Abstract
This study examined the association between prenatal exposure to cocaine and physiological regulation across the first 7 months of age. Measures of respiratory sinus arrhythmia (RSA) were obtained from 169 (82 cocaine-exposed and 87 nonexposed) infants during baseline periods at 1 month and 7 months of age and during tasks designed to elicit positive and negative affect at 7 months of age. After controlling for maternal age, gestational age, and obstetrical risk, structural equation modeling indicated that the association between prenatal exposure to cocaine and baseline RSA at 7 months of age was direct even in the presence of an indirect effect through baseline RSA at 1 month of age. There were no indirect effects through maternal affect during mother–infant interactions assessed at 1 month of age. Analyses also indicated a direct association between prenatal exposure to cocaine and RSA regulation to negative affect at 7 months of age.
Recently, prenatal exposure to cocaine has been linked to an increased risk of problems with regulatory processes in infancy and early childhood. Regulation is the ability to modulate emotional and physiological responses to environmental stimulation. During the neonatal period, cocaine-exposed neonates display signs of altered neurobehavioral regulation including state lability, altered sleep patterns, deficits in orienting and attention, and increased irritability (Chasnoff, Griffith, MacGregor, Dirkes, & Burns, 1989; Coles, Platzman, Smith, James, & Falek, 1992; Karmel & Gardner, 1996; Regalado, Schechtman, Del Angel, & Bean, 1995). Such difficulties in arousal regulation may impact the ability of cocaine-exposed infants to modulate emotional and behavioral responses to demands from the environment. Beyond the neonatal period, deficits in arousal regulation during cognitive tasks have been noted in cocaine-exposed infants (Alessandri, Sullivan, Imaizumi, & Lewis, 1993; Mayes & Bornstein, 1995). Individual differences in emotion regulation have also been noted. For instance, studies have found that cocaine-exposed infants demonstrated higher negative affect in response to novel stimulation including a “still-face” procedure at 3 to 4 months of infant age (Bendersky & Lewis, 1998) and in response to the presentation of novel visual stimuli at 3 months of age (Mayes, Bornstein, Chawarska, & Granger, 1996). Similarly, older cocaine-exposed infants have demonstrated higher negative affect in response to arm restraint and toy retraction paradigms at 7 months of age (Eiden, Lewis, Croff, & Young, 2002) and increased irritability in the context of standardized testing at 36 months of age (Azuma & Chasnoff, 1993). Although the preponderance of studies beyond the neonatal period has focused on the behavioral regulation of cocaine-exposed infants in response to task demands, few studies have examined biological substrates of regulation in cocaine-exposed infants.
One of the most well studied and theoretically developed biological substrates is parasympathetic regulation as measured by respiratory sinus arrhythmia (RSA). RSA is a measure of the variability in heart rate (HR) that occurs at the frequency of respiration and is believed to index the parasympathetic influence of heart rate variability (HRV) via the vagus nerve (Porges & Byrne, 1992) which Porges terms vagal tone (Vna). Two commonly used measures of RSA include RSA at rest (baseline RSA) and changes in RSA during environmental demands (RSA regulation; Bornstein & Suess, 2000; Calkins, 1997). Both of these measures are indexes of physiological regulation that are believed to reflect emotion regulation (Porges, 1995). Baseline RSA is a measure of the ability of an individual to maintain physiological homeostasis during periods of minimal external stimulation and the capacity of the nervous system to react. Infants with higher RSA are believed to have more organized stress responses and, therefore, the ability to more optimally react to environmental demands (Porges, 1991, 1992). During challenges to homeostasis as a result of exogenous stimulation, the myelinated vagal system optimally responds by functioning as a brake (vagal brake; Porges, Doussard-Roosevelt, Portales, & Greenspan, 1996) and decreasing RSA (RSA suppression). Suppression of RSA during periods of environmental challenge is believed to reflect the infant’s ability to appropriately engage or disengage with the environment (Bornstein & Suess, 2000; Huffman et al., 1998; Porges, 1996). Specifically, a negative change in RSA from baseline to environmental challenge is associated with more optimal state regulation in infancy (DeGangi, DiPietro, Greenspan, & Porges, 1991), decreased behavior problems in preschool-aged children (Porges et al., 1996), and more adaptive behavior during attention- and affect-eliciting tasks in both preschool and school-aged children (Calkins, 1997; Suess, Porges, & Plude, 1994), and during social approach (Stifter & Corey, 2001). Thus, the measurement of change in RSA from baseline to challenging situations is an important concurrent and predictive index of parasympathetic regulation in infants.
The majority of studies examining parasympathetic regulation as a function of cocaine exposure have focused on neonates and findings have been somewhat mixed. For instance, studies have reported lower heart rates (Silvestri, Long, Weese-Mayer, & Barkov, 1991), greater high-frequency power as a portion of total spectral power (Mehta et al., 1993), and greater overall HRV (Regalado, Schechtman, Del Angel, & Bean, 1996; Regalado, Schechtman, Khoo, & Bean, 2001) in cocaine-exposed compared to non-cocaine-exposed neonates. These studies suggest increased parasympathetic activity during rest among cocaine-exposed neonates. Conversely, other studies have not found any effects of maternal cocaine use on baseline HR or RSA (DiPietro, Suess, Wheeler, Smouse, & Newlin, 1995; Mehta et al., 1993), perhaps due to lower sample sizes or lack of examination of dose–response effects. Baseline indexes of HR and RSA are not as indicative of an infant’s ability to modulate arousal beyond the neonatal period (DiPietro, Porges, & Uhly, 1992). However, few studies have examined changes in physiological regulation in response to environmental challenge among cocaine-exposed infants. One exception is a study by Bard, Coles, Platzman, and Lynch (2000), who reported that prenatal cocaine exposure accounted for significant unique variance in arousal as measured by HR responses to stress among 8-week-old infants.
In addition to lack of studies examining the association between prenatal cocaine exposure and parasympathetic regulation beyond the neonatal period, there have been no published reports on the developmental changes in baseline RSA during early infancy among cocaine-exposed infants, or on the association of baseline RSA in cocaine-exposed neonates to RSA regulation (RSA suppression) later in infancy. It is important to have a clearer picture of individual differences in RSA in this population if these measures are to be used in any meaningful way as indicators or predictors of behavioral or emotional outcomes. Because measures of RSA are widely accepted indexes of emotional or self-regulation and are believed to be stable traits associated with temperament (Posner & Rothbart, 2000), it is reasonable to expect stability in measures of RSA across time. Several studies have provided evidence that RSA displays moderate stability during infancy among nonexposed infants. Bornstein and Suess (2000) reported marginal stability of baseline RSA between 2 months and 5 years of age and Porter, Bryan, and Hsu (1995) reported moderate stability (r = .39) of baseline RSA between 3 and 6 months of life. Similarly, Fracasso and colleagues (1994) found higher stability of baseline RSA during the second half of the first year of life (rs = .50–.60). However, it is not clear from these studies if high-risk, cocaine-exposed infants would exhibit similar patterns of stability in physiological regulation during the first part of infancy. Thus, one purpose of this study was to examine the stability of baseline RSA from 1 to 7 months of age in a sample of cocaine-exposed and demographically similar non-cocaine-exposed infants.
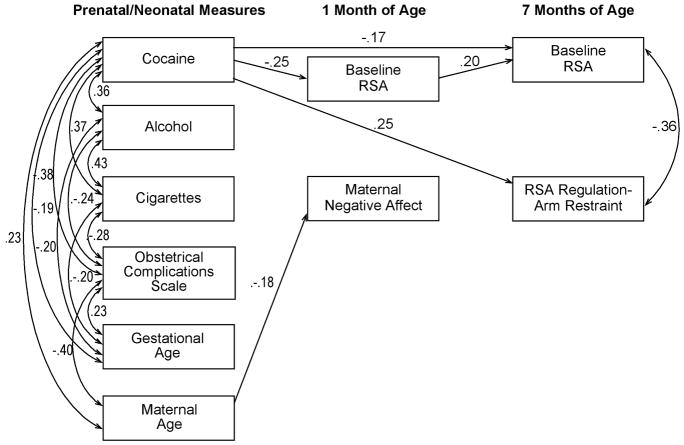
A second goal of the study was to test a conceptual model examining both direct and indirect pathways from cocaine exposure to physiological measures of regulation at 7 months of age (see Figure 1). We were specifically interested in examining whether any association between prenatal exposure to cocaine and measures of RSA at 7 months of age were direct effects or indirect effects through physiological regulation at 1 month of age.
