Abstract
The neuron-specific K-Cl cotransporter KCC2 maintains the low intracellular chloride concentration required for the fast hyperpolarizing actions of inhibitory neurotransmitters. The KCC2 gene codes for two isoforms, KCC2a and KCC2b, which differ in their N termini. The relative expression and cellular distribution of the two KCC2 protein isoforms are unknown. Here, we characterize an antibody against the KCC2a isoform and show that a previously described antibody against KCC2 is specific for the KCC2b isoform (Hubner, C. A., Stein, V., Hermans-Borgmeyer, I., Meyer, T., Ballanyi, K., and Jentsch, T. J. (2001) Neuron 30, 515–524). Immunostaining of dissociated hippocampal cultures confirms that both KCC2 isoforms are neuron-specific. Immunoblot analysis indicates that KCC2b is the major KCC2 isoform in the adult brain, whereas in the neonatal mouse central nervous system, half of total KCC2 protein is KCC2a. At this stage, the two KCC2 isoforms are largely colocalized and show similar patterns of distribution in the brain. When coexpressed in HEK293 cells, KCC2a and KCC2b proteins form heteromeric complexes. Moreover, the two isoforms can be coimmunoprecipitated from the neonatal brain, suggesting the presence of endogenous KCC2a-KCC2b heteromers. Consistent with this, native gel analysis shows that a substantial part of endogenous KCC2 isoforms in the neonatal brain constitute dimers.
The neuron-specific K+-Cl- cotransporter KCC2 extrudes potassium and chloride ions from neurons, thus maintaining the low intracellular chloride concentration [Cl]i necessary for the fast hyperpolarizing actions of inhibitory neurotransmitters γ-aminobutyric acid (GABA) and glycine (1). KCC2 is an ∼140-kDa plasma membrane protein that belongs to the cation chloride cotransporter (CCC)4 family (2, 3). CCCs, including KCC2, are thought to exist in the plasma membrane as functional oligomers, although the mechanisms whereby oligomerization affects their transport activity are unclear (4–8).
We have recently shown that the KCC2 gene (alias Slc12a5) generates two mRNAs, KCC2a and KCC2b, by an alternative promoter and first exon usage (9). The difference between the KCC2a and KCC2b proteins lies in the most N-terminal part; the 40 unique amino acids in KCC2a include a putative binding sequence for the Ste20-related proline-alanine-rich kinase (SPAK). KCC2 null mutant mice deficient for both KCC2 isoforms show a disrupted breathing rhythm and die immediately after birth (10, 11), whereas selective KCC2b isoform knock-out mice exhibit spontaneous seizures but can survive up to 3 weeks after birth (9, 12). Thus, KCC2a obviously supports some vital functions of lower brain structures.
In general, KCC2 expression in the CNS follows neuronal maturation; it is first detected in the postmitotic neurons of the spinal cord and brainstem and is then gradually increased in higher brain structures (13, 14). Our previous work has shown that during postnatal development, KCC2a mRNA expression remains relatively constant, whereas KCC2b mRNA is strongly up-regulated in the cortex during postnatal development (9). This indicates that KCC2b is responsible for the developmental shift from depolarizing to hyperpolarizing GABAergic responses.
Here, we study the relative expression and cellular distribution of KCC2a and KCC2b proteins in the mouse brain. We characterize a new antibody specific for the KCC2a isoform and demonstrate that a previously described antibody against KCC2 (11) is specific for the KCC2b isoform. The relative expression of the KCC2 protein isoforms determined by immunoblot analysis correlates well with their mRNA levels (9). KCC2a and KCC2b proteins have a similar level and pattern of expression in the neonatal mouse brain and are colocalized in most neurons in non-cortical lower brain areas. Coimmunoprecipitation (coIP) experiments and coexpression followed by native gel analysis indicate that KCC2a-KCC2b heteromers can form in vitro and may exist in vivo.
EXPERIMENTAL PROCEDURES
Expression Constructs—KCC2a and KCC2b expression constructs were described previously (9). For GFP-KCC2b and Myc-KCC2b fusion constructs, the tags were fused in-frame to the N terminus of rat KCC2b using the EGFP-C1 (Clontech-Takara) and pcDNA3.1 (Invitrogen) expression vectors, respectively. Hemagglutinin-tagged SPAK (15) was kindly provided by Dr. B. Forbush.
Production of Anti-KCC2a Polyclonal Antibodies—A polyclonal rabbit antiserum was raised against a polypeptide comprising the predicted 21 N-terminal residues of rat KCC2a protein (DPESRRHSVADPRRLPREDVK). The peptide was conjugated to keyhole limpet hemocyanin via a terminal cysteine and maleimide cross-linker. The anti-KCC2a antiserum was produced and purified by Innovagen AB (Lund, Sweden).
Cell Culture, Transfection, and Immunocytochemistry—Human embryonic kidney (HEK) 293 cells (CLR-1573, American Type Culture Collection, Manassas, VA) were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 mm l-glutamine. Cells were transfected using jetPEI cationic polymer transfection reagent (Polyplus Transfection Inc., New York, NY) according to the manufacturer's protocol. Standard dissociated hippocampal neuronal cultures were prepared from embryonic day 17 (E17) mice, as described previously (16), with small modifications. Briefly, hippocampal cells were dissociated by enzymatic treatment (0.25% trypsin for 15 min at 37 °C) and plated on poly-dl-ornithine-coated coverslips (50,000 cells/cm2). The cells were grown in Neurobasal medium containing B27 supplement (Invitrogen) and preincubated on astroglial cultures for 24 h. After the indicated period of time, the neuronal cultures were fixed for 10 min in -20 °C cold methanol, washed in PBS, and blocked for 1 h at 24 °C in blocking solution (4% bovine serum albumin, 3% sheep serum, 0.2% Triton X-100, in PBS). The cells were first incubated in 2% bovine serum albumin, 1.5% sheep serum, 0.2% Triton X-100 in PBS containing anti-KCC2a rabbit polyclonal (1:1000) and anti-neuronal class III β-tubulin (TUJ-1) mouse monoclonal (Covance Research Products, Berkeley, CA; 1:5000) or anti-GFAP (glial fibrillary acidic protein) mouse monoclonal (Chemicon-Millipore, Billerica, MA; 1:1000) antibodies at 24 °C for 2 h and then washed three times for 10 min with PBS and incubated for 30 min at 37 °C with Alexa Fluor 488 donkey anti-mouse (1:400) and Alexa Fluor 568 donkey anti-rabbit (1:400) secondary antibodies (Molecular Probes, Eugene, OR). After three washings for 10 min in PBS and one washing in water, coverslips were mounted in VECTASHIELD HardSet mounting medium (Vector Laboratories, Burlingame, CA).
SDS-PAGE—For the analysis of KCC2 protein levels, HEK293 cells or tissue samples were homogenized on ice in lysis buffer (50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 1% Triton X-100, 0.5% deoxycholic acid, 0.1% SDS) supplemented with the Complete protease inhibitor mixture (Roche Diagnostics, Mannheim, Germany). After centrifugation for 10 min (15,000 × g) at 4 °C, protein concentrations of the supernatants were determined by using a DC protein assay kit (Bio-Rad Laboratories). The standard 2× Laemmli loading buffer contained (4% SDS, 10% β-mercaptoethanol, 20% glycerol, and 0.004% bromphenol blue in 125 mm Tris-HCl, pH 6.8). The samples were not heated because this may cause transmembrane proteins to form unspecific higher order complexes (17). Protein samples were separated using 10% Criterion XT precast Bis-Tris gels (Bio-Rad) and transferred onto Hybond-ECL nitrocellulose membrane (Amersham Biosciences, Buckinghamshire, UK).
