Abstract
The Ca2+ release-activated Ca2+ channel is a principal regulator of intracellular Ca2+ rise, which conducts various biological functions, including immune responses. This channel, involved in store-operated Ca2+ influx, is believed to be composed of at least two major components. Orai1 has a putative channel pore and locates in the plasma membrane, and STIM1 is a sensor for luminal Ca2+ store depletion in the endoplasmic reticulum membrane. Here we have purified the FLAG-fused Orai1 protein, determined its tetrameric stoichiometry, and reconstructed its three-dimensional structure at 21-Å resolution from 3681 automatically selected particle images, taken with an electron microscope. This first structural depiction of a member of the Orai family shows an elongated teardrop-shape 150Å in height and 95Å in width. Antibody decoration and volume estimation from the amino acid sequence indicate that the widest transmembrane domain is located between the round extracellular domain and the tapered cytoplasmic domain. The cytoplasmic length of 100Å is sufficient for direct association with STIM1. Orifices close to the extracellular and intracellular membrane surfaces of Orai1 seem to connect outside the molecule to large internal cavities.
Ca2+ is an intracellular second messenger that plays important roles in various physiological functions such as immune response, muscle contraction, neurotransmitter release, and cell proliferation. Intracellular Ca2+ is mainly stored in the endoplasmic reticulum (ER).2 This ER system is distributed through the cytoplasm from around the nucleus to the cell periphery close to the plasma membrane. In non-excitable cells, the ER releases Ca2+ through the inositol 1,4,5-trisphosphate (IP3) receptor channel in response to various signals, and the Ca2+ store is depleted. Depletion of Ca2+ then induces Ca2+ influx from outside the cell to help in refilling the Ca2+ stores and to continue Ca2+ rise for several minutes in the cytoplasm (1, 2). This Ca2+ influx was first proposed by Putney (3) and was named store-operated Ca2+ influx. In the immune system, store-operated Ca2+ influx is mainly mediated by the Ca2+ release-activated Ca2+ (CRAC) current, which is a highly Ca2+-selective inwardly rectified current with low conductance (4, 5). Pathologically, the loss of CRAC current in T cells causes severe combined immunodeficiency (6) where many Ca2+ signal-dependent gene expressions, including cytokines, are interrupted (7). Therefore, CRAC current is necessary for T cell functions.
Recently, Orai1 (also called CRACM1) and STIM1 have been physiologically characterized as essential components of the CRAC channel (8–12). They are separately located in the plasma membrane and in the ER membrane; co-expression of these proteins presents heterologous CRAC-like currents in various types of cells (10, 13–15). Both of them are shown to be expressed ubiquitously in various tissues (16–18). STIM1 senses Ca2+ depletion in the ER through its EF hand motif (19) and transmits a signal to Orai1 in the plasma membrane. Although Orai1 is proposed as a regulatory component for some transient receptor potential canonical channels (20, 21), it is believed from the mutation analyses to be the pore-forming subunit of the CRAC channel (8, 22–24). In the steady state, both Orai1 and STIM1 molecules are dispersed in each membrane. When store depletion occurs, STIM1 proteins gather into clusters to form puncta in the ER membrane near the plasma membrane (11, 19). These clusters then trigger the clustering of Orai1 in the plasma membrane sites opposite the puncta (25, 26), and CRAC channels are activated (27).
Orai1 has two homologous genes, Orai2 and Orai3 (8). They form the Orai family and have in common the four transmembrane (TM) segments with relatively large N and C termini. These termini are demonstrated to be in the cytoplasm, because both N- and C-terminally introduced tags are immunologically detected only in the membrane-permeabilized cells (8, 9). The subunit stoichiometry of Orai1 is as yet controversial: it is believed to be an oligomer, presumably a dimer or tetramer even in the steady state (16, 28–30).
Despite the accumulation of biochemical and electrophysiological data, structural information about Orai1 is limited due to difficulties in purification and crystallization. In this study, we have purified Orai1 in its tetrameric form and have reconstructed the three-dimensional structure from negatively stained electron microscopic (EM) images.
EXPERIMENTAL PROCEDURES
Expression Constructs and Transfection of Cells—Full-length mouse Orai1 encoding sequence (GenBank™: NM_175423) was cloned from mouse brain Marathon-Ready cDNA (Clontech, Mountain View, CA) using PCR and was subcloned into pCMV-Tag2B or pCMV-Tag4B vector (Stratagene, La Jolla, CA) to introduce a FLAG tag sequence at the N or C terminus (N-FLAG or C-FLAG Orai1). For functional analysis, Orai1 encoding sequence was also subcloned into pCI-neo (Promega, Madison, WI), and mouse STIM1 encoding sequence was subcloned from pApuro-FLAG-tagged mouse STIM1 (31) into pCI-neo.
HEK293 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 100 μg/ml kanamycin, and incubated at 37 °C in a 5% CO2 atmosphere. For protein purification, HEK293 cells were transfected with 0.18 μg/cm2 of the N-FLAG Orai1 DNA construct for 6 h using the calcium phosphate precipitation method. After 48 h, the cells were washed twice with a phosphate-buffered saline (137 mm NaCl, 2.7 mm KCl, 4.3 mm Na2HPO4, 1.5 mm KH2PO4, pH 7.4), harvested with a cell scraper, and collected by centrifuging. Cells were stored at -80 °C for further protein purification. C-FLAG Orai1 proteins were similarly expressed.
Measurement of Intracellular Ca2+ Concentration ([Ca2+]i) Change and Current Recording—HEK293 cells were transfected using SuperFect Transfection Reagent (Qiagen) according to the manufacturer's instructions. The cells were seeded on polylysine-coated coverslips, and were loaded with fura-2 by incubation in Dulbecco's modified Eagle's medium containing 1 μm fura-2/AM (Dojindo Laboratories, Kumamoto, Japan), 10% fetal bovine serum, 30 units/ml penicillin, and 30 μg/ml streptomycin at 37 °C for 40 min. The coverslips were then placed in a perfusion chamber mounted on the stage of the microscope. The fura-2 fluorescence images of the cells were recorded in the HEPES-buffered saline (HBS) (in mm): 107 NaCl, 6 KCl, 1.2 MgSO4, 2 CaCl2, 1.2 KH2PO4, 11.5 glucose, 20 HEPES (pH 7.4 adjusted with NaOH) or Ca2+-free HBS containing 0.5 mm EGTA. The fluorescent images were analyzed with a video image analysis system (AQUACOSMOS, Hamamatsu Photonics, Hamamatsu, Japan). These images were converted to Ca2+ concentrations by in vivo calibration (32). Thapsigargin (Calbiochem) was diluted to the final concentration (2 μm) in Ca2+-free HBS and applied to the cells by perfusion, as previously described (33).
Whole cell currents were recorded at room temperature using the conventional whole cell mode of the patch clamp technique (34) with EPC9 amplifier (HEKA, Pfalz, Germany). Voltage ramps of 50-ms duration spanning a range of -100 to +100 mV were delivered from a holding potential of 0 mV at a rate of 0.5 Hz over a period of 100–400 s. The recordings were sampled at 2.0 kHz and filtered at 2.9 kHz. The external solutions contained (in mm): 145 NaCl, 2.8 KCl, 10 CsCl, 2 MgCl2, 10 CaCl2, 10 glucose, 10 HEPES, 10 tetraethylammonium chloride (adjusted to pH 7.2 with NaOH). The pipette solution contained (in mm): 120 CsCl, 8 NaCl, 10 Cs-1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis, 3 MgCl2, 10 HEPES, 0.04 IP3 (adjusted to pH 7.2 with CsOH). All data are expressed as mean ± S.E. The data were accumulated under each condition from at least three independent experiments. The p values are the results of Student's t tests.