FIGURE 1.
Hypothetical model. This figure includes the hypothesized indirect paths, which were examined first. Direct paths from prenatal substance use to the 7-month variables were then added to this model and tested in the presence of the indirect paths.
We were also interested in exploring the possibility that another pathway to poor regulation among cocaine-exposed infants may be through problematic maternal behavior. For instance, cocaine-using mothers have been reported to display higher negative affect during interactions with their infants (Burns, Chetnik, Burns, & Clark, 1991, 1997; Eiden, Stevens, Schuetze, & Dombkowski, 2006; LaGasse et al., 2003), and to be more disengaged and passive during mother–infant interactions (Gottwald & Thurman, 1994; Molitor, Mayes, & Ward, 2003). These aspects of parenting behavior have direct implications for infants’ reactivity and regulation in affect-arousing situations. Mothers who are able to read their child’s emotional signals and respond with appropriate levels of soothing or stimulation play a critical role in helping children manage their arousal and keep distress within tolerable limits in infancy (Feldman, Greenbaum, & Yirmiya, 1999; Schore, 1994; Tronick, 1989). Parents who exhibit poor sensitivity to infant cues, high negative affect, and low warmth toward their infants are more likely to have infants who have difficulty regulating arousal in affect-arousing situations. Maternal positive affect or warmth may help the infant regulate his or her arousal under stressful situations, and maternal negative affect may have the opposite effect. Thus, we anticipated that positive maternal behavior would mediate the association between cocaine exposure and 7-month RSA.
Finally, it is important to note that cocaine is a polydrug issue. The majority of women using cocaine also use alcohol, cigarettes, and marijuana. Alcohol and cigarettes have been known to have similar effects on infant regulation (e.g., Fried & Makin, 1987; Jacobson, Fein, Jacobson, Schwartz, & Dowler, 1984; Picone, Allen, Olsen, & Ferris, 1982; Saxton, 1978; Schuetze & Eiden, 2006; Streissguth, Barr, & Martin, 1983). Thus, the associations between cocaine exposure and infant regulation can only be examined in the context of associations with other substance use. For this reason, we considered the possible impact of prenatal exposure to other substances in all analyses.
METHOD
Participants
Participants consisted of mother–infant dyads recruited postpartum from two local area hospitals into a longitudinal study of maternal substance use and child development. Of the 220 infants in the study, only the 170 with complete data at 7 months of age were included in these analyses. One additional family was excluded from analyses because it was a multivariate outlier (cocaine, alcohol, and cigarette use were more than 3 SD above the sample mean and gestational age was more than 3 SD below the sample mean). 1 Thus, the final sample was 169 (82 cocaine-exposed, 87 nonexposed) infant–mother pairs.
Mothers ranged in age from 18 to 42 (M = 29.97, SD = 5.74). Seventy-five percent of mothers were African American, 16% were White, 7% were Hispanic American, and the remaining were of other ethnic groups. Of mothers, 71% were receiving Temporary Assistance for Needy Families at the time of their first laboratory visit (2001–2004) and 60% were single. Women were classified as either cocaine-using or “abstainers” and the two groups were matched on maternal education, age, race or ethnicity, and infant gender. Fifty-one of the infants were male. Eighty-six percent of the cocaine-exposed (ranged from 33–41 weeks) and 97% of the comparison infants (ranged from 36–42 weeks) were full term (> 37 weeks gestational age). Cocaine-exposed infants were significantly more likely to have been preterm than nonexposed infants, χ2 (1, N = 169) = 4.27, p < .05. Because there are significant age-related changes in HRV during early infancy (Fox, 1989a), all testing was conducted after age corrected for prematurity. Infants ranged from 1,531 to 5,072 g at birth (M = 3160.87, SD = 540.81). Additional exclusionary criteria for all participants consisted of the following: maternal age less than 18 years, use of illicit substances other than cocaine or marijuana, and significant medical problems in the infant (e.g., genetic disorders, major perinatal complications, baby in critical care for over 48 hr).
The study received approval from the institutional review boards of the hospitals as well as the primary institutions with which the authors are affiliated. Informed written consent was obtained from all recruited participants. Participants received $35 in monetary incentives at the 1-month visit and $50 in the form of gift certificates, checks, and infant toys at the 7-month visit for their participation.
Procedure
All mothers were screened after delivery for initial eligibility and matching criteria. Interested and eligible mothers were given detailed information about the study and asked to sign consent forms. About 2 weeks after delivery, mothers were contacted and scheduled for their first laboratory visit, which took place at the time that their infant was 1 month old. A second visit was scheduled when the infant was 7 months old. All visits consisted of a combination of maternal interviews, observations of mother–infant interactions, and physiological and observational assessments of infant arousal and arousal regulation. To ensure that complete data could be obtained from the maximum number of infants, the assessment of infant arousal commenced as soon as the infant fell asleep during the laboratory visit. As a result, the order of the mother–infant interaction assessments and arousal assessments varied across infants. In the circumstance of a change in custody arrangements, the person who had legal guardianship of the child was contacted and asked to participate. Biological mothers were interviewed at the 1-month assessment to obtain accurate information about prenatal substance use. For the current analyses, the data from the caregiver interview and the physiological assessment of infants at both time points were used. In addition, measures of maternal behavior were obtained during mother–infant interactions observed at the 1-month assessment.
Once a family was recruited into the cocaine group, the closest matching noncocaine family was recruited. However, a significantly higher proportion of mothers in the noncocaine group declined participation or withdrew before formal enrollment, resulting in a smaller number of families in the control group. Of the 4,800 women screened at delivery, 340 were eligible for participation in either group. Of these 340 women, 35% either declined participation or were not enrolled in the study because they expressed initial interest but later withdrew. Of the 220 mother–infant dyads who completed the 1-month laboratory visit, 7 declined to participate in the 7-month assessment or did not show up after repeated reschedules (5 exposed, 2 nonexposed), 10 were unable to be located (5 exposed, 5 nonexposed), 2 (1 exposed, 1 nonexposed) were dropped from the study (one because of a diagnosis of fetal alcohol syndrome, and another because of a diagnosis of Shaken Baby Syndrome), 5 were in the middle of new custody arrangements (5 exposed, 0 nonexposed), and 7 mothers no longer had custody of their children and the foster parent was uninterested in participating (6 exposed, 1 nonexposed). An additional 19 infants did not have complete physiological data at 7 months due to equipment failure (n = 8), research assistant error (n = 3), and infant irritability (n = 8; 5 exposed and 3 nonexposed). Thus, the final sample consisted of 169 (82 cocaine-exposed, 87 nonexposed) mother–infant dyads. There were no significant differences between families with complete versus missing data at 7 months on any demographic or substance use variable. From 1 to 7 months of age, 5 of these infants experienced a change in their primary caregiver. Two infants went from their mothers’ care into foster care and 3 began residing with their grandmothers. All analyses were run both including and excluding these infants.
Assessment of Growth and Risk Status
Three measures of growth were used in this study: birth weight (gm), birth length (cm), and head circumference (cm). All measurements were taken by obstetrical nurses in the delivery room and recorded in the infant’s medical chart. Research staff recorded this information from the charts after recruiting the mother–infant dyad. Medical chart review at the time of recruitment also was used to complete the Obstetrical Complications Scale (OCS; Littman & Parmelee, 1978), a scale designed to assess the number of perinatal risk factors experienced by the infant. Higher numbers on this scale indicate a more optimal obstetric score.
Identification of Substance Use
Cocaine status was determined by a combination of maternal self-report, chart review that provided information about results of the urine toxicology, and maternal hair analysis. Urine toxicologies were routinely conducted at the first prenatal visit on maternal urine or at delivery (for those mothers who tested positive prenatally, obtained prenatal care elsewhere, or did not receive any prenatal care) on infant and maternal urine by participating hospitals. Mothers were included in the cocaine group if self-reports were positive, regardless of urine toxicology or hair sample results. Similarly, mothers who reported that they did not use cocaine but had positive urine toxicology or hair samples were included in the cocaine group.