PFO-PAGE—Analysis of protein complexes in native gels was done as described (18), with small modifications. Briefly, cell and tissue samples were prepared in TNE buffer (1% Nonidet P-40, 50 mm Tris-HCl, pH 8.0, 140 mm NaCl, 5 mm EDTA), supplemented with the Complete protease inhibitor mixture (Roche Diagnostics) and 25 mm iodoacetamide (Sigma) to prevent formation of artificial disulfide bonds. The 2× sample buffer contained 8% (w/v) sodium perfluoro-octanoate (NaPFO, Fluorochem Ltd., Derbyshire, UK), 25 mm dithiothreitol (Sigma), 0.004% bromphenol blue, 20% (v/v) glycerol, and 100 mm Tris base. Proteins were separated using 5% Criterion Tris-HCl gels (Bio-Rad) in PFO-glycine buffer (25 mm Tris, 192 mm glycine, 0.5% NaPFO, pH 8.5).
Immunoblotting—Blots were probed with the following primary antibodies (all diluted 1:2000) made in rabbit (anti-KCC2a (this study), anti-KCC2b (11), anti-KCC2pan (19), anti-SPAK (20) (a kind gift from Dr. H. Ushiro), and anti-GFP (Invitrogen)) or in mouse (anti-GAPDH (Chemicon-Millipore) and anti-Myc tag (clone 9E10, Sigma)) with subsequent probing with horseradish peroxidase-linked ECL anti-rabbit (GE Healthcare) or anti-mouse (DAKO, Glostrup, Denmark) antibodies. Blots were developed with Pierce ECL Western blotting substrate (Pierce Biotechnology), visualized with the luminescent image analyzer LAS-3000 (Fuji Photo Film, Tokyo, Japan), and analyzed with Advanced Image Data Analysis (AIDA) imaging software (Raytest, Straubenhardt, Germany). All measurements were within the linear range of the sensitivity of the camera.
To estimate the relative contribution of KCC2a to total KCC2 protein (KCC2a/total KCC2) in different brain areas and time points, we included known dilutions of KCC2a-expressing HEK293 cell lysates as standards. Membranes were stained with the KCC2a antibody, stripped, and then stained with the KCC2pan antibody. A standard curve for each antibody was made by plotting the concentration (-fold dilution) of the KCC2a standard on the x axis and measuring band intensity on the y axis and then fitting a line to the data using the “least squares” method. The ratio between their slopes was used as a coefficient to account for the different ability of the KCC2a and KCC2pan antibodies to bind KCC2a. The method is also based on the fact that the KCC2pan antibody is directed against the C terminus that is identical in both KCC2a and KCC2b. Similarly, by using KCC2b standards, we estimated the ratio of KCC2b/total KCC2 in the same samples (data not shown). Summation of the obtained KCC2a/total KCC2 and KCC2b/total KCC2 percentage values for each sample resulted in ∼100%.
Immunohistochemistry—Brains obtained from E18 wild-type or KCC2 null mutant mouse embryos were embedded unfixed in O.C.T. compound (Tissue-Tek, Sakura Finetek, Zoeterwoude, the Netherlands). Cryosections were dried at 24 °C for 30 min, postfixed in ice-cold methanol-acetone (1:1) for 10 min, and washed three times for 10 min with PBS. Sections were blocked in 10% normal donkey serum in PBS + 0.5% Triton X-100 for 2 h at 24 °C. Sections were incubated overnight at 4 °C with primary antisera against KCC2a (1:500), KCC2b (1:200), or KCC2pan (1:800) dissolved in 5% normal donkey serum in PBS + 0.5% Triton X-100. After washing three times in PBS, sections were incubated for 2 h at 24 °C with Cy3-conjugated donkey anti-rabbit antibody (1:300, Jackson ImmunoResearch Europe, Suffolk, UK) dissolved in 5% normal donkey serum in PBS + 0.5% Triton X-100. After three more washings in PBS, coverslips were mounted in PBS-glycerol.
For detection of KCC2a and KCC2b in the same section using two primary antibodies from the same species, we used the method of tyramine signal amplification followed by heating after the first secondary antibody step that prevents binding of the second secondary antibody to the first primary antibody (21). The sections were first blocked for 1 h with a blocking buffer (0.1 m Tris, 0.15 m NaCl, pH 7.5) supplemented with 0.5% blocking reagent (supplied by the TSA kit, PerkinElmer Life Sciences), supplemented with 5% bovine serum albumin and 1% normal donkey serum followed by staining with the KCC2b antibody (1:3000) overnight at 4 °C. Then, a SuperPicture horseradish peroxidase polymer conjugate for rabbit primary antibodies (Zymed Laboratories Inc., Invitrogen) was applied at a 1:2 dilution for 1 h followed by the tyramine-Cy3 reaction (TSA Plus system; PerkinElmer Life Sciences) according to the manufacturer's instructions. The sections were then heated in PBS to boiling point in a microwave oven for 10 min. This was followed by blocking for 1 h with 10% normal donkey serum, staining with the KCC2a antibody (1:1000) overnight at 4 °C, and finally labeling with an Alexa Fluor 488-conjugated donkey anti-rabbit secondary antibody (Invitrogen). Similar results were obtained when sections were first labeled with the KCC2a antibody and detected with tyramine-Cy3, and then heated and stained for KCC2b with Alexa Fluor 488 detection. In both cases, no specific Alexa Fluor 488 labeling was seen in control sections when the second primary antibody was omitted (data not shown).
coIP—CoIP experiments were performed according to Cai et al. (22), with small modifications. HEK293 cells, grown on 10-cm plates and transfected with appropriate expression constructs, were rinsed twice with ice-cold PBS, lysed for 15 min in ice-cold TNE buffer, supplemented with the Complete protease inhibitor mixture and the PhosStop phosphatase inhibitor mixture (Roche Diagnostics), and centrifuged for 10 min (12,000 × g) at 4 °C. Supernatant was preclarified for 1 h at 4 °C with blocked protein-G-Sepharose beads (GammaBind G-Sepharose, GE Healthcare) and incubated overnight with 2 μg of the appropriate antibody. Thereafter, the blocked beads were added again, and incubation continued for 2 h. After washing of the beads twice with the ice-cold TNE buffer for 10 min and twice with PBS for 5 min, the bound proteins were eluted by SDS sample buffer (1% SDS, 100 mm dithiothreitol, 50 mm Tris, pH 7.5) and separated by SDS-PAGE.
CoIP using E18 rat brainstem as a starting material was performed as above, with small modifications. Lysis time was increased to 1 h, and 1% Triton X-100 was added into the lysis buffer. The lysis was followed by a prolonged (30 min, 6000 × g) centrifugation at 4 °C. For each coIP reaction, 5 mg of the total protein was used. The Sepharose beads were washed four times in TNE buffer and once in PBS.
