Abstract
Previously we have shown that tyrosine 718 of ASK1 when phosphorylated is critical for SOCS1 binding and SOCS1-mediated degradation of ASK1. However, the kinase and phosphatase responsible for phosphorylation and dephosphorylation of ASK1 at Tyr-718 are unknown. In this study, we identified JAK2 and SHP2 as a Tyr-718-specific kinase and phosphatase, respectively. Interferon-γ (IFN-γ) induced degradation of ASK1 in normal but not in SOCS1-KO endothelial cells (EC). IFN-γ-induced tyrosine phosphorylation of ASK1 at Tyr-718 was blocked by a JAK2-specific inhibitor. IFN-γ enhanced the association between JAK2 and ASK1, and the ASK1-JAK2 complex was labile and was stabilized by the proteasomal inhibitor MG132. Furthermore, JAK2, but not JAK1, directly bound to and phosphorylated ASK1 at Tyr-718, leading to an enhanced association of ASK1 with SOCS1 and subsequent ASK1 degradation. Next, we showed that overexpression of the SH2-containing protein-tyrosine phosphatase-2 (SHP2) augmented, whereas a phosphatase-inactive mutant of SHP2 inhibited, TNF-induced ASK1 dephosphorylation. SHP2 associated with ASK1 in response to tumor necrosis factor in EC. An SHP-2 substrate-trapping mutant formed a complex with tyrosine-phosphorylated ASK1, suggesting that ASK1 is a direct SHP2 substrate. Moreover, SHP2 wild type, but not a catalytically inactive mutant, dissociated SOCS1 from ASK1. IFN-γ-induced ASK1 Tyr(P)-718 was enhanced in mouse EC deficient in SHP2 (SHP2-KO). In contrast, tumor necrosis factor-induced dephosphorylation of ASK1 at Tyr(P)-718 and activation of ASK1-JNK signaling, as well as EC apoptosis, are significantly reduced in SHP2-KO EC. Our data suggest that JAK2-SOCS1 and SHP2 reciprocally regulate ASK1 phosphorylation and stability in response to cytokines.
Myocardial infarction due to atherosclerosis of coronary arteries remains the leading cause of death in the United States. It has become clear that increases in inflammatory mediators represent a common pathogenic mechanism for atherosclerosis (1). The vascular cell that normally limits the inflammatory and atherosclerotic process is the EC.3 Proinflammatory stimuli induce EC dysfunction, which is characterized by an enhanced sensitivity of vascular cells to proinflammatory and proapoptotic stimuli. Studies from our laboratory and others have demonstrated that ASK1 (apoptosis signal-regulating kinase-1), a member of MAP3K family (2, 3), is an effector of inflammation in EC (4–8). Almost all inflammatory stimuli such as tumor necrosis factor-α (TNF), interleukin-1 (IL-1), and reactive oxygen species activate ASK1. Activated ASK1 subsequently recruits and activates its downstream target MAP2Ks (MKK3/7 and MKK4/7), which in turn activate MAPKs (JNK and p38). Studies from ASK1-deficient mice have also linked ASK1 to cardiovascular pathogenesis. ASK1 deletion in mice attenuated angiotensin II-induced cardiac hypertrophy and remodeling. Neointimal formation due to proliferation of smooth muscle cells in a cuff injury model is also attenuated by ASK1 deletion in mice (9, 10).
Although the linkage of ASK1 to inflammation is very strong, the mechanism by which inflammatory stimuli, including TNF, activate ASK1 is not fully understood. The identification of proteins associated with ASK1 and their regulation on ASK1 have provided some insights into the mechanism for ASK1 activation. ASK1 is a 170-kDa protein that is composed of an inhibitory N-terminal domain, an internal kinase domain, and a C-terminal regulatory domain. One important regulatory mechanism of ASK1 activity is its Ser/Thr phosphorylation and dephosphorylation by kinases and phosphatases. ASK1 is basally phosphorylated at Ser-967 by an unidentified kinase, and 14-3-3 binds to this site and inhibits ASK1 activity (11, 12). TNF activates ASK1 in part by dissociating these cellular inhibitors from ASK1 (4, 7). Recently, we have identified PP2A as a phosphatase in TNF-induced dephosphorylation of ASK1 Ser(P)-967 (13). In addition to the 14-3-3-binding site, Ser(P)-967, ASK1 is phosphorylated at Ser-83 by Akt, leading to inhibition of ASK1 activity. In contrast, autophosphorylation of ASK1 at Thr-838 leads to oligomerization and activation (14). Phosphorylation of Thr-845 can be negatively regulated by the phosphatase PP5 (15). Similarly, we found that the ASK1 autophosphorylation at Thr-813 and Thr-842 also positively regulates ASK1 signaling (16).
In contrast to Ser/Thr phosphorylation, regulation of ASK1 by tyrosine phosphorylation is less well understood. We have recently shown that ASK1 is phosphorylated at Tyr-718, and this phosphorylation is critical for the binding to suppressor of cytokine signaling-1 (SOCS1), a subunit of ubiquitin ligase responsible for ASK1 degradation (17). Tyrosine phosphorylation of ASK1 is up-regulated in response to growth factors and cytokines such as IFN-γ, whereas this phosphorylation can be down-regulated by TNF treatment, resulting in ASK1 dissociation from SOCS1. However, the kinase and phosphatase responsible for phosphorylation and dephosphorylation of ASK1 at Tyr-718 are not known.
The cytoplasmic tyrosine kinase, JAK2, autophosphorylates in response to growth factors and cytokines, including IFN-γ. JAK2 then activates cytokine receptors and other cytoplasmic proteins such as the STATs by phosphorylating their key tyrosine residue. The JAK/STAT pathway can be regulated by SH2-containing protein-tyrosine phosphatases such as SHP2 (18–20). SHP2 is ubiquitously expressed and composed of two SH2 domains on the N-terminal and C-terminal protein-tyrosine phosphatase (PTP) domain. The SH2 domain of SHP2 mediates the association with phosphotyrosine-containing proteins present on activated receptors as well as on activated JAKs and STATs; this association triggers activation of the tyrosine phosphatase domain and subsequent dephosphorylation of substrates. SHP2 signals downstream of receptor tyrosine kinases and cytokine receptors, and in most cases it serves to positively transduce signals from these receptors. In other instances SHP2 has been shown to exhibit inhibitory signaling properties by negatively regulating the JAK-STAT pathway (19).