Protein Purification—The transfected cells were homogenized with a Potter Teflon homogenizer in Tris-buffered saline (TBS, 20 mm Tris-HCl, pH 7.4 at 4 °C, 140 mm NaCl, 0.02% sodium azide) containing a protease inhibitor mixture (Wako Pure Chemicals, Osaka, Japan). All procedures were performed on ice or at 4 °C. Cell debris was eliminated from the homogenate by centrifuging at 10,000 × g for 15 min. The supernatant was re-centrifuged at 100,000 × g for 1 h to sediment membrane fractions.
The membrane fraction was solubilized in the same buffer supplemented with 25 mm n-dodecyl β-d-maltoside (DDM, Sigma). After centrifuging at 100,000 × g for 30 min, the supernatant containing FLAG-tagged Orai1 was applied to an anti-FLAG M2 affinity gel (Sigma) column, equilibrated in advance. The column was washed with 15 column bed volumes of buffer A (TBS containing 1 mm DDM, 750 mm MgCl2, and 5% glycerol) supplemented with the protease inhibitors. The bound Orai1 proteins were eluted with buffer A containing 125 μg/ml FLAG peptide (Sigma). The eluate containing Orai1 protein was concentrated 15 times with a Microcon centrifuge filter unit YM-100 (Millipore, Billerica, MA) and further purified by Superdex 200 (3.2/30) size exclusion chromatography (SEC) in a Smartsystem (GE Healthcare) using buffer A. The elution of protein was profiled at 280 nm absorbance and subfractionated into 20-μl fractions.
Deglycosylation and Chemical Cross-linking—To remove the N-linked glycan, purified Orai1 protein was treated with 0.08 unit/μl of peptidyl N-glycosidase F (PNGase F) at 25 °C for 1 h. The deglycosylated Orai1 was then separated from PNGase F using Superdex 200 (3.2/30) SEC.
For chemical cross-linking, the TBS component in the buffer was substituted in advance with phosphate-buffered saline by SEC. Glutaraldehyde was mixed with the Orai1-containing solution to the indicated final concentrations, at 25 °C for 30 min. Cross-linking was terminated by incubation with an equal volume of SDS-sample buffer at 25 °C for 15 min. Proteins were separated in 3–10% polyacrylamide gel and visualized by silver staining.
SDS-PAGE—Samples were mixed with an SDS-sample buffer containing 62.5 mm Tris-HCl (pH 6.8), 25% glycerol, 2% SDS, 0.04 m dithiothreitol, and 0.01% bromphenol blue, and incubated at 25 °C for 15 min. Proteins were separated in 10–20% polyacrylamide gel and visualized by silver staining. For Western blots, proteins were transferred to the polyvinylidene difluoride membrane and analyzed with alkaline phosphatase-labeled anti-FLAG antibodies (Sigma).
Native Gel Electrophoresis—Each sample was mixed with an equal volume of sample buffer (62.5 mm Tris-HCl, pH 6.8, 25% glycerol, and 0.01% bromphenol blue) and electrophoresed in 3–10% polyacrylamide gel using a running buffer containing 25 mm Tris-HCl, pH 8.4, 192 mm glycine, and 1 mm DDM. Electrophoresis was performed at 3.4 V/cm at 4 °C. Proteins were visualized by silver staining.
Estimation of Stokes Radius by SEC—High molecular weight standard proteins (GE Healthcare) and purified Orai1 protein were analyzed by Superdex 200 (3.2/30) SEC in a Smartsystem (35). The elution profile of each protein was obtained at 280 nm. The distribution coefficient (Kav) was calculated from the equation Kav = (Ve - Vo)/(Vt - Vo), where Ve is the elution volume of each standard protein or Orai1-detergent complex. Column void volume (Vo) was determined by the elution volume of blue dextran 2000, whereas Vt represents the total bed volume (2.40 ml). The Stokes radius (Rs) of Orai1 was determined by interpolation using a calibration curve, which was constructed by plotting the Rs of reference proteins versus (-log Kav)1/2 according the relationship (-log Kav)1/2 = α (β + Rs). The Orai1 data are presented as mean ± S.E. The standard proteins used were: thyroglobulin (Rs, 85 Å), ferritin (Rs, 61 Å), catalase (Rs, 52 Å), and aldolase (Rs, 48 Å).
Transmission Electron Microscopy—Purified Orai1 proteins were adsorbed to thin carbon films supported by copper mesh grids, which were rendered hydrophilic in advance by glow discharge in low air pressure. Samples were washed with 10 drops of double-distilled water, negatively stained with 2% uranyl acetate solution for 30 s twice, blotted, and dried in air. Micrographs of negatively stained particles were imaged using a JEM-100CX transmission electron microscope (JEOL, Tokyo, Japan) at ×52,100 magnification with 100 kV acceleration voltage. Images were recorded on Kodak SO163 electron microscopy film (Eastman Kodak, Rochester, NY), developed with D19 developer (Kodak), and digitized with a Scitex Leafscan 45 scanner (Leaf Systems Inc., Westborough, MA) at a pixel size of 1.92 Å at the specimen level.
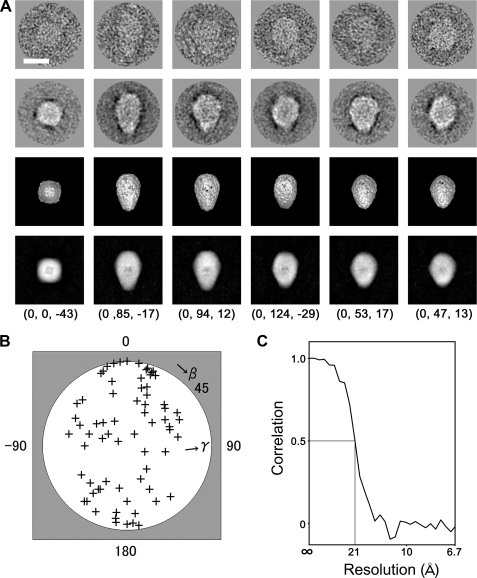
Automated Particle Selection and Image Analysis—We have developed a single particle image analysis method using neural network (36–38) and simulated annealing (39, 40) named SPINNS (41); image analysis was performed using SPINNS and IMAGIC V (42). Orai1 projections were first picked up using the auto-accumulation method with simulated annealing (SA) (39). 289 particles in 180 × 180 pixel subframes were selected and used to train a three-layer pyramid-type neural network (NN) (36, 37). Using the trained NN, 3681 particles were selected. After background subtraction, the particles selected by NN were aligned rotationally and translationally (43, 44) by the reference-free method (37). The aligned images were sorted into 120 classes by the modified growing neural gas network method (38). Their class averages were adopted as new references, and this cycle, from alignment to averaging, was repeated 41 times.
The Euler angles of the class averages were automatically determined by the echo-correlated three-dimensional reconstruction method with SA (40) assuming a C4 symmetry, and used to calculate a primary three-dimensional structure by the simultaneous iterative reconstruction technique (45). The reprojections from the volume were employed as references for multireference alignment, and raw images in the library were aligned and further clustered, providing improved cluster averages. From the averages, a new three-dimensional map was generated by the echo-correlated reconstruction method without a three-dimensional reference.