RESULTS
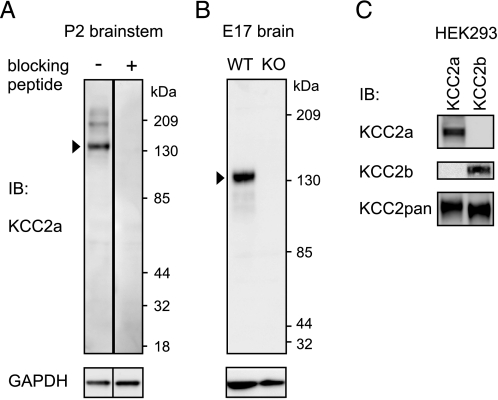
Characterization of Antibodies Specific for the KCC2a and KCC2b Isoforms—To study KCC2a protein expression in vivo, we developed a polyclonal antibody against the N-terminal part of KCC2a (amino acids 20–40 of mouse KCC2a sequence, see “Experimental Procedures”). Immunoblot analysis of neonatal (postnatal day 2 (P2)) mouse brain lysates indicated that the antibody detects a protein product of the size (∼140 kDa) expected for endogenous KCC2a monomer (Fig. 1A). This signal was absent when the antibody was preincubated with the immunizing peptide. The ∼140-kDa band corresponding to the KCC2 protein was absent from brain lysates of KCC2 knock-out (10) embryos (Fig. 1B).
FIGURE 1.
Specific antibodies against the KCC2a and KCC2b isoforms. A, whole-brain lysates obtained from P2 mice were analyzed by standard SDS-PAGE and immunoblotting (IB) with affinity-purified KCC2a antiserum (see “Experimental Procedures”). The KCC2a antiserum (diluted 1: 2000) detects a major product of ∼140 kDa (arrowhead, corresponding to KCC2a monomer), and occasionally (cf. B), a minor band of ∼200 kDa. A similar, higher molecular mass band observed with other KCC2 antibodies was suggested to represent the interaction of KCC2 with other proteins (28). These bands are not observed when the antiserum is preadsorbed with 1 μg/ml immunizing peptide. As a control, the membrane was reblotted with an antibody against GAPDH. B, whole-brain lysates of embryonic day 17.5 (E17) wild-type (WT) mice and their KCC2 null mutant (KO) littermates were analyzed as above. The KCC2a antiserum detected the expected product of ∼140 kDa from the WT but not from the KO lysate. C, lysates of HEK293 cells, transiently transfected with KCC2a or KCC2b expression plasmids, were analyzed as above with KCC2a, KCC2b, or KCC2pan antibodies (see “Experimental Procedures”). The KCC2a antiserum detected a product of ∼140 kDa from lysates of HEK293 cells transfected with the KCC2a plasmid but not with the KCC2b expression vector. Similarly, the KCC2b antiserum stained a product of ∼140 kDa from lysates of KCC2b-expressing but not KCC2a-expressing cells. The KCC2b antibody was raised against a 15-amino-acid peptide corresponding to the N terminus of the KCC2b isoform (11). Because the last five amino acids in the peptide are common to both KCC2 isoforms, the specificity of the antibody for the KCC2b isoform is notable.
To further verify the specificity of the KCC2a antibody, we analyzed protein lysates of HEK293 cells (which do not express KCC2 endogenously) transfected with either KCC2a or KCC2b expression vectors. A previously described KCC2pan antibody against a C-terminal peptide common for both KCC2 isoforms (19) detected both KCC2a and KCC2b proteins, as expected, whereas the KCC2a antibody revealed a protein product of the expected size from cells transfected with the KCC2a but not with the KCC2b plasmid (Fig. 1C).
In addition, we found that a previously published antibody against KCC2 (11) specifically recognized the KCC2b but not the KCC2a isoform from HEK293 lysates transfected with the corresponding expression plasmids (Fig. 1C). Here, this antibody is renamed as the KCC2b antibody.
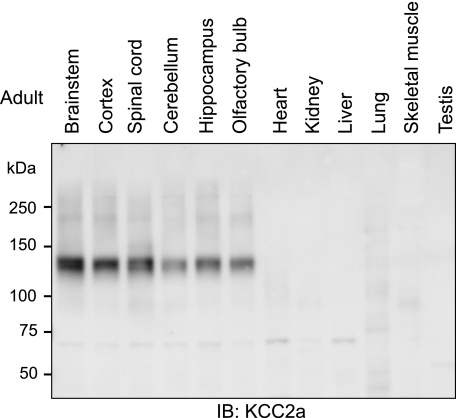
KCC2a Protein Is Expressed Only in the CNS—To study the distribution of KCC2a in adult mouse tissues, equal amounts of protein lysates from five brain regions, the spinal cord, and six non-neural tissues were analyzed by immunoblotting using the KCC2a antibody. A product of 140 kDa, close to the predicted molecular mass of 126 kDa for the KCC2a protein, was detected in the spinal cord and in all brain regions analyzed, being the strongest in the brainstem (Fig. 2). No bands corresponding to the KCC2a protein were seen in any of the non-neural tissues (Fig. 2). This result is consistent with our previous data showing that KCC2a mRNA is detectable exclusively in the CNS (9).
FIGURE 2.
CNS-specific expression of KCC2a protein in adult mouse tissues. Equal amounts (20 μg) of total protein lysates obtained from different CNS regions, as well as from several non-neural tissues of adult (48-week-old) mice, were subjected to standard immunoblotting (IB) procedure with the KCC2a antibody. A product of ∼140 kDa was detected in all CNS but not in non-neural tissues. Similar results were obtained in two independent experiments.
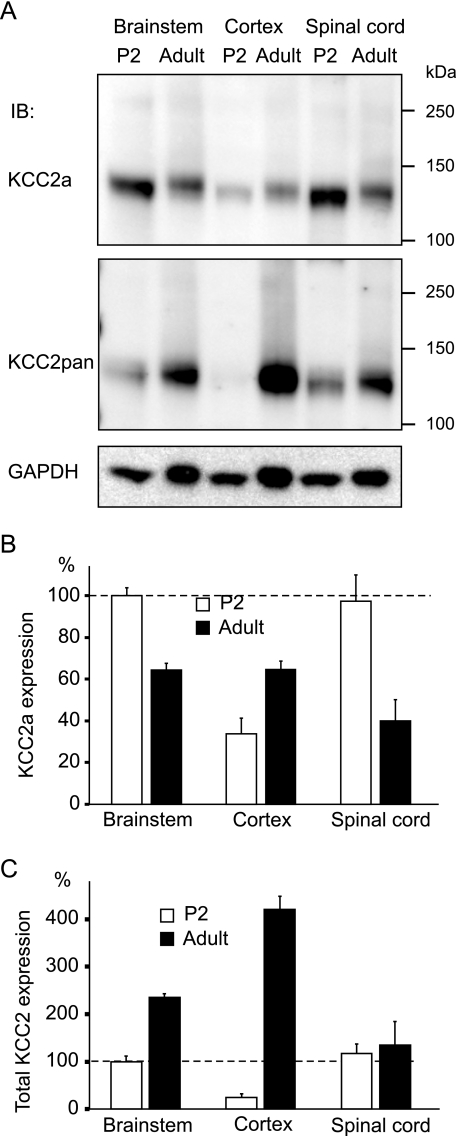
KCC2a Protein Expression Remains Relatively Constant during Postnatal Development—KCC2a and KCC2b isoforms are transcribed from different promoters, and thus, their expression is differently regulated (9). To study the expression of the KCC2 isoforms during postnatal development, we analyzed protein lysates of the brainstem, cortex, and spinal cord taken from P2 and adult (48-week-old) mice by immunoblotting. The samples were prepared and separated under standard reducing and denaturing conditions that resulted in the KCC2 proteins migrating as monomers in the gel. The blots were probed sequentially with the KCC2a, KCC2pan, and GAPDH antibodies (Fig. 3). KCC2a expression in the P2 brainstem and spinal cord was similar and ∼3-fold higher than expression in the P2 cortex (Fig. 3, A and B). When compared with P2, KCC2a protein levels in adults were ∼2-fold lower in the brainstem and spinal cord but ∼2-fold higher in the cortex, resulting in comparable KCC2a protein levels between the adult brain areas. KCC2pan antibody staining indicated an ∼15-fold higher expression of total KCC2 (KCC2a+KCC2b) in the adult than in the P2 cortex. Total KCC2 expression, by contrast, was increased only moderately (2-fold) in the brainstem and not significantly in the spinal cord between the P2 and adult time points (Fig. 3, A and C). In summary, KCC2a protein expression shows only moderate changes during postnatal mouse development, whereas total KCC2 (and thus KCC2b) expression is strongly up-regulated especially in the cortex.
