Abstract
Sphingosine 1-phosphate (S1P) is a bioactive sphingolipid implicated in diverse cellular functions including survival, proliferation, tumorigenesis, inflammation, and immunity. Sphingosine kinase (SphK) contributes to these functions by converting sphingosine to S1P. We report here that the nonstructural protein NS3 from bovine viral diarrhea virus (BVDV), a close relative of hepatitis C virus (HCV), binds to and inhibits the catalytic activity of SphK1 independently of its serine protease activity, whereas HCV NS3 does not affect SphK1 activity. Uncleaved NS2-3 from BVDV was also found to interact with and inhibit SphK1. We suspect that inhibition of SphK1 activity by BVDV NS3 and NS2-3 may benefit viral replication, because SphK1 inhibition by small interfering RNA, chemical inhibitor, or overexpression of catalytically inactive SphK1 results in enhanced viral replication, although the mechanisms by which SphK1 inhibition leads to enhanced viral replication remain unknown. A role of SphK1 inhibition in viral cytopathogenesis is also suggested as overexpression of SphK1 significantly attenuates the induction of apoptosis in cells infected with cytopathogenic BVDV. These findings suggest that SphK is targeted by this virus to regulate its catalytic activity.
Bovine viral diarrhea virus (BVDV)2 is an enveloped, positive-sense single-stranded RNA virus classified in the genus Pestivirus of the family Flaviviridae. BVDV establishes persistent infections in cattle populations worldwide. Because BVDV shares virological and molecular properties with the Flaviviridae family member hepatitis C virus (HCV), which chronically infects an estimated 200 million patients worldwide (1), BVDV is regarded as a surrogate model for HCV (2). Both HCV and BVDV encode a single large precursor polyprotein that is processed by cellular and viral proteases into mature structural and nonstructural (NS) proteins.
BVDV NS3 exhibits serine protease and helicase/ATPase activities that require its cofactor NS4A (3). NS3/4A protease is essential for generating mature NS proteins that are required for viral replication. HCV NS3/4A is well characterized and has been shown to suppress type-I interferons by cleaving the cellular interferon mediators IPS-1 and TRIF (4, 5). However, neither interferon suppression nor cellular targets have been identified for the BVDV NS3/4A protease (6).
Lytic and persistent BVDV infections depend on the virus biotype. Cytopathogenic (CP) BVDV causes cytopathic effects via apoptosis, whereas noncytopathogenic (NCP) BVDV does not induce obvious changes in cell morphology and viability. These features are distinguished by NS2-3 processing differences; free NS3 produced by NS2-3 cleavage is generated continuously following CP BVDV infections, whereas NS3 is detected only until ∼9 h postinfection (p.i.) for NCP BVDV due to down-regulation of NS2-3 cleavage by this biotype (7). The CP biotype is characterized by dramatic up-regulation of viral RNA synthesis that could be correlated with the induction of cytopathic effect (7–9). Because free NS3, but not NS2-3, can form an active viral replicase complex with other NS proteins, increased viral RNA synthesis promoted through the release of free NS3 has been suggested to be a determinant of the characteristic lytic phenotype of CP BVDV infections (10). However, little is known about the regulation of cellular signaling by BVDV NS2-3, NS3, and NS3/4A, which is crucial for the control of both viral replication and biotype.
Recent studies on the mechanisms of viral replication revealed that HCV RNA synthesis occurs on a lipid raft membrane structure where the active viral replicase complex is found (11, 12). The significance of the lipid raft as a scaffold for viral replication is further demonstrated by the identification of a novel HCV replication inhibitor, NA255, which prevents the biosynthesis of sphingolipids, the major components of lipid rafts (13). Administration of NA255 results in disruption of the HCV replicase complexes from the lipid rafts. This report proposes that the interaction between HCV NS5B and sphingomyelin on lipid rafts plays a crucial role for HCV RNA replication. Cellular sphingolipid metabolism is regulated by a large number of converting enzymes that maintain a homeostasis (14) but viral mechanisms that affect the sphingolipid metabolism to facilitate viral replication have yet to be identified.
In a search for potential host proteins that interact with BVDV NS3, we identified sphingosine kinase 1 (SphK1) as a binding partner of NS3 using the yeast two-hybrid system. SphK1 is a lipid kinase that catalyzes the phosphorylation of sphingosine to form sphingosine 1-phosphate (S1P), a bioactive sphingolipid implicated in diverse cellular functions, including proliferation, survival, tumorigenesis, development, inflammation, and immunity (14, 15). Here, we analyze the biological significance of the SphK1 interaction with NS3, NS2-3, and NS3/4A. Using purified recombinant SphK1 and NS3, SphK activity was inhibited by NS3 in a dose-dependent manner, independently of its serine protease activity. The inhibition appears to be specific for BVDV NS3 because HCV NS3 had no effect on SphK activity. Using specific chemical inhibitors, small interfering RNA (siRNA), and a catalytically inactive mutant of SphK1, we investigated the significance of SphK inhibition in the viral replication. The present study is the first report demonstrating that SphK1 is targeted by a virus to inhibit its catalytic activity, and this mechanism may contribute to the efficient replication and pathogenesis of BVDV.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies—d-erythro-Sphingosine and sphingosine kinase inhibitor (SKI) (16) were purchased from Calbiochem (La Jolla, CA). S1P was obtained from Cayman Chemical (Ann Arbor, MI). Anti-FLAG M2 monoclonal antibody (mAb; IgG1), anti-Myc mAb (IgG1), and isotype control IgG1 mAb were from Sigma. Anti-BVDV NS3 (IgG2a) and anti-GAPDH mAbs were from TropBio (Townsville, Australia) and Ambion (Austin, TX), respectively. Rabbit polyclonal antibodies (pAbs) against calnexin and SphK1 were from Stressgen (Victoria, Canada) and Exalpha (Maynard, MA), respectively. Mouse pAb against junctional adhesion molecule 1 was described previously (17). Goat anti-mouse IgG, IgG1, IgG2a, or anti-rabbit IgG antibodies conjugated with Alexa 488, Alexa 488, Alexa 594, or Alexa 568, respectively, were from Invitrogen. Rabbit pAb against HCV NS3 was kindly provided by Dr. Michinori Kohara (Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan). Protein concentrations in samples were determined with the Protein Quantification Kit-Rapid (Dojindo, Rockville, MD) using bovine serum albumin as a standard. Molybdenum blue spray was from Sigma. Recombinant human SphK1 (hSphK1) was purchased from BPS Bioscience (San Diego, CA).
Cells and Viruses—MDBK, LB9.K, and human embryonic kidney HEK293 cell lines were obtained from the American Type Culture Collection (ATCC) and maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5, 10, and 10% fetal calf serum (FCS), respectively, at 37 °C in a humidified 5% CO2 atmosphere. MDBK and LB9.K cells were confirmed to be free of BVDV by reverse transcriptase-polymerase chain reaction (RT-PCR). BVDV strains KS86-1cp, KS86-1ncp, and Nose have been described previously (18). Unless otherwise indicated, MDBK and LB9.K cells were infected with BVDV using a multiplicity of infection (m.o.i.) of 5 for 1 h, washed twice with FCS-free DMEM, and incubated in DMEM containing 5 or 10% FCS, respectively. End point viral titration was performed with four replicates on MDBK cells and the 50% tissue culture infective dose (TCID50) determined as described previously (7). The intracellular synthesis of virus-specific proteins at 72 h p.i. was detected by indirect immunofluorescence analysis using anti-BVDV NS3 mAb (TropBio) and a secondary fluorescein isothiocyanate-labeled antibody as described below under “Immunofluorescence Microscopy.”
RNA Extraction—Total RNA was extracted using the SV Total RNA Isolation System (Promega, Madison, WI) according to the manufacturer's protocol.
Plasmids—The bovine SphK1 complementary DNA (cDNA; GenBank™ data base accession number XM_870939.1) was generated from total RNA extracted from MDBK cells by RT-PCR using primers that incorporated EcoRI and EcoRV sites at the 5′ and 3′ ends, respectively. Mammalian expression vector of N-terminal FLAG-tagged bovine SphK1, designated pFlag-SphK1, was generated by cloning the SphK1 cDNA into pFLAG-CMV2 (Sigma) using EcoRI and EcoRV sites. The fragments encoding a series of deletion mutants of SphK1 were generated by PCR-mediated site mutagenesis using pFlag-SphK1 as a template. The fragment of catalytically inactive SphK1G177D was generated by PCR-mediated mutagenesis using pFlag-SphK1 as a template with the mutagenic primer 5′-TCGTGGATCAGCCCATCATCGGACATGACCACCAG-3′ to substitute Gly177 to Asp.
