response to bombesin stimulation, this PKD mutant displayed unimpaired Ser744 phosphorylation (indicative of transphosphorylation) but showed a striking decrease in the level of Ser748 phosphorylation, consistent with PKD-mediated autophosphorylation. In addition, PKD (either recombinant or isolated from Swiss 3T3 cells) incubated in vitro with ATP autophosphorylated preferentially on Ser748 rather than on Ser744. Extending our recent results (22), we propose that PKD transphosphorylation is the major mechanism targeting Ser744, whereas PKD autophosphorylation is the predominant mechanism for Ser748.
A novel feature of the results presented here is that the mechanisms leading to the phosphorylation of each of these residues depended on the time of Gq-coupled receptor stimulation. PKD phosphorylation on Ser744 was mediated primarily by PKC transphosphorylation at early times of bombesin stimulation, whereas a PKC-independent transphosphorylation of this residue was evident in Swiss 3T3 cells stimulated with bombesin or vasopressin for longer times (>60 min). These findings imply that, in addition to PKCs, another upstream protein kinase(s) is responsible for a substantial level of Ser744 phosphorylation at later times of Gq-coupled receptor stimulation. Thus, PKD provides a unique example of a protein kinase in which the phosphorylation of the key serines in its activation loop, Ser744 and Ser748, is regulated by both transphosphorylation (Ser744) and autophosphorylation (Ser748) mechanisms.
Having established biphasic PKD activation in Swiss 3T3 cells, a major objective of the present studies was to test the hypothesis that the sustained phase of PKD activation contributes to mediate long term biological responses stimulated by Gq-coupled receptor agonists, including prolonged ERK signaling and re-initiation of DNA synthesis. In support of this hypothesis, we found that prolonged ERK signaling and stimulation of DNA synthesis induced by GPCR agonists in Swiss 3T3 cells expressing PKD were only slightly diminished by treatment with preferential PKC inhibitors, indicating that these events were greatly augmented by PKD signaling through a PKC-independent pathway. In contrast, ERK activation and DNA synthesis induced by PDBu remained sensitive to preferential PKC inhibitors, in line with the fact that PDBu did not induce a PKC-independent phase of Ser744 and Ser748 PKD phosphorylation and catalytic activation in Swiss 3T3 cells. Thus, Gq-coupled receptor activation induces prolonged ERK signaling and enhances DNA synthesis through a PKD-dependent but PKC-independent pathway in Swiss 3T3 cells, whereas PDBu stimulates these responses via a PKC/PKD-dependent cascade in the same cell type.
In a variety of cell types, stimulation of GPCRs induces EGFR phosphorylation (55, 56) that leads to ERK activation via the Ras-Raf-MEK pathway (37). In agreement with our previous results (57), bombesin stimulation of Swiss 3T3 cells induced rapid ERK activation through an EGFR-independent pathway. In striking contrast, we show here for the first time that the sustained phase of ERK activation and subsequent DNA synthesis induced by Gq-coupled receptor agonists in PKD-overexpressing 3T3 cells requires EGFR-tyrosine kinase activity. These results identify a novel role of EGFR in PKD-induced enhancement of mitogenic signaling triggered by Gq-coupled receptor agonists in Swiss 3T3 cells.
In conclusion, Gq-coupled receptor agonists, in contrast to phorbol esters, specifically induce PKD activation and multisite phosphorylation through early PKC-dependent and late PKC-independent mechanisms in Swiss 3T3 cells. Further results demonstrate the involvement of the PKC-independent phase of PKD activation in prolonged ERK signaling and stimulation DNA synthesis induced by Gq-coupled receptor agonists through an EGFR-dependent pathway. Thus, our results identify a novel mechanism of Gq-coupled receptor-induced mitogenesis mediated by sustained PKD activation through a PKC-independent pathway and demonstrate that sustained PKD activation can be induced through PKC-dependent and -independent mechanisms within the same cell type.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grant R0-1 DK 55003, R0-1 DK56930, and P30 DK41301 (to E. R.).
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–7.