FIGURE 3.
Although total KCC2 expression is increased in the cortex during postnatal development, KCC2a levels remain constant. A, equal amounts (20 μg) of total protein lysates obtained from brainstems, cortices, and spinal cords of P2 or adult (48-week-old) mice were analyzed by standard SDS-PAGE and detected sequentially with the KCC2a (upper panel) and KCC2pan (middle panel) antibodies. A protein of 140 kDa, close to the predicted molecular mass for KCC2a, was observed in all samples analyzed. IB, immunoblot. B, shown is the result of quantification of four independent experiments, one of which is presented in A. Expression of KCC2a in the mouse brainstem and spinal cord was about 2-fold higher in P2 than in adult mice. By contrast, KCC2a expression in the cortex was about 2-fold lower in P2 than in adult mice. Values are mean ± S.E. (error bars) of four independent experiments. C, total KCC2 expression (detected with KCC2pan antibody) was dramatically up-regulated in the cortex between P2 and adulthood but increased only moderately in the brainstem. In the spinal cord, total KCC2 expression did not change significantly between the P2 and adult time points. Values are mean ± S.E. (error bars) of four independent experiments.
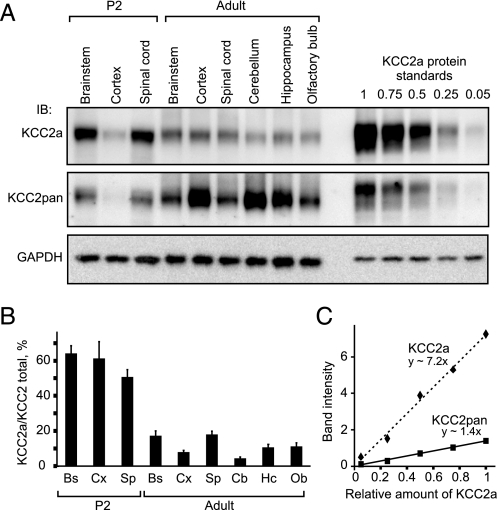
Approximately Half of Total KCC2 Protein in Neonatal Mouse CNS Is KCC2a—Expression of KCC2 mRNA and protein is high in mouse brainstem and spinal cord neurons already at birth (11, 23). Our previous study indicated that KCC2a contributes up to 50% of total KCC2 mRNA expression in these areas (9). To estimate the relative abundance of KCC2a versus total KCC2 protein in different regions of neonatal and adult mouse CNS, we exploit the KCC2pan antibody that is directed against the common C terminus and is thus expected to recognize the two KCC2 isoforms with equal affinity. Lysates of spinal cord and several brain regions from P2 and adult mice were analyzed by immunoblotting in parallel with KCC2a standards (known dilutions of a lysate of HEK293 cells overexpressing KCC2a). Membranes were stained with the KCC2a antibody, stripped, and then stained with the KCC2pan antibody (Fig. 4A). Standard curves for the KCC2a and KCC2pan antibodies were derived from measured band intensities of known KCC2a amounts (Fig. 4C) and used to quantify the ratio of KCC2a to total KCC2 protein in the CNS samples. In newborn mice, KCC2a protein made up about 60% of the total KCC2 expression in the brainstem and cortex and about 50% in the spinal cord (Fig. 4B). By contrast, the KCC2b isoform was the prevalent KCC2 form in all adult CNS areas analyzed. The lowest relative expression of KCC2a in the adult mouse CNS was seen in the cerebellum (∼5% of total KCC2 protein), and the highest (∼20%) was seen in the brainstem and spinal cord (Fig. 4B). Similar results were obtained by analyzing the ratio of KCC2b to total KCC2 protein from the same samples including KCC2b protein standards using the KCC2b and KCC2pan antibodies (data not shown). To sum up, the KCC2a and KCC2b protein levels are comparable in the neonatal mouse CNS, whereas most of the KCC2 protein in the adult mouse is of the KCC2b isoform.
FIGURE 4.
KCC2a contributes at least half of the total KCC2 protein in the neonatal mouse, whereas KCC2b is the predominant isoform in the adult brain. A, equal amounts (20 μg) of total protein lysates obtained from spinal cords and different brain regions of P2 or adult (48-week-old) mice were analyzed in parallel with KCC2a protein standards by immunoblotting. Membrane was first immunoblotted (IB) with the KCC2a antibody (upper panel), stripped, and then processed with KCC2pan (middle panel) antibody. GAPDH expression in the samples is shown as a loading control (lower panel). B, relative (percentage) contribution of KCC2a to total KCC2 protein expression in different CNS regions of neonatal (P2) and adult mice. Shown is the result of quantification of five independent experiments, one of which is presented in A. Band intensities of the ∼140-kDa protein product, corresponding to the predicted molecular mass for KCC2a and KCC2b, were measured, and the ratio of KCC2a to total KCC2 was calculated by using standard curves (panel C, see “Experimental Procedures”). KCC2a contributes about 50–65% of the total KCC2 expression in the neonatal mouse brainstem (Bs), cortex (Cx), and spinal cord (Sp). By contrast, KCC2a is a minor KCC2 isoform in the adult CNS; the percentage of KCC2a of the total KCC2 ranges from 4% in the cerebellum (Cb) to 17–18% in the brainstem and spinal cord. Intermediate values are seen in the cortex (8%), hippocampus (Hc, 10%), and olfactory bulb (Ob, 11%). Values are mean ± S.E. (error bars) of five independent experiments. C, standard curves for the KCC2a and KCC2pan antibodies were made by plotting the concentration of the KCC2a standard (= different dilutions, from undiluted up to 1/20, of a KCC2a-expressing HEK293 cell lysate) on the x axis and the measured band intensities on the y axis and then fitting a line to the data.