Self-reports of maternal substance use before, during, and after pregnancy were obtained using the Timeline Follow-Back Interview administered to the biological mother (TLFB; Sobell, Sobell, Klajner, Pavan, & Basian, 1986) at the 1-month visit. Participants were provided a calendar and asked to identify events of personal interest (i.e., holidays, birthdays, vacations, etc.) as anchor points to aid recall. This method has been established as a reliable and valid method of obtaining longitudinal data on substance use patterns, has good test–retest reliability, and is highly correlated with other intensive self-report measures (Brandon, Copeland, & Saper, 1995; Brown et al., 1998). The TLFB yielded data about the average number of days per week cocaine was used, the average number of joints of marijuana and the average number of cigarettes (tobacco) smoked per week, and the average number of standard drinks consumed per week, as well as the mean standard drinks per drinking day and number of alcohol binges (five or more standard drinks) during pregnancy and for the postnatal period.
Urine toxicologies consisted of standard urine screening for drug level or metabolites of cocaine, opiates, benzodiazepines, and tetrahydrocannabinol. Urine was rated positive if the quantity of drug or metabolite was ≥ 300 g/ml. Hair samples were collected from the mothers at the first laboratory visit and sent to the Psychemedics Corporation for radioimmunoanalyses (RIAH). Hair samples were screened for cocaine followed by a gas chromatography/mass spectrometry (GC/MS) confirmation for positive cocaine screens. Drugs and their metabolites are absorbed into the hair and can be extracted and measured. As hair grows at an average rate of 0.5 in. per month, it can record a pattern of drug consumption related to the amount and frequency of use (see Baumgartner, Hill, & Blahd, 1989). Thus, a 2-in. length of hair could contain a record of approximately 4 months of use, and given adequate hair length (i.e., about 4–5 in.), use per trimester can be recorded. Drugs become detectable in hair about 3 to 4 days after use, a time when cocaine is rendered undetectable by urinalysis. RIAH is the most well established hair-analysis technique and has been replicated by independent laboratories across the world (see Magura, Freeman, Siddiqi, & Lipton, 1992). GC/MS confirmations of RIAH have not revealed any false positives because of testing errors (see Magura et al., 1992).
Approximately 32% (n = 54) of mothers in the study (55% of the mothers in the cocaine group) had positive urine toxicologies at delivery, and 25% (n = 42) of mothers (79% of the mothers in the cocaine group) had hair samples that tested positive for cocaine during pregnancy. Eighty-five percent (n = 70) of mothers in the cocaine group admitted having used cocaine in the brief self-report screening instrument administered after delivery. Ten percent (n = 8) of mothers in the cocaine group were identified as cocaine users during pregnancy by medical record (that included urine toxicology information) alone or from the brief screening interview alone. One mother in the cocaine group was identified on the basis of the TLFB alone and 16% (n = 13) were identified by hair analysis alone. Thus, the majority of mothers met more than one criterion for inclusion in the cocaine use group. Mothers who were included in the cocaine group reporting using cocaine an average of .60 times per week during pregnancy (SD = 1.22). Mothers in the comparison group reported not having used any illicit substances other than marijuana and did not test positive for cocaine on any biomarker.
Assessment of Infant Reactivity and Regulation
At the 1-month assessment, infants were tested in a quiet examining room by examiners blind to group status. Because behavioral state can impact HR and to maximize the likelihood of obtaining 10 min of uninterrupted data of HR at rest, all testing was conducted while infants were in a sleep state, which was assessed using a 6-point scale for behavioral state (Brazelton, 1984). Physiological assessment did not begin until infants were in States 1 (Quiet Sleep) and 2 (Active Sleep). To ensure that infants remained in a sleep state throughout the testing session, behavioral state was time-sampled and recorded every 60 sec.
A five-channel Bioamp (James Long Company, Caroga Lake, NY) recorded respiration and electrocardiograph (ECG) data. Disposable electrodes were triangulated on the infant’s chest. A respiration bellows was placed at the bottom of the sternum (zyphoid process) to measure inspiration and expiration. The infant was then placed in a bassinet. Following a 5- min period of undisturbed acclimation to the equipment and bassinet, 10 min of undisturbed physiological data were recorded online directly into a data acquisition computer.
IBI Analysis software (James Long Company, Caroga Lake, NY) was used to process the HR data and to calculate RSA. HR samples, which were collected every 10 msec, were used to calculate mean HR per 1-sec period. A level detector was triggered at the peak of each R-wave. The interval between sequential R-waves was calculated to the nearest millisecond. Data files of R-wave intervals were later manually edited to remove incorrect detection of the R-wave or movement artifacts that occurred in less than 1% of cases because infants were asleep during data collection. The software computes RSA using respiration and interbeat interval (IBI) data as suggested by Grossman (1983). The difference between maximum IBI during expiration and the minimum IBI during inspiration was calculated. The difference, which is measured in seconds, is considered to be a measure of RSA, and is measured twice for each respiration cycle (once for each inspiration and once for each expiration). The time for inspirations and expirations is assigned as the midpoint for each. The time for each arrhythmia sample is assigned as the midpoint between an inspiration time and an expiration time. The software synchronizes with respiration and is, thus, relatively insensitive to arrhythmia due to tonic shifts in HR, thermoregulation, and baroreceptor. Average RSA was calculated for the 10-min period of sleep.
At the 7-month assessment, the physiological assessment of reactivity and regulation was recorded during a 3-min baseline period, a 2-min puppet show, a 3-min intertask interval, and the two arm restraint trials (2 min) by examiners blind to infant group status. Infants were tested while seated in a high-chair. Recording of the physiological data began once the infant was observed to be in a stable, quiet, alert state. A resting state was induced by having the infant watch a 3-min segment of a neutral “Baby Einstein” videotape (see Calkins, 1997, for similar procedures for inducing rest). Although this condition was not a true baseline because infant attention was engaged, it served to keep the infant seated quietly without eliciting affect, thereby minimizing movement artifact. All physiological data were recorded continuously online directly into a data acquisition computer.
Two different paradigms designed to elicit positive affect (PA) and negative affect (NA) were used. The PA paradigm consisted of a puppet show that measured PA in response to social stimulation using puppets for a standardized presentation. A scripted dialogue was used, which took approximately 90 sec to present, followed by 30 sec during which the child was allowed to play with the puppets. The NA paradigm consisted of a gentle arm restraint episode, which is a widely used, well-validated measure of anger and frustration used to assess infant regulation and reactivity (Goldsmith & Rothbart, 1999; Stifter & Braungart, 1995). In this episode, the child was allowed to play with an attractive toy for 30 sec, until the child was engaged with the toy. The caregiver was asked to stand behind the child, place her hands on the child’s forearms, move them to the child’s sides, and hold them there for 30 sec, while maintaining a neutral expression. After the first trial, the caregiver was again asked to play with the child for 30 sec, followed by a second trial. The session was stopped at the caregiver’s request or if the child reached a maximum distress code, defined as the child reaching the highest intensity of negative affect of a full cry. As described earlier, this occurred for 8 infants (5 nonexposed and 3 exposed), who were not included in this sample. The child was allowed to play with the toy at the end of the two trials.
Average RSA was calculated for the 3-min baseline period, for the puppet show, and for each arm restraint trial. Because there were no significant differences in RSA between the two NA trials, we created mean RSA for the two trials. This composite variable was used in all subsequent analyses. To assess physiological regulation during the affect-eliciting procedures, change scores for RSA between paradigms were calculated. Thus, we calculated a change score for RSA from baseline to the PA task and from baseline to the NA task. These were termed RSA regulation to PA and RSA regulation to NA. Negative scores indicate a decrease in RSA and are reflective of more optimal parasympathetic regulation.
Maternal Affect During Feeding Interactions
The 1-month visit was scheduled around a time when the infant was likely to be hungry. Mothers were asked to feed their infants in a comfortable, living-room-type setting as they normally would at home. All infants were bottle-fed. This feeding session was videotaped. The first 10 min of these interactions were coded by two research assistants blind to group status, using the Mother–Infant Feeding Scale (Chatoor, 1986). For the purposes of this study, 18 of the original 26 maternal items were coded at this visit. The remaining items were not coded due to low variability during the neonatal period. For the coded items, the scale requires attention to affect (body posture, facial expression), sensitivity to infant cues, nonverbal communication (positioning the infant for eye contact), verbal communication, and feeding behaviors. This scale has been used by several researchers to measure mother–infant feeding interactions among children with eating disorders (Chatoor & Egan, 1986; Chatoor, Schaefer, Dickson, & Egan, 1984) and in previous studies of mother–infant feeding interactions among substance-abusing mothers (Eiden, 2001).
