Abstract
Trypanosome lytic factor (TLF) is a subclass of human high density lipoprotein (HDL) that mediates an innate immune killing of certain mammalian trypanosomes, most notably Trypanosoma brucei brucei, the causative agent of a wasting disease in cattle. Mechanistically, killing is initiated in the lysosome of the target trypanosome where the acidic pH facilitates a membrane-disrupting activity by TLF. Here we utilize a model liposome system to characterize the membrane binding and permeabilizing activity of TLF and its protein constituents, haptoglobin-related protein (Hpr), apolipoprotein L-1 (apoL-1), and apolipoprotein A-1 (apoA-1). We show that TLF efficiently binds and permeabilizes unilamellar liposomes at lysosomal pH, whereas non-lytic human HDL exhibits inefficient permeabilizing activity. Purified, delipidated Hpr and apoL-1 both efficiently permeabilize lipid bilayers at low pH. Trypanosome lytic factor, apoL-1, and apoA-1 exhibit specificity for anionic membranes, whereas Hpr permeabilizes both anionic and zwitterionic membranes. Analysis of the relative particle sizes of susceptible liposomes reveals distinctly different membrane-active behavior for native TLF and the delipidated protein components. We propose that lysosomal membrane damage in TLF-susceptible trypanosomes is initiated by the stable association of the TLF particle with the lysosomal membrane and that this is a property unique to this subclass of human HDL.
High density lipoproteins (HDL)2 are complex yet ordered macromolecules consisting of characteristic proteins embedded in a phospholipid monolayer that surrounds a hydrophobic core of esterified cholesterol and triglycerides. A subclass of HDL is responsible for an innate immune killing of the African blood stream parasite Trypanosoma brucei brucei (1–3), and very recently, has been shown to be cytotoxic to intracellular Leishmania promastigotes (4). The trypanolytic HDL particle, termed trypanosome lytic factor (TLF), is characterized by the presence of two proteins, apolipoprotein L-1 (apoL-1) and haptoglobin-related protein (Hpr), as well as the HDL ubiquitous apolipoprotein A-1 (apoA-1) (1, 5–7). Killing of the susceptible parasite involves high affinity binding to a cell-surface receptor, endocytosis, and trafficking of the TLF particle to the lysosome (8–12). The acidic lysosomal environment facilitates a membrane-disrupting activity by the TLF particle and subsequent cell death (9, 13). It has been shown that purified, delipidated apoL-1 or Hpr are sufficient for trypanosome killing. When these proteins are incorporated into the same lipoprotein particle, a several hundredfold increase in killing activity is exhibited (5). In addition, Molina-Portela et al. (14) show that maximal protection against T. b. brucei in a transgenic mouse model requires the expression of human Hpr, apoL-1, and apoA-1, supporting a synergistic mode of action.
Haptoglobin-related protein evolved during primate evolution and is restricted to apes, old world monkeys, and humans (15). Haptoglobin-related protein is highly similar (92%) to the acute phase serum protein haptoglobin (Hp) (16). All mammals use Hp as a scavenger of hemoglobin (Hb) released during hemolysis associated with infection or trauma. Haptoglobin binds cell-free Hb with high affinity and facilitates its removal from the circulation through a receptor-mediated process in the liver (17). Like Hp, Hpr binds free Hb, yet this Hpr·Hb complex is not recognized by the requisite receptors in mammals and is thus not removed from the circulation (18). TLF uptake by susceptible trypanosomes requires specific binding to an Hpr·Hb complex that facilitates trafficking of the TLF particle to the lysosome (10). It has been proposed that once inside the lysosomal compartment, Hpr·Hb contributes directly to membrane disruption through the generation of oxygen radicals with the bound Hb providing the iron necessary for Fenton chemistry (7, 10, 19).
Apolipoprotein L-1 is a unique member of the apolipoprotein L protein family in that, unlike the remaining apoL proteins, it possesses an N-terminal signal sequence and is thus secreted from cells. As is the case for Hpr, apoL-1 appeared during primate evolution (20–22). Within the circulation of primates, apoL-1 is exclusively associated with HDL, and the majority of the protein is in the TLF subclass (5). The apoL family members are all predicted to adopt amphipathic α-helical conformations, suggesting that their physiological role involves membrane interaction (20). Apolipoprotein L-1 shares limited homology with channel-forming colicins and, consistent with this observation, has been shown to function as an ion channel when incorporated into lipid bilayers (23).
The ultimate fate of TLF-targeted lysosomal membranes is not firmly established. Several studies employing both in vivo cellular analysis and artificial membrane systems address this point with conflicting results. Electron microscopy studies with gold-conjugated TLF revealed accumulation of TLF in intracellular vesicles and subsequent vesicle membrane breakdown and appearance of gold particles in the cytoplasm (9). Widener et al. (10) observed efflux of lysosomally localized large molecular mass dextrans (500 kDa) in TLF-treated T. b. brucei. These data suggest that the lysosomal membrane experiences large scale disruption. In contrast, Perez-Morga et al. (23) and Vanhollebeke et al. (24) report uncontrollable lysosomal swelling in susceptible trypanosomes treated with normal human serum, suggesting stability of the lamellar structure of the lysosomal membrane after TLF attack. Swelling is attributed to apoL-1-mediated influx of Cl– ions and concomitant osmotic flow of water into the lysosome. However, Molina-Portela et al. (25) observed the formation of cation-selective pores in TLF-treated planar lipid bilayers composed of trypanosome lipids. The diversity of activities reported for TLF and normal human serum may reflect the packaging of multiple toxins within the same complex that can act synergistically to provide optimal killing activity (5, 14).