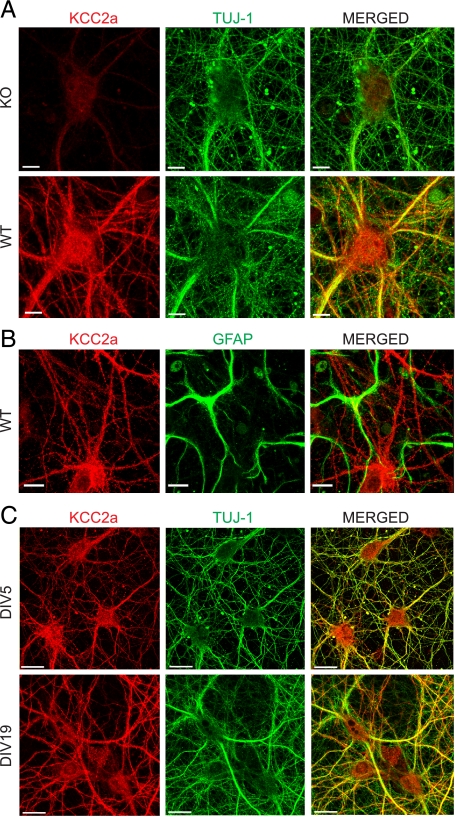
Neuron-specific Expression and Subcellular Localization of the KCC2a Isoform—To test whether the KCC2a antibody is able to detect endogenous KCC2a in neurons, parallel cultures of hippocampal neurons (cultured for 11 days in vitro, DIV11) from wild-type and KCC2 null mutant (10) embryos were fixed in methanol and double-immunostained with the KCC2a and TUJ-1 (neuron-specific β-III-tubulin) antibodies. A strong KCC2a immunoreactive signal was detected in the TUJ1-positive neurons from wild-type embryos, whereas only weak background labeling was observed in the TUJ1-positive neurons from the KCC2 null mutant embryos (Fig. 5A). To confirm that the KCC2a antibody labeling is neuron-specific, co-cultures of hippocampal neurons and astroglial cells were double-stained with the KCC2a and GFAP (marker for astroglial cells) antibodies. All of the GFAP-positive glial cells were KCC2a-negative (Fig. 5B). To study the subcellular localization of the KCC2a protein during neuronal development, DIV5 and DIV19 neuron cultures were fixed and double-stained for the KCC2a and TUJ-1 as above and analyzed by confocal microscopy. KCC2a immunoreactivity was located throughout the cell soma and dendritic shafts already in DIV5 neurons (Fig. 5C). We did not find any apparent change in KCC2a expression levels or localization between DIV5 and the more mature DIV19 cultures; the KCC2a signal was always neuron-specific and was equally distributed in the cell bodies and dendritic shafts (Fig. 5C). To summarize, the results indicate that the KCC2a antibody can detect the endogenous KCC2a isoform in methanol-fixed cultures and confirm the exclusively neuron-specific expression of KCC2a protein.
FIGURE 5.
Localization of the KCC2a isoform in cultured neurons. Shown are maximum intensity projections of confocal optical images. A, hippocampal neurons, derived from E17 WT or KCC2 null mutant (KO) mice and cultured for 11 days in vitro (DIV11), were double-immunostained (see “Experimental Procedures”) with antibodies against KCC2a (red) and neuron-specific β-III tubulin (TUJ-1) (green). WT and KO cultures were treated in parallel, and the same settings were applied during the imaging procedures, thus allowing a direct comparison of the KCC2a staining in WT and KO neurons. KCC2a antibody revealed a strong KCC2a protein expression in WT neurons, whereas only residual background staining was found in KO neurons. B, DIV11 hippocampal cultures from WT mice were double-stained with antibodies against KCC2a (red) and GFAP (green). Non-overlapping patterns revealed by these antibodies confirmed an exclusively neuron-specific expression of the KCC2a protein. C, mouse hippocampal neuronal cultures, prepared similar to A and cultured for 5 or 19 days, were double-immunostained with KCC2a (red) and TUJ-1 (green) antibodies. KCC2a expression was exclusively neuronal at all time points analyzed. KCC2a immunostaining was present in cell bodies and dendritic shafts already at DIV5 and remained similar in DIV19 neurons. Scale bar is 10 μmin A and B and 20 μmin C.
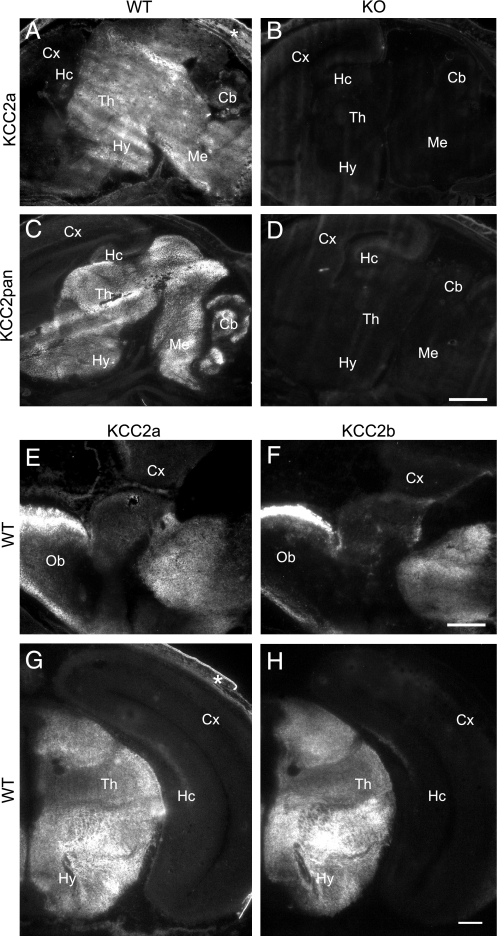
KCC2a and KCC2b Have Similar Expression Patterns in Perinatal Mouse Brain—Applicability of the KCC2a antibody for immunohistochemistry was tested on cryosections from E18 wild-type and KCC2 null mutant mice. A standard 4% paraformaldehyde fixation protocol led to a high background (data not shown), whereas staining of sections fixed in cold methanol-acetone (1:1) gave a clear signal in the wild-type mice (Fig. 6A) and little or no signal in the KCC2 null mutant mice (Fig. 6B). As expected, no staining above background was seen in sections stained with the KCC2a antibody that was preadsorbed with the peptide used for immunization (data not shown). Strong KCC2a immunoreactivity was seen in non-cortical regions of E18 mouse brain (including olfactory bulb, basal forebrain, hypothalamus, thalamus, midbrain, and hindbrain), whereas labeling of cerebral cortex and hippocampus was close to the background level (Fig. 6, A, E, and G). Parallel sections stained with the KCC2pan antibody, which recognizes both KCC2a and KCC2b isoforms, produced a largely similar pattern of staining (Fig. 6C). Specificity of the KCC2pan antibody staining was further confirmed on sections from the KCC2 null mutant mice (Fig. 6D). To compare the cellular distributions of the KCC2a and KCC2b proteins in E18 mouse brain, we stained adjacent sections with KCC2a- and KCC2b-specific antibodies. Specificity of the KCC2b antibody was confirmed on sections from the KCC2 null mutant mice (data not shown). Interestingly, the distribution of KCC2a and KCC2b immunoreactivities was remarkably similar (Fig. 6, E–H), suggesting that the two isoforms are colocalized in the E18 mouse brain.
FIGURE 6.