Footnotes
The abbreviations used are: PKD and PKC, protein kinase D and C, respectively; PDBu, phorbol 12,13-dibutyrate; GPCR, G protein-coupled receptor; EGFR, epidermal growth factor (EGF) receptor; DMEM, Dulbecco's modified Eagle's medium; PBS, phosphate-buffered saline; MEK, mitogen-activated protein kinase/extracellular signal-regulated kinase kinase; IVK, in vitro kinase; ERK, extracellular signal-regulated kinase; PDGF, platelet-derived growth factor; GFP, green fluorescent protein; RSK, ribosomal S6 kinase; siRNA, small interfering RNA; PBS, phosphate-buffered saline; PLC, phospholipase C; MARCKS, myristoylated alanaine-rich C kinase substrate.
References
- 1.Rozengurt, E., Rey, O., and Waldron, R. T. (2005) J. Biol. Chem. 280 13205–13208 [DOI] [PubMed] [Google Scholar]
- 2.Iglesias, T., and Rozengurt, E. (1998) J. Biol. Chem. 273 410–416 [DOI] [PubMed] [Google Scholar]
- 3.Iglesias, T., and Rozengurt, E. (1999) FEBS Lett. 454 53–56 [DOI] [PubMed] [Google Scholar]
- 4.Waldron, R. T., and Rozengurt, E. (2003) J. Biol. Chem. 278 154–163 [DOI] [PubMed] [Google Scholar]
- 5.Zugaza, J. L., Sinnett-Smith, J., Van Lint, J., and Rozengurt, E. (1996) EMBO J. 15 6220–6230 [PMC free article] [PubMed] [Google Scholar]
- 6.Zugaza, J. L., Waldron, R. T., Sinnett-Smith, J., and Rozengurt, E. (1997) J. Biol. Chem. 272 23952–23960 [DOI] [PubMed] [Google Scholar]
- 7.Iglesias, T., Waldron, R. T., and Rozengurt, E. (1998) J. Biol. Chem. 273 27662–27667 [DOI] [PubMed] [Google Scholar]
- 8.Zhukova, E., Sinnett-Smith, J., and Rozengurt, E. (2001) J. Biol. Chem. 276 40298–40305 [DOI] [PubMed] [Google Scholar]
- 9.Zhukova, E., Sinnett-Smith, J., Wong, H., Chiu, T., and Rozengurt, E. (2001) J. Cell. Physiol. 189 291–305 [DOI] [PubMed] [Google Scholar]
- 10.Chiu, T., and Rozengurt, E. (2001) FEBS Lett. 489 101–106 [DOI] [PubMed] [Google Scholar]
- 11.Chiu, T., and Rozengurt, E. (2001) Am. J. Physiol. Cell Physiol. 280 929–942 [DOI] [PubMed] [Google Scholar]
- 12.Chiu, T., Wu, S. S., Santiskulvong, C., Tangkijvanich, P., Yee, H. F., Jr., and Rozengurt, E. (2002) Am. J. Physiol. Cell Physiol. 282 434–450 [DOI] [PubMed] [Google Scholar]
- 13.Guha, S., Rey, O., and Rozengurt, E. (2002) Cancer Res. 62 1632–1640 [PubMed] [Google Scholar]
- 14.Sinnett-Smith, J., Zhukova, E., Hsieh, N., Jiang, X., and Rozengurt, E. (2004) J. Biol. Chem. 279 16883–16893 [DOI] [PubMed] [Google Scholar]
- 15.Paolucci, L., Sinnett-Smith, J., and Rozengurt, E. (2000) Am. J. Physiol. Cell Physiol. 278 33–39 [DOI] [PubMed] [Google Scholar]
- 16.Yuan, J., Slice, L. W., Gu, J., and Rozengurt, E. (2003) J. Biol. Chem. 278 4882–4891 [DOI] [PubMed] [Google Scholar]
- 17.Yuan, J. Z., Slice, L., Walsh, J. H., and Rozengurt, E. (2000) J. Biol. Chem. 275 2157–2164 [DOI] [PubMed] [Google Scholar]
- 18.Yuan, J., Slice, L. W., and Rozengurt, E. (2001) J. Biol. Chem. 276 38619–38627 [DOI] [PubMed] [Google Scholar]