The three-dimensional map was further refined by projection matching (46) followed by an optimization using echo-correlated reconstruction. This cycle was repeated 19 times. Particle images correlating poorly with the three-dimensional projections were automatically rejected using the cross-correlation function. The final reconstruction includes 2940 particles, 79.9% of all the selected images. The resolution of the final three-dimensional map was assessed using the Fourier shell correlation (FSC) function (47) at the threshold of 0.5.
Formation of the Complexes between Orai1 and Antibody or between Orai1 and Wheat Germ Agglutinin—Anti-FLAG antibodies were added to purified Orai1 proteins in binding buffer (TBS containing 1 mm DDM, 350 mm MgCl2, and 5% glycerol) and incubated at 4 °C for 20 min. Unbound antibodies were then separated from the Orai1-antibody complex using Superdex 200 (3.2/30) SEC, and the complexes were negatively stained and imaged as described above.
Fab fragments of anti-FLAG antibodies were generated by papain digestion (Pierce) and conjugated with colloidal gold (5 nm in diameter, BB International, Cardiff, UK). Non-reacted Fab fragments were removed using 10–30% glycerol gradient centrifugation. The Fab-gold conjugates were mixed with purified Orai1 in binding buffer and incubated at 4 °C for 20 min. Orai1-Fab-gold complexes were separated from unbound Fab-gold by Superose 6 (3.2/30) SEC, negatively stained, and observed by EM.
Wheat germ agglutinin-gold conjugates (EY Laboratories, San Mateo, CA) were mixed with purified Orai1 in binding buffer at 4 °C for 1 h. This mixture was applied to an anti-FLAG M2 affinity gel column. The column was washed with 6 column bed volumes of binding buffer. The complexes between Orai1 and wheat germ agglutinin-gold were eluted with FLAG peptide in binding buffer, negatively stained, and observed by EM.
RESULTS
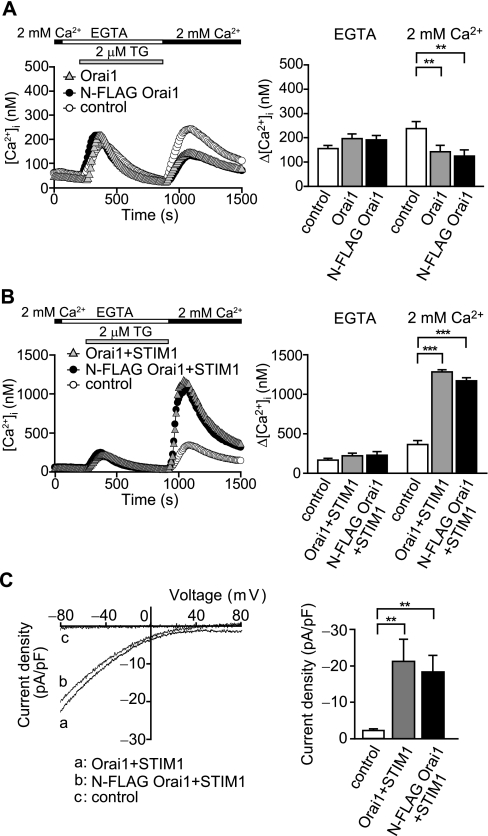
Expression and Purification of Orai1 Protein—Because endogenous Orai1 protein is scarce and not sufficient for purification, we constructed N-terminally FLAG-tagged Orai1 recombinant (N-FLAG Orai1) and expressed it in HEK293 cells. The channel function of wild-type and N-FLAG Orai1 was measured by the fluorescence of fura-2 and by the whole cell current. In comparison to the control experiment, overexpression of wild-type Orai1 reduced cytoplasmic [Ca2+]i rises when the store depletion was induced by thapsigargin, an inhibitor of the sarcoendoplasmic reticulum Ca2+-ATPase pump (Fig. 1A). The [Ca2+]i rises were similarly reduced with the overexpression of N-FLAG Orai1. In contrast, STIM1 co-expressed with wild-type or N-FLAG Orai1 substantially increased [Ca2+]i (Fig. 1B) (13, 15). The whole cell currents in response to IP3-induced Ca2+ store depletion were also measured. Co-expression of STIM1 with wild-type or N-FLAG Orai1 generated large currents with typical inwardly rectifying current-voltage relationships in CRAC current (Fig. 1C). These results confirm that the N-FLAG Orai1 protein forms a fully functional CRAC channel with STIM1. We also constructed C-terminally FLAG-tagged Orai1 and examined its function in [Ca2+]i regulation. We could not find any significant difference in the channel function from either the wild-type or the N-FLAG Orai1 (data not shown).
FIGURE 1.
Functional analysis of the N-FLAG-tagged Orai1 channel. A, thapsigargin (TG)-induced [Ca2+]i rises in Orai1-expressing HEK293 cells. Left, average time course of Ca2+ responses induced by 2 μm TG in Orai1 (gray triangles)-, N-FLAG-tagged Orai1 (black circles)-, and control vector (white circles)-transfected HEK293 cells (n = 27–28). The perfusion solution was first changed to 0.5 mm EGTA containing Ca2+-free HBS (EGTA), and 2 μm TG was applied to the cells in the absence of extracellular Ca2+. Eleven minutes after the application of TG, 2 mm Ca2+ was added to the extracellular solution. Right, maximum [Ca2+]i rises (Δ[Ca2+]i) induced by TG in EGTA and those after re-addition of 2 mm external Ca2+. B, TG-induced [Ca2+]i rises in Orai1 and STIM1-coexpressing HEK293 cells. Left, average time course of Ca2+ responses induced by 2 μm TG in Orai1 plus STIM1 (gray triangles)- and N-FLAG-tagged Orai1 plus STIM1 (black circles)-cotransfected HEK293 cells and control vector (white circles)-transfected HEK293 cells (n = 25–41). Right, Δ[Ca2+]i induced by TG in EGTA and those after re-addition of 2 mm external Ca2+. C, activation of CRAC current induced by 40 μm IP3 in Orai1- and STIM1-coexpressing cells. Left, representative current-voltage relationships in Orai1 plus STIM1 (a)- and N-FLAG-tagged Orai1 plus STIM1 (b)-cotransfected HEK293 cells and control vector (c)-transfected HEK293 cells. Data represent leak-subtracted current densities (pA/picofarad) evoked by 50-ms voltage ramps from -100 to +100 mV. Right, average CRAC current densities at -80 mV in Orai1 plus STIM1 (n = 11), N-FLAG-tagged Orai1 plus STIM1 (n = 9) and control (n = 9) cells. **, p < 0.01; and ***, p < 0.001.