KCC2a and KCC2b protein isoforms have a similar pattern of expression in E18 mouse brain. Sagittal (A–D) or coronal (E–H) cryosections of E18 WT (A, C, and E–H) or KCC2 null mutant (KO, B and D) mice were analyzed by immunohistochemistry (see “Experimental Procedures”) with KCC2a (A, B, E, and G), KCC2pan (C and D), and KCC2b (F and H) antibodies. Immunoreactivity for KCC2a was widely distributed in non-cortical brain areas (A) of wild-type mice and was absent in the KCC2 null mutant littermates (B). The KCC2pan antibody produced a pattern similar to KCC2a antibody in wild-type mice (C) and no staining in sections from KCC2 null mutant embryos (D). Immunostaining of adjacent sections with the KCC2a and KCC2b antibodies revealed a similar distribution of the two KCC2 isoforms in the olfactory bulb (compare E and F) and thalamus (compare G and H). All three KCC2 antibodies detected only a weak signal in the cerebral cortex and hippocampus. The signal in the skin (asterisk in A and G) was unspecific. Cb, cerebellum; Cx, neocortex; Hc, hippocampus; Hy, hypothalamus; Me, medulla; Ob, olfactory bulb; Th, thalamus. Scale bar is 1 m min A–D; 0.5 mm in E and F, and 0.2 mm in G and H.
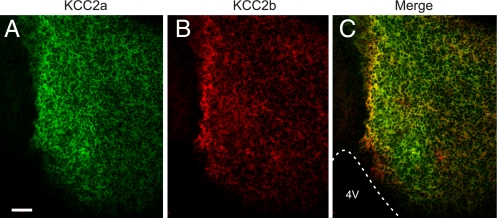
KCC2a and KCC2b Are Colocalized in Perinatal Mouse Brain Neurons—To demonstrate that the two KCC2 isoforms are colocalized in the E18 mouse brain, we applied a method for simultaneous visualization of multiple antigens using primary antibodies from the same species (21). Consistent with the data from adjacent sections (Fig. 6, E and F), most non-cortical brain areas were positive for both KCC2a and KCC2b, although the relative expression of the two isoforms showed some regional variability (Fig. 7, A–C, and data not shown). Analysis of merged images demonstrated that many of the positive neurons coexpressed both isoforms.
FIGURE 7.
KCC2a and KCC2b are colocalized in E18 mouse brain neurons. Shown are confocal optical section images of a coronal cryosection through E18 mouse midbrain double-stained with the KCC2a (green) and KCC2b (red) antibodies (see “Experimental Procedures”). C is the merged image of A and B. Most neurons in this area coexpress KCC2a and KCC2b, although the distribution is not identical. 4V, fourth ventricle. Scale bar is 50 μm.
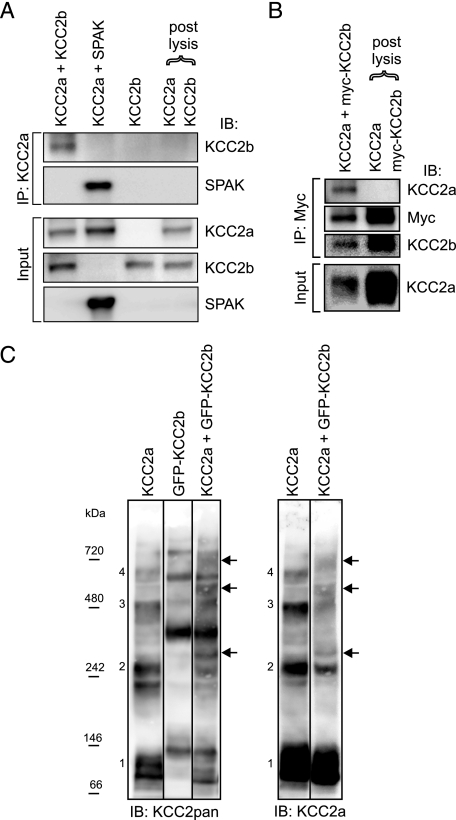
KCC2a-KCC2b Heteromers Can Be Formed in HEK293 Cells— To address whether KCC2a and KCC2b isoforms form hetero-oligomers when coexpressed in HEK293 cells, we performed reciprocal coIP experiments. After precipitation with the KCC2a antibody, KCC2b protein was detected in lysates of HEK293 cells coexpressing both isoforms simultaneously but not in a combined lysate of cells expressing these proteins separately (Fig. 8A). As a positive control, KCC2a antibody successfully precipitated overexpressed SPAK (Fig. 8A), consistent with the consensus SPAK/OSR1 binding site in KCC2a (9), thus demonstrating that SPAK can interact with KCC2a. Similarly, after immunoprecipitation of Myc-tagged KCC2b, KCC2a protein was detected in lysates of cells coexpressing both proteins but not in the combined lysate of cells expressing these proteins separately (Fig. 8B). Similar results were obtained by coIP using GFP-tagged KCC2b and KCC2a (data not shown).
FIGURE 8.
KCC2a can interact with KCC2b in vitro. HEK293 cells were transfected with the indicated plasmids and lysed 48 h later. A and B, the lysates were immunoprecipitated (IP) with the indicated antibodies and analyzed for interacting partners using standard SDS-PAGE and immunoblotting (IB). A, after precipitation with the KCC2a antibody, immunoblotting with the KCC2b antibody detected a band of ∼140 kDa (corresponding to KCC2b) from cells coexpressing KCC2a and KCC2b but not from the combined lysate of cells expressing KCC2a and KCC2b separately (upper panel of IP:KCC2a part). Immunoblotting with the SPAK antibody confirmed that KCC2a is able to interact with SPAK (lower panel of IP: KCC2a part). Analysis of the lysates (Input) ensured that the proteins were successfully expressed. B, after precipitation with an anti-Myc antibody, immunoblotting with the KCC2a antibody detected a band corresponding to KCC2a from cells coexpressing KCC2a and Myc-KCC2b fusion construct but not from the combined lysate of cells expressing KCC2a and Myc-KCC2b separately (upper panel of IP:Myc part). Expression of KCC2a in the cell lysates is also shown (Input). For simplicity, only the monomer bands of KCC2 are shown in A and B. C, the lysates were separated in native PFO gel and analyzed by immunoblotting sequentially with KCC2pan (left panel) and KCC2a (right panel) antibodies. Bands corresponding to the expected size of monomeric (band 1) and homo-oligomeric (band 2–4) forms of KCC2a are numbered. The corresponding bands for the GFP-KCC2b fusion protein are larger due to the additional GFP moiety (∼30 kDa). Bands corresponding to the expected size of hetero-oligomers between KCC2a and GFP-KCC2b (marked by arrows) are seen between the KCC2a and GFP-KCC2b homo-oligomeric bands, and they appear more prominent in the left than in the right panel because the KCC2pan antibody can bind both KCC2a and GFP-KCC2b in the hetero-oligomers.
As an independent method to demonstrate the ability of KCC2a and KCC2b to form heteromers in the HEK293, we used native gel electrophoresis to separate the homo- and hetero-oligomers of KCC2a and GFP-KCC2b by size. When expressed alone, the KCC2a and GFP-KCC2b proteins ran as monomers and as higher order species corresponding to dimers, trimers, and tetramers (Fig. 8C). In contrast, when KCC2a and GFP-KCC2b were coexpressed, distinct bands corresponding to the expected size of hetero-oligomers between KCC2a and GFP-KCC2b were detected in addition to the KCC2a and GFP-KCC2b homo-oligomers (Fig. 8C). Taken together, these data indicate the ability of KCC2a and KCC2b, when coexpressed in vitro, to form heteromers.