Here we utilize model liposomes to monitor the membrane activity of TLF and its protein constituents. We describe the effects of TLF, delipidated Hpr, apoL-1, and apoA-1 on the permeability of unilamellar liposomes. Additionally, we show that TLF, apoL-1, and apoA-1 exhibit lipid specificity and that Hpr, apoL-1, and apoA-1 induce large scale changes in the geometry of liposomes. These results provide a molecular basis for the recognition of lysosomal membranes by this toxic HDL and support a multicomponent mechanism for trypanosome killing.
EXPERIMENTAL PROCEDURES
Lipids and Reagents—All lipids were purchased from Avanti Polar Lipids (Alabaster, AL). These include soy azolectin (catalog number 541601G), phosphatidylcholine (PC) from egg (catalog number 840051), phosphatidylethanolamine (PE) from egg (catalog number 841118), and phosphatidylserine (PS) from brain (catalog number 840032), phosphatidic acid (PA) from egg (catalog number 840101), cardiolipin from heart (catalog number 840012). Calcein (catalog number C481) was purchased from Molecular Probes.
Purification of TLF and Its Constituent Proteins—Isolation of TLF was performed as described previously in detail (5, 10). Briefly, total human HDLs were purified by density gradient centrifugation. Intact TLF was isolated by immunoaffinity chromatography utilizing antibodies against Hpr. The lipid-free protein constituents of TLF, Hpr, apoL-1, and apoA-1 were purified in a similar fashion from HDL particles solubilized in 10 mm CHAPS with the corresponding antibody. Eluted TLF and proteins were dialyzed in phosphate-buffered saline and stored at –80 °C. The integrity and purity of TLF, Hpr, apoL-1, and apoA-1 preparations were assessed by non-reducing SDS-PAGE visualized with silver staining and Western blotting with the appropriate antibody. Additionally, the trypanolytic activity was confirmed for each preparation as described previously (2). A non-trypanolytic fraction of human HDL was prepared from the unbound material passaged over the anti-Hpr immunoaffinity chromatography (>99% of the total human HDL). This HDL fraction was depleted in Hpr and lacked trypanosome-killing activity.
Other Proteins—Human Hp, phenotype 1-1, was purchased from Sigma (catalog number H0138).
Liposome Permeabilization Assays—The integrity of unilamellar liposomes was monitored in a time-resolved fashion by fluorescently observing the leakage of entrapped calcein from liposomal interiors. Dry lipid films were hydrated in 10 mm Hepes, 30 mm calcein to a final lipid concentration of 10 mg/ml. Unilamellar liposomes were prepared by extrusion through a 0.1-μm polycarbonate filter. Untrapped calcein was separated from liposomes by gel filtration (Sephacryl S-300 HR, GE Healthcare). Assays were performed by diluting liposomes 1:1000 into the appropriate buffer and monitoring fluorescence with an LS-55 spectrofluorometer from PerkinElmer Life Sciences. Excitation and emission wavelengths were 484 and 513 nm, respectively, with 10 nm excitation and 5 nm emission slit widths. Data are presented as representative points obtained with a single TLF and liposome preparation. To achieve increasing ionic strengths, NaCl was added to a buffer consisting of 50 mm Tris-maleate, pH 5.0. The ionic strength thus contains contributions from both the NaCl and the buffer, and was calculated according to the formula I = ½ ∑ cnzn2, where I is the ionic strength, c is the molar concentration of ion n, and z is the charge of that ion.
Liposome Binding Assays—Liposomes were constructed by hydration of dry lipid films in 10 mm Hepes. Liposomes were left multilamellar to facilitate pelleting by moderate centrifugation. Assays were performed by incubating 100 μg of lipid with 2.5 μg of TLF in a final volume of 50 μl at 37 °C for 30 min in the indicated buffer. Liposomes were pelleted by centrifugation at 16,000 × g for 10 min. In some instances, lipid pellets were washed with buffer containing 1 m KCl. Supernatant, wash, and pellet fractions were analyzed by non-reducing gel electrophoresis on a 10% polyacrylamide gel stained with silver nitrate.
90° Light Scattering—Assay conditions were the same as those described for the permeabilization assays. Liposomes were constructed from soy bean azolectin and extruded with a 0.1-μm polycarbonate filter. The 90° light-scattering intensity was monitored over time with the PerkinElmer Life Sciences LS55. Excitation and emission wavelengths were both set to 405 nm with 10-nm slit widths.
Electron Microscopy—Unilamellar liposomes were constructed from soy bean azolectin and prepared by extrusion (0.1 μm). 100 μg/ml lipid was incubated with 200 nm TLF or the indicated delipidated protein for ∼15 min. Samples were adsorbed to glow-discharged Formvar-coated copper grids, stained with 2% uranyl acetate, and imaged with a FEI Tecnai 20 transmission electron microscope.