In this study, we demonstrate that the IFN-γ-activated kinase JAK2 and TNF-activated SHP2 are the tyrosine kinase and phosphatase for Tyr-718 on ASK1, respectively. The actions of both JAK2 and SHP2 affect protein turnover of ASK1 and thus regulate ASK1/JNK-dependent proinflammatory and proapoptotic pathways in EC.
EXPERIMENTAL PROCEDURES
Plasmid Construction—Expression plasmids for human SOCS1 and ASK1 were described previously (4, 17, 21). Human JAK1 and JAK2 expression plasmids were constructed by cloning the respective cDNA into pcDNA3. The mutant ASK1 and SHP2 were constructed by site-directed mutagenesis using QuikChange™ site-directed mutagenesis kit (Stratagene) according to the protocol of the manufacturer and were verified by DNA sequencing. Adenoviral and GST vectors expressing SHP2-WT, SHP2-RM, and SHP2-DAQA were described previously (22). Briefly, SHP-2 expression was established by subcloning wild type, catalytically inactive, and nonsubstrate trapping (Arg-465 to Met-465 (RM)) and the double substrate-trapping mutant of SHP-2 (Asp-425 to Ala-425, Gln-506 to Ala-506 (DAQA)) into the pIRES-green fluorescent protein vector (Invitrogen). SHP-2 adenoviruses were generated by using the pAdEasy method. For GST substrate-trapping experiments, GST fusion proteins that represented the PTP domain alone of SHP-2 (amino acids 218–528) were constructed by PCR amplification with wild type SHP-2 as a template. The resultant PCR product was subcloned into the pGEX-2TK vector to generate pGEX-2TK-PTP-WT (GST-PTP-WT). The substrate-trapping variant of SHP-2, Asp-425 to Ala-425, was generated by performing site-directed mutagenesis on GST-PTP-WT to generate pGEX-2TK-PTP-DA (GST-PTP-DA).
Cells, Cytokines, and Inhibitors—SOCS1+/– mice were from Dr. James Ihle (St. Jude Children's Research Hospital, Memphis, TN) (23). SHP2-lox/lox mice were from Dr. Ben Neel and were described previously (24). Isolation of SOCS1- and SHP2-deficient mouse lung EC (MLEC) was performed as we described recently (25). Human aorta EC (HAEC) were purchased from Clonetics (San Diego, CA). Human and murine recombinant TNF-α, interleukin-1β (IL-1), IL-6, IFN-β, and IFN-γ were purchased from R & D Systems (Minneapolis, MN). Chemicals, including proteasomal inhibitor MG132 (20 μm), JAK inhibitor AG-490 (tyrphostin B42, 10 μm), and protein synthesis inhibitor cycloheximide (10 μg/ml), were purchased from Calbiochem.
Cell Transfection—Transfection of EC was performed by Lipofectamine 2000 according to the manufacturer's protocol (Invitrogen). Cells were cultured at 90% confluence in 6-well plates and were transfected with total of 4 μg of plasmid constructs as indicated. Cells were harvested at 36–48 h post-transfection, and cell lysates were used for protein assays.
Adenoviral Expression and Preparation—Replication-deficient adenovirus expressing β-galactosidase (LacZ), Cre recombinase (Cre), SHP2, and its mutants under the control of the cytomegalovirus promoter were generated using pAdTrack-CMV vector and the AdEasy System. The viruses were amplified in HEK293 cells, purified using CsCl, and titered using cytopathic effects. Multiplicities of infection of 50 of virus resulted in close to 100% of the cells expressing the gene of interest with no signs of toxicity.
Generation of Antibodies against Phospho-ASK1 (Tyr(P)-718)-specific Antibody—Polyclonal antibodies directed against specific phospho-ASK1 (Tyr(P)-718) were produced from Cell Signaling by immunizing rabbits with synthetic phosphopeptides corresponding to residues surrounding ASK1 Tyr-718. The peptide sequences is AC-CERDSRpYSQPLH-amide, where pY indicates phospho-Tyr-718. The peptide was synthesized with N-terminal cysteine residues and coupled to keyhole limpet hemocyanin for immunization. The antibodies were affinity-purified from rabbit antisera by affinity chromatography steps using protein A columns to purify immunoglobulins followed by specific phosphopeptide (immunogen) columns.
Immunoprecipitation and Immunoblotting—EC (HAEC or MLEC) after various treatments were washed twice with cold phosphate-buffered saline and lysed in 1.5 ml of cold lysis buffer (50 mm Tris-HCl, pH 7.6, 150 mm NaCl, 0.1% Triton X-100, 0.75% Brij 96, 1 mm sodium orthovanadate, 1 mm sodium fluoride, 1 mm sodium pyrophosphate, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 2 mm phenylmethylsulfonyl fluoride, 1 mm EDTA) for 20 min on ice. Protein concentrations were determined with a Bio-Rad kit. For immunoprecipitation to analyze protein interaction in vivo, 400 μg of cell lysate supernatant were precleared by incubating with 5 μg of normal rabbit serum plus protein A/G-agarose beads on a rotator at 4 °C overnight. The lysates were then incubated with 5 μg of the first protein-specific antiserum (e.g. anti-ASK1 H300, Santa Cruz Biotechnology) for 2 h with 50 μl of protein A/G-agarose beads. The immune complexes were collected after each immunoprecipitation by centrifugation at 14,000 × g for 10 min followed by four washes with lysis buffer. The immune complexes were subjected to SDS-PAGE followed by immunoblot (Immobilon P, Millipore, Milford, MA) with the second protein-specific antibody (e.g. JAK2 from Cell Signaling). The chemiluminescence was detected using an ECL kit according to the instructions of the manufacturer (Amersham Biosciences). Antibodies against STAT1, ASK1, TRAF1, SHP2, JNK1, and β-tubulin were purchased from Santa Cruz Biotechnology. Phosphospecific antibodies recognizing ASK1 (Tyr(P)-718), ASK1 (Thr(P)-845), p-JAK1 or -2, p-STAT1, p-JNK1/2, p-SHP2 (Tyr(P)-542), and anti-phosphotyrosine (4G10) were purchased from Cell Signaling. Anti-p-JAK1/2 was from BIOSOURCE, and anti-SOCS1 was from Medical and Biological Laboratory.