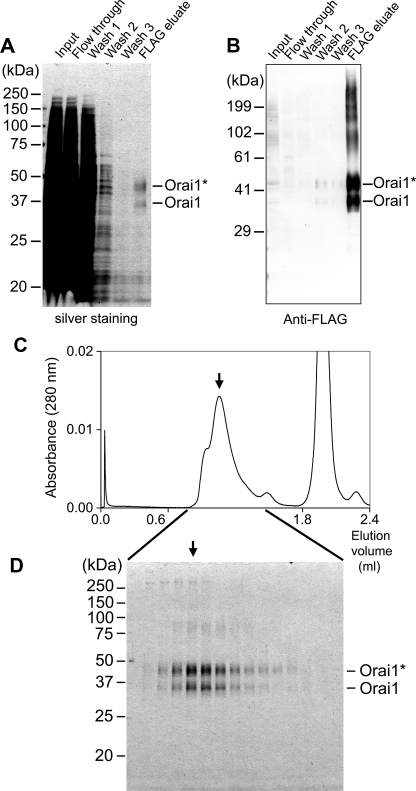
From the membranes of transiently expressed HEK293 cells, N-FLAG Orai1 proteins were solubilized with nonionic detergent DDM and purified using immunoaffinity chromatography and SEC. The Orai1 proteins bound to FLAG affinity column were competitively eluted with 125 μg/ml FLAG peptide. Each fraction was analyzed by SDS-PAGE, followed by silver staining (Fig. 2A) or by Western blotting using anti-FLAG antibody (Fig. 2B). Orai1 was observed as two broad bands at 36 and 44 kDa (Fig. 2A, right end lane), both of which were recognized by anti-FLAG antibodies (Fig. 2B, right end lane). The lower band at 36 kDa corresponds to the molecular size of the Orai1 polypeptide 35.3 kDa (33.1 kDa accompanied by an additional 2.2-kDa linker tag), whereas the upper band at 44 kDa seems to represent the glycosylated form (22). The glycosylated form of Orai1 is dominant. Especially in Western blotting, broad bands of larger than 60 kDa were frequently observed (Fig. 2B, right end lane). These bands are considered to be aggregations of Orai1 proteins caused by SDS treatment. Contaminants of mostly lower molecular weights, which were not positive in the Western blot, were also observed (Fig. 2A, right end lane). To remove them, the eluate from the anti-FLAG column was concentrated using a Microcon YM-100 filter unit and further purified by Superdex 200 SEC (Fig. 2C). Each fraction was analyzed by SDS-PAGE followed by silver staining (Fig. 2D).
FIGURE 2.
Purification of Orai1 protein from transiently expressed HEK293 cells. N-terminally FLAG-tagged Orai1 was solubilized using DDM, then purified with immunoaffinity chromatography followed by SEC. A, SDS-PAGE of aliquots at each step in anti-FLAG affinity chromatography visualized by silver staining, and B, Western blotting using anti-FLAG antibody. Orai1 proteins are demonstrated as glycosylated (indicated as Orai1*, 44 kDa) and unglycosylated (Orai1, 36 kDa) forms. C, SEC profile using Superdex 200 column. The Orai1-rich eluates from the immunoaffinity column were concentrated using the Microcon YM-100 filter unit and analyzed by Superdex 200 SEC. Orai1 was mainly eluted in a peak at 1.04 ml elution (indicated by an arrow). Another peak at 1.96 ml contains FLAG-peptide. D, SDS-PAGE analysis of the SEC fractions, visualized by silver staining. Intensity of the bands of Orai1 in the gel corresponds well to the absorbance in SEC. The peak fraction at 1.04 ml (arrow) was used for electron microscopic analysis.
By measuring UV absorbance at 280 nm, elution of Orai1 protein was monitored during SEC. Orai1 molecules were eluted at 1.04 ml with a shoulder of higher molecular weight (Fig. 2C). An additional peak at 1.96 ml is an elution of the FLAG peptides used to dissociate proteins from the anti-FLAG column. The Orai1 protein was again detected as two bands in SDS-PAGE (Fig. 2D), where their intensities basically correspond to the UV profile in SEC. The shoulder at 0.94 ml does not accompany the increase in the density of Orai1 or accumulation of contaminated proteins. Therefore, it is speculated to be absorption due to micelles of lipids derived from the plasma membrane. Both the glycosylated and non-glycosylated subunits are considered to be able to form a functional CRAC channel complex (16). An aliquot at 1.04-ml elution (Fig. 2C, arrow) was used for the following analysis and EM.
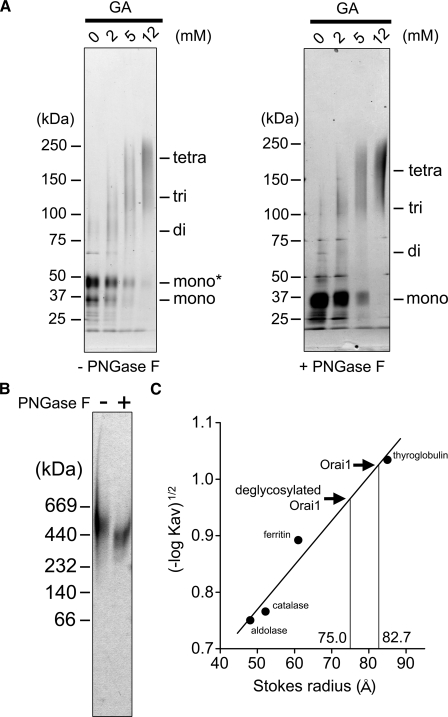
Chemical Cross-linking and Native PAGE of Orai1—Purified Orai1 was treated with various concentrations of glutaraldehyde and analyzed by SDS-PAGE followed by silver staining (Fig. 3A, left panel). After cross-linking, the original bands at 36 and 44 kDa were shifted to a broad band of ∼200 kDa (Fig. 3A, left panel, fourth lane), which is similar in size to the tetramer (141–176 kDa). This agrees with the report that the functional Orai1 molecule forms a tetramer (28, 29). To eliminate glycan, purified Orai1 was treated with PNGase F and analyzed with SDS-PAGE (Fig. 3A, right panel). After treatment, the intensity of the band at 44 kDa decreased, and the intensity at 36 kDa increased, confirming that the upper band is glycosylated (Fig. 3A, right panel, first lane). The PNGase F-treated Orai1 was further cross-linked with glutaraldehyde. The size decreased to ∼170 kDa (Fig. 3A, right panel, fourth lane), which is comparable to the molecular mass of the deglycosylated tetramer (141 kDa).
FIGURE 3.
Chemical cross-linking, native PAGE and Stokes radius of Orai1. A, chemical cross-linking of Orai1. Purified proteins with (right panel) or without (left panel) PNGase F treatment were reacted with glutaraldehyde (GA) at indicated concentrations and separated by SDS-PAGE followed by silver staining. Left, the bands of Orai1 at 36 kDa and 44 kDa were shifted upward to ∼200 kDa, suggesting glycosylated tetramer formation (44 × 4 = 176 kDa). Right, the deglycosylated band at 36 kDa was also shifted upward by the cross-linking to ∼170 kDa. B, native gel electrophoresis of Orai1. Purified proteins were separated in native gel and visualized by silver staining. The Orai1 proteins were detected as a broad band at 520 kDa, and shifted downward to 380 kDa after PNGaseF treatment. This much larger estimation of the molecular weight may suggest the swollen structure of the Orai1 molecule. C, Stokes radius (Rs) of purified Orai1 calculated from SEC. From the calibration curve obtained from the standards, the Rs of glycosylated and deglycosylated Orai1 were calculated to be 82.7 ± 1.1Å(n = 5) and 75.0 ± 1.0Å(n = 3), respectively. Standard proteins are thyroglobulin (Rs, 85 Å), ferritin (Rs, 61 Å), catalase (Rs, 52 Å), and aldolase (Rs, 48 Å).
To estimate the molecular size of Orai1, we performed native PAGE analysis (Fig. 3B). The Orai1 band was detected between the 440- and 669-kDa standards, and its molecular mass was estimated to be 520 kDa. This is three times larger than the estimation derived from tetrameric amino acid composition, including tag and glycan (176 kDa). Although PNGase F treatment decreased the size to 380 kDa (Fig. 3B, second lane), it was still much larger than the estimation from the sequence (141 kDa), and larger than the estimation from cross-linking (∼170 kDa). This inconsistency in size may be attributed to the bulky three-dimensional structure of Orai1 molecule, as described below.