RESULTS
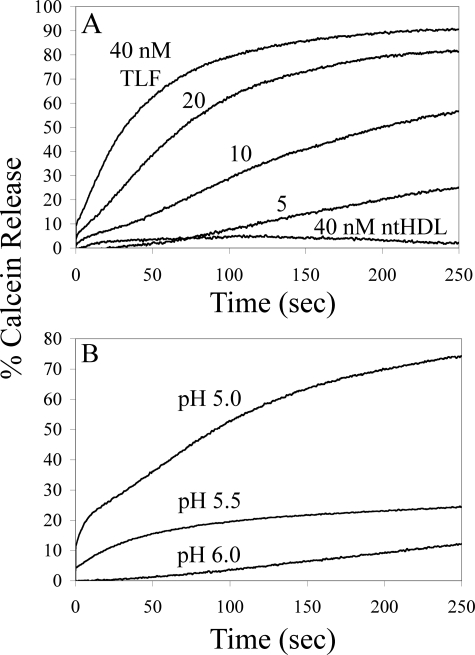
TLF Permeabilizes Lipid Bilayers in a pH-dependent Fashion—To study the interaction of TLF with lipid bilayers, we utilized a model system in which we monitor leakage of a liposomally entrapped fluorescent dye. Nanomolar concentrations of purified TLF effectively permeabilized unilamellar soy bean azolectin liposomes (Fig. 1A). Consistent with the necessity for localization of TLF in an acidic intracellular vesicle in vivo, permeabilizing activity in these in vitro assays is highly dependent on acidic pH (Fig. 1B). Permeabilizing activity was greatest at pH 5.0, the lowest pH tested, and unobservable at pH 6.5. Non-trypanolytic HDL, i.e. HDL depleted of TLF by passage over an α-Hpr affinity resin, did not exhibit permeabilizing activity against these particular liposomes at any pH (Fig. 1A and data not shown).
FIGURE 1.
Membrane permeabilization by TLF. A, unilamellar soy bean azolectin liposomes were assayed for calcein release against nanomolar concentrations of TLF. Rapid release of calcein is seen upon the addition of 5–40 nm TLF in 50 mm Tris-maleate, pH 5.0. TLF exhibits dramatically different membrane behavior than non-trypanolytic HDL (ntHDL); 40 nm non-trypanolytic HDL is incapable of permeabilizing soy bean azolectin liposomes. B, the permeabilizing activity of TLF is highly dependent on acidic pH. Calcein leakage assays were performed with 20 nm TLF in 50 mm of the appropriate buffer species at varying pH.
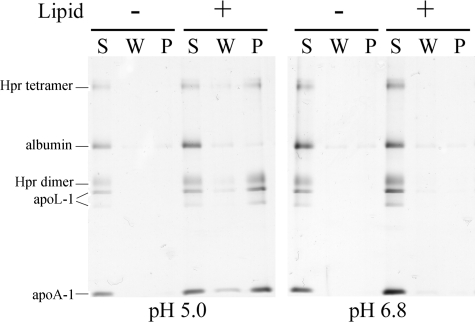
TLF Membrane Binding Is Facilitated by Low pH and Mediated by Ionic Interactions—The targeting of TLF to lipid bilayers was investigated with liposome binding assays. SDS-PAGE analysis of supernatant and pellet fractions reveals that TLF binds soy bean azolectin bilayers at pH 5.0 but not pH 6.8 (Fig. 2). Interestingly, the entire protein component of TLF appears in the liposomal pellet, whereas contaminating albumin remains in the supernatant. Non-trypanolytic HDLs do not exhibit binding in these assays (supplemental Fig. 1). To determine whether TLF was peripherally bound to the interface of the liposomal bilayers or stably incorporated into the acyl chain region, liposomes were washed with a high ionic strength buffer (1 m KCl, 50 mm Tris-maleate). When TLF is allowed to interact with bilayers in a low ionic strength buffer, a subsequent high ionic strength wash does not remove membrane-associated TLF proteins.
FIGURE 2.
Proton concentration mediates binding of TLF to target membranes. Liposome binding assays were performed with soy bean azolectin liposomes in 50 mm Tris-maleate, pH 5.0, or 50 mm Tris, pH 6.8. Supernatant (S), 1 m KCl wash (W), and pellet (P) fractions were analyzed by non-reducing SDS-PAGE. The entire TLF protein component appears in the pellet fraction at pH 5.0 but not pH 6.8. The dimeric and tetrameric forms of Hpr (indicated by Hpr dimer and Hpr tetramer) and the full-length and truncated apoL-1 bands (both indicated by apoL-1) are indicated.
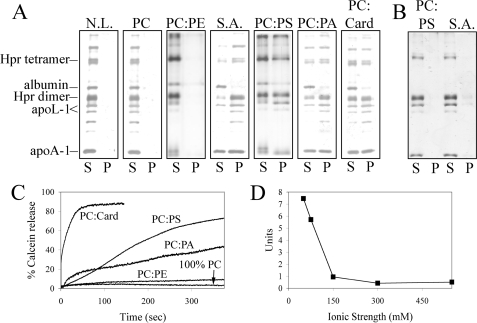
We further investigated the role of ionic interactions by analyzing TLF binding to liposomes possessing a net neutral or a net negative surface charge. TLF readily binds bilayers containing anionic lipids, i.e. PC:PS, PC:PA, PC:cardiolipin but not zwitterionic PC or PC:PE (all 3:1 by mass) (Fig. 3A). Consistent with an electrostatic mode of membrane targeting, the presence of 1 m NaCl inhibits binding of TLF to target bilayers (Fig. 3B). In all cases, membrane binding is consistent with permeabilization, i.e. TLF readily permeabilizes anionic but not zwitterionic liposomes (Fig. 3C) and is inhibited by high salt (Fig. 3D). The pH dependence for interaction with anionic liposomes is identical to that for soy bean azolectin, i.e. no permeabilization is seen at neutral pH (supplemental Fig. 2). Minor permeabilizing activity was detected from non-trypanolytic HDL against anionic liposomes (supplemental Fig. 3); in all cases, TLF exhibits significantly higher membrane-permeabilizing activity, 12–65 times that of non-trypanolytic HDL at equimolar concentrations (40 nm).
FIGURE 3.