The mammalian expression vectors of BVDV NS proteins were generated using SRα promoter vector, pME18S. The Myc tag sequence together with the multiple cloning site from pGBKT7 was amplified by PCR using pGBKT7-NS3 as a template and cloned between XhoI and PstI sites of pME18S vector. A DNA fragment encoding BVDV NS3, NS2-3, NS3/4A, and NS5A was generated from the BVDV Nose strain (genotype 1a; GenBank data base accession number AB078951) by SuperScript III One-Step RT-PCR System with Platinum Taq High Fidelity (Invitrogen) using primers that incorporated NdeI and PstI sites at the 5′ and 3′ ends, respectively. The fragments were then cloned into pME18S-Myc using NdeI and PstI sites to generate pME-NS3, pME-NS2-3, pME-NS3/4A, and pME-NS5A. A series of N-terminal deletion mutants of NS3 was generated by PCR-mediated site mutagenesis using pME-NS3 as a template. The fragment of serine protease-negative NS3/4AS2051A was generated by PCR-mediated mutagenesis using pME-NS3/4A as a template with the mutagenic primer 5′-AATATAGGCAGGCCCGCCCATCCCTTCAAGTT-3′ to substitute Ser2051 to Ala. Hybrid cytomegalovirus enhancer/chicken β-actin (CAG) promoter-driven pME18S vectors, termed pCAG, encoding BVDV NS proteins were constructed by a replacement of the SRα promoter with the CAG promoter fragment from the pCAGGS vector using SspI and XhoI sites of pME18S vectors. pEF-HCV NS3/4A, which contains the HCV NS3/4A cDNA of the HCV HCR6 strain (genotype 1b; GenBank data base accession number AY045702) cloned into pEF-1 vector (Invitrogen), was kindly provided by Dr. Michinori Kohara. All constructs were confirmed by sequencing with an ABI PRISM 3150 genetic analyzer (Applied Biosystems, Tokyo, Japan).
Yeast Two-hybrid Screening—Potential interacting partners of NS3 were sought using the yeast two-hybrid system according to the manufacturer's manual for the MATCHMAKER Library Construction and Screening Kit (Clontech, Palo Alto, CA). The N-terminal domain of NS3 (amino acids 1889 to 2032) derived from the BVDV Nose strain was amplified by RT-PCR using primers that incorporated NdeI and EcoRI sites at the 5′ and 3′ ends, respectively, and cloned into NdeI and EcoRI sites of pGBKT7 in-frame with the Gal4 DNA-binding domain to express N-terminal Myc-tagged partial NS3, designated pGBKT7-NS3. For the construction of the MDBK cDNA library, first strand cDNA was synthesized using random primers from 0.6 μg of mRNA, which was purified from total RNA using the oligotex-dT30<Super> mRNA Purification Kit (TaKaRa, Shiga, Japan), and double-stranded cDNA amplified by 22 cycles long distance (LD) PCR as described in manufacturer's protocol. Saccharomyces cerevisiae strain AH109 was transformed with the bait plasmid pGBKT7-NS3, and selected in synthetic medium lacking tryptophan. A positive clone harboring pGBKT7-NS3 was confirmed to express N-terminal 142 amino acids of the NS3 protein with anti-Myc mAb by Western blot analysis (data not shown). The MDBK cell double-stranded cDNA library together with SmaI-linearized pGADT7-Rec (Clontech) was cotransformed in an AH109 clone harboring pGBKT7-NS3 to clone cDNA into the GAL4 AD expression vector pGADT7-Rec by homologous recombination. The transformed yeast cells were grown on agar plates of synthetic medium lacking histidine, leucine, and tryptophan containing 20 μg/ml 5-bromo-4-chloro-3-indolyl-α-O-galactopyranoside (X-α-gal). A total of 145 clones were identified from 1 × 106 colonies screened in the library. The insert DNA fragments of isolated clones were amplified by PCR using LD-Insert Screening Amplimer Sets (Clontech) according to the manufacturer's protocol, and then determined by sequencing.
Transfection and Immunoprecipitation—LB9.K cells were transiently transfected using Lipofectamine 2000 (Invitrogen) as described in the manufacturer's protocol. Transfection efficiency of LB9.K cells was typically 80–90%. LB9.K cells were seeded onto 6-well tissue culture plates 24 h before transfection. Cells were then transfected with 4 μg of plasmids per well. At 24 h post-transfection, cells were washed twice with ice-cold phosphate-buffered saline (PBS) and scraped into 0.2 ml of lysis buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1% Triton X-100, 20 mm sodium fluoride, 1 mm Na3VO4) supplemented with Complete protease inhibitor mixture (Roche Diagnostics). The lysates equalized with the same amount of proteins were immunoprecipitated with 3 μg of anti-FLAG, anti-Myc, anti-BVDV NS3, or control mouse IgG1 mAbs for 2 h at 4 °C, respectively. The immune complexes were precipitated by incubation with protein G-Sepharose beads (GE Healthcare) for another 1 h. The agarose beads were washed four times with 1 ml of wash buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, 0.1% Triton X-100, 20 mm sodium fluoride, 1 mm Na3VO4). The immunoprecipitates were separated by SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad), probed with antibodies, and immunocomplexes detected by enhanced chemiluminescence (ECL). Antibodies used were: horseradish peroxidase-conjugated mAbs against FLAG (1:1000 dilution; Sigma) and Myc (1:1000; Santa Cruz Biotechnology), and a rabbit pAb against NS3 (1:3000). Images were taken by LAS-4000mini image analyzer system (Fujifilm, Tokyo, Japan).
Subcellular Fractionation—Cells were harvested into lysis buffer lacking Triton X-100, sonicated, and centrifuged at 1,000 × g for 10 min. Subcellular fractionation was performed by sequential centrifugation as described previously (19). In brief, postnuclear supernatants were centrifuged at 17,000 × g for 15 min to obtain the inner membrane fraction. The resulting supernatants were centrifuged at 100,000 × g for 1 h to obtain cytosolic and pelleted plasma membrane fractions. The pellet containing inner or plasma membrane was resuspended in lysis buffer (volume comparable with supernatant) and sonicated.
Sphingosine Kinase Assay—Sphingosine kinase activity was determined as described previously (20). The labeled S1P was separated by TLC on Silica Gel G-60 (Whatman) with 1-butanol/ethanol/acetic acid/water (80:20:10:20, v/v) and visualized by autoradiography. The radioactive spots corresponding to S1P were scraped and counted in a scintillation counter.
Generation of Recombinant Bovine Sphingosine Kinase 1— pFlag-SphK1 was transfected into HEK293 cells using Lipofectamine 2000 (Invitrogen) to express the Flag-SphK1, which was subsequently purified by binding to FLAG(M2)-Sepharose (Sigma), followed by elution with the FLAG peptide (0.2 mg/ml). The eluted Flag-SphK1 was concentrated using an Ultrafree-0.5 Centrifugal Filter Device (50,000 Da cutoff; Millipore, Billerica, MA) and diluted in the sphingosine kinase buffer (20 mm Tris-HCl, pH 7.4, 20% glycerol, 1 mm β-mercaptoethanol, 1 mm EDTA, 15 mm sodium fluoride, 20 mm Na3VO4, and 0.5 mm 4-deoxypyridoxine) supplemented with a Complete protease inhibitor mixture (Roche Diagnostics). This procedure was repeated five times to reduce the concentration of the FLAG peptide.
Generation of Glutathione S-Transferase (GST) Fusion Proteins—NS3, NS3/4A, and NS5A sequences of BVDV Nose strain were amplified by PCR using plasmid pME-NS3/4A or pME-NS5A as a template. The PCR product of NS3, NS3/4A, or NS5A was cloned into bacterial expression vector pGEX5X-2 using SmaI and NotI sites (NS3 and NS3/4A) or EcoRI and NotI sites (NS5A). HCV NS3 and NS3/4A sequences were amplified by PCR using plasmid pEF-HCV NS3/4A as a template. The PCR products were then cloned into pGEX5X-1 using EcoRI and XhoI sites. BVDV and HCV NS proteins were expressed in Escherichia coli BL21 as GST fusion proteins at the N terminus. Overnight cultures were grown with shaking at 37 °C in Luria-Bertani broth containing 50 μg/ml ampicillin and 20 μg/ml chloramphenicol. The culture was then diluted into fresh Luria-Bertani broth containing 50 μg/ml ampicillin and 20 μg/ml chloramphenicol, and grown with shaking at 37 °C to an A600 of 0.6–1.0. Expression of the GST fusion proteins was then induced with 1.2 mm isopropyl β-d-thiogalactopyranoside, and the cultures were incubated with shaking at 37 °C for a further 3 h. The bacterial cells were then harvested by centrifugation at 6,000 × g for 10 min at 4 °C, resuspended in 10 ml of GST-soluble buffer (40 mm Tris-HCl, pH 7.5, 5 mm EDTA, 0.5% Triton X-100), and lysed by sonication. The lysates were mixed well and centrifuged at 20,000 × g for 20 min at 4 °C. The resultant clarified bacterial lysate was then incubated with GSH-Sepharose 4B for 2 h at 4 °C with constant mixing. Subsequently, the GSH-Sepharose beads (with bound protein) were pelleted by centrifugation at 3,000 × g for 5 min at 4 °C and washed five times in GST-soluble buffer. These beads were then either used directly in a pull-down assay, or the GST fusion proteins were eluted by incubation with cold PBS containing 10 mm GSH for 30 min with constant mixing. This elution procedure was repeated three times. Eluted proteins were concentrated by using Ultrafree-0.5 Centrifugal Filter Device (10,000 Da cutoff; Millipore).