The hydrated size (Stokes radius, Rs) of Orai1 was further calculated from the elution volume in the SEC, where Orai1 was eluted between ferritin (Rs is 61.0 Å, molecular mass is 440 kDa) and thyroglobulin (Rs is 85.0 Å, molecular mass is 669 kDa). The distribution coefficient (Kav) of Orai1 was calculated as 0.089 ± 0.004 (mean ± S.E., n = 5), and the Rs was determined to be 82.7 ± 1.1 Å from the calibration curve (Fig. 3C). Considering the small molecular mass estimated from the sequence (141–176 kDa), this result again indicates the bulky nature of the molecule. To assess the contribution of the glycan moiety, purified Orai1 was treated with PNGase F and analyzed by SEC. The Rs of deglycosylated Orai1 was 75.0 ± 1.0 Å, slightly smaller than that of glycosylated Orai1. Therefore, the bulkiness of Orai1 is not attributable to glycosylation.
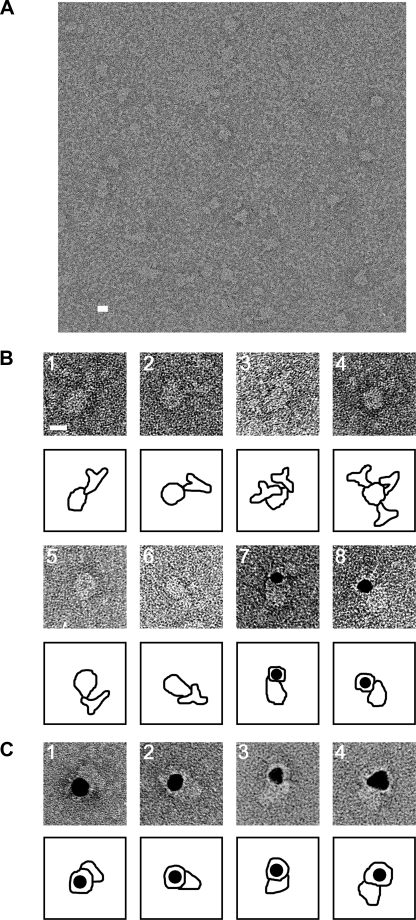
Electron Microscopy and Three-dimensional Reconstruction of Orai1—Purified Orai1 proteins were blotted onto a glow-discharged carbon film supported by a copper mesh grid, negatively stained with 2% uranyl acetate and imaged using an electron microscope. Variously shaped particles of uniform size were observed (Fig. 4A). Most particles were ellipse- or tear-drop-shape, which likely represent side views. Squared particles with round corners were occasionally observed, interpreted to be top views of tetrameric Orai1. The variation in shape is interpreted to reflect different orientations of the same molecule on the grid.
FIGURE 4.
Electron microscopy of negatively stained Orai1. A, Orai1 particles were observed as variously shaped but uniformly sized projections of the molecule. For statistical analysis, 3681 particles were picked up by a combination of the auto-accumulation method (39) and the three-layer neural network (NN) auto-picking system (36, 37). B, assignment of the cytoplasmic domain of Orai1. Gallery of negatively stained complexes between N-FLAG Orai1 and anti-FLAG antibodies (panels 1–4) and between C-FLAG Orai1 and anti-FLAG antibodies (panels 5 and 6). Both the cytoplasmic N and C termini were assigned at the smaller end of the Orai1 molecule. The binding of multiple antibodies to a single Orai1 molecule is frequently observed (panels 3 and 4). Gallery of complex between N-FLAG Orai1 and Fab-gold (panels 7 and 8). Fab fragments of anti-FLAG antibodies are conjugated with colloidal gold, and then mixed with purified Orai1. The gold conjugate binds to similar positions as indicated in panels 1–4. C, assignment of the extracellular glycan moiety of Orai1. Gallery of complex between N-FLAG Orai1 and wheat germ agglutinin-gold (panels 1–4). The gold conjugate binds to the larger end of the Orai1 molecule. Contours are shown below each image. Scale bars represent 100 Å.
Molecular orientation of Orai1 to the plasma membrane was determined by decorating cytoplasmic N-terminal FLAG tag with anti-FLAG antibody. The Orai1/anti-FLAG antibody complex was negatively stained and observed using EM (Fig. 4B, panels 1–4). The antibodies attached to the smaller side of Orai1, indicating that this narrower domain locates inside the cell (Fig. 4B, panels 1 and 3). Orai1 bearing multiple antibodies was frequently observed (Fig. 4B, panels 3 and 4). This also supports the tetrameric subunit stoichiometry of Orai1, which allows a maximum of four antibody bindings. Antibody binding was more clearly observed using the Fab fragment conjugated with colloidal gold. Gold conjugates were again observed at the smaller side of Orai1 (Fig. 4B, panels 7 and 8). To assign the location of the C terminus in the molecule, we purified C-FLAG Orai1 proteins and decorated them with anti-FLAG antibodies. The antibodies again bound to the smaller side (Fig. 4B, panels 5 and 6), confirming the C terminus is also located in the narrower domain. Wheat germ agglutinin-gold was also used to assign the extracellular glycan moiety of Orai1 (Fig. 4C, panels 1–4). Gold conjugates were observed at the larger side, indicating that this domain is extracellular.
The three-dimensional reconstruction was performed using our method SPINNS (36–41) and IMAGIC V (42). The projections were obtained by a combination of two automatic pickup programs: the auto-accumulation method using SA (39) and the three-layer neural network method (36, 37). The 3681 images selected by the network were aligned rotationally and translationally (43, 44) using the reference-free method (37). The aligned images were then classified into 120 clusters using the modified growing neural gas network method (38). Images in each cluster were averaged, and the averages were used as new references. This cycle, from alignment to averaging, was repeated until convergence.
Euler angles of the class averages were determined using the echo-correlated three-dimensional reconstruction method with SA (40). In this step, C4 symmetry was imposed because tetrameric subunit stoichiometry was indicated by cross-linking experiments and because the top projections and their class average exhibited 4-fold symmetry (Fig. 5A, first and second rows, left end column). Using the angles, a primary three-dimensional density map was calculated by the simultaneous iterative reconstruction technique method (45). The reprojections from the volume were employed as new references for multireference alignment, and each image in the library was aligned and classified, providing improved cluster averages. A new three-dimensional map was then generated by the echo-correlated method without a three-dimensional reference.
FIGURE 5.
Three-dimensional reconstruction of Orai1. A, raw images of Orai1 with different Euler angles (row 1) are compared with their corresponding class averages (row 2), the surface representations of the three-dimensional reconstruction (row 3) and the reprojections from the reconstruction (row 4) along the corresponding Euler directions. The Euler angle (α, β, γ) is denoted below each column. Protein is displayed in bright shades. Scale bar represents 100 Å. B, plot of the Euler (β, γ) angles of 70 adopted class averages. Orai1 molecules are almost randomly oriented on the carbon surface. C, FSC function indicates a resolution limit of 21 Å by the FSC > 0.5 criterion.
The density map was further refined by the projection matching (46) followed by an optimization using the echo-correlated reconstruction. This cycle was repeated until convergence. The final reconstruction included 2940 particles, 79.9% of all the selected images.