Two coders blind to group status were trained to code mother–infant interactions by the second author until interrater reliability criterion was reached (percentage agreement = 90% or higher). Subsequent interrater reliability was established on 19% of the tapes, with both coders viewing these tapes separately and comparing codes subsequently. If the coders disagreed by more than 1 point on any dimension, they arrived at a final code by viewing the tape again and resolving discrepancies by mutual agreement. Interrater reliability on individual items ranged from Pearson r = .71 (Cohen’s kappa κ = .53) to r = .98 (κ = .95). Following Eiden et al. (2006), we computed two composite indexes of maternal affect, one labeled maternal positive affect and the other labeled maternal negative affect. Maternal positive affect included items such as makes positive remarks to infant, demonstrates pleasure toward infant, and appears cheerful; maternal negative affect included items such as misses infant cues, makes critical remarks to infant, appears angry, and handles the infant roughly.
RESULTS
Group Differences
Descriptive characteristics of cocaine-exposed and nonexposed groups were examined using multivariate analysis of variance (MANOVA; see Table 1). As expected, mothers in the cocaine group were heavier drinkers and smokers than non-users and these variables were used as covariates in all substantive analyses. There were no group differences in marijuana use during pregnancy and, consequently, it was not considered further as a covariate. Cocaine-using mothers tended to be older, have less education, and have more children than abstainers.
TABLE 1.
Group Differences
| Abstainersa |
Usersb |
|||
|---|---|---|---|---|
| M | SD | M | SD | |
| Maternal characteristics | ||||
| Age (years)** | 27.87 | 5.83 | 31.89 | 7.06 |
| Parity** | 3.21 | 1.73 | 4.43 | 2.35 |
| Socioeconomic status | 1.49 | 0.50 | 1.61 | 0.49 |
| Maternal education (years)** | 12.19 | 1.80 | 11.46 | 1.91 |
| Prenatal care (no. of visits)+ | 15.61 | 7.55 | 13.1 | 8.91 |
| Prenatal no. of cigarettes/week** | 9.98 | 21.47 | 38.52 | 44.91 |
| Prenatal no. of standard drinks/week** | 0.13 | 0.62 | 3.88 | 9.28 |
| Prenatal no. of binge drinking episodes/week** | 0.02 | 0.09 | 0.33 | 1.00 |
| Prenatal no. of joints/week | 1.05 | 5.99 | 1.34 | 4.41 |
| Maternal positive affect | 10.63 | 4.25 | 11.24 | 4.39 |
| Maternal negative affect+ | 7.18 | 2.28 | 7.36 | 2.89 |
| Infant characteristics | ||||
| Gestational age* | 39.34 | 1.24 | 38.59 | 1.93 |
| Birth weight (g)** | 3313.06 | 480.81 | 2961.21 | 572.33 |
| Birth length (cm)** | 49.90 | 2.99 | 47.97 | 3.29 |
| Head circumference at birth (cm) | 33.52 | 1.31 | 33.27 | 2.32 |
| OCS** | 100.61 | 17.81 | 85.59 | 15.95 |
| Baseline RSA: 1 month** | .0299 | .0185 | .0201 | .0181 |
| Baseline RSA: 7 months** | .0269 | .0149 | .0207 | .0126 |
| RSA regulation: Positive affect+ | −.0051 | .0125 | .0021 | .0156 |
| RSA regulation: Negative affect** | −.0010 | .0143 | .0023 | .0095 |
Note. OCS= Obstetrical Complications Scale; RSA = respiratory sinus arrhythmia. High scores on OCS reflect more optimal obstetric scores.
n = 86.
n = 83.
p < .10.
p < .05.
p < .01.
The cocaine-exposed infants had lower birth weights, gestational age, and more obstetrical complications. Eighty-six percent of the cocaine-exposed and 97% of the comparison infants were full term (> 37 weeks gestational age). Cocaine-exposed infants also had lower baseline RSA at 1 and 7 months of age and more RSA change during arousal than infants not exposed to cocaine. However, no differences between groups were found for 1 month maternal affect variables. Preliminary analyses examined whether there were gender differences for any of the RSA measures. No such differences were found and gender was, consequently, not considered further in analyses.
Potential Confounds: Demographics and Perinatal Risk
To examine potential confounds, all study variables were correlated with demographic and infant risk variables, including parity, maternal education, maternal age, infant gestational age, birth weight, infant age at testing, and infant obstetrical complication score. Correlations between study variables can be seen in Table 2. Maternal age was significantly associated with baseline RSA at 7 months of age and scores on the OCS were marginally associated with measures of RSA. As a result, both maternal age and OCS were included in the models as covariates. Because gestational age is associated with the development of the nervous system including vagal-respiratory maturation (Lester, Boukydis, & LaGasse, 1996; Suess et al., 2000), gestational age was also included as an additional covariate in all models. Infant age at testing for both the 1- and 7-month laboratory visits was not associated with outcome variables and was, therefore, not included in the models.
TABLE 2.
Associations Among Study Variables
| Variable | Maternal Education | Maternal Age | Parity | Gestational Age | Birth Weight | Obstetrical Complications Scale |
|---|---|---|---|---|---|---|
| Prenatal cocaine exposure | −.19* | .30*** | .29*** | −.22** | −.30*** | −.41*** |
| Prenatal alcohol exposure | −.21** | .09 | .14+ | −.16* | −.08 | −.21** |
| Prenatal cigarette exposure | −.30*** | .11 | .18* | −.19* | −.23** | −.31*** |
| Maternal positive affect | .04 | .19* | .04 | .08 | .12 | −.17* |
| Maternal negative affect | −.11 | −.19* | −.11 | −.03 | −.03 | .04 |
| Baseline RSA: 1 month | .10 | −.03 | −.02 | .09 | .04 | .13 |
| Baseline RSA: 7 months | .04 | −.17* | .01 | .07 | .02 | .13 |
| RSA regulation: Positive affect | −.06 | −.03 | −.12 | −.05 | −.08 | −.13+ |
| RSA regulation: Negative affect | −.07 | .11 | .03 | −.11 | −.06 | −.17* |
Note. RSA = respiratory sinus arrhythmia.
p < .10.
p < .05.
p < .01.
p < .001.
Stability of RSA from 1 to 7 Months
Correlational analyses were used to examine the stability of RSA from 1 to 7 months of infant age. Moderate stability in RSA between the two visits was found (r = .29, n = 145). Separate analyses for the two groups indicated a significant association between baseline RSA at 1 and 7 months of age for nonexposed infants (r = .33, n = 71), but not cocaine-exposed infants (r = .13, n = 74). Fisher’s r to z transformation was used to test if this difference in correlation coefficients was statistically significant. This analysis revealed that although the size of the correlation for exposed infants was smaller, it was not significantly different from the correlation for nonexposed infants (z = 1.25).
Data Preparation and Screening
Prior to model testing, data were examined for missingness, normality, multi-collinearity, outliers, and relative variance according to standards suggested by Kline (2005). Such screening is important, as missing data and distribution issues can adversely affect model fitting, specification, and parameter estimates.
Missing data
As would be expected of longitudinal studies involving substance-abusing mothers, there were incomplete data for some participants at one or more of the assessment points included in this study. Families from the initial sample (n = 220) missing 7-month data (n = 50) were excluded from the current analyses, but it is important to note that they did not differ significantly from the rest of the sample on substance use, maternal affect, RSA, gestational age, or mother’s education. Nor did those families that had 7-month data but were missing 1-month data (n = 23) differ from those families with data at both time points. To take advantage of the data from this second group, we used full information maximum likelihood (FIML) to estimate parameters in our models (Arbuckle, 1996). This missing data approach includes all cases in the analysis, even those with missing data. When data are missing at random, FIML produces good estimates of population parameters. Even if the data are not missing at random, FIML is thought to produce more accurate estimates of population parameters than if listwise deletion was used. Thus, the final sample used in the structural equation modeling (SEM) analyses consisted of 82 cocaine-exposed and 87 nonexposed (n = 169) mother–infant dyads.
Distributional and Relational Issues
Normality of all variables was assessed, as skewed distributions can lead to excessively large chi-squares. Alcohol use was significantly kurtotic and skewed and was therefore transformed using normal log. No issues of multicollinearity (r > .85) were identified. Relative variance was also assessed and cigarette smoking appeared to be problematic. If the ratio of the smallest to largest variance is greater than 10 it can cause problems with model convergence. The variance of cigarette smoking was 1,311.70 (compared to other variables, which were generally less than 10) and was rescaled by dividing it by 100 to make it more comparable to other variables.