Immunofluorescence Microscopy—LB9.K cells were seeded on an eight-well chamber slide (Nunc, Roskilde, Denmark) at 2 × 104 per well 24 h before transfection. Nontransfected cells or the cells transfected with Flag-SphK1 were inoculated with BVDV as described in the figure legends. At 18 h p.i., cells were washed twice with PBS, fixed with PBS containing 4% paraformaldehyde, permeabilized with PBS containing 0.5% Triton X-100, and blocked with PBS containing 10% bovine serum albumin for 10 min. Nontransfected cells were then incubated with anti-calnexin pAb and mouse anti-BVDV NS3 mAb for 1 h followed by incubation with Alexa 568-conjugated goat anti-rabbit IgG and Alexa 488-conjugated goat anti-mouse IgG antibodies for 1 h at room temperature. Transfected cells were double-stained with mouse anti-FLAG mAb (IgG1) and mouse anti-BVDV NS3 mAb (IgG2a) followed by Alexa 488-conjugated goat anti-mouse IgG1 and Alexa 594-conjugated goat anti-mouse IgG2a antibodies, or with mouse anti-FLAG mAb and anti-calnexin pAb followed by Alexa 488-conjugated goat anti-mouse IgG and Alexa 568-conjugated goat anti-rabbit IgG antibodies. Cells incubated with secondary antibodies were then washed three times with PBS, mounted in Dako fluorescent mounting medium (Dako Corporation, Carpinteria, CA), then sealed and observed under an LSM 510 microscope (Carl Zeiss, Tokyo, Japan).
Measurement of S1P Synthesis—LB9.K cells transiently transfected with pCAG vectors encoding BVDV NS proteins or MDBK cells infected with BVDV were incubated for 4 h in phosphate-free DMEM (Invitrogen), then labeled with fresh phosphate-free DMEM containing [32P]orthophosphate (0.2 mCi/ml) and incubated for 4 h at 37 °C in a humidified 5% CO2 atmosphere. Cells were then scraped on ice into 400 μl of methanol, 1 m NaCl, 5 m NaOH (100:100:3, v/v), then 200 μl of chloroform added. Samples were vortexed thoroughly and centrifuged at 14,000 × g for 5 min. The upper aqueous phase containing S1P was transferred to a new tube, and acidified through addition of 20 μl of 1 m HCl and 400 μl of chloroform/methanol/HCl (100/200/1, v/v). Samples were vortexed thoroughly and phases separated by addition of 120 μl of chloroform and 120 μl of 2 m KCl. After centrifugation, the lower organic phase was dried under vacuum and resuspended in chloroform, and resolved by TLC as described above. The radioactive spots corresponding to S1P were scraped from the plates and counted in a scintillation counter.
RNA Interference—Duplex siRNAs were purchased from Invitrogen. The siRNA sequence targeting SphK1 was 5′-GCAGUGGCCGCUUCUUUGAACUAUU-3′ (sense) and 5′-AAUAGUUCAAAGAAGCGGCCACUGC-3′ (antisense), corresponding to 634–658 relative to the first nucleotide of the start codon. The sequence used for scrambled control siRNA was 5′-GCAGGCCCGUUUCUUAGCAUUGAUU-3′ (sense) and 5′-AAUCAAUGCUAAGAAACGGGCCUGC-3′ (antisense). LB9.K cells were transfected with 20 nm siRNA using siLentfect (Bio-Rad) according to the manufacturer's protocol.
Quantitative Real-time RT-PCR—cDNA synthesis was performed with the PrimeScript RT Reagent Kit (TaKaRa) according to the manufacturer's protocol. GAPDH mRNA and viral RNA were quantified using Power SYBR Green PCR Master Mix (Applied Biosystems) as previously described (21).
Apoptosis Assay—The DEVDase activity assay was performed as described previously (22) using Ac-DEVD-AMC as a substrate.
RESULTS
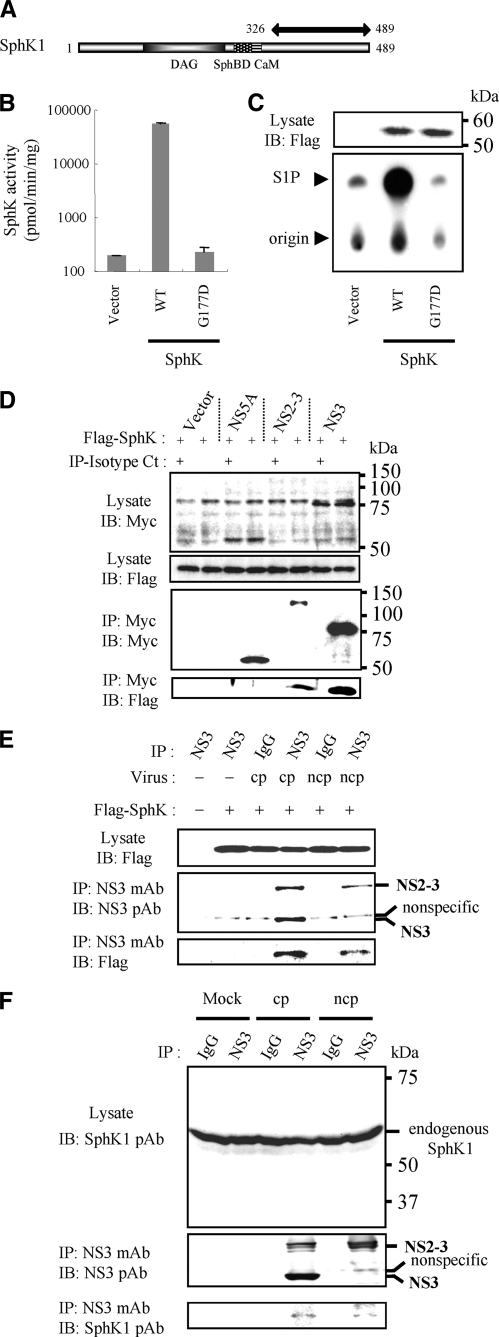
Identification of SphK1 as a Binding Partner of BVDV NS3 and NS2-3— To identify potential cellular binding partners of BVDV NS3, we conducted a yeast two-hybrid screen using the N-terminal 142 amino acids of NS3 as bait, and isolated a cDNA clone encoding partial SphK1 from 1 of 145 total positive colonies. The cDNA sequence encoded 164 C-terminal amino acids of SphK1 (Fig. 1A). Employing the cDNA sequence for bovine SphK1 from the GenBank data base, specific primers were designed and used to clone a bovine SphK1 by RT-PCR cloning using total RNA isolated from MDBK cells. Subsequently, a catalytically inactive form of bovine SphK1 was constructed by substituting aspartic acid (D) for glycine (G) at position 177 in the ATP-binding site of the diacylglycerol catalytic domain, according to the previous study (23). This SphK1G177D was used for further functional studies. To investigate whether the cloned bovine SphK1 encodes a bona fide SphK, LB9.K cells were transiently transfected with expression vectors containing FLAG-tagged SphK1. Similar to the previous study (24), SphK activity in cell lysates from LB9.K cells transiently transfected with SphK1 was increased ∼300-fold (Fig. 1, B and C). By comparison, expression of catalytically inactive SphK1G177D produced no detectable increase in SphK activity. Western blot analysis using anti-FLAG antibody revealed a specific protein band with an apparent molecular mass consistent with the predicted size (∼55 kDa) of FLAG-tagged SphK1, which was absent in vector-transfected cells (Fig. 1C).
FIGURE 1.