GST-SOCS1 Pulldown Assay—GST fusion protein preparation and GST pulldown assay were performed as described previously (4, 21). Briefly, GST-SOCS1 fusion proteins expressed in Escherichia coli XL-1 Blue were affinity-purified on glutathione-Sepharose beads (Amersham Biosciences). 400 μg of cell lysates expressing JAK1 or JAK2 or together with WT-ASK or Y718F ASK1 were incubated overnight at 4 °C with 10 μg of GST-SOCS1 bound to glutathione-Sepharose in the lysis buffer. The beads were washed four times with the lysis buffer before the addition of boiling Laemmli sample buffer. Bound ASK1 or JAK proteins were resolved on SDS-PAGE and detected by Western blot with antibodies.
Quantitation of Apoptotic Cell—Cell killing assay was performed as described previously with a modification (7, 21, 26, 27). The propidium iodide (PI)-exclusion method for loss of integrity of cell membranes was used to assess viability. In brief, cells were suspended in phosphate-buffered saline containing 25 μg/ml PI for 5 min at 37 °C and then subjected to analytic flow cytometry on a FACSort (BD Biosciences) immediately after labeling. A light scatter gate was set up to eliminate cell debris from the analysis. The PI fluorescence signal was recorded on the FL3 channel and analyzed by using CellQuest software. Phosphatidylserine translocation, which precedes loss of PI exclusion in apoptotic cell death, was assessed by an annexin V-488 staining kit (Invitrogen) following the manufacturer's protocol. For nuclear morphology, cells were stained with DAPI, and apoptotic cells (nuclei condensation) were visualized under a UV microscope.
RESULTS
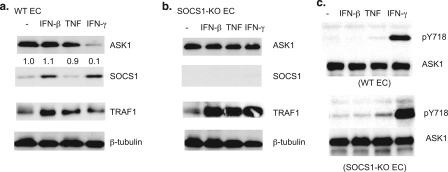
IFN-γ Induces ASK1 Phosphorylation and Degradation in EC—To identify tyrosine kinases responsible for phosphorylation of ASK1 at Tyr(P)-718, which mediates SOCS1 association and ASK1 degradation, we examined the effects of various cytokines on ASK1 protein stability. MLEC were treated with various cytokines (TNF, IL-1, IL-6, IFN-β, or IFN-γ at 10 ng/ml) for 16 h, and ASK1 expression was determined by Western blotting using an anti-ASK1 antibody. Among these cytokines, only IFN-γ strongly induced ASK1 degradation (Fig. 1a for IFN-γ, TNF, and IFN-β). To confirm these cytokines function in EC, cytokine-inducible proteins, including TRAF1 and SOCS1, were tested for their expression. As expected, IFN-β and IFN-γ induced SOCS1 expression. TNF up-regulated TRAF1 as reported previously (28). Interestingly, IFN-β and IFN-γ also induced TRAF1 expression in EC. To determine whether IFN-γ-induced ASK1 degradation is SOCS1-dependent, as we demonstrated previously, we examined the effects of cytokines in SOCS1-KO EC. As expected, SOCS1 was not detected in SOCS1-KO EC (Fig. 1b). Cytokine-induced TRAF1 expression was augmented in SOCS1-KO EC, consistent with the observation that SOCS1 is a suppressor in IFN-β, IFN-γ, and TNF signaling (17, 23). Importantly, IFN-γ did not induce ASK1 degradation in SOCS1-KO EC (Fig. 1b), suggesting that IFN-γ-induced ASK1 degradation is SOCS1-dependent.
FIGURE 1.
IFN-γ induces ASK1 phosphorylation and degradation in EC. a and b, IFN-γ induces ASK1 degradation in WT but not in SOCS-KO EC. WT (a) and SOCS1-KO (b) MLEC were treated with TNF, IFN-β, or IFN-γ at 10 ng/ml for 16 h. Cell lysates were subjected to Western blot with antibodies against ASK1, SOCS1, TRAF1, and tubulin. c, IFN-γ induces ASK1 phosphorylation at Tyr-718. WT and SOCS1-KO EC were serum-starved for 12 h followed by treatment with TNF, IFN-β, or IFN-γ (10 ng/ml for 15 min). Phospho- and total ASK1 were determined by Western blot with anti-Tyr(P)-718 (pY718) and anti-ASK1 antibody, respectively.
Because both IFN-β and IFN-γ induce SOCS1 up-regulation, and only IFN-γ treatment leads to ASK1 degradation, we reasoned that IFN-γ but not IFN-β induces tyrosine phosphorylation of ASK1 at Tyr-718 (Tyr(P)-718), which has been shown to be required for ASK1 degradation. To this end, WT and SOCS1-KO EC were treated with TNF, IFN-β, or IFN-γ (10 ng/ml for 15 min), and ASK1 Tyr(P)-718 was determined by Western blot with an ASK1 Tyr(P)-718-specific antibody. IFN-γ, but not TNF or IFN-β, strongly induced ASK1 Tyr(P)-718 in both WT and SOCS1-KO EC (Fig. 1c).
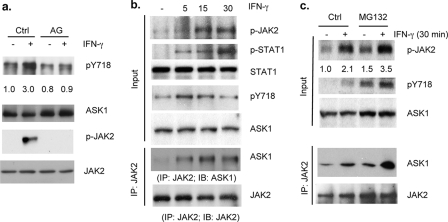
JAK2 Associates with ASK1 and Mediates IFN-γ-induced ASK1 Phosphorylation—IFN-γ activates JAK1 and JAK2, whereas IFN-β activates JAK1 and Tyk2. To determine whether IFN-γ-specific kinase JAK2 is responsible for ASK1 phosphorylation, HAEC were treated with IFN-γ (10 ng/ml) for 15 min with or without prior treatment with the JAK2-specific inhibitor AG490 for 60 min. As a control, IFN-γ-induced activation of JAK2, as determined by Western blot with a phosphospecific antibody, was blocked by AG490 (Fig. 2a). IFN-γ-induced ASK1 phosphorylation was completely diminished by AG490. These results suggest that JAK2 kinase mediates IFN-γ-induced tyrosine phosphorylation of ASK1. To determine whether JAK2 and ASK1 form a complex in response to IFN-γ, HAEC were treated with IFN-γ for the indicated times. As controls, IFN-γ-induced phosphorylation of JAK2 and STAT1 was determined by phospho-specific antibodies. Consistent with previous results, IFN-γ induced activation of the JAK-STAT pathway in EC in a time-dependent manner, peaking at 15–30 min (Fig. 2b). ASK1-JAK2 complex formation was determined by immunoprecipitation assay with anti-JAK2 followed by Western blot with anti-ASK1. ASK1-JAK2 complex was weakly detected in untreated EC and was increased in response to IFN-γ, correlating with JAK2 activation and ASK1 phosphorylation (Fig. 2b). However, phosphorylation of ASK1 and JAK2-ASK1 complex declined by 30 min, suggesting that JAK2-ASK1 complex is unstable. To determine whether the JAK2-ASK1 complex could be stabilized by the proteasomal inhibitor MG132, EC were pretreated with MG312 (20 μm) for 60 min followed by IFN-γ treatment for 30 min. As shown in Fig. 2c, IFN-γ-induced JAK2 and ASK1 phosphorylation and subsequently JAK2-ASK1 complex formation were increased in the presence of MG132.