Model Testing
SEM was used to examine relationships among variables. All SEM analyses were conducted using AMOS (Arbuckle, 1997). Maximum likelihood estimation procedures were used and standardized parameter estimates are presented. The goodness-of-fit of the models was examined by using the normed fit index (NFI) and the root mean square error of approximation (RMSEA). The NFI is utilized when running saturated models without latent variables as it corrects for model complexity. The NFI varies between zero and 1.0 and values of .90 or higher indicate acceptable fit (Hu & Bentler, 1995). The RMSEA is bounded by zero and will take on that value when a model exactly reproduces a set of observed data. A value of .05 to .06 is indicative of close fit, a value of .08 is indicative of marginal fit, and higher values are indicative of poor fit (Browne & Cudeck, 1994). The chi-square difference test was used to compare the fit of successive nested models.
Indirect Pathways
In the first model, the indirect path from maternal cocaine use to RSA at 7 months via 1-month RSA and maternal affect was tested. All predictor variables were covaried on paths from maternal substance use, maternal age, gestational age, and infant obstetrical risk to 1-month maternal affect and baseline RSA and from 1-month maternal affect and baseline RSA to 7-month RSA and RSA regulation were included. Cocaine, alcohol, and cigarette use, obstetrical complications, and gestational age all significantly covaried in the expected directions (r = .19–.43). Mother’s age was associated with both cocaine use and obstetrical complications, such that younger mothers were more likely to use cocaine and have more complications. As expected, cocaine exposure was associated with lower levels of 1-month baseline RSA. However, no associations between maternal substance use and maternal affect were found. Alcohol and cigarette use were not associated with RSA. Maternal age was negatively associated with maternal NA, indicating that as the age of the mothers increased, their levels of NA decreased. Some evidence of stability of RSA was evidenced by the association of 1-month and 7-month RSA. Low levels of 1-month RSA were associated with low levels of baseline RSA at 7 months and greater change in RSA during the arm restraint procedure, χ2(19, N = 169) = 16.56, p = .62, NFI = .95, RMSEA = .00. The model was again estimated after trimming nonsignificant paths and is depicted in Figure 2. This final model continued to fit the data fairly well, χ2(28, N = 169) = 31.18, p = .31, NFI = .88, RMSEA = .00.
FIGURE 2.
Indirect effects. Only significant paths were included in the figure. The model tested included covariances between residuals for the 1-month variables.
Direct Pathways
In the next model, direct paths from maternal prenatal cocaine use to 7-month baseline RSA and RSA regulation were added, χ2(17, N = 169) = 15.73, p = .54, NFI = .95, RMSEA = .00. The model was again estimated after trimming non-significant paths and is depicted in Figure 3. The fit of this model was also quite good, χ2(16, N = 169) = 9.18, p = .91, NFI = .97, RMSEA = .002 Prenatal cocaine exposure was directly associated with lower levels of both 1- and 7-month RSA and change in RSA in response to arousal during the arm restraint procedure. The path from 1-month RSA to 7-month baseline RSA remained significant as well. This suggests that cocaine-exposed infants have higher physiological reactivity, as measured by baseline RSA, and less ability to physiologically regulate themselves during periods of negative arousal. The path from 1-month RSA to 7-month RSA regulation during arm restraint was no longer significant. After the nonsignificant paths were trimmed, the final model was reestimated and it continued to fit the data well, χ2(27, N = 169) = 23.8373, p = .64, NFI = .90, RMSEA = .00. Compared with the trimmed indirect effects model, the trimmed direct effects model resulted in a significant improvement in fit, Δχ2(1, N = 169) = 7.35, p < .05, suggesting that 1-month RSA mediates the association between prenatal cocaine exposure and 7-month RSA and RSA regulation (Sobel mediation test statistic = −2.48, p = .01).
FIGURE 3.
Direct effects. Only significant paths were included in the figure. The model tested included covariances between residuals for the 1-month variables.
DISCUSSION
Recent studies have suggested that prenatal exposure to cocaine is associated with regulatory problems during infancy. Although this has been demonstrated in a large number of behavioral outcomes from the neonatal period through early childhood, there is less information on physiological regulation in this high-risk population. Because cocaine crosses the fetal blood–brain barrier, it has the potential to alter neurotransmitter systems. Indeed, cocaine is known to inhibit the reuptake of monoamines at the presynaptic junction, leading to higher concentrations of norepinephrine, serotonin, and dopamine in the synaptic cleft and higher levels of activation in the catecholaminergic systems (Gawin & Ellinwood, 1988; Nassogne, Evrard, & Courtoy, 1998). The regions of the brain that are rich in monoamines are the very centers involved in regulatory activities and reactivity to stress (Robbins, 1997; Tucker & Williamson, 1984). The potential of prenatal cocaine exposure to alter these regulatory processes is especially critical given that monaminergic systems appear early in fetal brain development (by the second month of gestation) and influence the ontogeny of other brain cells (see Mayes, Grillon, Granger, & Schottenfeld, 1998). As such, one of the major goals of this study was to examine RSA reactivity and regulation across the first 7 months of life among infants prenatally exposed to cocaine. In particular, we were interested in examining if there were direct associations between prenatal exposure to cocaine and RSA reactivity and regulation at 7 months of age or were indirect through baseline RSA or maternal affect at 1 month of age.
Previous studies have reported mixed findings regarding the stability of RSA during the first year of life. Whereas some previous studies have reported moderate stability in baseline RSA during both early infancy (Bornstein & Suess, 2000) and in the second half of the first year of life (Fracasso, Porges, Lamb, & Rosenberg, 1994), other studies have not reported stability for baseline RSA during infancy (Porter, Bryan, & Hsu, 1995; Stifter, Fox, & Porges, 1989). In this study, we found that baseline RSA exhibits moderate stability across the first 7 months of life for nonexposed infants, providing additional evidence that baseline RSA may be a relatively stable measure of physiological regulation among infants not exposed to cocaine. The findings regarding stability for baseline RSA among the cocaine-exposed infants, however, were less clear. A comparison of the magnitude of the association between baseline RSA at 1 month and 7 months of age for the exposed and nonexposed infants indicated no significant difference in levels of stability. However, the association between baseline RSA at 1 and 7 months of age was significant for the nonexposed infants but not for the exposed infants. The equivocal nature of these findings makes it difficult to draw a firm conclusion regarding the presence of stability in baseline RSA for exposed infants. It is imperative to resolve this issue if RSA is to be used as an index of concurrent behavioral regulation or a predictor of later developmental outcomes in this high-risk sample. Consequently, additional research is needed to determine if the lack of a significant association between baseline RSA measures in this study is an issue related to this specific sample or to the larger population of cocaine-exposed infants.
There are a couple of explanations that should be considered when attempting to interpret our findings regarding the stability of baseline RSA in this population. First, previous studies have found that there are significant changes in autonomic functioning that occur during the first 6 months of life, with the most pronounced fluctuations occurring during the neonatal period indicating the possibility of a substantial biological reorganization early in infancy (e.g., Fox, 1988; Harper et al., 1978; Richards, 1989; Schechtman et al., 1989). The failure of the exposed infants to display a significant association between baseline RSA measures from 1 month to 7 months of age may, therefore, indicate a delayed biological reorganization of their autonomic system relative to the nonexposed infants. A second explanation for the lack of stability in baseline RSA for cocaine-exposed infants may reflect the fact that baseline RSA was collected under different circumstances at the two laboratory visits. At 1 month of age, baseline RSA was assessed during sleep, whereas at 7 months of age, it was assessed while infants watched an emotionally neutral video. Although both contexts had very low affective demands for the infants, the task during which baseline RSA was assessed at 7 months of age tapped attentional processes, whereas at 1 month of age, RSA was measured in a context with minimal external stimulation of any kind. Thus, it is possible that exposed infants may have been more sensitive to the differences in context used to assess baseline RSA relative to nonexposed infants. Future studies should further explore the stability of RSA among exposed infants using similar measurement contexts and explore the possible relationship between baseline RSA during early infancy and attentional capacities later in infancy.