Specific interaction between SphK1 and BVDV NS3 or NS2-3. A, schematic representation of bovine SphK1. Functional domains (28, 37), diacylglycerol (DAG) catalytic domain, sphingosine-binding domain (SphBD), and calmodulin-binding domain (CaM) are shown. Partial SphK1 cDNA sequence isolated from a positive clone is indicated by a two-headed arrow. B, LB9.K cells were transiently transfected with empty vector or SphK1 expression vector (WT or G177D). At 24 h post-transfection, cells were harvested and cytosolic SphK activity measured as described under “Experimental Procedures.” Data in the graph are the mean ± S.D. from three independent experiments, each performed in duplicate. C, shown on the lower panel is an autoradiogram of a TLC plate used for separation of S1P. The arrowhead indicates the location of S1P visualized with molybdenum blue spray. The upper panel shows the expression of Flag-SphK1 by Western blotting with anti-FLAG mAb. D, Flag-SphK1 expression vector in combination with either an empty vector, Myc-tagged BVDV NS5A, NS2-3, or NS3 were cotransfected into LB9.K cells. Proteins immunoprecipitated (IP) with anti-Myc mAb (even lanes) or isotype control IgG (Isotype Ct, odd lanes) were subjected to Western blotting (IB) using anti-FLAG mAb. E, 1 h after inoculation with either KS86-1cp or KS86-1ncp at an m.o.i. of 5, Flag-SphK1 expression vector was transfected into LB9.K cells. At 24 h p.i., BVDV NS3 and/or NS2-3 protein was immunoprecipitated with isotype control IgG or anti-NS3 mAb as indicated. Immunoprecipitated proteins were subjected to Western blotting using anti-FLAG mAb. F, 24 h after infection with either KS86-1cp or KS86-1ncp at an m.o.i. of 5 in MDBK cells, NS3 and/or NS2-3 protein was immunoprecipitated with isotype control IgG or anti-NS3 mAb as indicated. Immunoprecipitated proteins were subjected to Western blotting using anti-SphK1 pAb. These data were representative of at least three independent experiments.
To confirm the interaction between NS3 and SphK1, we cotransfected Flag-SphK1 and Myc-NS3 in LB9.K cells, immunoprecipitated NS3 using an anti-Myc mAb, and determined whether SphK1 was coprecipitated with NS3 by Western blotting. We also cotransfected an empty vector or NS5A as a negative control and uncleaved NS2-3. Both NS3 and NS2-3, but neither NS5A nor vector control, coprecipitated with SphK1 (Fig. 1D). We attempted to express Myc-tagged NS2 in the same way as above to demonstrate that NS2 does not mediate the binding of NS2-3 to SphK1, but failed to express detectable levels of NS2 protein, most likely due to its instability in LB9.K cells (data not shown).
To confirm the specific interaction of SphK1 with NS3 and/or NS2-3 expressed in the context of BVDV infection, we transfected an expression vector encoding Flag-SphK following virus infections, immunoprecipitated NS3 and NS2-3 with anti-NS3 mAb, and determined binding to SphK1 by Western blotting. When expressed in the context of BVDV infection, Flag-SphK1 coprecipitated with NS3 and NS2-3 expressed in CP BVDV-infected cells as well as NS2-3 in NCP BVDV-infected cells (Fig. 1E). To further demonstrate whether NS3 and NS2-3 from BVDV infection interacts with endogenous SphK1, we immunoprecipitated NS3 and NS2-3 with anti-NS3 mAb from the lysates of the BVDV-infected MDBK cells, and determined binding to SphK1 by Western blotting using anti-SphK1 pAb. As shown in Fig. 1F, specific interaction of endogenous SphK1 with NS3 and NS2-3 was detected.
NS3 and NS2-3 Colocalize with SphK1 in Membrane Fractions—Previous studies indicated that BVDV NS3 and NS2-3 are predominantly localized to the endoplasmic reticulum in cultured cells (25) and SphK1 is diffusely distributed in the cytoplasm (19). To investigate the localization of these proteins in BVDV-infected LB9.K cells, we transfected the cells with an expression vector encoding Flag-SphK1 and performed immunostaining using anti-FLAG and anti-NS3 mAbs. For positive identification of the endoplasmic reticulum, we stained cells with a calnexin pAb. The staining patterns of NS3 and NS2-3 (CP BVDV) or NS2-3 (NCP BVDV) overlapped with those of calnexin (Fig. 2B) and partially with those of SphK1 (Fig. 2A). SphK1 was also partially colocalized with calnexin (Fig. 2C). We further examined the localization of endogenous SphK1 and NS3 by subcellular fractionation. The majority of SphK1 and NS3 were found to localize in inner and plasma membrane fractions in MDBK cells (Fig. 2D). Although previous studies have shown that SphK1 in many different cell types is mainly cytosolic (26, 27), these data suggest that bovine SphK1 localizes to both cytosolic and membrane fractions.
FIGURE 2.
Colocalization of SphK1 with NS3 or NS2-3 in membrane fractions of mammalian cells. LB9.K cells were transiently transfected with Flag-SphK1 expression vector. After 6 h, cells were inoculated with CP BVDV or NCP BVDV and further incubated for 18 h. Transfected cells were immunostained with (A) anti-FLAG mAb (green) and anti-BVDV NS3 mAb (red), (B) anti-BVDV NS3 mAb (green) and anti-calnexin pAb (red), and (C) anti-FLAG mAb (green) and anti-calnexin pAb (red). D, MDBK cells were mock-infected or infected with either KS86-1cp or KS86-1ncp at an m.o.i. of 5. At 20 h p.i., cells were lysed and subcellularly fractionated into cytosol (S), inner membrane (IM) (mitochondria, endoplasmic reticulum, and Golgi), and plasma membrane (PM), as described under “Experimental Procedures.” Equal volumes of lysates were subjected to Western blotting with anti-SphK1 pAb and antibodies against GAPDH, Calnexin, or junctional adhesion molecule (JAM-1) as specific organelle markers.
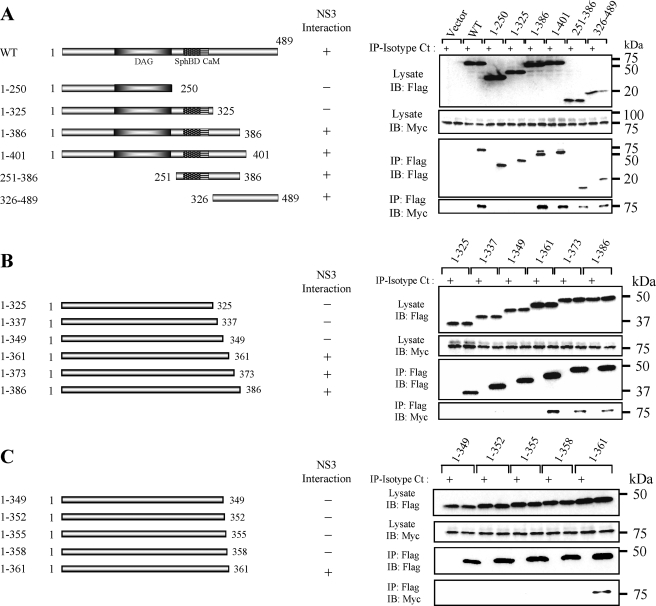
Mapping the NS3-binding Domain on SphK1—Deletion mutagenesis of SphK1 was performed to identify the NS3-binding domain on SphK1 (Fig. 3A). FLAG-tagged SphK1 mutants were coexpressed with Myc-NS3 and immunoprecipitated with anti-FLAG mAb. Myc-NS3 coprecipitated with SphK1 mutants containing a region downstream of calmodulin, which consisted of amino acid residues 326–386. This region was also present in the partial SphK1 cDNA sequence isolated from the positive yeast colony in our yeast two-hybrid screen (Fig. 1A). FLAG-tagged SphK1 C-terminal deletion mutants of this region were constructed to further determine the NS3-binding domain. The SphK1 mutant consisting of amino acid residues 1–361 coprecipitated with Myc-NS3 but the mutant with residues 1–349 did not (Fig. 3B). Furthermore, the deletion mutant containing amino acids 1–361, but not 1–358, coprecipitated with NS3 (Fig. 3C), suggesting that amino acids 359–361 of SphK1, or the protein folding affected by these residues, are critical for its binding to NS3.
FIGURE 3.
Identification of the NS3-binding site in SphK1. A, schematic representation of SphK1 and its deletion mutants. Myc-NS3 expression vector in combination with expression vectors for Flag-SphK1 and its mutants were cotransfected into LB9.K cells. Proteins immunoprecipitated (IP) with anti-FLAG mAb (even lanes) or isotype control IgG (Isotype Ct; odd lanes) were subjected to Western blotting (IB) using anti-Myc mAb. B, to further determine the critical amino acids of SphK1 for specific binding to NS3, deletion mutants ranging from the N-terminal 325–386 amino acids were immunoprecipitated with Flag-SphK1. C, according to the result obtained in B, deletion mutants ranging from the N-terminal 349–361 amino acids were immunoprecipitated with Flag-SphK1. The data are representative of at least three independent experiments.