TLF targets lipid bilayers via electrostatic interaction. A, binding assays were performed with liposomes of various composition (no lipid control (N.L.); zwitterionic bilayers, PC and PC:PE; and anionic bilayers, soy bean azolectin (S.A.), PC:PS, PC:PA, and PC:cardiolipin (PC:card)) in 50 mm Tris-maleate, pH 5.0. S, supernatant. P, pellet. B, the presence of 1 m NaCl in assay buffer inhibits binding of TLF to target bilayers. C, calcein leakage assays were performed with liposomes of identical composition as in A. D, TLF-mediated membrane permeabilization is inhibited by increasing ionic strength (achieved by the addition of NaCl), and the rate of calcein release is expressed in units of 5% increase in fluorescence intensity per minute relative to the 100% fluorescence intensity.
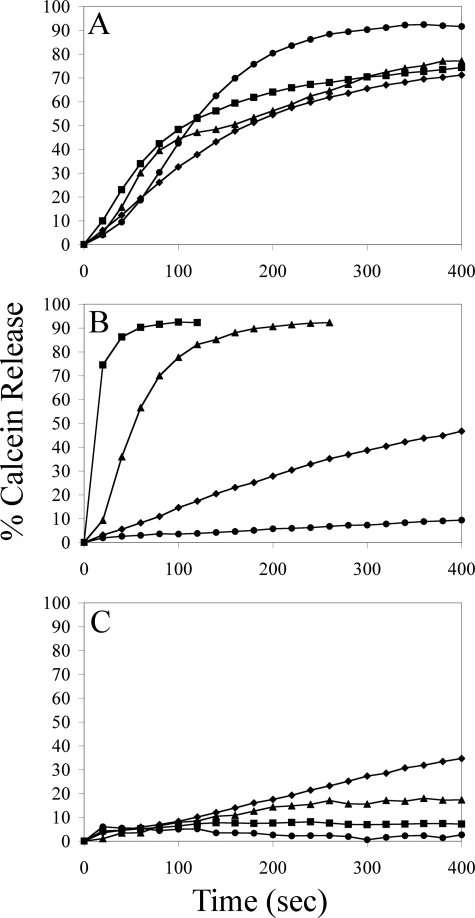
The Protein Components of TLF Permeabilize Lipid Bilayers—In an effort to identify the TLF components responsible for the membrane-permeabilizing activity, we assayed apoA-1, Hpr, and apoL-1, purified from detergent-solubilized TLF particles, for the ability to induce calcein leakage. Each purified, lipid-free protein elicits calcein leakage at acidic pH and nanomolar concentrations (Fig. 4). Similar to native TLF, the membrane-permeabilizing activity of the individual proteins is attenuated at increasing pH (supplemental Fig. 4). The TLF-specific proteins, Hpr and apoL-1, exhibit more robust membrane-permeabilizing activity on a molar basis than apoA-1. Indeed, at molar concentrations double those of apoL-1 or Hpr, i.e. 40 nm apoA-1 and 20 nm apoL-1/Hpr, calcein release is only observable for liposomes composed of PC:PS. The lipid specificity for apoL-1 is identical to the native TLF molecule (Fig. 3C), i.e. apoL-1 permeabilizes liposomes containing anionic lipids but not liposomes composed entirely of zwitterionic lipids. Hpr does not exhibit a requirement for anionic lipids, permeabilizing both zwitterionic and negatively charged liposomes. We found no liposome-permeabilizing activity from human haptoglobin under any conditions (data not shown). The activities of the individual proteins are indeed less than that of native TLF on an equimolar basis; however, the synergy observed for TLF colocalized apoA-1, apoL-1, and Hpr in vitro (5) and in vivo (14), i.e. a several hundredfold increase in trypanosome killing activity, is not reflected in this model system.
FIGURE 4.
The delipidated protein components of TLF permeabilize liposomes. Calcein leakage assays were performed at pH 5.0 with 20 nm (0.9 μg/ml) Hpr (A), 20 nm (0.84 μg/ml) apoL-1 (B), and 40 nm (1.12 μg/ml) apoA-1 (C), all of which were affinity-purified from detergent-solubilized TLF, against liposomes constructed from 100% PC (•), PC:PS (♦), PC:PA (▴), and PC: cardiolipin (▪).
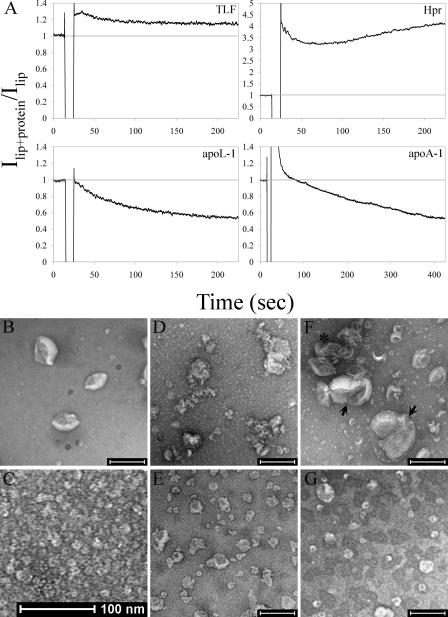
Hpr, ApoL-1, and ApoA-1 Remodel Liposomes—To discern the mode of membrane permeabilization by TLF and its protein components, we analyzed the morphology of target liposomes. The 90° light-scattering intensity of liposomal suspensions was monitored as an indicator of relative particle size (Fig. 5A). Under conditions that facilitate membrane permeabilization, TLF induced a minor increase in the hydrodynamic diameter of susceptible liposomes. However, when the individual proteins were assayed, marked changes in the light-scattering intensity were observed.
FIGURE 5.