Representative raw images are presented (Fig. 5A, first row), with their corresponding class averages (second row), and with the surface representations and the projections of the reconstructed three-dimensional structure (third and fourth rows). A high level of consistency was observed in size, shape, and inner structure among these datasets (Fig. 5A), indicating successful three-dimensional reconstruction from the original particle images. A plot of the Euler angles of the 70 adopted class averages (Fig. 5B) shows that Orai1 is almost randomly oriented on the grid surface. According to the FSC function (47), the resolution limit is 21 Å by the correlation coefficient > 0.5 criterion (Fig. 5C).
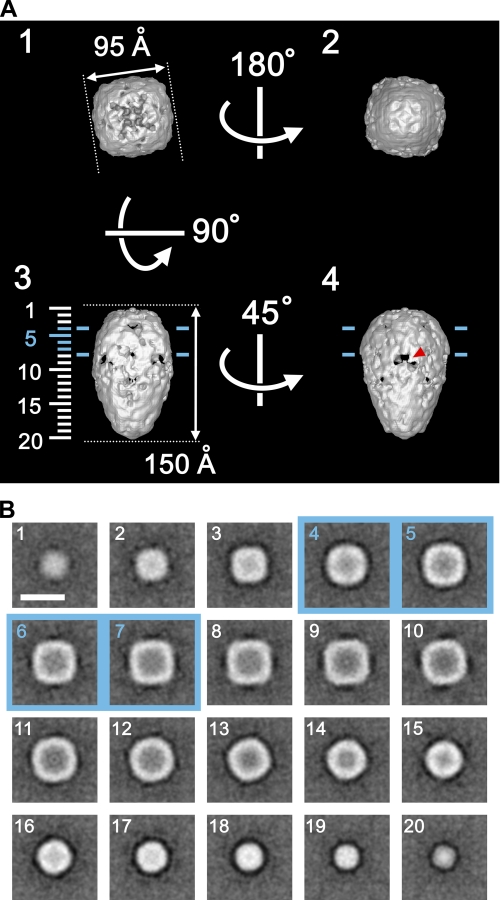
Structural Features of the Orai1 Molecule—The surface representation demonstrates that Orai1 is a teardrop-shaped molecule 150 Å in height, 95 Å in side length, and 105 Å diagonally at the widest square-shaped TM region (Fig. 6A). The cytoplasmic domain, from the widest part of the molecule to the narrow end of the teardrop, extends ∼100 Å. The three-dimensional map was contoured at an isosurface containing a volume corresponding to 210 kDa assuming a protein density of 1.37 g/cm3, which is 149% of the tetrameric Orai1 mass (141 kDa) calculated from the amino acid composition. The putative position of the TM region is indicated by two blue lines 30 Å apart in Fig. 6A, panels 3 and 4, so that the ratio of each region is close to the prediction derived from the hydropathy plot (i.e. the volumes of TM, extracellular, and cytoplasmic domains are 23, 17, and 60%, respectively, Fig. 7A).
FIGURE 6.
Structural features of Orai1. A, surface representations of Orai1 viewed from four different Euler angles (α, β, γ): 1 (0, 180, -45), 2 (0, 0, -45), 3 (0, 90, -45), and 4 (0, 90, 0). The molecular mass enclosed by the isosurface is 210 kDa, corresponding to 149% of the tetrameric Orai1 protein. Protein is displayed in bright shades. Two blue lines, ∼30 Å apart in panels 3 and 4, indicate the putative position of the lipid bilayer. A red arrowhead in panel 4 indicates one of inverted-V-shaped orifices in the cytoplasmic domain. B, horizontal sections parallel to the membrane plane. Sections at 7.7-Å intervals through the molecule are indicated by numbers 1–20 on the left side of panel A3. The internal structure of the Orai1 molecule is sparse. Scale bar represents 100 Å.
FIGURE 7.
Predicted topology of Orai1 and a docking model with STIM1. A, schematic diagram of N-FLAG Orai1. Orai1 is a highly glycosylated protein with glycan moiety of ∼8 kDa per subunit. The Orai1 polypeptide has a cytosolic N terminus, four TM segments (yellow columns: S1–S4), and a cytosolic C terminus with a putative coiled-coil sequence (green box) involved in binding with STIM1. The FLAG tag (red box) was introduced in the N terminus. B, illustration of putative docking between Orai1 and STIM1. The three-dimensional reconstruction in this study suggests that the cytoplasmic domain of Orai1 is long enough for direct association with STIM1. STIM1 contains a coiled-coil motif (green box) in the cytoplasm, a single TM segment (yellow column), a sterile α motif domain (purple box), and an EF-hand motif (blue crescent) in the ER lumen.
Between the hemispheric extracellular domain and the tapered cytoplasmic domain, the putative TM region formed a seamless outer shell (Fig. 6, A (panels 3 and 4) and B (panels 4–7)), which is similar to those of other ion channels such as the sodium channel, the IP3 receptor, the transient receptor potential channels, and the cystic fibrosis transmembrane conductance regulator channel (35, 48–51). Variously shaped orifices are prominent close to the extracellular and intracellular membrane surfaces. Inverted V-shaped orifices 20 Å in height and 28 Å in width are especially conspicuous in the cone-shaped cytoplasmic domain (indicated by a red arrowhead in Fig. 6A, panel 4).
Sections parallel to the membrane plane demonstrate the internal structure (Fig. 6B). Both the TM and extracellular domains have a delicate internal structure surrounded by a dense outer shell 20 Å thick (Fig. 6B, panels 1–7). Cross-sections of this outer shell were square with round corners. The inner structure in the TM region is interpreted as an ion permeation apparatus. This reconstruction is considered to be in the closed state, because STIM1 was not expressed in this system. In contrast, the internal structure of the cytoplasmic domain is less complex and is surrounded by a round-shaped outer shell. In total, the two-layered structure of Orai1 molecule includes a considerable amount of internal water-filled space.
DISCUSSION
In this study, we have purified tetrameric Orai1 and reconstructed the three-dimensional structure at 21-Å resolution, providing the first depiction of a member of the Orai family. The gross dimensions of the reconstruction agree well with the molecular size estimated using SEC or native gel electrophoresis. The two-layered structure is common among some of the transient receptor potential channels, the sodium channel, and the IP3 receptor (48–51). However, the inner core in the TM region of Orai1 is smaller than the corresponding cores of six-TM type channels, which may be a major difference between the four- and six-TM types.
In the reconstruction, orifices close to the extracellular and intracellular membrane surfaces are prominent. These orifices connect to internal cavities, and this, together with the two-layered structure, seems to support the hypothesis that Orai1 is a pore component of the CRAC channel. The orifices close to the intracellular membrane surface are considered to be outlets for permeated Ca2+ ions. Because phosphate residues of plasma membrane are negatively charged, the released Ca2+ may be arrested on the membrane surface, and if so, this Ca2+ arrest can create a spatially confined Ca2+ signal: a sub-plasma membrane Ca2+ rise rather than a bulky Ca2+ elevation in the cytoplasm. Such local Ca2+ signaling is known to up-regulate both the intracellular messenger arachidonic acid and the pro-inflammatory molecule leukotriene C4 in mast cells (52). This Ca2+ arrest may also enable efficient signal transmission using a small amount of Ca2+. In fact, the CRAC channel has very low Ca2+ conductance (5).
Orai1 dynamically associates with STIM1 to form a functional CRAC channel complex (11, 19, 25, 26, 53), the Orai1 subcomplex of which is reported to be a homotetramer (28). However, the subunit stoichiometry of Orai1 alone has not been clearly understood, although several reports suggest Orai1 exists as an oligomer form (16, 30). The biochemical analysis of purified Orai1 in this study strongly supports the tetrameric nature of Orai1 even when STIM1 is not present; monomeric Orai1 was rarely observed during purification. The relatively tight, continuous surface of the TM domain of Orai1 agrees well with a report that the TM region plays an important role in subunit-subunit interaction (54).