A second goal of the study was to examine the possibility that the association between prenatal exposure to cocaine and measures of RSA at 7 months was indirect through RSA during rest at 1 month or through maternal affect during interactions at 1 month of age. As expected, cocaine exposure was associated with lower levels of baseline RSA at 1 month of age. Baseline RSA at 1 month was, in turn, associated with baseline RSA and RSA regulation during a negative affect paradigm at 7 months of age. Higher baseline RSA has been associated with appropriate positive and negative emotional reactivity (Fox, 1989b; Stifter & Fox, 1990; Stifter et al., 1989) and with behavioral reactivity (DiPietro & Porges, 1991; Gunnar, Porter, Wolf, Rigatuso, & Larson, 1995). Thus, the lower baseline RSA for cocaine-exposed infants at 1 month of age in this study suggests reduced competence in the ability to regulate internal bodily processes (Porges, 1996) and less organized responses to stress (Porges, 1991). These deficits in physiological reactivity during early infancy are, in turn, associated with continued deficits in physiological reactivity and regulation at 7 months of age. Specifically, cocaine-exposed infants had lower RSA at 1 month of age, which was, in turn, associated with lower RSA at 7 months and with less RSA suppression during the arm restraint procedure. These results suggest that deficits in physiological reactivity during the neonatal period predict continued deficits in both physiological reactivity and regulation later in infancy.
Contrary to our hypothesis, we did not find that maternal affect mediated the association between prenatal exposure to cocaine and physiological regulation at 7 months of age. Unlike previous studies that have found associations between maternal cocaine use during pregnancy and maternal affect during mother–infant interactions (Blackwell, Kirkhart, Schmitt, & Kaiser, 1998; Eiden, 2001; Hans, Bernstein, & Henson, 1999; Mayes et al., 1997), we did not find an association between cocaine use and maternal affect. We did, however, find that older mothers exhibited less NA during mother–infant interactions than younger mothers. This is similar to the results of previous studies that have found a positive association between maternal age and maternal sensitivity, structuring during infant interactions, and parenting satisfaction (Bornstein et al., 2003). Although this was an objective measure of maternal affect, as opposed to self-reports of their own affect during parenting, it is important to note that maternal affect was only assessed once in this sample at 1 month of age within a single context. Thus, our failure to find an association between maternal affect and cocaine use during pregnancy or infant physiological regulation may be an artifact of the limited timing and context of the assessment of maternal affect.
We also found that prenatal exposure to cocaine had a direct effect on RSA regulation during an NA-eliciting paradigm at 7 months of age. Whereas RSA levels for cocaine-exposed infants remained relatively stable from baseline to the NA task, nonexposed infants showed the expected RSA suppression during the NA task. This pattern of RSA suppression is consistent with earlier reports of decreased RSA during frustrating tasks and arm restraint among low-risk, non-exposed infants (Stifter & Fox, 1990; Stifter & Jain, 1996). RSA suppression (applying the vagal brake) during NA tasks is indicative of adaptive autonomic nervous system regulation for strong NA (Porges, 1995, 1997) and is believed to reflect the ability of infants to shift their focus from the internal need to maintain homeostasis to the need to employ strategies for engaging or disengaging with environmental demands. These results, therefore, indicate appropriate physiological regulation during environmental challenge among the nonexposed infants.
Infants in the cocaine exposure group, however, did not display RSA suppression during the arm restraint task. Instead their RSA remained consistently high throughout the remainder of the data collection procedure. One explanation for these findings is that the cocaine-exposed infants were not able to regulate themselves in response to an environmental challenge designed to elicit NA. Previous studies have found that infants with regulatory disorders did not regulate RSA from baseline to a task (DeGangi et al., 1991). Furthermore, there is a positive association between RSA regulation and both concurrent and future behavioral regulation during frustration (Calkins, 1997) and social tasks (Doussard-Roosevelt, Porges, Scanlon, Alemi, & Scanlon, 1997; Stifter & Corey, 2001; Suess & Bornstein, 2000). Taken together, these studies suggest that the deficits in physiological regulation in these cocaine-exposed infants may predict continued problems with regulatory behaviors. RSA regulation is associated with more optimal state regulation and an increased ability to self-soothe during infancy (DeGangi et al., 1991; Huffman et al., 1998), to reduced behavior problems and more optimal emotion regulation during the preschool years (Calkins, 1997), and to increased attention in school-aged children (Suess et al., 1994). Thus, the findings reported here may explain, in part, the frequent findings in previous studies of nonoptimal and disrupted neurobehavioral functioning, regulatory behaviors, and attentional abilities among cocaine-exposed infants and children. Future research should explore the potential link between deficits in physiological regulation during the first year of life and emotional and behavioral problems beyond infancy. However, a second explanation for the failure of the exposed infants to suppress RSA during the NA task is the possibility that their lower baseline RSA is functioning as a floor effect. Thus, the failure of the cocaine-exposed infants to display RSA suppression during environmental challenges may indicate that there was no need for them to regulate themselves during the NA challenge rather than an inability to regulate.
Finally, it is important to note two additional limitations of this study. First, although care was taken in this study to identify substance use in this sample, the accurate assessment of substance use is difficult. Pregnant women are often hesitant to divulge information regarding the use of substances during pregnancy, particularly illicit substances such as cocaine. To address this issue, multiple indexes of illicit substance use were used, including self-report using the reliable TLFB, as well as analysis of hair and urine samples. Each of these measures has its own limitations, although, when used in combination, the likelihood of accurately identifying cocaine use is increased. There were, however, no biological markers used in this study for assessing prenatal alcohol consumption or cigarette smoking. It is possible that mothers may have misrepresented their use of these substances as a result of inaccurate memory or hesitance to divulge usage. This might, in turn, explain our finding of no association between prenatal alcohol and cigarette exposure and physiological regulation. Second, maternal cocaine use during pregnancy is a marker variable for a number of other risk variables that may adversely impact the regulatory system of the infant (Mayes, 2002). These risk variables include postnatal substance use, maternal characteristics such as increased levels of psychopathology, and higher environmental risk. These factors should be systematically explored in future research examining possible pathways between prenatal cocaine exposure and infant regulation,
In conclusion, infants who were prenatally exposed to cocaine had lower baseline RSA at both 1 and 7 months of age, indicating disrupted physiological functioning that continues at least through the first half of the first year. These RSA levels were moderately stable across time. A direct association between prenatal exposure to cocaine and RSA regulation at 7 months of age was also found, suggesting that maternal cocaine use during pregnancy may be associated with disrupted physiological regulation during environmental stimulation later in infancy. Because failure to physiologically regulate to environmental challenge during infancy is associated with both concurrent and future problems in behavioral regulation, these findings suggest that one pathway to the problematic regulatory behaviors consistently seen in cocaine-exposed children may be through disrupted physiological regulation.
Footnotes
Analyses were also run after removing eight families who were too young at the time of the 1-month assessment after correcting for prematurity (n = 161), again after removing those missing 1-month data (n = 138), and again after removing the 5 infants that experienced a child care change between the 1- and 7-month laboratory visits. There were no differences in path structure or significance in these models from the model reported in the text.
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re‐distribution, re‐selling, loan or sub‐licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Contributor Information
Pamela Schuetze, Department of Psychology, State University of New York College at Buffalo and Research Institute on Addictions Department of Pediatrics, University at Buffalo.