TLF and its protein components exhibit dramatically different membrane altering activities. A, the 90° light-scattering intensity of susceptible liposomes (lip) was monitored as an indicator of relative particle size at pH 5.0 with the addition (indicated by the drop in signal) of 20 nm TLF (10μg/ml), Hpr (0.9μg/ml), apoL-1 (0.84 μg/ml), or 60 nm apoA-1 (1.68 μg/ml). B–G, transmission electron micrographs of negatively stained preparation of soy bean azolectin liposomes (B), TLF alone (C), liposomes treated with TLF (D), liposomes treated with apoL-1 (E), liposomes treated with Hpr (F), and liposomes treated with apoA-1 (G). Scale bars are 200 nm, except in C. The liposomes in D appear decorated with TLF particles. Apparent fusion products and agglutinated liposomes in F are indicated by an arrow and asterisk, respectively.
The addition of apoL-1 to suspensions of unilamellar liposomes containing anionic lipids resulted in a decrease in the light-scattering intensity, indicating a reduction in the relative size of the liposome particles (Fig. 5A). Consistent with the known membrane microsolubilizing activity of apoA-1 (26, 27), a decrease in the light-scattering intensity was also observed for susceptible liposomes (Fig. 5A). Interestingly, the addition of nanomolar concentrations of Hpr resulted in a large increase in the light-scattering intensity of liposomal suspensions (factor 5A).
To determine the nature of the particle size changes revealed by light scattering, we performed transmission electron microscopy on negatively stained preparations of liposomes treated with TLF, Hpr, apoL-1, or apoA-1 (Fig. 5, B–G). Target unilamellar liposomes treated with TLF at pH 5.0 appear similar in size to untreated control liposomes (Fig. 5, A and D, respectively). TLF particles are readily visible, both in solution and decorating the surface of liposomes (TLF particles, Fig. 5C; TLF with liposomes, Fig. 5D). The addition of Hpr results in clumping and a dramatic redistribution of particle sizes (Fig. 5F). Agglutinated intact liposomes, tightly packed together (Fig. 5F, asterisk), and large vesicles (200–250 nm), most likely fusion products, are readily apparent (Fig. 5F, arrows). Additionally, small diameter vesicles and micellar-like structures are also present. Presumably, the large liposome aggregates and fusion products dominate the light-scattering signal. Liposomes treated with apoL-1 appear as smaller 40–70-nm vesicles, distorted in shape (Fig. 5E). Apolipoprotein A-1 induces 50-nm spherical lipid structures, again consistent with the formation of nascent discoidal HDL (Fig. 5G) (27). In each case, the appearance of treated liposomes is consistent with the light-scattering data, i.e. TLF does not induce large scale morphological changes, Hpr induces an enlargement of particle size, and apoL-1 and apoA-1 induce a decrease in particle size.
DISCUSSION
The ultimate step in trypanosome killing by TLF is permeabilization of the lysosomal membrane. We have addressed this particular step with the use of simplified model liposomes, a technique utilized to study myriad membrane-interacting proteins and peptides, including human HDL (see for example Refs. 28–33). Our in vitro system recapitulates data from extensive in vivo analysis of TLF killing and elucidates the role of pH and membrane surface charge in TLF bilayer targeting (9, 13). Permeabilization of liposomes is highly dependent on acidic pH. We have shown here that a high proton concentration facilitates binding of TLF to lipid bilayers that possess a net negative surface charge but not to bilayers that are electrically neutral. These data suggest that protonation of acidic groups, most likely of the TLF molecule, facilitates the interaction of TLF with target membranes. It may be the case that protonation effectively dissipates electrostatic repulsion between aspartic and glutamic acid residues and the target bilayer, allowing basic residues of the TLF proteins to mediate an interaction with anionic phospholipids. The lysosome presents an attractive membrane for such a targeting mechanism as, relative to other cellular organelles, it is highly enriched in anionic phospholipids (34, 35). Interestingly, preferential binding to anionic bilayers is a strategy employed by myriad antimicrobial peptides to differentiate between more negatively charged prokaryotic membranes, which may represent potential pathogens, and zwitterionic membranes, characteristic of the plasma membrane of metazoans (36).
A growing body of evidence suggests that TLF, and indeed HDL in general, function as innate immune factors active against a broad spectrum of microbes (4, 37). Recently Samanovic et al. (4) reported that TLF effectively kills Leishmania promastigotes within the parasitophorous vacuole of macrophages. Additionally, it is known that apoA-1 binds the bacterial outer membrane component lipopolysaccharide (38–40), protects against the toxic effects of this molecule in vivo (41), and inhibits the growth of Escherichia coli in vitro (42). Thus it is intriguing to consider the possibility that the membrane-destabilizing activity of TLF is also an effective mechanism of combating bacterial infection. The obvious impediment to this scenario is the neutral pH of the internal milieu. However, it is known that locally, acidic conditions occur, and that antimicrobial responses are amplified at sites of inflammation (43–46).
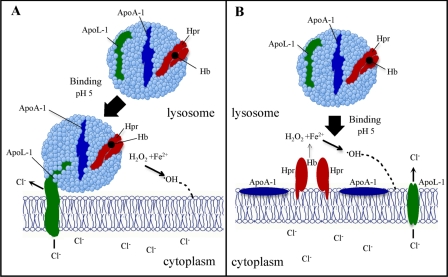
The initial binding of TLF is followed by stable association of the entire TLF particle with the target liposome. The appearance of the total protein component of TLF in target bilayers and the insensitivity to high ionic strength after interacting with bilayers may be interpreted as either tethering of the TLF molecule to the target bilayer, where a proteinaceous extension from TLF embeds in the acyl chain region of the membrane, or fusion of the lipoprotein particle with the bilayer (Fig. 6). A large number of apolipoproteins associate with lipidic particles via embedding of the hydrophobic faces of amphipathic α-helices into the hydrophobic regions of the particle (47–49). These proteins are capable of partly dissociating from the parent lipoprotein, via the presence of hinge regions, and associating with opposing lipid bilayer structures (47, 48). In this regard, it is predicted that apoL-1 exhibits a predominantly α-helical structure (23), consistent with known apolipoproteins and, integrating our membrane targeting data, it may be the case that apoL-1 mediates the tethering of TLF to target lipid bilayers (Fig. 6A). Alternatively, it is well known that HDL particles fuse with one another, and transmission electron microscopy data indicate that Hpr induces fusion of lipid bilayers, suggesting the possibility that TLF fuses with the target membrane (Fig. 6B). In either case, it is apparent that the TLF particle induces large scale disruption of the lamellar packing of bilayer lipids such that a significant increase in permeability is achieved.