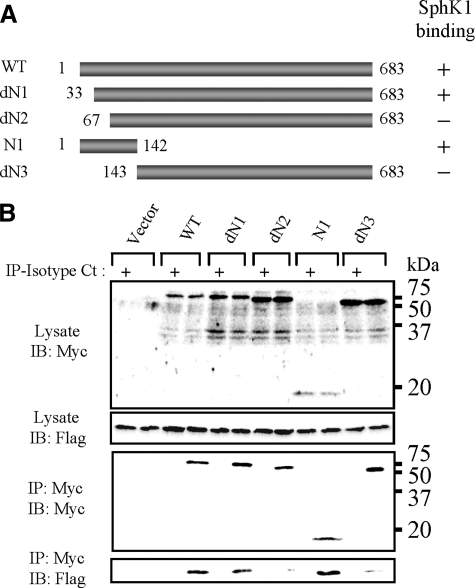
Mapping the SphK1-binding Domain on NS3—Because the N-terminal 142 amino acid residues of NS3 were sufficient to bind to SphK1 in our yeast two-hybrid screen, we constructed N-terminal deletion mutants of NS3 to determine the region of NS3 that bind to SphK1 (Fig. 4A). Myc-tagged NS3 mutants were coexpressed with Flag-SphK1 and immunoprecipitated using the anti-Myc mAb. Flag-SphK1 coimmunoprecipitated with the dN1 mutant lacking amino acids 1–32, but not with the dN2 mutant lacking amino acids 1–66 (Fig. 4, A and B). As expected from the yeast two-hybrid screen, the N1 mutant containing NS3 amino acids 1–142 coimmunoprecipitated with SphK1, suggesting that amino acids 33–66 of NS3 are critical for its binding to SphK1.
FIGURE 4.
Mapping of the SphK1-binding site on BVDV NS3. A, schematic representation of NS3 and its deletion mutants. B, SphK1 expression vector in combination with expression vectors of Myc-NS3 and its deleted mutants were cotransfected into LB9.K cells. Proteins immunoprecipitated (IP) with anti-Myc mAb (even lanes) or isotype control IgG (Isotype Ct; odd lanes) were subjected to Western blotting (IB) using anti-FLAG mAb. Data shown in each panel are representative of at least three independent experiments.
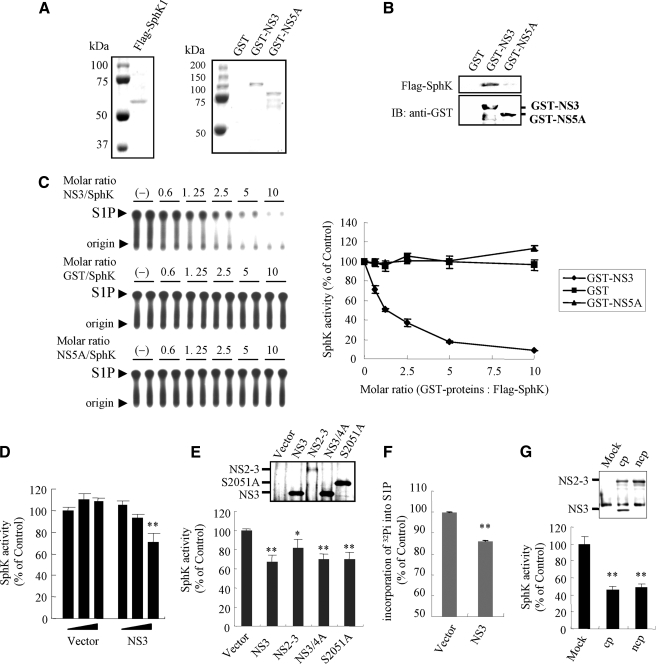
The Dose-dependent Inhibition of SphK1 Activity by NS3— We performed in vitro SphK assays in the presence and absence of NS3 to assess the functional significance of NS3 to SphK1. GST fusion proteins GST-NS3 and GST-NS5A, and GST alone were expressed and purified from E. coli strain BL21 (Fig. 5A). We failed to express GST-NS2-3, most likely due to toxicity of its highly hydrophobic NS2 domain (7). Recombinant bovine SphK1 (rSphK1) was purified from lysates of HEK293 cells transiently transfected with the FLAG-tagged SphK1 expression vector (Fig. 5A). Glutathione (GSH)-Sepharose beads were used to bind to and precipitate GST-NS3, GST-NS5A, or GST alone in the presence of rSphK1. The GST pull-down experiments confirmed a specific interaction between rSphK1 and GST-NS3 (Fig. 5B). Although GST-NS5A and GST alone showed no specific binding to rSphK1 (Fig. 5B) and had no significant effect on SphK1 activity, GST-NS3 inhibited SphK1 catalytic activity dose-dependently with maximal inhibition (∼90%) attained using above a 5-fold molar excess of NS3 to SphK1 (Fig. 5C).
FIGURE 5.
NS3 inhibits SphK1 activity in vitro. A, Coomassie Brilliant Blue-stained SDS-PAGE of the purified proteins as indicated above the lanes (Flag-SphK1 and GST-NS proteins). B, GST, GST-NS3, and GST-NS5A bound to GSH-Sepharose was incubated with 1 μm purified recombinant Flag-SphK1 for 2 h. Beads were washed four times with wash buffer, then SphK1 associated with GST-NS3 was resolved by SDS-PAGE and detected by Western blotting with anti-FLAG mAb (upper panel). GST-NS3 and GST-NS5A bound to GSH beads were visualized by blotting with anti-GST mAb (lower panel). C, the effect of GST-NS3, GST-NS5A, or GST control on the catalytic activity of purified recombinant bovine SphK1 (10 nm) was determined using the in vitro SphK assay over a range of increasing concentrations of GST-NS proteins or GST. Shown on the left panels are autoradiograms of TLC plates used for separation of S1P. The arrowhead indicates the location of S1P. Data in a graph on the right panel represent the means ± S.D. from three independent experiments. D, increasing doses (0.25, 0.5, and 1 μg) of pCAG empty vector or pCAG vector encoding Myc-tagged NS3 were transfected into LB9.K cells. At 24 h post-transfection, cell lysates were subjected to the in vitro SphK assay as described under “Experimental Procedures.” E, pCAG vector encoding Myc-tagged NS3, NS2-3, NS3/4A, or the S2051A NS3/4A mutant, or empty vector was transfected into LB9.K cells. At 24 h post-transfection, cell lysates were harvested and subjected to the in vitro SphK assay. Inset shows expression levels of NS proteins by Western blotting with anti-Myc mAb. F, LB9.K cells transfected with empty vector or pCAG-NS3 were labeled with [32P]orthophosphate at 24 h post-transfection for 4 h, then harvested and analyzed for endogenous S1P synthesis as described under “Experimental Procedures.” G, MDBK cells were mock-infected or infected with CP BVDV or NCP BVDV at an m.o.i. of 5. After 24 h p.i., cells were harvested into lysis buffer and immunoprecipitated by anti-NS3 mAb for 2 h followed by incubation with Protein G-Sepharose beads for 1 h. Beads were then extensively washed, mixed with 10 nm purified recombinant bovine SphK1, and subjected to the in vitro SphK assay. Inset shows expression levels of NS3 and NS2-3 proteins bound to beads by Western blotting with anti-NS3 mAb. Data (mean ± S.D.) shown in each panel are representative of at least three independent experiments. p values were determined for the experimental sample versus the control-treated sample using Student's t test. **, p < 0.01; *, p < 0.05.
Suppression of SphK1 Activity by NS3, NS2-3, and NS3/4A— Uncleaved NS2-3 and NS3 are expressed in CP BVDV-infected cells, whereas only NS2-3 is detectable in NCP BVDV-infected cells after 9 h p.i. (7). Therefore, we assessed possible differences in the regulation of SphK1 activity among NS3, NS2-3, and NS3/4A. Plasmids encoding BVDV NS3, NS2-3, and NS3/4A proteins were transiently transfected into LB9.K cells, and the endogenous SphK activity in the cell lysate was measured using the in vitro SphK assay. Transfection with various doses of plasmid encoding NS3 exhibited a dose-dependent reduction in SphK activity by up to 30% (Fig. 5D). Cells transfected with plasmids encoding NS2-3 and NS3/4A showed endogenous SphK activity that was reduced by ∼15 and 30%, respectively (Fig. 5E). It seemed that the expression of the NS4A cofactor does not affect the inhibitory effect of NS3 on SphK1 activity. To further determine whether inhibition of SphK1 activity by NS3 depends on its serine protease activity or not, we examined the effect of the S2051A NS3/4A mutant, whose serine protease activity is eliminated and the protease-dependent cleavage between NS3 and NS4A is deficient (3), on SphK1 inhibition. As shown in Fig. 5E, S2051A mutant efficiently inhibited endogenous SphK1 activity to the same extent as wild-type NS3/4A. In addition, SphK1 exhibited no putative NS3/4A recognition sites (3) and no detectable degradation when coexpressed with Myc-NS3/4A (data not shown), suggesting that SphK1 inhibition by NS3 is independent of its serine protease activity. In the cells transfected with the NS3 expression plasmid, the endogenous S1P synthesis was reduced by ∼15% (Fig. 5F). The weaker inhibition of SphK1 by NS2-3 appeared to be the result of lower expression levels of NS2-3 relative to NS3 and NS3/4A (Fig. 5E, inset). However, the higher level of NS2-3 obtained from lysates of NCP BVDV-infected MDBK cells by immunoprecipitation was shown to significantly inhibit SphK activity by ∼50%, similar to the inhibition observed with the NS2-3 and NS3 from CP BVDV-infected MDBK cell lysates (Fig. 5G). These results suggest that SphK1 activity is down-regulated by NS2-3 as well as NS3.