The signaling pathway from STIM1 to Orai1 is still controversial. Using co-immunoprecipitation analyses, some studies suggest direct coupling between Orai1 and STIM1 (23, 24), but another does not (16). The chemically inducible bridge formation technique predicts that the length of the cytoplasmic domain of Orai1 is longer than 8–9 nm but shorter than 12–14 nm, and that of the cytoplasmic domain of STIM1 is 4–6 nm (55). Our reconstruction demonstrating the elongated cytoplasmic domain of Orai1 (∼10 nm) coincides with the above prediction. In considering the EM study estimating the distance between the plasma membrane and the ER membrane to be 10–25 nm (27), the structure presented here seems to support the direct binding of Orai1 with STIM1 (Fig. 7B), although it does not exclude the possibility that additional adaptor proteins bridge between these two molecules.
The C-terminal coiled-coil motif of Orai1 is shown to be essential in communication with the C-terminal region of STIM1 (residues 233–685) and for the activation of the CRAC channel (54, 56). The antibody decoration experiment demonstrated that the coiled-coil motif locates near the cytoplasmic end in the Orai1 structure, because the FLAG tag locates near the motif on the amino acid sequence. The N-terminal region (residues 74–90) of Orai1 is also shown to be important for channel function, but not necessary for interaction with STIM1 (54, 56). Because this region is three amino acids upstream from the first TM segment on the sequence, it is expected to locate just beneath the membrane.
Herein we have provided the structure of Orai1 alone. To understand the gating and regulation mechanisms as a CRAC channel, the structure of the Orai1-STIM1 molecular complex should be further clarified.
Acknowledgments
We thank M. Mio, S. Abe, and M. Yanagihara (National Institute of Advanced Industrial Science and Technology) for technical assistance in biochemistry and image analysis.
This work was supported by a Grant-in-Aid for Science Research on Priority Areas, Structure of Biological Macromolecular Assemblies, by a grant from the Japan New Energy and Industrial Technology Development Organization (to C. S.), by a grant from Precursory Research for Embryonic Science and Technology of the Japan Science Agency (to T. O.), and by grants from the National Institute of Advanced Industrial Science and Technology (AIST).
Footnotes
The abbreviations used are: ER, endoplasmic reticulum; CRAC, Ca2+ release-activated Ca2+; DDM, n-dodecyl β-d-maltoside; EM, electron microscopy; FSC, Fourier shell correlation; HEK, human embryonic kidney; HBS, HEPES-buffered saline; IP3, inositol 1,4,5-trisphosphate; NN, neural network; PNGase F, peptidyl N-glycosidase F; Rs, Stokes radius; SA, simulated annealing; SEC, size exclusion chromatography; SPINNS, single particle image analysis method using neural network and simulated annealing; TBS, Tris-buffered saline; TM, transmembrane; TG, thapsigargin.
References
- 1.Berridge, M. J., Bootman, M. D., and Roderick, H. L. (2003) Nat. Rev. Mol. Cell Biol. 4 517-529 [DOI] [PubMed] [Google Scholar]
- 2.Parekh, A. B., and Putney, J. W., Jr. (2005) Physiol. Rev. 85 757-810 [DOI] [PubMed] [Google Scholar]
- 3.Putney, J. W., Jr. (1986) Cell Calcium 7 1-12 [DOI] [PubMed] [Google Scholar]
- 4.Hoth, M., and Penner, R. (1992) Nature 355 353-356 [DOI] [PubMed] [Google Scholar]
- 5.Zweifach, A., and Lewis, R. S. (1993) Proc. Natl. Acad. Sci. U. S. A. 90 6295-6299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Partiseti, M., Le Deist, F., Hivroz, C., Fischer, A., Korn, H., and Choquet, D. (1994) J. Biol. Chem. 269 32327-32335 [PubMed] [Google Scholar]
- 7.Feske, S., Giltnane, J., Dolmetsch, R., Staudt, L. M., and Rao, A. (2001) Nat. Immunol. 2 316-324 [DOI] [PubMed] [Google Scholar]
- 8.Feske, S., Gwack, Y., Prakriya, M., Srikanth, S., Puppel, S. H., Tanasa, B., Hogan, P. G., Lewis, R. S., Daly, M., and Rao, A. (2006) Nature 441 179-185 [DOI] [PubMed] [Google Scholar]
- 9.Vig, M., Peinelt, C., Beck, A., Koomoa, D. L., Rabah, D., Koblan-Huberson, M., Kraft, S., Turner, H., Fleig, A., Penner, R., and Kinet, J. P. (2006) Science 312 1220-1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang, S. L., Yeromin, A. V., Zhang, X. H., Yu, Y., Safrina, O., Penna, A., Roos, J., Stauderman, K. A., and Cahalan, M. D. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 9357-9362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liou, J., Kim, M. L., Heo, W. D., Jones, J. T., Myers, J. W., Ferrell, J. E., Jr., and Meyer, T. (2005) Curr. Biol. 15 1235-1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roos, J., DiGregorio, P. J., Yeromin, A. V., Ohlsen, K., Lioudyno, M., Zhang, S., Safrina, O., Kozak, J. A., Wagner, S. L., Cahalan, M. D., Velicelebi, G., and Stauderman, K. A. (2005) J. Cell Biol. 169 435-445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mercer, J. C., Dehaven, W. I., Smyth, J. T., Wedel, B., Boyles, R. R., Bird, G. S., and Putney, J. W., Jr. (2006) J. Biol. Chem. 281 24979-24990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peinelt, C., Vig, M., Koomoa, D. L., Beck, A., Nadler, M. J., Koblan-Huberson, M., Lis, A., Fleig, A., Penner, R., and Kinet, J. P. (2006) Nat. Cell Biol. 8 771-773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soboloff, J., Spassova, M. A., Tang, X. D., Hewavitharana, T., Xu, W., and Gill, D. L. (2006) J. Biol. Chem. 281 20661-20665 [DOI] [PubMed] [Google Scholar]
- 16.Gwack, Y., Srikanth, S., Feske, S., Cruz-Guilloty, F., Oh-hora, M., Neems, D. S., Hogan, P. G., and Rao, A. (2007) J. Biol. Chem. 282 16232-16243 [DOI] [PubMed] [Google Scholar]
- 17.Gross, S. A., Wissenbach, U., Philipp, S. E., Freichel, M., Cavalié, A., and Flockerzi, V. (2007) J. Biol. Chem. 282 19375-19384 [DOI] [PubMed] [Google Scholar]