FIGURE 2.
JAK2 associates with ASK1 and mediates IFN-γ-induced ASK1 phosphorylation. a, HAEC were untreated or pretreated with AG490 (AG) (10 μm) for 60 min followed by IFN-γ (10 ng/ml) for 15 min. Phosphorylation of JAK2 and ASK1 was determined by Western blot with phospho-specific antibodies. Ctrl, control. b, JAK2 associates with ASK1. HAEC were treated with IFN-γ for the indicated times. Phospho- and total JAK2, STAT1, and ASK1 were determined by Western blot with respective antibodies. ASK1-JAK2 complex was determined by immunoprecipitation (IP) assay with anti-JAK2 followed by Western blot with anti-ASK1. c, JAK2-ASK1 complex is stabilized by proteasomal inhibitor MG132. HAEC were pretreated with MG312 for 60 min followed by IFN-γ treatment for 30 min. JAK2-ASK1 complex was determined as in b.
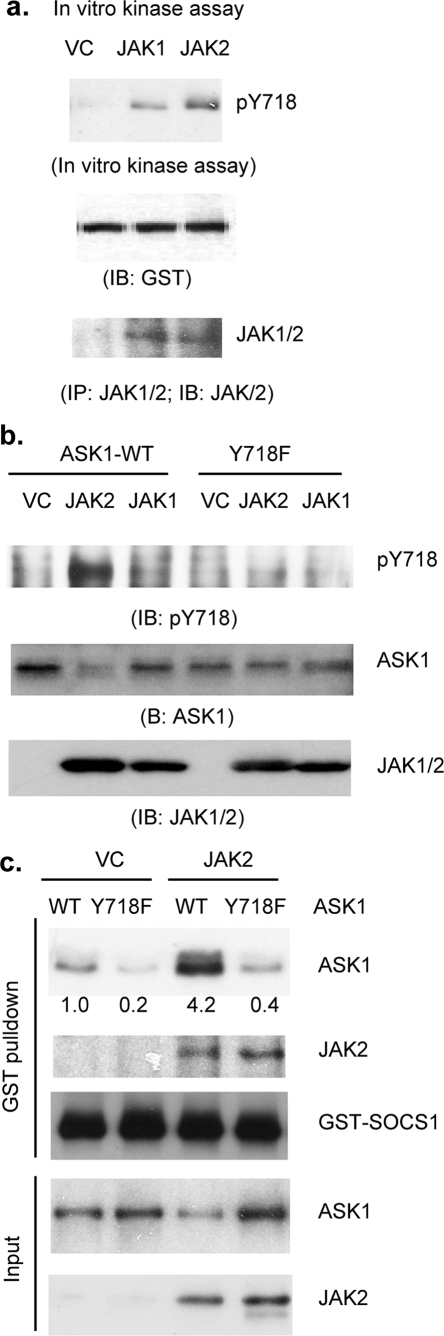
JAK2 Directly Phosphorylates ASK1 at Tyr(P)-718—To test if JAK2 phosphorylates ASK1, we first performed an in vitro kinase assay. JAK2 or the JAK1-expressing cell lysate was immunoprecipitated with anti-JAK1/2, and the immunoprecipitates were used for an in vitro kinase using GST-ASK1 as a substrate. Phosphorylation of GST-ASK1 at Tyr-718 was determined by Western blot with anti-Tyr(P)-718. JAK2 strongly but JAK1 only weakly phosphorylated GST-ASK1 (Fig. 3a). To determine whether JAK2 phosphorylates ASK1 in cells, we employed an overexpression system by expressing JAK2 together with ASK1. JAK1 was used as a control. Co-expression of ASK1 with JAK2, but not with JAK1, induced tyrosine phosphorylation of ASK1 at Tyr-718, indicating JAK2 specifically phosphorylates ASK1 in vivo. Consistent with previous findings, increased ASK1 tyrosine phosphorylation correlated with decreased total ASK1 protein levels. In contrast, ASK1-Y718F was not phosphorylated and degraded upon overexpression of JAK2 (Fig. 3b), suggesting that Tyr(P)-718 is a major site that can be phosphorylated by JAK2. To determine whether JAK2-mediated phosphorylation increases ASK1-SOCS1 association, ASK1 was co-transfected with JAK2, and cell lysates were used for an in vitro pulldown assay with GST-SOCS1. As a control, ASK1-Y718F failed to bind to GST-SOCS1 (data not shown). As expected, JAK2 also bound to GST-SOCS1. More importantly, consistent with increased ASK1-Tyr(P)-718 by JAK2 overexpression, JAK2 strongly enhanced association of ASK1 to GST-SOCS1 (Fig. 3c).
FIGURE 3.
JAK2 directly phosphorylates ASK1 at Tyr(P)-718 (pY718). a, in vitro phosphorylation assay. JAK2 or JAK1-expressing cell lysate was immunoprecipitated (IP) with anti-JAK1/2, and the immunoprecipitates were used for an in vitro kinase using GST-ASK1 as a substrate. Phosphorylation of GST-ASK1 at Tyr-718 was determined by Western blot with anti-Tyr(P)-718. GST-ASK1 was detected by Western blot with anti-GST. IB, immunoblot. b, in vivo phosphorylation assay. HAEC were transfected with JASK1-WT or ASK1-Y718F in the presence of JAK2 or JAK1. Phosphotyrosine of ASK1 was determined by Western blot with anti-ASK1-Tyr(P)-718. Total ASK1 and JAK2 were also determined by respective antibodies. c, JAK2 increases ASK1-SOCS1 association. Cell lysates co-expressing ASK1 and JAK2 were used for an in vitro pulldown assay with GST-SOCS1. Bound ASK1 or JAK2 was determined by Western blot with respective antibodies. VC, vector control.