FIGURE 6.
Models of the interaction of TLF with the lysosomal membrane and proposed mechanism of TLF disruption of trypanosome lysosomal membranes. The stable integration of TLF into lipid bilayers may be facilitated by either a tethering (A) or a fusion (B) of the TLF particle into the membrane. Reduced Fe2+ from Hb, at low pH, in the presence of H2O2 results in the formation of membrane-reactive •OH. Insertion of apoL-1 into the membrane allows Cl– to enter from the cytoplasm. The synergistic activity of Hb, carried by Hpr, and the pore-forming activity of apoL-1 provides maximal lysosomal destabilization as based upon published results (5, 6). The dashed line represents the reaction of •OH with the lysosomal membrane.
TLF molecules are physically and functionally distinct from non-trypanolytic HDL in several respects, namely 1) trypanolytic activity, 2) protein composition, 3) size (TLF molecules are larger (15–20 nm) than non-trypanolytic HDL (10–11 nm) (2)), and 4) membrane-permeabilizing activity (we have shown that TLF exhibits distinctly different membrane-permeabilizing activity than non-trypanolytic HDL). Liposomes composed of soy bean azolectin are efficiently permeabilized by TLF, whereas non-trypanolytic HDL are incapable of evoking calcein leakage from this particular composition. Minor permeabilizing activity is observed when non-trypanolytic HDL is assayed against liposomes of a defined composition, e.g. egg PC in combination with any of several anionic phospholipids. In all cases, the activity of equimolar concentrations of TLF against these liposomes is significantly greater. Binding assays performed with non-trypanolytic HDL and susceptible liposomes, i.e. PC:PS, do not reveal a pelletable product as is the case for TLF. These data indicate that TLF interacts with lipid bilayers in a fundamentally different manner than human non-trypanolytic HDL.
The complexity of the TLF particle has hindered a clear understanding of the molecular events specifically involved in lysosome membrane disruption. To address this question, we assayed the purified, lipid-free protein components of TLF for membrane-permeabilizing activity. We found that each of the protein components of TLF, Hpr, apoL-1, and indeed the HDL ubiquitous protein apoA-1 elicited the leakage of calcein from liposomes. The activity of apoA-1 was not unexpected as it is known to interact with cellular membranes with the concomitant formation of structures reminiscent of nascent HDL (26, 27). Both of the unique protein components of TLF, apoL-1 and Hpr, efficiently permeabilized target liposomes. The mode of membrane permeabilization employed by each protein is different not only from each other but from the native TLF particle itself. The interaction of Hpr with liposomes results in a heterogeneous distribution of lipid particles. Agglutination and apparent fusion of the liposomes readily occur; however, the appearance of much smaller lipid particles indicates that fission and/or micellization is also taking place. Interaction of apoL-1 with target liposomes may resemble the membrane microsolubilizing activity of apoA-1, where the amphipathic helices wrap, belt-like, around the acyl chains of bilayer lipids. The light-scattering data are consistent with this interpretation; however, when visualized by transmission electron microscopy, lipid particles resulting from apoL-1 treatment do not appear uniformly discoidal, as is the case for apoA-1. The appearance of pockets of uranyl acetate on the apoL-1 particles suggests that they retain a vesicular structure. It has been shown that the purified, delipidated apoL-1 and Hpr are, although attenuated in relationship to native TLF, readily cytotoxic to T. b. brucei (5). Our data suggest that a membrane-destabilizing mechanism is relevant to the mode of killing by apoL-1 or Hpr, but this mechanism may be distinct from native TLF. It may be the case that association with lipids modifies subsequent membrane behavior of these proteins, and thus, lysosomal membrane disruption requires downstream events, i.e. peroxidase and ion channel activities from Hpr (7) and apoL-1 (23), respectively.
The nature of the trypanolytic activity of human serum has not been well resolved. Central to the debate is the question of whether the natural toxin is a single protein, i.e. apoL-1 (6, 23), or a complex containing multiple contributing proteins. Previously, we have reported that the association of apoL-1 and Hpr with bound Hb provided maximal killing activity (10). Consistent with these findings, the Hpr·Hb complex has been identified as the necessary ligand on TLF that binds a high affinity trypanosome cell-surface receptor facilitating endocytosis, directly demonstrating the requirement for a multicomponent complex (11). The remaining issue then becomes whether the mechanism of lysosomal membrane disruption by TLF is solely due to any single protein component of TLF. We have demonstrated here that TLF binds to liposomal membranes at low pH and that this binding is mediated by electrostatic interactions with anionic bilayer lipids. This binding is followed by the association of all of the TLF proteins, with equal efficiency, with the target liposomal bilayer. These findings contrast predictions, based on the proposed structural properties of apoL-1, that argued that at low pH, apoL-1 dissociates from the HDL prior to integration into membranes (50). Based upon the findings reported here, we propose a model for the synergistic activity of Hpr·Hb and apoL-1 that is dependent on the association of the entire TLF particle with the trypanosome lysosomal membrane (Fig. 6).