- 18.Williams, R. T., Manji, S. S., Parker, N. J., Hancock, M. S., Van Stekelenburg, L., Eid, J. P., Senior, P. V., Kazenwadel, J. S., Shandala, T., Saint, R., Smith, P. J., and Dziadek, M. A. (2001) Biochem. J. 357 673-685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang, S. L., Yu, Y., Roos, J., Kozak, J. A., Deerinck, T. J., Ellisman, M. H., Stauderman, K. A., and Cahalan, M. D. (2005) Nature 437 902-905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao, Y., Erxleben, C., Yildirim, E., Abramowitz, J., Armstrong, D. L., and Birnbaumer, L. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 4682-4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng, K. T., Liu, X., Ong, H. L., and Ambudkar, I. S. (2008) J. Biol. Chem. 283 12935-12940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prakriya, M., Feske, S., Gwack, Y., Srikanth, S., Rao, A., and Hogan, P. G. (2006) Nature 443 230-233 [DOI] [PubMed] [Google Scholar]
- 23.Yeromin, A. V., Zhang, S. L., Jiang, W., Yu, Y., Safrina, O., and Cahalan, M. D. (2006) Nature 443 226-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vig, M., Beck, A., Billingsley, J. M., Lis, A., Parvez, S., Peinelt, C., Koomoa, D. L., Soboloff, J., Gill, D. L., Fleig, A., Kinet, J. P., and Penner, R. (2006) Curr. Biol. 16 2073-2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu, P., Lu, J., Li, Z., Yu, X., Chen, L., and Xu, T. (2006) Biochem. Biophys. Res. Commun. 350 969-976 [DOI] [PubMed] [Google Scholar]
- 26.Luik, R. M., Wu, M. M., Buchanan, J., and Lewis, R. S. (2006) J. Cell Biol. 174 815-825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu, M. M., Buchanan, J., Luik, R. M., and Lewis, R. S. (2006) J. Cell Biol. 174 803-813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mignen, O., Thompson, J. L., and Shuttleworth, T. J. (2008) J. Physiol. 586 419-425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji, W., Xu, P., Li, Z., Lu, J., Liu, L., Zhan, Y., Chen, Y., Hille, B., Xu, T., and Chen, L. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 13668-13673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penna, A., Demuro, A., Yeromin, A. V., Zhang, S. L., Safrina, O., Parker, I., and Cahalan, M. D. (2008) Nature 456 116-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baba, Y., Hayashi, K., Fujii, Y., Mizushima, A., Watarai, H., Wakamori, M., Numaga, T., Mori, Y., Iino, M., Hikida, M., and Kurosaki, T. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 16704-16709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hara, Y., Wakamori, M., Ishii, M., Maeno, E., Nishida, M., Yoshida, T., Yamada, H., Shimizu, S., Mori, E., Kudoh, J., Shimizu, N., Kurose, H., Okada, Y., Imoto, K., and Mori, Y. (2002) Mol. Cell 9 163-173 [DOI] [PubMed] [Google Scholar]
- 33.Mori, Y., Wakamori, M., Miyakawa, T., Hermosura, M., Hara, Y., Nishida, M., Hirose, K., Mizushima, A., Kurosaki, M., Mori, E., Gotoh, K., Okada, T., Fleig, A., Penner, R., Iino, M., and Kurosaki, T. (2002) J. Exp. Med. 195 673-681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inoue, R., Okada, T., Onoue, H., Hara, Y., Shimizu, S., Naitoh, S., Ito, Y., and Mori, Y. (2001) Circ. Res. 88 325-332 [DOI] [PubMed] [Google Scholar]
- 35.Mio, K., Ogura, T., Mio, M., Shimizu, H., Hwang, T. C., Sato, C., and Sohma, Y. (2008) J. Biol. Chem. 283 30300-30310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogura, T., and Sato, C. (2001) J. Struct. Biol. 136 227-238 [DOI] [PubMed] [Google Scholar]
- 37.Ogura, T., and Sato, C. (2004) J. Struct. Biol. 145 63-75 [DOI] [PubMed] [Google Scholar]
- 38.Ogura, T., Iwasaki, K., and Sato, C. (2003) J. Struct. Biol. 143 185-200 [DOI] [PubMed] [Google Scholar]
- 39.Ogura, T., and Sato, C. (2004) J. Struct. Biol. 146 344-358 [DOI] [PubMed] [Google Scholar]
- 40.Ogura, T., and Sato, C. (2006) J. Struct. Biol. 156 371-386 [DOI] [PubMed] [Google Scholar]
- 41.Yazawa, M., Ferrante, C., Feng, J., Mio, K., Ogura, T., Zhang, M., Lin, P. H., Pan, Z., Komazaki, S., Kato, K., Nishi, M., Zhao, X., Weisleder, N., Sato, C., Ma, J., and Takeshima, H. (2007) Nature 448 78-82 [DOI] [PubMed] [Google Scholar]
- 42.van Heel, M., Harauz, G., Orlova, E. V., Schmidt, R., and Schatz, M. (1996) J. Struct. Biol. 116 17-24 [DOI] [PubMed] [Google Scholar]
- 43.Frank, J. (2006) Three-Dimensional Electron Microscopy of Macromolecular Assemblies: Visualization of Biological Molecules in Their Native State, pp. 91-109, Oxford University Press, New York
- 44.van Heel, M., Gowen, B., Matadeen, R., Orlova, E. V., Finn, R., Pape, T., Cohen, D., Stark, H., Schmidt, R., Schatz, M., and Patwardhan, A. (2000) Q. Rev. Biophys. 33 307-369 [DOI] [PubMed] [Google Scholar]
- 45.Penczek, P., Radermacher, M., and Frank, J. (1992) Ultramicroscopy 40 33-53 [PubMed] [Google Scholar]
- 46.Penczek, P. A., Grassucci, R. A., and Frank, J. (1994) Ultramicroscopy 53 251-270 [DOI] [PubMed] [Google Scholar]
- 47.Harauz, G., and Van Heel, M. (1986) Optik 73 146-156 [Google Scholar]
- 48.Sato, C., Ueno, Y., Asai, K., Takahashi, K., Sato, M., Engel, A., and Fujiyoshi, Y. (2001) Nature 409 1047-1051 [DOI] [PubMed] [Google Scholar]
- 49.Sato, C., Hamada, K., Ogura, T., Miyazawa, A., Iwasaki, K., Hiroaki, Y., Tani, K., Terauchi, A., Fujiyoshi, Y., and Mikoshiba, K. (2004) J. Mol. Biol. 336 155-164 [DOI] [PubMed] [Google Scholar]
- 50.Mio, K., Ogura, T., Kiyonaka, S., Hiroaki, Y., Tanimura, Y., Fujiyoshi, Y., Mori, Y., and Sato, C. (2007) J. Mol. Biol. 367 373-383 [DOI] [PubMed] [Google Scholar]
- 51.Maruyama, Y., Ogura, T., Mio, K., Kiyonaka, S., Kato, K., Mori, Y., and Sato, C. (2007) J. Biol. Chem. 282 36961-36970 [DOI] [PubMed] [Google Scholar]
- 52.Chang, W. C., Di Capite, J., Singaravelu, K., Nelson, C., Halse, V., and Parekh, A. B. (2008) J. Biol. Chem. 283 4622-4631 [DOI] [PubMed] [Google Scholar]
- 53.Luik, R. M., Wang, B., Prakriya, M., Wu, M. M., and Lewis, R. S. (2008) Nature 454 538-542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li, Z., Lu, J., Xu, P., Xie, X., Chen, L., and Xu, T. (2007) J. Biol. Chem. 282 29448-29456 [DOI] [PubMed] [Google Scholar]
- 55.Várnai, P., Tóth, B., Tóth, D. J., Hunyady, L., and Balla, T. (2007) J. Biol. Chem. 282 29678-29690 [DOI] [PubMed] [Google Scholar]
- 56.Muik, M., Frischauf, I., Derler, I., Fahrner, M., Bergsmann, J., Eder, P., Schindl, R., Hesch, C., Polzinger, B., Fritsch, R., Kahr, H., Madl, J., Gruber, H., Groschner, K., and Romanin, C. (2008) J. Biol. Chem. 283 8014-8022 [DOI] [PubMed] [Google Scholar]