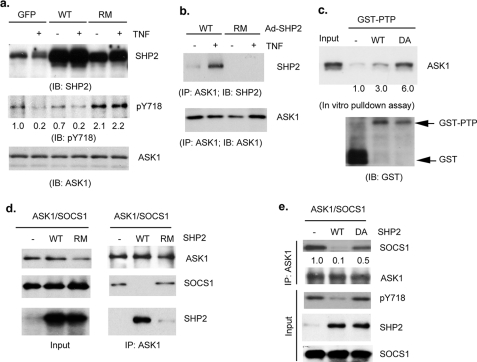
SHP2 Binds to and Dephosphorylates ASK1—SHP2, an SH2-containing protein-tyrosine phosphatase, has been shown to share the same binding phosphotyrosine motif as SOCS proteins (29). To determine whether SHP2 could dephosphorylate the SOCS1-binding site on ASK1 (Tyr(P)-718), human EC were infected with adenovirus expressing SHP2 wild type (WT) or a phosphatase-inactive/nonsubstrate-trapping mutant of SHP2 (SHP2-RM) followed by TNF treatment (10 ng/ml for 15 min). Overexpression of SHP2-WT decreased, but SHP2-RM enhanced, ASK1 tyrosine phosphorylation. More importantly, TNF-induced dephosphorylation of ASK1 Tyr(P)-718 was inhibited by SHP2-RM (Fig. 4a). SHP2-ASK1 interaction was also determined by immunoprecipitation with anti-ASK1 followed by Western blot with anti-SHP2. Consistent with the effect of SHP2 on ASK1 phosphorylation, SHP2-WT, but not SHP2-RM, associated with ASK1 in response to TNF (Fig. 4b). To determine whether SHP2 directly targets ASK1 for dephosphorylation, we examined SHP2-ASK1 interaction in an in vitro GST pulldown assay using a substrate-trapping mutant of SHP2 (SHP2-DAQA) containing a mutation in the catalytic domain (PTP). The application of PTP “substrate-trapping” mutants has been widely used as a tool to define PTP-substrate interactions, and such an interaction is direct evidence of a PTP-substrate based interaction based on the fact this complex is mediated by the catalytic cysteine of the PTP (20). Significantly, we found that ASK1 bound more tightly to SHP2-DAQA than SHP2-WT in the pulldown assay (Fig. 4c), suggesting ASK1 is a direct substrate of SHP-2.
FIGURE 4.
SHP2 binds to and dephosphorylates ASK1. a and b, HAEC were infected with adenovirus expressing SHP2-WT or SHP2-R465M. 24 h post-infection, cells were treated by TNF (10 ng/ml for 15 min). SHP2 expression, ASK1-Tyr(P)-718, and total ASK1 were determined by Western blot with respective antibodies (a). SHP2-ASK1 interaction was also determined by immunoprecipitation (IP) with anti-ASK1 followed by Western blot with anti-SHP2 (b). IB, immunoblot; GFP, green fluorescent protein. c, SHP2-ASK1 interaction in vitro. ASK1-expressing cell lysate was subjected to an in vitro GST pulldown assay using GST-SHP1-PTP domain or a trapping mutant SHP2-DAQA. Bound ASK1 was determined by Western blot with anti-ASK1. GST was used as a control. d and e, SHP2 dephosphorylates ASK1 and dissociates SOCS1 from ASK1. ASK1 and SOCS1 (FLAG tag) were co-transfected into 293T cells in the presence or absence of SHP2-WT, SHP2-RM, or SHP2-DA. ASK1-SOCS1 association was determined by co-immunoprecipitation with anti-ASK1 followed by Western blot with anti-FLAG. ASK1-associated SHP2 was also determined. Dephosphorylation of ASK by SHP2 was determined by Western blot with anti-Tyr(P)-718 (pY718) (e).
Based on these observations, we reasoned that SHP2 binds to and dephosphorylates ASK1, leading to dissociation of ASK1 from SOCS1. To test this hypothesis, ASK1 and SOCS1 were co-transfected into 293T cells in the presence of SHP2-WT, SHP2-RM (inactive in substrate binding and catalysis), or SHP2-DA (catalytic inactive/substrate trapping). ASK1-SOCS1 association was determined by co-immunoprecipitation with anti-ASK1 followed by Western blot with anti-FLAG. As reported previously, ASK1 associated with SOCS1 in the absence of SHP2. Expression of SHP2-WT, but not SHP2-RM, disrupted the ASK1-SOCS1 complex (Fig. 4d). SHP2-DA, which lacks significant catalytic activity, could not dephosphorylate ASK1-Tyr(P)-718 but still partially disrupted the ASK1-SOCS1 complex (Fig. 4e). Taken together, these data suggest that both the phosphatase activity and the ASK1 binding activity of SHP2 are required to disrupt ASK1-SOCS1 complex formation.
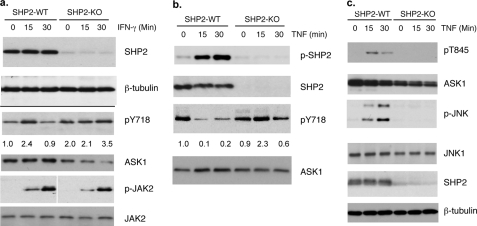
SHP2 Knock-out EC (?) Blunted TNF-induced Dephosphorylation of ASK1 and ASK1-JNK Signaling—To determine the role of endogenous SHP2 in ASK1 dephosphorylation and stability, we isolated MLEC from SHP2-lox/lox mice (24) followed by infection with LacZ or Cre-expressing adenovirus. Deletion of SHP2 mediated by Cre expression was verified by Western blot with anti-SHP2 (Fig. 5a). SHP2-lox/lox (WT) and SHP2-lox/lox/Cre (KO) EC were first examined for IFN-γ-induced phosphorylation of ASK1 by Western blot with a phospho-specific antibody (Tyr(P)-718). Consistent with the observation shown in Fig. 1, IFN-γ-induced tyrosine phosphorylation of ASK1 in SHP2-WT EC peaked at 15 min followed by a decline at 30 min. However, the basal level of ASK-pY 718 was increased, and IFN-γ-induced ASK1-Tyr(P)-718 was prolonged in SHP2-KO EC compared with normal EC. Consistent with this, phospho-ASK1 was unstable, and the level of total ASK1 protein was decreased in SHP2-KO EC in response to IFN-γ (Fig. 5a). As a control, IFN-γ-induced phosphorylation of JAK2 was slightly reduced in SHP2-KO EC, suggesting that increased ASK1-Tyr(P)-718 in SHP2-KO EC was primarily due to lack of SHP2. We then determined the role of endogenous SHP2 in TNF-induced dephosphorylation of ASK1-Tyr(P)-718. EC were treated with TNF (10 ng/ml) as indicated, and TNF induced SHP2 activation as determined by a phospho-specific antibody recognizing an active form of SHP1 (Tyr(P)-542). TNF induced a reduction of ASK1-Tyr(P)-718 in SHP2-WT cells as determined by a phospho-specific antibody. In contrast, TNF failed to induce dephosphorylation of ASK1-Tyr(P)-718 in SHP2-KO EC (Fig. 5b). These data suggest that SHP2 is a critical regulator controlling basal and TNF-induced dephosphorylation of ASK1.