- 19.Needham, L. K., and Rozengurt, E. (1998) J. Biol. Chem. 273 14626–14632 [DOI] [PubMed] [Google Scholar]
- 20.Matthews, S. A., Pettit, G. R., and Rozengurt, E. (1997) J. Biol. Chem. 272 20245–20250 [DOI] [PubMed] [Google Scholar]
- 21.Waldron, R. T., Rey, O., Iglesias, T., Tugal, T., Cantrell, D., and Rozengurt, E. (2001) J. Biol. Chem. 276 32606–32615 [DOI] [PubMed] [Google Scholar]
- 22.Jacamo, R., Sinnett-Smith, J., Rey, O., Waldron, R. T., and Rozengurt, E. (2008) J. Biol. Chem. 283 12877–12887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brandlin, I., Hubner, S., Eiseler, T., Martinez-Moya, M., Horschinek, A., Hausser, A., Link, G., Rupp, S., Storz, P., Pfizenmaier, K., and Johannes, F. J. (2002) J. Biol. Chem. 277 6490–6496 [DOI] [PubMed] [Google Scholar]
- 24.Sinnett-Smith, J., Zhukova, E., Rey, O., and Rozengurt, E. (2007) J. Cell. Physiol. 211 781–790 [DOI] [PubMed] [Google Scholar]
- 25.Wang, Y., Waldron, R. T., Dhaka, A., Patel, A., Riley, M. M., Rozengurt, E., and Colicelli, J. (2002) Mol. Cell. Biol. 22 916–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matthews, S. A., Liu, P., Spitaler, M., Olson, E. N., McKinsey, T. A., Cantrell, D. A., and Scharenberg, A. M. (2006) Mol. Cell. Biol. 26 1569–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liljedahl, M., Maeda, Y., Colanzi, A., Ayala, I., Van Lint, J., and Malhotra, V. (2001) Cell 104 409–420 [DOI] [PubMed] [Google Scholar]
- 28.Prigozhina, N. L., and Waterman-Storer, C. M. (2004) Curr. Biol. 14 88–98 [DOI] [PubMed] [Google Scholar]
- 29.Storz, P., and Toker, A. (2003) EMBO J. 22 109–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mihailovic, T., Marx, M., Auer, A., Van Lint, J., Schmid, M., Weber, C., and Seufferlein, T. (2004) Cancer Res. 64 8939–8944 [DOI] [PubMed] [Google Scholar]
- 31.Chiu, T. T., Leung, W. Y., Moyer, M. P., Strieter, R. M., and Rozengurt, E. (2007) Am. J. Physiol. Cell Physiol. 292 767–777 [DOI] [PubMed] [Google Scholar]
- 32.Rozengurt, E. (1986) Science 234 161–166 [DOI] [PubMed] [Google Scholar]
- 33.Rozengurt, E. (1998) J. Cell. Physiol. 177 507–517 [DOI] [PubMed] [Google Scholar]
- 34.Rozengurt, E. (2002) Trends Endocrinol. Metab. 13 128–134 [DOI] [PubMed] [Google Scholar]
- 35.Seufferlein, T., Withers, D. J., Mann, D., and Rozengurt, E. (1996) Mol. Biol. Cell 7 1865–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Lint, J. V., Sinnett-Smith, J., and Rozengurt, E. (1995) J. Biol. Chem. 270 1455–1461 [DOI] [PubMed] [Google Scholar]
- 37.Rozengurt, E. (2007) J. Cell. Physiol. 213 589–602 [DOI] [PubMed] [Google Scholar]
- 38.Toullec, D., Pianetti, P., Coste, H., Bellevergue, P., Grandperret, T., Ajakane, M., Baudet, V., Boissin, P., Boursier, E., Loriolle, F., Duhamel, L., Charon, D., and Kirilovsky, J. (1991) J. Biol. Chem. 266 15771–15781 [PubMed] [Google Scholar]