Rina D. Eiden, Research Institute on Addictions and Departments of Psychology and Pediatrics, University at Buffalo
Ellen P. Edwards, Research Institute on Addictions, University at Buffalo
References
- Alessandri SM, Sullivan MW, Imaizumi S, Lewis M. Learning and emotional responsivity in cocaine-exposed infants. Developmental Psychology. 1993;29:989–997. [Google Scholar]
- Arbuckle JL. Full information estimation in the presence of incomplete data. In: Marcoulides GA, Schumacker RE, editors. Advanced structural equation modeling techniques. Mahwah, NJ: Lawrence Erlbaum Associates, Inc; 1996. pp. 243–277. [Google Scholar]
- Arbuckle JL. AMOS users’ guide (Version 4.0) Chicago: SmallWaters; 1997. [Google Scholar]
- Azuma SD, Chasnoff IJ. Outcome of children prenatally exposed to cocaine and other drugs: A path analysis of three-year data. Pediatrics. 1993;92:396–402. [PubMed] [Google Scholar]
- Bard KA, Coles CD, Platzman KA, Lynch M. The effects of prenatal drug exposure, term status, and caregiving on arousal and arousal modulation in 8-week-old infants. Developmental Psychobiology. 2000;36:194–212. [PubMed] [Google Scholar]
- Baumgartner WA, Hill VA, Blahd WH. Hair analysis for drugs of abuse. Journal of Forensic Science. 1989;34:1433–1453. [Google Scholar]
- Bendersky M, Lewis M. Arousal modulation in cocaine-exposed infants. Developmental Psychology. 1998;34:555–564. [PMC free article] [PubMed] [Google Scholar]
- Blackwell P, Kirkhart K, Schmitt D, Kaiser M. Cocaine/polydrug affected dyads: Implications for infant cognitive development and mother–infant interaction during the first six postnatal months. Journal of Applied Developmental Psychology. 1998;19:235–248. [Google Scholar]
- Bornstein MH, Hendricks C, Hahn CS, Haynes OM, Painter KM, Tamis-LeMonda CS. Contributors to self-perceived competence, satisfaction, investment, and role balance in maternal parenting: A multivariate ecological analysis. Parenting: Science and Practice. 2003;3:285–326. [Google Scholar]
- Bornstein M, Suess P. Physiological self-regulation and information-processing in infancy: Cardiac vagal tone and habituation. Child Development. 2000;71:273–287. doi: 10.1111/1467-8624.00143. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Copeland AL, Saper ZL. Programmed therapeutic messages as a smoking treatment adjunct: Reducing the impact of negative affect. Health Psychology. 1995;14:41–47. doi: 10.1037//0278-6133.14.1.41. [DOI] [PubMed] [Google Scholar]
- Brazelton TB. Neonatal behavioral assessment scale, Clinics in Developmental Medicine, No. 50. 2. Philadelphia: Lippincott; 1984. [Google Scholar]
- Brown RA, Burges ES, Sales SD, Whitely JA, Evans DM, Miller I. Reliability and validity of a smoking timeline followback interview. Psychology of Addictive Behaviors. 1998;12:101–112. [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long J, editors. Testing structural equation models. Newbury Park, CA: Sage; 1994. pp. 136–162. [Google Scholar]
- Burns KA, Chetnik L, Burns WJ, Clark R. Dyadic disturbances on cocaine-abusing mothers and their infants. Journal of Clinical Psychology. 1991;47:316–319. doi: 10.1002/1097-4679(199103)47:2<316::aid-jclp2270470220>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Burns KA, Chetnik L, Burns WJ, Clark R. The early relationship of drug abusing mothers and their infants: An assessment at eight to twelve months of age. Journal of Clinical Psychology. 1997;53:279–287. doi: 10.1002/(sici)1097-4679(199704)53:3<279::aid-jclp11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Calkins SD. Cardiac vagal tone indices of temperamental reactivity and behavioral regulation in young children. Developmental Psychobiology. 1997;31:125–142. doi: 10.1002/(sici)1098-2302(199709)31:2<125::aid-dev5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Chasnoff IJ, Griffith DR, MacGregor S, Dirkes K, Burns KA. Temporal patterns of cocaine use in pregnancy: Perinatal outcome. Journal of the American Medical Association. 1989;261:1741–1744. [PubMed] [Google Scholar]
- Chatoor I. Mother–infant/toddler feeding scale. Washington, DC: Children’s Research Institute and George Washington University; 1986. Unpublished manuscript. [Google Scholar]
- Chatoor I, Egan J. Diagnosis and treatment of feeding disorders in infants and young children. Abstract in Scientific Proceedings presented at the American Academy of Child and Adolescent Psychiatry 33rd annual meeting; Los Angeles. 1986. May, [Google Scholar]
- Chatoor I, Schaefer S, Dickson L, Egan J. Non-organic failure to thrive: A developmental perspective. Pediatric Annals. 1984;13:829–835. [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Smith I, James M, Falek A. Effects of cocaine and alcohol use in pregnancy on neonatal growth and neurobehavioral status. Neurotoxicology and Teratology. 1992;14:23–33. doi: 10.1016/0892-0362(92)90025-6. [DOI] [PubMed] [Google Scholar]
- DeGangi GA, DiPietro JA, Greenspan SI, Porges SW. Psychophysiological characteristics of the regulatory disordered infant. Infant Behavior and Development. 1991;14:37–50. [Google Scholar]
- DiPietro JA, Porges SW. Vagal responsiveness to gavage feeding as an index of preterm status. Pediatric Research. 1991;29:231–236. doi: 10.1203/00006450-199103000-00003. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Porges SW, Uhly B. Reactivity and developmental competence in preterm and full-term infants. Developmental Psychology. 1992;28:1–11. [Google Scholar]
- DiPietro JA, Suess PE, Wheeler JS, Smouse PH, Newlin DB. Reactivity and regulation in cocaine-exposed neonates. Infant Behavior and Development. 1995;18:407–414. [Google Scholar]
- Doussard-Roosevelt JA, Porges SW, Scanlon JW, Alemi B, Scanlon K. Vagal regulation of heart rate in the prediction of outcome for very low birth weight preterm infants. Child Development. 1997;68:173–186. [PubMed] [Google Scholar]
- Eiden RD. Maternal substance use and mother–infant feeding interactions. Infant Mental Health Journal. 2001;22:497–511. [Google Scholar]
- Eiden RD, Lewis A, Croff S, Young E. Maternal cocaine use and infant behavior. Infancy. 2002;3:77–96. [Google Scholar]
- Eiden RD, Stevens A, Schuetze P, Dombkowski LE. A conceptual model for maternal behavior among poly-drug cocaine using mothers. Psychology of Addictive Behaviors. 2006;20:1–10. doi: 10.1037/0893-164X.20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Greenbaum CW, Yirmiya N. Mother–infant affect synchrony as an antecedent of the emergence of self-control. Developmental Psychology. 1999;35:223–231. doi: 10.1037//0012-1649.35.1.223. [DOI] [PubMed] [Google Scholar]
- Fox NA. Heart-rate variability and behavioral reactivity: Individual differences in autonomic patterning and their relation to infant and child temperament. In: Reznick JS, editor. Perspectives on behavioral inhibition: The John D. and Catherine T. MacArthur Foundation series on mental health and development. Chicago: University of Chicago Press; 1989a. pp. 177–195. [Google Scholar]
- Fox NA. Psychophysiological correlates of emotional reactivity during the first year of life. Developmental Psychology. 1989b;25:364–372. [Google Scholar]
- Fracasso MP, Porges SW, Lamb ME, Rosenberg AA. Cardiac activity in infancy: Reliability and stability of individual differences. Infant Behavior and Development. 1994;17:277–284. [Google Scholar]
- Fried PA, Makin JE. Neonatal behavioural correlates of prenatal exposure to marihuana, cigarettes, and alcohol in a low-risk population. Neurotoxicology and Teratology. 1987;9:1–7. doi: 10.1016/0892-0362(87)90062-6. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Ellinwood EH. Cocaine and other stimulants: Actions, abuse, and treatment. New England Journal of Medicine. 1988;318:1173–1182. doi: 10.1056/NEJM198805053181806. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Rothbart MK. Laboratory Temperament Assessment Battery. Madison: University of Wisconsin–Madison; 1999. (technical manual) [Google Scholar]
- Gottwald SR, Thurman SK. The effects of prenatal cocaine exposure on mother–infant interaction and infant arousal in the newborn period. Topics in Early Childhood Special Education. 1994;14:217–231. [Google Scholar]
- Grossman P. Respiration, stress, and cardiovascular function. Psychophysiology. 1983;20:284–299. doi: 10.1111/j.1469-8986.1983.tb02156.x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Porter FL, Wolf CM, Rigatuso J, Larson MC. Neonatal stress reactivity: Predictions to later emotional temperament. Child Development. 1995;66:1–13. doi: 10.1111/j.1467-8624.1995.tb00851.x. [DOI] [PubMed] [Google Scholar]