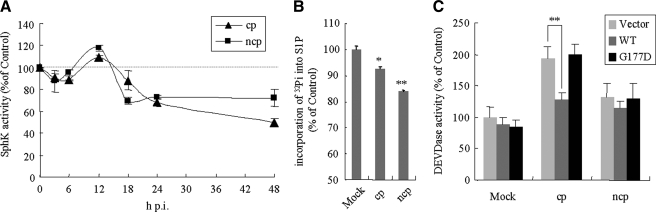
Down-regulation of SphK1 Activity and S1P Synthesis in BVDV-infected Cells—The kinetics of endogenous SphK1 activity was examined in the context of BVDV infection. At 6 and 12 h p.i., SphK activity in MDBK cells infected with both BVDV biotypes was unaffected or slightly up-regulated. However, at later than 18 h p.i., a time-dependent decrease in SphK activity was observed, which culminated to maximal reductions of 30 and 50% at 48 h p.i. for cells infected with NCP BVDV and CP BVDV, respectively (Fig. 6A). No change in SphK1 mRNA levels was observed in MDBK cells at 24 h p.i. with either BVDV biotype (data not shown). Therefore, it appears unlikely that SphK1 activity is down-regulated at the transcriptional level; it probably occurs concomitantly with the accumulation of newly synthesized NS3 and NS2-3 proteins in the cells. Cellular S1P synthesis levels were reduced by 10 and 20% in CP BVDV- and NCP BVDV-infected cells, respectively, as SphK activity decreased (Fig. 6B).
FIGURE 6.
Kinetics of sphingosine kinase activity and the effect of SphK1 overexpression in BVDV-infected cells. A, MDBK cells infected with either KS86-1cp or KS86-1ncp at an m.o.i. of 5 were harvested at the indicated time points p.i., and SphK activity was determined by the in vitro SphK assay as described under “Experimental Procedures.” B, MDBK cells infected with CP BVDV or NCP BVDV were labeled with [32P]orthophosphate at 18 h p.i. for 4 h, harvested, and analyzed for endogenous S1P synthesis as described under “Experimental Procedures.” Data are expressed as percent relative to the mock-infected samples. C, LB9.K cells were transiently transfected with empty vector, or SphK1 (WT) or SphK1G177D (G177D) expression vector. After 24 h, cells were infected as described above in B. At 24 h p.i., cells were harvested and the cell lysates assessed for DEVDase activity. Data are expressed as percent relative to the mock-infected samples transfected with empty vector. The data represent the mean ± S.D. from at least three independent experiments. **, p < 0.01; *, p < 0.05.
Overexpression of SphK1 Reduced Apoptosis Induced by CP BVDV Infection—Our previous studies showed that apoptotic cell death induced by CP BVDV infection is mediated through activation of DEVDase activity (9, 22). Because several previous studies have demonstrated anti-apoptotic effects of SphK1 (24, 28, 29), we tested whether inhibition of SphK1 by BVDV had any influence in the context of apoptotic induction by overexpression of SphK1 and catalytically inactive SphK1G177D. Overexpression of SphK, but not SphK1G177D, resulted in an ∼40% reduction in DEVDase activity in CP BVDV-infected cells, whereas almost no reduction was observed in mock-infected or NCP BVDV-infected cells (Fig. 6C). Because overexpression of SphK1 had no significant effect on viral replication (Fig. 7, E and F) but counteracted the activation of DEVDase, these data suggest that apoptotic cell death in CP BVDV-infected cells may be exaggerated by the inhibition of SphK.
FIGURE 7.
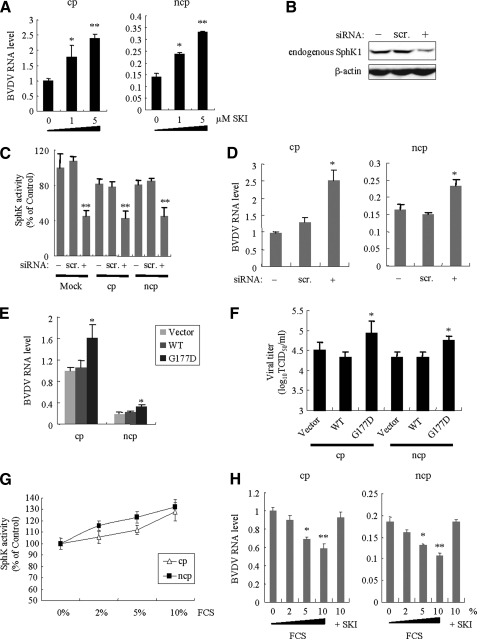
Effects of SphK1 activity on BVDV replication. A, the effect of SphK1 inhibitor (SKI) on BVDV RNA replication. After infection with either KS86-1cp or KS86-1ncp at an m.o.i. of 5 for 1 h, MDBK cells were treated with 0, 1, and 5 μm SKI. After 48 h p.i., cells were harvested and the BVDV RNA level determined by real-time PCR analysis. B–D, SphK1 knockdown with siRNA enhanced BVDV replication. LB9.K cells were mock-transfected (-) or transfected with siRNA targeted to SphK1 (+) or scrambled control siRNA (scr.) at a final concentration of 20 nm. At 24 h post-transfection, cells were mock-infected or infected with BVDV as described above. Cells were further incubated for 24 h, then harvested and assessed for (B) the endogenous SphK1 expression by Western blotting using anti-SphK1 pAb, (C) the endogenous SphK1 activity, and (D) the BVDV RNA level by real-time PCR analysis. E, LB9.K cells were transiently transfected with empty vector or SphK1 expression vector (wild-type (WT) or G177D). After 24 h, cells were infected as described above. At 24 h p.i., cells were harvested and BVDV RNA level determined by real-time PCR analysis. At the same time, the viral titer (F) in the culture supernatant was assessed. G and H, the effect of SphK1 activation by FCS on BVDV RNA replication. MDBK cells were infected as described above, and incubated with DMEM containing 0, 2, 5, or 10% FCS, or 10% FCS with 5 μm SKI. After 24 h p.i., cells were harvested and assessed for (G) the endogenous SphK1 activity and (H) the BVDV RNA level determined by real-time PCR analysis. The data are expressed relative to the control treated data, which were set to 1.0, and represent the mean ± S.D. from at least three independent experiments, each performed in duplicate. **, p < 0.01; *, p < 0.05.
Inhibition of SphK1 Activity Significantly Enhanced Viral Replication—To examine the influence of SphK activity on BVDV replication, we measured intracellular viral RNA levels in the presence of SKI, which was identified as a highly selective, noncompetitive inhibitor of SphK (16). SKI treatment increased viral RNA levels of both CP BVDV and NCP BVDV infections ∼2-fold in a dose-dependent manner (Fig. 7A). To further analyze the effect of SphK1 inhibition on BVDV replication more specifically, LB9.K cells were either depleted of SphK1 by siRNA-mediated knockdown or by overexpression of the catalytically inactive SphK1G177D, and then infected with BVDV. Transfection with siRNA significantly inhibited endogenous SphK1 expression levels (Fig. 7B) as well as endogenous SphK1 activity in LB9.K cells by ∼50% compared with levels in scrambled siRNA-transfected cells (Fig. 7C). Viral RNA levels from both CP BVDV and NCP BVDV infections increased ∼2-fold compared with mock-treated or scrambled siRNA-transfected cells (Fig. 7D). Transfection with SphK1G177D also increased viral RNA levels and titers concomitantly by over 160% (Fig. 7, E and F). At 48 h p.i., the viral RNA level from CP BVDV infections was 2.5-fold in cells overexpressing SphK1G177D (data not shown). In contrast, transfection with wild-type SphK1, reaching a 300-fold increase in SphK activity, did not affect viral RNA levels and titers (Fig. 7, E and F). We next examined the effect of FCS addition on BVDV replication as FCS is known to mediate SphK activation (30). FCS addition resulted in a dose-dependent increase in endogenous SphK1 activity (Fig. 7G), and the viral RNA level was reduced by up to 40% in the presence of 10% FCS and the effect was reversible with the addition of SKI (Fig. 7H). We also examined the effect of S1P, a metabolite of SphK, on viral RNA replication. However, addition of 1–10 μm S1P did not affect the viral RNA levels (data not shown). These data collectively suggest that SphK inhibition may allow for enhanced BVDV replication, whereas it appears that S1P does not regulate BVDV replication.