- 39.Sinnett-Smith, J., Zachary, I., Valverde, A. M., and Rozengurt, E. (1993) J. Biol. Chem. 268 14261–14268 [PubMed] [Google Scholar]
- 40.Matthews, S. A., Rozengurt, E., and Cantrell, D. (1999) J. Biol. Chem. 274 26543–26549 [DOI] [PubMed] [Google Scholar]
- 41.Rozengurt, E., Rodriguez-Pena, M., and Smith, K. A. (1983) Proc. Natl. Acad. Sci. U. S. A. 80 7244–7248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brooks, S. F., Herget, T., Erusalimsky, J. D., and Rozengurt, E. (1991) EMBO J. 10 2497–2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brooks, S. F., Herget, T., Broad, S., and Rozengurt, E. (1992) J. Biol. Chem. 267 14212–14218 [PubMed] [Google Scholar]
- 44.Young, L. H., Balin, B. J., and Weis, M. T. (2005) Cardiovasc. Drug Rev. 23 255–272 [DOI] [PubMed] [Google Scholar]
- 45.Zachary, I., and Rozengurt, E. (1985) Proc. Natl. Acad. Sci. U. S. A. 82 7616–7620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zachary, I., and Rozengurt, E. (1987) J. Biol. Chem. 262 3947–3950 [PubMed] [Google Scholar]
- 47.Poulin, B., Sekiya, F., and Rhee, S. G. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 4276–4281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gschwendt, M., Dieterich, S., Rennecke, J., Kittstein, W., Mueller, H. J., and Johannes, F. J. (1996) FEBS Lett. 392 77–80 [DOI] [PubMed] [Google Scholar]
- 49.Geiges, D., Meyer, T., Marte, B., Vanek, M., Weissgerber, G., Stabel, S., Pfeilschifter, J., Fabbro, D., and Huwiler, A. (1997) Biochem. Pharmacol. 53 865–875 [DOI] [PubMed] [Google Scholar]
- 50.Rey, O., Reeve, J. R., Jr., Zhukova, E., Sinnett-Smith, J., and Rozengurt, E. (2004) J. Biol. Chem. 279 34361–34372 [DOI] [PubMed] [Google Scholar]
- 51.Zhao, Y., Bjorbak, C., and Moller, D. E. (1996) J. Biol. Chem. 271 29773–29779 [DOI] [PubMed] [Google Scholar]
- 52.Frodin, M., and Gammeltoft, S. (1999) Mol. Cell. Endocrinol. 151 65–77 [DOI] [PubMed] [Google Scholar]
- 53.Dalby, K. N., Morrice, N., Caudwell, F. B., Avruch, J., and Cohen, P. (1998) J. Biol. Chem. 273 1496–1505 [DOI] [PubMed] [Google Scholar]
- 54.Hunger-Glaser, I., Salazar, E. P., Sinnett-Smith, J., and Rozengurt, E. (2003) J. Biol. Chem. 278 22631–22643 [DOI] [PubMed] [Google Scholar]
- 55.Gschwind, A., Zwick, E., Prenzel, N., Leserer, M., and Ullrich, A. (2001) Oncogene 20 1594–1600 [DOI] [PubMed] [Google Scholar]
- 56.Schafer, B., Gschwind, A., and Ullrich, A. (2004) Oncogene 23 991–999 [DOI] [PubMed] [Google Scholar]
- 57.Santiskulvong, C., Sinnett-Smith, J., and Rozengurt, E. (2001) Am. J. Physiol. Cell Physiol. 281 886–898 [DOI] [PubMed] [Google Scholar]
- 58.Levitzki, A., and Gazit, A. (1995) Science 267 1782–1788 [DOI] [PubMed] [Google Scholar]
- 59.Bridges, A. J., Zhou, H., Cody, D. R., Rewcastle, G. W., McMichael, A., Showalter, H. D., Fry, D. W., Kraker, A. J., and Denny, W. A. (1996) J. Med. Chem. 39 267–276 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.