The studies presented in this report were designed to examine the interaction of TLF and its major protein components, Hpr, apoL-1, and apoA-1, with membranes using a simplified bilayer system of model liposomes. Because the proposed activities of Hpr and apoL-1 require specific conditions, bound Hb and an ionic membrane gradient, respectively, we have not addressed the potential mechanisms of Hpr or apoL-1 action within the trypanosome lysosome. However, our new findings raise important questions about the consequence of TLF apolipoprotein insertion on membrane stability and add a new dimension to our considerations of the mechanism of trypanosome killing by TLF.
Supplementary Material
Acknowledgments
We thank all of the members of the Hajduk laboratory for helpful discussions concerning these studies. In particular, we appreciate critical input from April Shiflett, Justin Widener, and Rudo Kieft on this manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant AI39033 (to S. L. H.).
The on-line version of this article (available at http://www.jbc.org) contains four supplemental figures.
Footnotes
The abbreviations used are: HDL, high density lipoprotein; apoL-1, apolipoprotein L-1; apoA-1, apolipoprotein A-1; TLF, trypanosome lytic factor; Hp, haptoglobin; Hpr, haptoglobin-related protein; Hb, hemoglobin; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PS, phosphatidylserine; PA, phosphatidic acid; CHAPS, 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid.
References
- 1.Rifkin, M. R. (1978) Proc. Natl. Acad. Sci. U. S. A. 75 3450–3454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hajduk, S. L., Moore, D. R., Vasudevacharya, J., Siqueira, H., Torri, A. F., Tytler, E. M., and Esko, J. D. (1989) J. Biol. Chem. 264 5210–5217 [PubMed] [Google Scholar]
- 3.Hajduk, S. L., Hager, K., and Esko, J. D. (1992) Parasitol. Today 8 95–98 [DOI] [PubMed] [Google Scholar]
- 4.Samanovic, M., Molina-Portela, M. P., Chessler, A. D., Burleigh, B. A., and Raper, J. (2009) PLoS Pathog. 5 e1000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiflett, A. M., Bishop, J. R., Pahwa, A., and Hajduk, S. L. (2005) J. Biol. Chem. 280 32578–32585 [DOI] [PubMed] [Google Scholar]
- 6.Vanhamme, L., Paturiaux-Hanocq, F., Poelvoorde, P., Nolan, D. P., Lins, L., Van Den Abbeele, J., Pays, A., Tebabi, P., Van Xong, H., Jacquet, A., Moguilevsky, N., Dieu, M., Kane, J. P., De Baetselier, P., Brasseur, R., and Pays, E. (2003) Nature 422 83–87 [DOI] [PubMed] [Google Scholar]
- 7.Smith, A. B., Esko, J. D., and Hajduk, S. L. (1995) Science 268 284–286 [DOI] [PubMed] [Google Scholar]
- 8.Oli, M. W., Cotlin, L. F., Shiflett, A. M., and Hajduk, S. L. (2006) Eukaryot. Cell 5 132–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hager, K. M., Pierce, M. A., Moore, D. R., Tytler, E. M., Esko, J. D., and Hajduk, S. L. (1994) J. Cell Biol. 126 155–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Widener, J., Nielsen, M. J., Shiflett, A., Moestrup, S. K., and Hajduk, S. (2007) PLoS Pathog. 3 1250–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanhollebeke, B., De Muylder, G., Nielsen, M. J., Pays, A., Tebabi, P., Dieu, M., Raes, M., Moestrup, S. K., and Pays, E. (2008) Science 320 677–681 [DOI] [PubMed] [Google Scholar]
- 12.Drain, J., Bishop, J. R., and Hajduk, S. L. (2001) J. Biol. Chem. 276 30254–30260 [DOI] [PubMed] [Google Scholar]
- 13.Lorenz, P., Barth, P. E., Rudin, W., and Betschart, B. (1994) Trans. R. Soc. Trop. Med. Hyg. 88 487–488 [DOI] [PubMed] [Google Scholar]
- 14.Molina-Portela, M. P., Samanovic, M., and Raper, J. (2008) J. Exp. Med. 205 1721–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McEvoy, S. M., and Maeda, N. (1988) J. Biol. Chem. 263 15740–15747 [PubMed] [Google Scholar]
- 16.Maeda, N. (1985) J. Biol. Chem. 260 6698–6709 [PubMed] [Google Scholar]
- 17.Kristiansen, M., Graversen, J. H., Jacobsen, C., Sonne, O., Hoffman, H. J., Law, S. K., and Moestrup, S. K. (2001) Nature 409 198–201 [DOI] [PubMed] [Google Scholar]
- 18.Nielsen, M. J., Petersen, S. V., Jacobsen, C., Oxvig, C., Rees, D., Moller, H. J., and Moestrup, S. K. (2006) Blood 108 2846–2849 [DOI] [PubMed] [Google Scholar]
- 19.Bishop, J. R., Shimamura, M., and Hajduk, S. L. (2001) Mol. Biochem. Parasitol. 118 33–40 [DOI] [PubMed] [Google Scholar]
- 20.Page, N. M., Butlin, D. J., Lomthaisong, K., and Lowry, P. J. (2001) Genomics 74 71–78 [DOI] [PubMed] [Google Scholar]
- 21.Duchateau, P. N., Pullinger, C. R., Cho, M. H., Eng, C., and Kane, J. P. (2001) J. Lipid Res. 42 620–630 [PubMed] [Google Scholar]