FIGURE 5.
SHP2 knock-out blunts TNF-induced dephosphorylation of ASK1. MLEC from SHP2-lox/lox mice were infected with adenovirus-expressing LacZ or Cre. a, 36 h post-infection, cells were serum-starved for 12 h followed by treatment with IFN-γ (10 ng/ml) for the indicated times. Cre-mediated deletion of SHP2, IFN-γ-induced phosphorylation of JAK2, and ASK1 was determined by the respective antibodies. b, 48 h post-infection, EC were treated with TNF (10 ng/ml) for the indicated times. TNF-induced SHP2 activation (pY542) and ASK1 dephosphorylation at Tyr(P)-718 (pY718) were determined by phospho-specific antibodies. c, ASK1-JNK signaling was determined by Western blot with phospho-specific antibodies recognizing active forms of ASK1 (pT845) and p-JNK1/2.
To determine the role of endogenous SHP2 in TNF-induced ASK1-JNK signaling, SHP2-lox/lox (WT) and SHP2-lox/lox/Cre (KO) EC were generated and treated with TNF as indicated. ASK1-JNK signaling was determined by Western blot with phospho-specific antibodies recognizing active forms of ASK1 (Thr(P)-845) and JNK1/2. Phosphorylation of ASK1 Thr-845 is indicative of ASK1 activation, resulting from ASK1 oligomerization and autophosphorylation. As observed in our previous studies (17), TNF induced ASK1-JNK signaling, peaking at 15 min. In contrast, deletion of SHP2 blunted TNF-induced activation of ASK1 and its downstream target JNK (Fig. 5c). Therefore, dephosphorylation of ASK1 at Tyr-718 is crucial for TNF-induced activation ASK1-JNK signaling. Taken together, these results suggest that dephosphorylation of Tyr(P)-718 not only increases ASK1 stability but also is critical for the activation of ASK1 kinase.
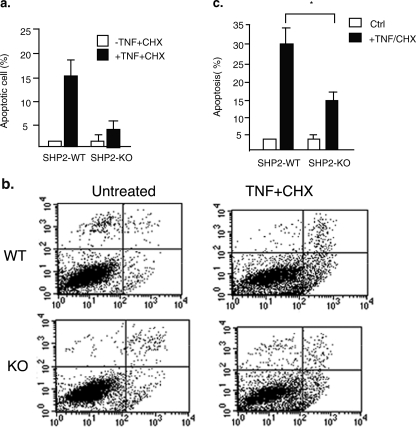
Critical Roles of SHP2 in TNF-induced EC Apoptosis—ASK1-JNK signaling has been shown to be critical for TNF-induced apoptosis (7, 21, 26, 27), and we determined TNF (plus cycloheximide, CHX)-induced EC apoptosis in WT and SHP2-KO EC. MLEC were treated with TNF (10 ng/ml) in the presence of CHX (10 μg/ml) for 6 h when EC apoptosis is initiated (26). EC apoptosis, characterized by nuclear fragmentation, was determined by DAPI staining followed by fluorescence microscopy. TNF (+CHX) strongly induced apoptosis in WT EC (∼16%) (Fig. 6a). Similar to TNF-induced ASK1-JNK signaling, TNF-induced apoptosis was also significantly reduced in SHP2-KO MLEC (∼5%) (Fig. 6a). Some of the dead cells cannot be visualized using this assay as they detached from the culture dish. Therefore, EC apoptosis was further determined by PI exclusion and annexin V staining followed by FACS analysis. TNF (plus CHX) significantly induced apoptosis in WT EC (∼30%). However, TNF(+CHX)-induced apoptosis was significantly blunted in SHP2-KO EC (∼14%) (Fig. 6, b with quantification apoptosis in c). These data support the critical roles of SHP2 in TNF-induced ASK1-JNK activation and EC apoptosis.
FIGURE 6.
Critical roles of SHP2 in TNF-induced ASK1-JNK signaling and EC apoptosis. a, SHP2 deletion reduces EC apoptosis. MLEC were treated with TNF (10 ng/ml) in the presence of CHX (10 μg/ml) for 6 h, and EC apoptosis characterized by nuclear fragmentation was determined by DAPI staining followed by fluorescence microscopy. b, EC apoptosis was determined by PI exclusion and annexin V staining followed by FACS analysis. Representative FACS is shown in b, and the % of apoptotic cells (both upper and lower right quadrants in FACS) are quantified in c. Data are presented as mean of duplicates from two independent experiments. * indicates statistically significance between WT and SHP2-KO MLEC, p < 0.05. Ctrl, control.
DISCUSSION
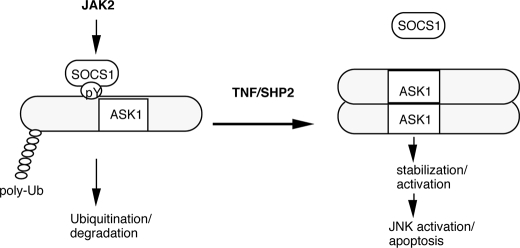
We have previously shown that the activity of ASK1 is negatively regulated by Tyr-718 phosphorylation, which is critical for SOCS1 binding and SOCS1-mediated proteasomal degradation. TNF induces dephosphorylation of ASK1, leading to dissociation of ASK1 from SOCS1 and ASK1 stabilization. However, the kinase and phosphatase modulating the phosphorylation status of Tyr-718 on ASK1 were unknown. In this study, we identified that JAK2, when overexpressed or activated by IFN-γ, can phosphorylate Tyr-718 of ASK1. JAK2-induced Tyr(P)-718 triggers interaction between SOCS1 and ASK1, leading to subsequent ASK1 proteasomal degradation. In contrast, SHP2, an SH2 domain-containing protein-tyrosine phosphatase that is engaged by TNF, dephosphorylates ASK1 at Tyr-718 and dissociates ASK1 from SOCS1, stabilizing ASK1 and activating ASK1-JNK apoptotic pathway in EC (Fig. 7).