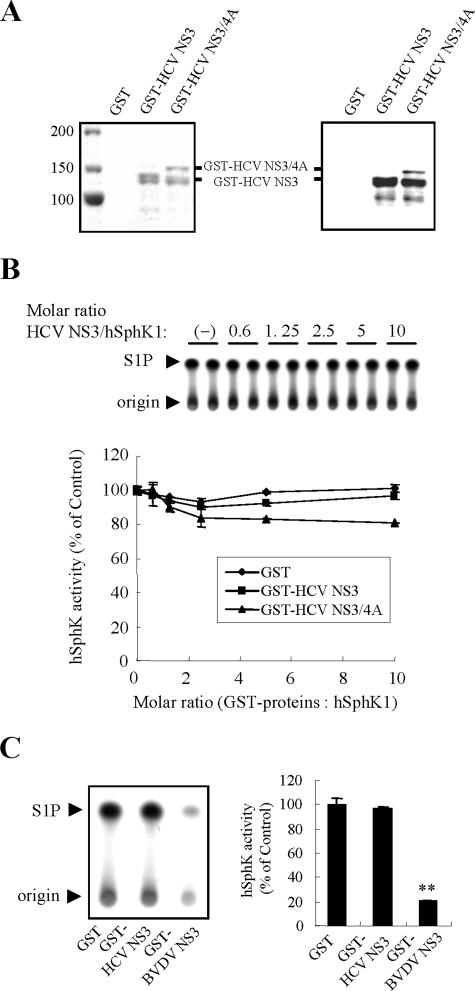
HCV NS3 Does Not Regulate SphK1 Activity—Because BVDV NS3 was shown to inhibit bovine SphK1 activity, it was of interest to determine whether NS3 from HCV, a close relative of BVDV, regulates the catalytic activity of hSphK1. We thus examined the effect of purified recombinant HCV NS3 and NS3/4A expressed in E. coli (Fig. 8A) on the catalytic hSphK1 activity in vitro. Neither GST-HCV NS3 nor NS3/4A had a significant effect on hSphK1 activity, even at 10-fold molar excess to hSphK1 (Fig. 8B). Furthermore, transient transfection into HEK293 cells with pEF-HCV NS3 or NS3/4A did not affect the endogenous SphK1 activity, compared with empty vector-transfected cells (data not shown). Taken together, hSphK1 activity appears to be regulated neither by HCV NS3 nor by HCV NS3/4A. In contrast, BVDV NS3 suppressed hSphK1 activity efficiently by ∼80% at 5-fold molar excess to hSphK1 (Fig. 8C). Accordingly, disruption of SphK1 activity appears to be specific to BVDV NS3.
FIGURE 8.
NS3 from BVDV, but not from HCV, inhibits catalytic activity of hSphK1 in vitro. A, Coomassie Brilliant Blue-stained SDS-PAGE of the purified proteins as indicated above the lanes (left panel), and corresponding Western blotting analysis using anti-HCV NS3 pAb are shown (right panel). B, the effect of GST-HCV NS3, GST-HCV NS3/4A, or the GST control on the catalytic activity of purified recombinant hSphK1 (10 nm) was determined by an in vitro SphK assay over a range of increasing concentrations of GST-HCV NS proteins or GST. Shown on the upper panel is an autoradiogram of a TLC plate used for separation of S1P. Data in the graph on the lower panel represent the mean ± S.D. from three independent experiments. C, the effect of GST-HCV NS3, GST-BVDV NS3, or the GST control on the catalytic activity of purified hSphK1 (10 nm) was determined by an in vitro SphK assay. GST-HCV NS3, GST-BVDV NS3, or GST was added to hSphK1 in 5-fold molar excess. Shown on the left panel is an autoradiogram of a TLC plate used for separation of S1P. The arrowhead indicates the location of S1P. Data in a graph on the right panel are the mean ± S.D. from three independent experiments. **, p < 0.01.
DISCUSSION
Sphingolipids are predominant components of biological membranes that also play pivotal roles in signal transduction pathways. In particular, the sphingolipid S1P is an intracellular second messenger and an extracellular ligand with diverse cellular functions including survival, proliferation, differentiation, tumorigenesis, inflammation, and immunity (14, 15). Despite the profound importance of S1P, no reports have described the direct regulation of SphK activity by a virus, although some studies showed that SphK activity is up-regulated after infection with human cytomegalovirus or respiratory syncytial virus, contributing to efficient viral replication or cell survival, respectively (31, 32). In the present study, we demonstrated that BVDV NS3 or NS2-3, but not HCV NS3, directly binds to and inhibits the catalytic activity of SphK1. Inhibition of SphK1 activity was also observed in the context of BVDV infection. This effect seems independent of additional cofactors or auxiliary proteins because the experiments were performed using purified proteins. Studies are currently underway to determine the amino acid residues of NS3 responsible for SphK1 inhibition.
The involvement of sphingolipids in HCV replication has been well described. Replicase complexes of HCV, a close relative of BVDV, associate with lipid rafts enriched in sphingolipids and cholesterol via NS4B, NS5A, and NS5B (12). Although the molecular mechanisms characterizing these raft-viral protein interactions are still largely unknown, a direct association of HCV NS5B with lipid rafts via its finger domain is considered essential for HCV replication (13). In the presence of a chemical inhibitor of sphingolipid biosynthesis, the association of NS5B with lipid rafts is disrupted and HCV replication is repressed. Because NS5B from HCV shares structural similarity with that from BVDV (33), it is tempting to speculate that BVDV utilizes similar cellular components for viral replication. Although the molecular mechanisms responsible for enhanced BVDV replication by SphK inhibition are unknown, one may postulate that BVDV NS3 may alter the proportion of intracellular sphingolipid species through inhibition of SphK1 activity so they are more suited for efficient viral replication. Although we showed that HCV NS3 does not inhibit SphK1 activity in this study, it will be important to determine whether SphK activity is regulated by HCV through other mechanisms or not.
BVDV NS3 possesses serine protease and helicase activities that are crucial for efficient viral replication. More importantly, NS3 is abundantly detected in cells infected with CP BVDV. We thus hypothesized that an inhibitory interaction between NS3 and SphK1 may be a determinant for the induction of cell death. Unexpectedly, uncleaved NS2-3 also interacted with and catalytically inhibited SphK1, and NCP BVDV infection suppressed SphK activity. Because inhibitory regulation of SphK1 is conserved between both the CP and NCP biotypes, SphK1 inhibition itself is not directly linked to the induction of cytopathic effects by BVDV. Previously, we showed that viral cytopathogenicity is mediated via up-regulation of DEVDase activity triggered by intracellular accumulation of double-stranded RNA, a replicative intermediate, resulting from increased viral RNA replication (9). Therefore, an inhibitory interaction of NS3 with SphK1 seems to have an indirect role in virus-induced cell death because inhibition of SphK1 activity enhances viral RNA replication, which leads to an accumulation of double-stranded RNA. The expression of NS3 by itself has been suspected to be correlated with induction of cell death because abundant levels of NS3 are detected exclusively in CP BVDV-infected cells. Indeed, a previous study suggested that ectopic expression of NS3 using an adenovirus-based vector is sufficient for the induction of apoptosis (34). However, in this study, we observed no changes in morphology or DEVDase activity in cells expressing more abundant NS3 relative to CP BVDV-infected cells using the CAG promoter-driven expression vector (data not shown). Thus, it appears that cell death occurs via intracellular events downstream of NS2-3 autoprocessing, presumably as a result of enhanced RNA replication controlled by NS3 expression (7–9).
A pro-survival role of S1P has been demonstrated for cells under stress induced by trophic deprivation or ceramide addition (24). Additionally, overexpression of SphK1 protects many different types of cells from apoptosis mediated by the corresponding stress induction (19, 24, 28). In the present study, reduced DEVDase activity was observed after overexpression of SphK in CP BVDV-infected cells. Note that oxidative and endoplasmic reticulum stresses are common characteristics of CP BVDV infections (35, 36) directly linked to apoptosis. SphK1 overexpression in CP BVDV-infected cells could function to counteract these pro-apoptotic stresses, thereby causing reduced activation of DEVDase. The combined data suggest that inhibitory interaction of NS3 with SphK1 may have a role in enhancing BVDV-induced apoptosis.