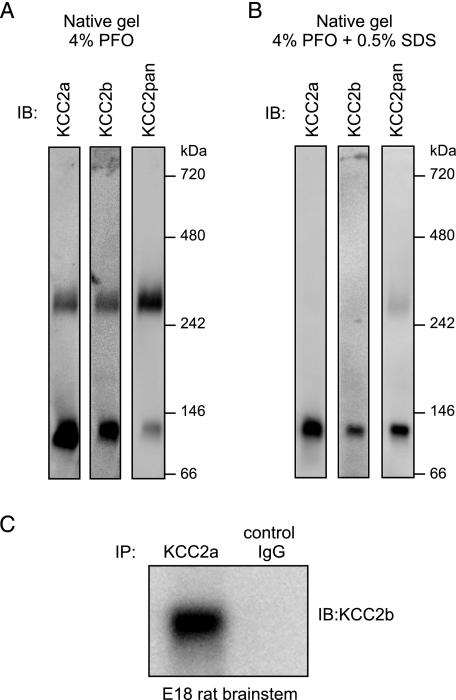
KCC2a-KCC2b Heteromers May Exist Endogenously in the Brain—Several members of the CCC family have been reported to form homo-oligomers in vivo (4, 6) and even heteromers, at least in vitro (24). To study whether endogenous KCC2 isoforms exist as oligomers, neonatal (P2) mouse brain lysates were analyzed by native gel electrophoresis. Immunoblotting with the KCC2 isoform-specific antibodies indicated that KCC2a and KCC2b exist both as monomers and as dimers in the neonatal mouse brain (Fig. 9A). No trimers or higher order complexes of KCC2 were detected under these conditions. The monomer/dimer ratio was similar between the KCC2a and KCC2b isoforms. Interestingly, the KCC2 dimer band appeared stronger than the KCC2 monomer band when the blot was detected with the KCC2pan antibody (directed against the common C terminus), in contrast to the isoform-specific antibodies (directed against the unique N termini). In comparison, when SDS was added up to 0.5% to the same sample 20 min before loading it, both KCC2a and KCC2b ran mostly as monomers in the native gel (Fig. 9B).
FIGURE 9.
The two KCC2 isoforms form dimers and interact with each other in vivo. A, native PFO-PAGE analysis of protein complexes present in neonatal (P2) mouse brain lysates (see “Experimental Procedures”). Immunoblotting (IB) with antibodies to KCC2a and KCC2b (directed against their unique N termini) indicates that a substantial part of both KCC2 isoforms exists as dimers in the neonatal brain. Note that the relative amount of KCC2 dimers versus monomers appears to be higher when detected with the KCC2pan antibody (directed against the common C terminus). B, the same sample as in A, but 0.5% SDS was included in the sample 20 min before loading to PFO-PAGE. C, a lysate of E18 rat brainstem was subjected to coIP with the KCC2a or a control antibody (normal rabbit IgG), and the precipitate was analyzed by immunoblotting with the KCC2b antibody. A band of 140 kDa, corresponding to the KCC2b, was detected in the sample after coIP with KCC2a but not with the control antibody.
To study whether endogenous KCC2a may form heteromers with KCC2b in vivo, coIP using the KCC2a antibody was performed with a lysate of E18 rat brainstem. KCC2b protein was detected in a complex with KCC2a only when the KCC2a but not a control antibody was used for coIP (Fig. 9C). This result indicates that the endogenous KCC2a and KCC2b proteins interact in vivo.
DISCUSSION
Here, we have characterized antibodies specific for the KCC2a and KCC2b isoforms. Although KCC2b is the major KCC2 isoform in the adult brain, we show that in the neonatal mouse brain, the two KCC2 isoforms have similar protein levels and distribution, are coexpressed in many neurons, and can form heterodimers.
To compare the relative protein levels of the KCC2 isoforms in mouse brain, we used standard denaturing conditions so that the isoforms appear as monomers in immunoblot analysis. Our method to estimate relative levels of KCC2a (or KCC2b) versus total KCC2 protein in different brain areas and in two age groups relied on the expected equal affinity of the KCC2pan antibody (directed against the common C terminus) on the two isoforms. The validity of this method is supported by the similar results obtained using either KCC2a-specific or KCC2b-specific antibodies. Moreover, the relative amount of the two KCC2 protein isoforms estimated in different brain areas and at two developmental time points reflects well their mRNA levels (9).
Immunostaining of adjacent sections of perinatal (E18) mouse brain with antibodies specific for KCC2a and KCC2b showed a similar, but not identical, pattern of expression for the two isoforms. Most neurons in non-cortical brain structures were positive for both KCC2a and KCC2b. Consistent with this, double staining with the KCC2a and KCC2b antibodies showed that many neurons in these areas coexpressed the two KCC2 isoforms. A more detailed mapping of their cellular distribution at different developmental stages is ongoing.
The ability of KCC2a and KCC2b to form hetero-oligomers was demonstrated in HEK293 cells using two different methods. The physical interaction between the KCC2a and KCC2b isoforms was shown by reciprocal coIP experiments. Control experiments verified that KCC2a-KCC2b complexes were not formed unspecifically after the lysis during the coIP procedure. Native gel analysis of protein lysates from cells expressing KCC2a and GFP-KCC2b showed distinct bands apparently corresponding to heteromers of KCC2a and GFP-KCC2b fusion protein migrating between the bands corresponding to the KCC2a and GFP-KCC2b homo-oligomers. Finally, coIP from neonatal rat brainstem lysates demonstrated association of KCC2a and KCC2b isoforms, suggesting that KCC2a/KCC2b heteromers may exist in vivo.
Endogenous KCC2 migrates generally as a monomer in standard SDS-PAGE (25). In contrast, native gel analysis of protein complexes demonstrated that a major part of endogenous KCC2a and KCC2b exists as dimers. The results are consistent with a previous report that endogenous KCC2 proteins exist as oligomers in the plasma membrane (4). However, in contrast to that study, our results indicate that the KCC2 dimers (from total brain lysates homogenized in 1% Triton) are sensitive to SDS, as described previously for other CCCs (4, 6). In contrast, KCC2 overexpressed in HEK293 cells appears in oligomeric form even in the presence of SDS, suggesting that the protein concentration (and probably sample preparation) may affect oligomerization. Another discrepancy concerns the relative amount of KCC2 oligomers versus monomers in the neonatal brain (4). Our observation that antibodies against the N and C termini of KCC2 seem to differ in detecting the monomeric versus dimeric forms of KCC2 suggests that the C terminus of the KCC2 monomer or the N termini in the dimer are masked. Further studies are warranted to resolve these issues.
Although we show that KCC2 isoforms can form heteromers in vitro and demonstrate that KCC2 exists as a dimer and may form heteromers in the neonatal brain, the functional role of this interaction remains elusive. There is indirect evidence that oligomerization leads to the functional activation of KCC2 (4) and KCC1 (5) proteins. In the neonatal brain where KCC2a and KCC2b are colocalized in many neurons, a substantial part of KCC2 oligomers may represent KCC2a-KCC2b heteromers, whereas KCC2b homomers would predominate in the mature CNS. Neonatal brainstem (23) and retinal (26) neurons are reported to express KCC2 protein, which is not, however, functional. This might be because KCC2 molecules are not properly transported to (26) or oligomerized (4) in the plasma membrane or require additional protein modifications (e.g. phosphorylation) (27). Consistent with the SPAK binding motif in KCC2a, coIP experiments in HEK cells showed that SPAK can physically bind KCC2a. Thus, KCC2b homomers could be regulated differently than the KCC2a-containing hetero- or homomers. Our work sets the stage for future studies on the endogenous KCC2a-KCC2b heteromers to dissect the specific physiological roles of the two KCC2 isoforms and their interactions.