FIGURE 7.
Model for regulation of ASK1 by JAK2 kinase and SHP2 phosphatase. In resting or IFN-γ-treated EC, JAK2 phosphorylates ASK1 at Tyr(P)-718, which enhances the interaction between SOCS1 and ASK1, leading to ASK1 proteasomal degradation. In contrast, SHP2, an SH2 domain-containing tyrosine phosphatase that can be activated by TNF, dephosphorylates ASK1 at Tyr-718 and dissociates ASK1 from SOCS1, stabilizing ASK1 and activating ASK1-JNK apoptotic pathway in EC. Ub, ubiquitin.
We have previously shown a single tyrosine residue Tyr-718 on ASK1 is responsible for binding to SOCS1, which in turn mediates a ubiquitin-dependent ASK1 degradation. Our current studies demonstrate that JAK2 is a specific kinase for ASK1 Tyr(P)-718. This is supported by the following evidence. 1) Overexpression of JAK2, but not JAK1, induces ASK1 Tyr(P)-718. 2) IFN-γ (activates JAK1 and JAK2), but not IFN-β (activates JAK1 and Tyk2), can induce ASK1 degradation. 3) IFN-γ-induced ASK1 Tyr(P)-718 and degradation can be blocked by a JAK2-specific inhibitor AG490. Interestingly, SOCS1 is a specific feedback inhibitor of IFN-γ, which acts by associating with JAK2 and its downstream signaling proteins to interfere with their kinase activity or binding to Tyr(P)-1007 on JAK2 to mediate its proteosome degradation. It is plausible that SOCS1 mediates degradation of the ASK1-JAK2 complex in response to IFN-γ. Consistent with this model, ASK1-JAK2 complex is labile and can be stabilized by proteasomal inhibitors. JAK2 can be activated by a variety of growth factors and cytokines, including IGF-1 and vascular endothelial growth factor. Consistently, we have previously shown that ASK1 Tyr(P)-718 is induced by vascular endothelial growth factor and IGF-1. Although the IGF-1 receptor is reported to directly bind to and tyrosine-phosphorylate ASK1, whether or not ASK1 tyrosine phosphorylation by IGF-1 receptor is mediated by the IGF-1 receptor-associated JAK2 has not been determined. Similarly, it needs to be further investigated whether JAK2 mediates vascular endothelial growth factor or other cytokine-induced ASK1 phosphorylation.
We have also investigated the mechanism by which TNF induces dephosphorylation of ASK1 Tyr(P)-718 and subsequent release of SOCS1 from ASK1, a critical step in TNF-induced ASK1-JNK signaling. Our data suggest that SHP2 is a tyrosine phosphatase critical for TNF-induced dephosphorylation of ASK1 Tyr(P)-718 and activation of the ASK1-JNK signaling pathway. Several lines of evidence support this conclusion. First, overexpression of the SHP2 wild type, but not a catalytically inactive form, induces dephosphorylation of ASK1 Tyr(P)-718. Second, a substrate-trapping mutant directly binds to ASK1 to a greater extent than SHP2-WT. More importantly, SHP2 deletion in EC blunts TNF-induced dephosphorylation of ASK1 Tyr(P)-718 and JNK activation. In SHP2-KO EC, basal and IFN-γ-induced ASK1 Tyr(P)-718 was enhanced, and TNF-induced dephosphorylation of ASK1 and activation of ASK1-JNK signaling as well as EC apoptosis are reduced. A similar role of SHP2 in facilitating SOCS1 release from JAK2 has also been reported (18). SHP2 dephosphorylates tyrosine (Tyr(P)-1007) of JAK2 kinase, a critical recruitment site for SOCS1, dissociating SOCS1 from JAK2 and stabilizing JAK2. Our data may reveal a common mechanism by which SOCS1-SHP2 regulates cytokine signaling and function.
ASK1 is phosphorylated at several Ser/Thr sites (Ser(P)-83, pThr-845, Ser(P)-967, and Ser(P)-1034) (15, 30, 31). Regulation of phosphorylation by kinase and/or dephosphorylation by phosphatase at Ser-83, Ser-967, and Thr-845 has been investigated (15). It is known that the protein kinase Akt in complex with Hsp90 phosphorylates ASK1 at Ser-83 to inhibit ASK1-induced apoptosis (30, 32). We have also identified PP2A as a Ser-967-specific phosphatase, activating TNF-induced ASK1-JNK signaling. In contrast, Thr(P)-845 positively regulates ASK1 activation. In response to upstream signals, ASK1 is activated by autophosphorylation at Thr-845 (14). PP5 and a member of PP2C (PP2Cε) have been implicated in dephosphorylation of ASK1 Thr-845 to negatively regulate ASK1 activity, functioning as a negative feedback inhibitor of ASK1 signaling (15, 33). Our current study has identified the first pair of kinase and phosphatase that regulates ASK1 tyrosine phosphorylation.
This work was supported, in whole or in part, by National Institutes of Health Grants P01HL070295-6 and R01 HL-65978-9 and AR46504 (to A. M. B.). This work was also supported by National Nature Science Foundation of China Grant 30828032 (to W. M.) and Scientific Development Grant 0835544N from the American Heart Association (to H. C.).
Footnotes
The abbreviations used are: EC, endothelial cell; IFN, interferon; SHP2, SH2-containing protein-tyrosine phosphatase-2; SH, Src homology; TNF, tumor necrosis factor; IL, interleukin; KO, knock-out; WT, wild type; MLEC, mouse lung EC; HAEC, human aorta EC; PTP, protein-tyrosine phosphatase; CHX, cycloheximide; FACS, fluorescence-activated cell sorter; PI, propidium iodide; GST, glutathione S-transferase; DAPI, 4,6-diamidino-2-phenylindole; JAK, Janus tyrosine kinase; STAT, signal transducers and activators of transcription; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase.