Interactions between S1P and its receptors are being increasingly recognized as important in many aspects of immune responses, including lymphocyte chemotaxis. Given the importance of S1P functions in immunity, BVDV-mediated down-regulation of SphK1 that catalyzes the formation of S1P might possibly contribute to BVDV pathogenesis. Further studies are required to analyze the details of SphK inhibition on lymphoid organ or immune cells in cattle persistently infected with BVDV. Moreover, identification of sphingolipid species that are crucial for viral replication or affected through SphK inhibition by NS3 or NS2-3 may provide new insights into the mechanisms of BVDV pathogenesis.
Acknowledgments
We thank Dr. Michinori Kohara for providing the pEF-HCV NS3 and NS3/4A vectors and anti-HCV NS3 antibody.
This work was supported in part by grants-in-aid from the Ministry of Agriculture, Forestry and Fisheries of Japan, the Ministry of Education, Culture, Sports, Science and Technology, Japan, and the Japan Society for the Promotion of Science.
Footnotes
The abbreviations used are: BVDV, bovine viral diarrhea virus; HCV, hepatitis C virus; CP, cytopathogenic; NCP, noncytopathogenic; S1P, sphingosine 1-phosphate; SphK1, sphingosine kinase 1; m.o.i., multiplicity of infection; SKI, sphingosine kinase inhibitor; DMEM, Dulbecco's modified Eagle's medium; p.i., postinfection; siRNA, small interfering RNA; mAb, monoclonal antibody; pAb, polyclonal antibody; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; FCS, fetal calf serum; RT, reverse transcriptase; MDBK, Madin-Darby bovine kidney; HEK, human embryonic kidney; PBS, phosphate-buffered saline.
References
- 1.Shepard, C. W., Finelli, L., and Alter, M. J. (2005) Lancet Infect. Dis. 5 558-567 [DOI] [PubMed] [Google Scholar]
- 2.Buckwold, V. E., Beer, B. E., and Donis, R. O. (2003) Antiviral Res. 60 1-15 [DOI] [PubMed] [Google Scholar]
- 3.Xu, J., Mendez, E., Caron, P. R., Lin, C., Murcko, M. A., Collett, M. S., and Rice, C. M. (1997) J. Virol. 71 5312-5322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li, K., Foy, E., Ferreon, J. C., Nakamura, M., Ferreon, A. C., Ikeda, M., Ray, S. C., Gale, M., Jr., and Lemon, S. M. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 2992-2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li, X. D., Sun, L., Seth, R. B., Pineda, G., and Chen, Z. J. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 17717-17722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, Z., Benureau, Y., Rijnbrand, R., Yi, J., Wang, T., Warter, L., Lanford, R. E., Weinman, S. A., Lemon, S. M., Martin, A., and Li, K. (2007) J. Virol. 81 964-976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lackner, T., Muller, A., Pankraz, A., Becher, P., Thiel, H. J., Gorbalenya, A. E., and Tautz, N. (2004) J. Virol. 78 10765-10775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vassilev, V. B., and Donis, R. O. (2000) Virus Res. 69 95-107 [DOI] [PubMed] [Google Scholar]
- 9.Yamane, D., Kato, K., Tohya, Y., and Akashi, H. (2006) J. Gen. Virol. 87 2961-2970 [DOI] [PubMed] [Google Scholar]
- 10.Lackner, T., Muller, A., Konig, M., Thiel, H. J., and Tautz, N. (2005) J. Virol. 79 9746-9755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aizaki, H., Lee, K. J., Sung, V. M., Ishiko, H., and Lai, M. M. (2004) Virology 324 450-461 [DOI] [PubMed] [Google Scholar]
- 12.Gao, L., Aizaki, H., He, J. W., and Lai, M. M. (2004) J. Virol. 78 3480-3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakamoto, H., Okamoto, K., Aoki, M., Kato, H., Katsume, A., Ohta, A., Tsukuda, T., Shimma, N., Aoki, Y., Arisawa, M., Kohara, M., and Sudoh, M. (2005) Nat. Chem. Biol. 1 333-337 [DOI] [PubMed] [Google Scholar]
- 14.Spiegel, S., and Milstien, S. (2003) Nat. Rev. Mol. Cell Biol. 4 397-407 [DOI] [PubMed] [Google Scholar]
- 15.Shida, D., Takabe, K., Kapitonov, D., Milstien, S., and Spiegel, S. (2008) Curr. Drug Targets 9 662-673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.French, K. J., Schrecengost, R. S., Lee, B. D., Zhuang, Y., Smith, S. N., Eberly, J. L., Yun, J. K., and Smith, C. D. (2003) Cancer Res. 63 5962-5969 [PubMed] [Google Scholar]
- 17.Makino, A., Shimojima, M., Miyazawa, T., Kato, K., Tohya, Y., and Akashi, H. (2006) J. Virol. 80 4482-4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagai, M., Sakoda, Y., Mori, M., Hayashi, M., Kida, H., and Akashi, H. (2003) J. Gen. Virol. 84 447-452 [DOI] [PubMed] [Google Scholar]
- 19.Maceyka, M., Sankala, H., Hait, N. C., Le Stunff, H., Liu, H., Toman, R., Collier, C., Zhang, M., Satin, L. S., Merrill, A. H., Jr., Milstien, S., and Spiegel, S. (2005) J. Biol. Chem. 280 37118-37129 [DOI] [PubMed] [Google Scholar]
- 20.Olivera, A., Kohama, T., Tu, Z., Milstien, S., and Spiegel, S. (1998) J. Biol. Chem. 273 12576-12583 [DOI] [PubMed] [Google Scholar]
- 21.Yamane, D., Kato, K., Tohya, Y., and Akashi, H. (2008) Vet. Microbiol. 129 69-79 [DOI] [PubMed] [Google Scholar]
- 22.Yamane, D., Nagai, M., Ogawa, Y., Tohya, Y., and Akashi, H. (2005) Microbes. Infect. 7 1482-1491 [DOI] [PubMed] [Google Scholar]
- 23.Pitson, S. M., Moretti, P. A., Zebol, J. R., Xia, P., Gamble, J. R., Vadas, M. A., D'Andrea, R. J., and Wattenberg, B. W. (2000) J. Biol. Chem. 275 33945-33950 [DOI] [PubMed] [Google Scholar]
- 24.Olivera, A., Kohama, T., Edsall, L., Nava, V., Cuvillier, O., Poulton, S., and Spiegel, S. (1999) J. Cell Biol. 147 545-558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang, G., Flick-Smith, H., and McCauley, J. W. (2003) Virus Res. 97 89-102 [DOI] [PubMed] [Google Scholar]
- 26.Kihara, A., Anada, Y., and Igarashi, Y. (2006) J. Biol. Chem. 281 4532-4539 [DOI] [PubMed] [Google Scholar]
- 27.Pitson, S. M., Moretti, P. A., Zebol, J. R., Lynn, H. E., Xia, P., Vadas, M. A., and Wattenberg, B. W. (2003) EMBO J. 22 5491-5500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taha, T. A., Hannun, Y. A., and Obeid, L. M. (2006) J. Biochem. Mol. Biol. 39 113-131 [DOI] [PubMed] [Google Scholar]
- 29.Xia, P., Wang, L., Gamble, J. R., and Vadas, M. A. (1999) J. Biol. Chem. 274 34499-34505 [DOI] [PubMed] [Google Scholar]
- 30.Olivera, A., and Spiegel, S. (1993) Nature 365 557-560 [DOI] [PubMed] [Google Scholar]
- 31.Machesky, N. J., Zhang, G., Raghavan, B., Zimmerman, P., Kelly, S. L., Merrill, A. H., Jr., Waldman, W. J., Van Brocklyn, J. R., and Trgovcich, J. (2008) J. Biol. Chem. 283 26148-26160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monick, M. M., Cameron, K., Powers, L. S., Butler, N. S., McCoy, D., Mallampalli, R. K., and Hunninghake, G. W. (2004) Am. J. Respir. Cell Mol. Biol. 30 844-852 [DOI] [PubMed] [Google Scholar]
- 33.Choi, K. H., Groarke, J. M., Young, D. C., Kuhn, R. J., Smith, J. L., Pevear, D. C., and Rossmann, M. G. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 4425-4430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.St-Louis, M. C., Massie, B., and Archambault, D. (2005) Vet. Res. 36 213-227 [DOI] [PubMed] [Google Scholar]
- 35.Jordan, R., Wang, L., Graczyk, T. M., Block, T. M., and Romano, P. R. (2002) J. Virol. 76 9588-9599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schweizer, M., and Peterhans, E. (1999) J. Gen. Virol. 80 1147-1155 [DOI] [PubMed] [Google Scholar]
- 37.Sutherland, C. M., Moretti, P. A., Hewitt, N. M., Bagley, C. J., Vadas, M. A., and Pitson, S. M. (2006) J. Biol. Chem. 281 11693-11701 [DOI] [PubMed] [Google Scholar]