- 22.Monajemi, H., Fontijn, R. D., Pannekoek, H., and Horrevoets, A. J. (2002) Genomics 79 539–546 [DOI] [PubMed] [Google Scholar]
- 23.Perez-Morga, D., Vanhollebeke, B., Paturiaux-Hanocq, F., Nolan, D. P., Lins, L., Homble, F., Vanhamme, L., Tebabi, P., Pays, A., Poelvoorde, P., Jacquet, A., Brasseur, R., and Pays, E. (2005) Science 309 469–472 [DOI] [PubMed] [Google Scholar]
- 24.Vanhollebeke, B., Lecordier, L., Perez-Morga, D., Amiguet-Vercher, A., and Pays, E. (2007) J. Eukaryot. Microbiol. 54 448–451 [DOI] [PubMed] [Google Scholar]
- 25.Molina-Portela, M. P., Lugli, E. B., Recio-Pinto, E., and Raper, J. (2005) Mol. Biochem. Parasitol. 144 218–226 [DOI] [PubMed] [Google Scholar]
- 26.Fukuda, M., Nakano, M., Sriwongsitanont, S., Ueno, M., Kuroda, Y., and Handa, T. (2007) J. Lipid Res. 48 882–889 [DOI] [PubMed] [Google Scholar]
- 27.Massey, J. B., and Pownall, H. J. (2008) Biochim. Biophys. Acta 1781 245–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andra, J., Herbst, R., and Leippe, M. (2003) Dev. Comp. Immunol. 27 291–304 [DOI] [PubMed] [Google Scholar]
- 29.Winkelmann, J., Leippe, M., and Bruhn, H. (2006) Mol. Biochem. Parasitol. 147 85–94 [DOI] [PubMed] [Google Scholar]
- 30.Harrington, J. M., Chou, H. T., Gutsmann, T., Gelhaus, C., Stahlberg, H., Leippe, M., and Armstrong, P. B. (2008) Biochem. J. 413 305–313 [DOI] [PubMed] [Google Scholar]
- 31.Fedders, H., Michalek, M., Grotzinger, J., and Leippe, M. (2008) Biochem. J. 416 65–75 [DOI] [PubMed] [Google Scholar]
- 32.Tall, A. R., and Small, D. M. (1977) Nature 265 163–164 [DOI] [PubMed] [Google Scholar]
- 33.Thuduppathy, G. R., Terrones, O., Craig, J. W., Basanez, G., and Hill, R. B. (2006) Biochemistry 45 14533–14542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolter, T., and Sandhoff, K. (2005) Annu. Rev. Cell Dev. Biol. 21 81–103 [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez-Paris, J. M., Nolta, K. V., and Steck, T. L. (1993) J. Biol. Chem. 268 9110–9116 [PubMed] [Google Scholar]
- 36.Matsuzaki, K. (October 8, 2008) Biochim. Biophys. Acta 10.1016/j.bbamem.2008.09.013 [DOI] [PubMed]
- 37.Vaisar, T., Pennathur, S., Green, P. S., Gharib, S. A., Hoofnagle, A. N., Cheung, M. C., Byun, J., Vuletic, S., Kassim, S., Singh, P., Chea, H., Knopp, R. H., Brunzell, J., Geary, R., Chait, A., Zhao, X. Q., Elkon, K., Marcovina, S., Ridker, P., Oram, J. F., and Heinecke, J. W. (2007) J. Clin. Investig. 117 746–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ulevitch, R. J., Johnston, A. R., and Weinstein, D. B. (1979) J. Clin. Investig. 64 1516–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ulevitch, R. J., Johnston, A. R., and Weinstein, D. B. (1981) J. Clin. Investig. 67 827–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baumberger, C., Ulevitch, R. J., and Dayer, J. M. (1991) Pathobiology 59 378–383 [DOI] [PubMed] [Google Scholar]
- 41.Levine, D. M., Parker, T. S., Donnelly, T. M., Walsh, A., and Rubin, A. L. (1993) Proc. Natl. Acad. Sci. U. S. A. 90 12040–12044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thaveeratitham, P., Plengpanich, W., Naen-Udorn, W., Patumraj, S., and Khovidhunkit, W. (2007) J. Endotoxin. Res. 13 58–64 [DOI] [PubMed] [Google Scholar]
- 43.Wright, J., Schwartz, J. H., Olson, R., Kosowsky, J. M., and Tauber, A. I. (1986) J. Clin. Investig. 77 782–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Zwieten, R., Wever, R., Hamers, M. N., Weening, R. S., and Roos, D. (1981) J. Clin. Investig. 68 310–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martinez, D., Vermeulen, M., Trevani, A., Ceballos, A., Sabatte, J., Gamberale, R., Alvarez, M. E., Salamone, G., Tanos, T., Coso, O. A., and Geffner, J. (2006) J. Immunol. 176 1163–1171 [DOI] [PubMed] [Google Scholar]
- 46.Zhang, J., Koh, J., Lu, J., Thiel, S., Leong, B. S., Sethi, S., He, C. Y., Ho, B., and Ding, J. L. (2009) PLoS Pathog. 5 e1000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bolanos-Garcia, V. M., and Miguel, R. N. (2003) Prog. Biophys. Mol. Biol. 83 47–68 [DOI] [PubMed] [Google Scholar]
- 48.Segrest, J. P., Jones, M. K., De Loof, H., Brouillette, C. G., Venkatachalapathi, Y. V., and Anantharamaiah, G. M. (1992) J. Lipid Res. 33 141–166 [PubMed] [Google Scholar]
- 49.Hristova, K., Wimley, W. C., Mishra, V. K., Anantharamiah, G. M., Segrest, J. P., and White, S. H. (1999) J. Mol. Biol. 290 99–117 [DOI] [PubMed] [Google Scholar]
- 50.Vanhollebeke, B., and Pays, E. (2006) CMLS Cell. Mol. Life Sci. 63 1937–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.