References
- 1.Libby, P. (2002) Nature 420 868–874 [DOI] [PubMed] [Google Scholar]
- 2.Ichijo, H. (1999) Oncogene 18 6087–6093 [DOI] [PubMed] [Google Scholar]
- 3.Matsuzawa, A., Nishitoh, H., Tobiume, K., Takeda, K., and Ichijo, H. (2002) Antioxid. Redox. Signal. 4 415–425 [DOI] [PubMed] [Google Scholar]
- 4.Liu, Y., Yin, G., Surapisitchat, J., Berk, B. C., and Min, W. (2001) J. Clin. Investig. 107 917–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Surapisitchat, J., Hoefen, R. J., Pi, X., Yoshizumi, M., Yan, C., and Berk, B. C. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 6476–6481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berk, B. C., Min, W., Yan, C., Surapisitchat, J., Liu, Y., and Hoefen, R. (2002) Drug News Perspect. 15 133–139 [DOI] [PubMed] [Google Scholar]
- 7.Zhang, R., He, X., Liu, W., Lu, M., Hsieh, J. T., and Min, W. (2003) J. Clin. Investig. 111 1933–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamawaki, H., Pan, S., Lee, R. T., and Berk, B. C. (2005) J. Clin. Investig. 115 733–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Izumi, Y., Kim, S., Yoshiyama, M., Izumiya, Y., Yoshida, K., Matsuzawa, A., Koyama, H., Nishizawa, Y., Ichijo, H., Yoshikawa, J., and Iwao, H. (2003) Circulation 108 2812–2818 [DOI] [PubMed] [Google Scholar]
- 10.Izumiya, Y., Kim, S., Izumi, Y., Yoshida, K., Yoshiyama, M., Matsuzawa, A., Ichijo, H., and Iwao, H. (2003) Circ. Res. 93 874–883 [DOI] [PubMed] [Google Scholar]
- 11.Zhang, L., Chen, J., and Fu, H. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 8511–8515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang, R., Al-Lamki, R., Bai, L., Streb, J. W., Miano, J. M., Bradley, J., and Min, W. (2004) Circ. Res. 94 1483–1491 [DOI] [PubMed] [Google Scholar]
- 13.Min, W., Lin, Y., Tang, S., Yu, L., Zhang, H., Wan, T., Luhn, T., Fu, H., and Chen, H. (2008) Circ. Res. 102 840–848 [DOI] [PubMed] [Google Scholar]
- 14.Tobiume, K., Saitoh, M., and Ichijo, H. (2002) J. Cell. Physiol. 191 95–104 [DOI] [PubMed] [Google Scholar]
- 15.Morita, K., Saitoh, M., Tobiume, K., Matsuura, H., Enomoto, S., Nishitoh, H., and Ichijo, H. (2001) EMBO J. 20 6028–6036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bunkoczi, G., Salah, E., Filippakopoulos, P., Fedorov, O., Muller, S., Sobott, F., Parker, S. A., Zhang, H., Min, W., Turk, B. E., and Knapp, S. (2007) Structure (Lond.) 15 1215–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He, Y., Zhang, W., Zhang, R., Zhang, H., and Min, W. (2006) J. Biol. Chem. 281 5559–5566 [DOI] [PubMed] [Google Scholar]
- 18.Ali, S., Nouhi, Z., Chughtai, N., and Ali, S. (2003) J. Biol. Chem. 278 52021–52031 [DOI] [PubMed] [Google Scholar]
- 19.Xu, D., and Qu, C. K. (2008) Front. Biosci. 13 4925–4932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiganis, T., and Bennett, A. M. (2007) Biochem. J. 402 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu, Y., and Min, W. (2002) Cir. Res. 90 1259–1266 [DOI] [PubMed] [Google Scholar]
- 22.Kontaridis, M. I., Eminaga, S., Fornaro, M., Zito, C. I., Sordella, R., Settleman, J., and Bennett, A. M. (2004) Mol. Cell. Biol. 24 5340–5352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marine, J. C., Topham, D. J., McKay, C., Wang, D., Parganas, E., Stravopodis, D., Yoshimura, A., and Ihle, J. N. (1999) Cell 98 609–616 [DOI] [PubMed] [Google Scholar]
- 24.Fornaro, M., Burch, P. M., Yang, W., Zhang, L., Hamilton, C. E., Kim, J. H., Neel, B. G., and Bennett, A. M. (2006) J. Cell Biol. 175 87–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang, H., He, Y., Dai, S., Xu, Z., Luo, Y., Wan, T., Luo, D., Jones, D., Tang, S., Chen, H., Sessa, W. C., and Min, W. (2008) J. Clin. Investig. 118 3904–3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang, H., Zhang, R., Luo, Y., D'Alessio, A., Pober, J. S., and Min, W. (2004) J. Biol. Chem. 279 44955–44965 [DOI] [PubMed] [Google Scholar]
- 27.Zhang, W., Zheng, S., Storz, P., and Min, W. (2005) J. Biol. Chem. 280 19036–19044 [DOI] [PubMed] [Google Scholar]
- 28.Wang, C. Y., Mayo, M. W., Korneluk, R. G., Goeddel, D. V., and Baldwin, A. S., Jr. (1998) Science 281 1680–1683 [DOI] [PubMed] [Google Scholar]
- 29.De Souza, D., Fabri, L. J., Nash, A., Hilton, D. J., Nicola, N. A., and Baca, M. (2002) Biochemistry 41 9229–9236 [DOI] [PubMed] [Google Scholar]
- 30.Kim, A. H., Khursigara, G., Sun, X., Franke, T. F., and Chao, M. V. (2001) Mol. Cell. Biol. 21 893–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujii, K., Goldman, E. H., Park, H. R., Zhang, L., Chen, J., and Fu, H. (2004) Oncogene 23 5099–5104 [DOI] [PubMed] [Google Scholar]
- 32.Zhang, R., Luo, D., Miao, R., Bai, L., Ge, Q., Sessa, W. C., and Min, W. (2005) Oncogene 24 3954–3963 [DOI] [PubMed] [Google Scholar]
- 33.Tamura, S., Toriumi, S., Saito, J., Awano, K., Kudo, T. A., and Kobayashi, T. (2006) Cancer Sci. 97 563–567 [DOI] [PMC free article] [PubMed] [Google Scholar]