Acknowledgments
We thank Peter Blaesse for comments, Seija Lågas, Outi Nikkilä, and Miika Palviainen for the neuronal cultures, Hiroshi Ushiro for the anti-SPAK antibody, and Biff Forbush for the SPAK expression construct.
This work was supported by grants from the Academy of Finland, the University of Helsinki, and the Sigrid Jusélius Foundation (to M. S. A. and C. R.).
Footnotes
The abbreviations used are: CCC, cation chloride cotransporter; CNS, central nervous system; SPAK, Ste20-related proline-alanine-rich kinase; GFP, green fluorescent protein; HEK, human embryonic kidney; PBS, phosphate-buffered saline; Bis-Tris, 2-(bis(2-hydroxyethyl)amino)-2-(hydroxymethyl)propane-1,3-diol; PFO, perfluoro-octanoate; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GFAP, glial fibrillary acidic protein; IP, immunoprecipitation; coIP, coimmunoprecipitation; E, embryonic day; DIV, days in vitro; P, postnatal day; KO, knockout; WT, wild type.
References
- 1.Blaesse, P., Airaksinen, M. S., Rivera, C., and Kaila, K. (2009) Neuron 61 820-838 [DOI] [PubMed] [Google Scholar]
- 2.Payne, J. A., Stevenson, T. J., and Donaldson, L. F. (1996) J. Biol. Chem. 271 16245-16252 [DOI] [PubMed] [Google Scholar]
- 3.Gamba, G. (2005) Physiol. Rev. 85 423-493 [DOI] [PubMed] [Google Scholar]
- 4.Blaesse, P., Guillemin, I., Schindler, J., Schweizer, M., Delpire, E., Khiroug, L., Friauf, E., and Nothwang, H. G. (2006) J. Neurosci. 26 10407-10419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casula, S., Shmukler, B. E., Wilhelm, S., Stuart-Tilley, A. K., Su, W., Chernova, M. N., Brugnara, C., and Alper, S. L. (2001) J. Biol. Chem. 276 41870-41878 [DOI] [PubMed] [Google Scholar]
- 6.Moore-Hoon, M. L., and Turner, R. J. (2000) Biochemistry 39 3718-3724 [DOI] [PubMed] [Google Scholar]
- 7.Parvin, M. N., Gerelsaikhan, T., and Turner, R. J. (2007) Biochemistry 46 9630-9637 [DOI] [PubMed] [Google Scholar]
- 8.Pedersen, M., Carmosino, M., and Forbush, B. (2008) J. Biol. Chem. 283 2663-2674 [DOI] [PubMed] [Google Scholar]
- 9.Uvarov, P., Ludwig, A., Markkanen, M., Pruunsild, P., Kaila, K., Delpire, E., Timmusk, T., Rivera, C., and Airaksinen, M. S. (2007) J. Biol. Chem. 282 30570-30576 [DOI] [PubMed] [Google Scholar]
- 10.Tornberg, J., Voikar, V., Savilahti, H., Rauvala, H., and Airaksinen, M. S. (2005) Eur. J. Neurosci. 21 1327-1337 [DOI] [PubMed] [Google Scholar]
- 11.Hubner, C. A., Stein, V., Hermans-Borgmeyer, I., Meyer, T., Ballanyi, K., and Jentsch, T. J. (2001) Neuron 30 515-524 [DOI] [PubMed] [Google Scholar]
- 12.Woo, N. S., Lu, J., England, R., McClellan, R., Dufour, S., Mount, D. B., Deutch, A. Y., Lovinger, D. M., and Delpire, E. (2002) Hippocampus 12 258-268 [DOI] [PubMed] [Google Scholar]
- 13.Li, H., Tornberg, J., Kaila, K., Airaksinen, M. S., and Rivera, C. (2002) Eur. J. Neurosci. 16 2358-2370 [DOI] [PubMed] [Google Scholar]
- 14.Stein, V., Hermans-Borgmeyer, I., Jentsch, T. J., and Hubner, C. A. (2004) J. Comp. Neurol. 468 57-64 [DOI] [PubMed] [Google Scholar]
- 15.Dowd, B. F. X., and Forbush, B. (2003) J. Biol. Chem. 278 27347-27353 [DOI] [PubMed] [Google Scholar]
- 16.Banker, G., and Goslin, K. (eds) (1998) Culturing Nerve Cells, 2nd Ed., pp. 339-370, MIT press, Cambridge, UK
- 17.Sagne, C., Isambert, M. F., Henry, J. P., and Gasnier, B. (1996) Biochem. J. 316 825-831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramjeesingh, M., Huan, L. J., Garami, E., and Bear, C. E. (1999) Biochem. J. 342 119-123 [PMC free article] [PubMed] [Google Scholar]
- 19.Ludwig, A., Li, H., Saarma, M., Kaila, K., and Rivera, C. (2003) Eur. J. Neurosci. 18 3199-3206 [DOI] [PubMed] [Google Scholar]
- 20.Ushiro, H., Tsutsumi, T., Suzuki, K., Kayahara, T., and Nakano, K. (1998) Arch. Biochem. Biophys. 355 233-240 [DOI] [PubMed] [Google Scholar]
- 21.Toth, Z. E., and Mezey, E. (2007) J. Histochem. Cytochem. 55 545-554 [DOI] [PubMed] [Google Scholar]
- 22.Cai, C., Coleman, S. K., Niemi, K., and Keinanen, K. (2002) J. Biol. Chem. 277 31484-31490 [DOI] [PubMed] [Google Scholar]
- 23.Balakrishnan, V., Becker, M., Lohrke, S., Nothwang, H. G., Guresir, E., and Friauf, E. (2003) J. Neurosci. 23 4134-4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simard, C. F., Bergeron, M. J., Frenette-Cotton, R., Carpentier, G. A., Pelchat, M. E., Caron, L., and Isenring, P. (2007) J. Biol. Chem. 282 18083-18093 [DOI] [PubMed] [Google Scholar]
- 25.Williams, J. R., Sharp, J. W., Kumari, V. G., Wilson, M., and Payne, J. A. (1999) J. Biol. Chem. 274 12656-12664 [DOI] [PubMed] [Google Scholar]
- 26.Zhang, L. L., Fina, M. E., and Vardi, N. (2006) J. Comp. Neurol. 499 132-143 [DOI] [PubMed] [Google Scholar]
- 27.Khirug, S., Huttu, K., Ludwig, A., Smirnov, S., Voipio, J., Rivera, C., Kaila, K., and Khiroug, L. (2005) Eur. J. Neurosci. 21 899-904 [DOI] [PubMed] [Google Scholar]
- 28.Lu, J., Karadsheh, M., and Delpire, E. (1999) J. Neurobiol. 39 558-568 [PubMed] [Google Scholar]