- Hans SL, Bernstein VJ, Henson LG. The role of psychopathology in parenting of drug dependent women. Development and Psychopathology. 1999;11:957–978. doi: 10.1017/s0954579499002400. [DOI] [PubMed] [Google Scholar]
- Hu LT, Bentler PM. Evaluating model fit. In: Hoyle RH, editor. Structural equation modeling: Concepts, issues, and applications. Thousand Oaks, CA: Sage; 1995. pp. 76–99. [Google Scholar]
- Huffman LC, Bryan Y, del Carmen R, Pederson F, Doussard-Roosevelt J, Porges W. Infant temperament and cardiac vagal tone: Assessments at 12 weeks of age. Child Development. 1998;69:624–635. [PubMed] [Google Scholar]
- Jacobson SW, Fein GG, Jacobson JL, Schwartz PM, Dowler JK. Neonatal correlates of prenatal exposure to smoking, caffeine, and alcohol. Infant Behavior and Development. 1984;7:253–265. [Google Scholar]
- James Long Company. IBI Analysis System reference guide. Caroga, NY: Author; 1999. [Google Scholar]
- Karmel BZ, Gardner JM. Prenatal cocaine exposure effects on arousal modulated attention during the neonatal period. Developmental Psychobiology. 1996;29:463–480. doi: 10.1002/(SICI)1098-2302(199607)29:5<463::AID-DEV5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practices in structural equation modeling. New York: Guilford; 2005. [Google Scholar]
- LaGasse LL, Messinger D, Lester BM, Seifer R, Tronick EZ, Bauer CR, et al. Prenatal drug exposure and maternal and infant feeding behavior. Archives of Disease in Childhood Fetal and Neonatal Edition. 2003;88:F391–F399. doi: 10.1136/fn.88.5.F391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester BM, Boukydis CFZ, LaGasse L. Cardiorespiratory reactivity during the Brazelton scale in term and preterm infants. Journal of Pediatric Psychology. 1996;21:771–783. doi: 10.1093/jpepsy/21.6.771. [DOI] [PubMed] [Google Scholar]
- Littman A, Parmelee B. Medical correlation of infant development. Pediatrics. 1978;61:470–474. doi: 10.1542/peds.61.3.470. [DOI] [PubMed] [Google Scholar]
- Magura S, Freeman RC, Siddiqi Q, Lipton DS. The validity of hair analysis for detecting cocaine and heroin use among addicts. International Journal of the Addictions. 1992;27:51–69. doi: 10.3109/10826089109063462. [DOI] [PubMed] [Google Scholar]
- Mayes LC. A behavioral teratogenic model of the impact of prenatal cocaine exposure on arousal regulatory systems. Neurotoxicology & Teratology. 2002;24:385–395. doi: 10.1016/s0892-0362(02)00200-3. [DOI] [PubMed] [Google Scholar]
- Mayes LC, Bornstein MH. Developmental dilemmas for cocaine-abusing parents and their children. In: Lewis M, Bendersky M, editors. Mothers, babies and cocaine: The role of toxins in development. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1995. pp. 251–272. [Google Scholar]
- Mayes LC, Bornstein MH, Chawarska K, Granger RH. Impaired regulation of arousal in three-month-old infants exposed to cocaine and other drugs. Developmental Psychopathology. 1996;8:29–42. [Google Scholar]
- Mayes LC, Feldman R, Granger RH, Haynes O, Bornstein MH, Schottenfeld R. The effects of polydrug use with and without cocaine on mother–infant interaction at 3 and 6 months. Infant Behavior and Development. 1997;20:489–502. [Google Scholar]
- Mayes LC, Grillon R, Granger R, Schottenfeld R. In: Cocaine: Effects on the developing brain. Harvey JA, Kosofsky BE, editors. New York: New York Academy of Sciences; 1998. pp. 126–143. [Google Scholar]
- Mehta SK, Finkelhor RS, Anderson RL, Harcar-Sevik RA, Wasser TE, Bahler RC. Transient myocardial ischemia in infants prenatally exposed to cocaine. Journal of Pediatrics. 1993;122:945–949. doi: 10.1016/s0022-3476(09)90025-7. [DOI] [PubMed] [Google Scholar]
- Molitor A, Mayes LC, Ward A. Emotion regulation behavior during a separation procedure in 18-month-old children of mothers using cocaine and other drugs. Development and Psychopathology. 2003;15:51–69. doi: 10.1017/s0954579403000038. [DOI] [PubMed] [Google Scholar]
- Nassogne MC, Evrard P, Courtoy PJ. Selective direct toxicity of cocaine on fetal mouse neurons: Teratogenic implications of neurite and apoptotic neuronal loss. In: Harvey JA, Kosofsky BE, editors. Cocaine: Effects on the developing brain. New York: New York Academy of Sciences; 1998. pp. 51–68. [PubMed] [Google Scholar]
- Picone TA, Allen LH, Olsen PN, Ferris ME. Pregnancy outcome in North American women: II. Effects of diet, cigarette smoking, stress, and weight gain on placentas, and on neonatal physical and behavioral characteristics. American Journal of Clinical Nutrition. 1982;36:1214–1224. doi: 10.1093/ajcn/36.6.1214. [DOI] [PubMed] [Google Scholar]
- Porges SW. Vagal tone: An automatic mediator of affect. In: Garber J, Dodge KA, editors. The development of emotion regulation and dysregulation. Cambridge, UK: Cambridge University Press; 1991. pp. 111–128. [Google Scholar]
- Porges SW. Vagal tone: A physiologic marker of stress vulnerability. Pediatrics. 1992;90:498–504. [PubMed] [Google Scholar]
- Porges SW. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage: A polyvagal theory. Psychophysiology. 1995;32:301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. Physiological regulation in high-risk infants: A model for assessment and potential intervention. Development & Psychopathology. 1996;8:43–58. [Google Scholar]
- Porges SW. Emotion: An evolutionary by-product of the neural regulation of the autonomic nervous system. In: Carter CS, Kirkpatrick B, Lederhendler II, editors. The integrative neurobiology of affiliation. Annals of New York Academy of Science. Vol. 807. 1997. pp. 62–77. [DOI] [PubMed] [Google Scholar]
- Porges SW, Byrne EA. Research methods for measurement of heart rate and respiration. Biological Psychology. 1992;34:93–130. doi: 10.1016/0301-0511(92)90012-j. [DOI] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales L, Greenspan S. Infant regulation of the vagal brake predicts child behavior problems: A psychobiological models of social behavior. Developmental Psychobiology. 1996;29:697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Porter CL, Bryan YE, Hsu HC. Physiological markers in early infancy: Stability of 1-to 6-month vagal tone. Infant Behavior and Development. 1995;18:363–367. [Google Scholar]
- Posner M, Rothbart MK. Developing mechanisms of self-regulation. Development and Psychopathology. 2000;12:427–441. doi: 10.1017/s0954579400003096. [DOI] [PubMed] [Google Scholar]
- Regalado M, Schechtman V, Del Angel AP, Bean X. Sleep disorganization in cocaine-exposed neonates. Infant Behavior and Development. 1995;18:319–327. [Google Scholar]
- Regalado M, Schechtman V, Del Angel AP, Bean X. Cardiac and respiratory patterns during sleep in cocaine-exposed neonates. Early Human Development. 1996;44:187–200. doi: 10.1016/0378-3782(95)01708-9. [DOI] [PubMed] [Google Scholar]
- Regalado MG, Schechtman VL, Khoo MCK, Bean XD. Spectral analysis of heart rate variability and respiration during sleep in cocaine-exposed neonates. Clinical Physiology. 2001;21:428–436. doi: 10.1046/j.1365-2281.2001.00353.x. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Arousal systems and attentional processes. Biological Psychology. 1997;45:57–71. doi: 10.1016/s0301-0511(96)05222-2. [DOI] [PubMed] [Google Scholar]
- Saxton DW. The behavior of infants whose mothers smoke in pregnancy. Early Human Development. 1978;2:363–369. doi: 10.1016/0378-3782(78)90063-4. [DOI] [PubMed] [Google Scholar]
- Schore AN. Affect regulation and the origin of self: The neurobiology of emotional development. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1994. [Google Scholar]
- Schuetze P, Eiden RD. The association between maternal cocaine use during pregnancy and physiological regulation in one-month old infants: An examination of possible mediators and moderators. The Journal of Pediatric Psychology. 2006;31:15–26. doi: 10.1093/jpepsy/jsj022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri JM, Long JM, Weese-Mayer DE, Barkov GA. Effect of prenatal cocaine on respiration, heart rate and sudden infant death syndrome. Pediatric Pulmonology. 1991;11:328–334. doi: 10.1002/ppul.1950110409. [DOI] [PubMed] [Google Scholar]
- Sobell MB, Sobell LC, Klajner F, Pavan D, Basian E. The reliability of a timeline method for assessing normal drinker college students’ recent drinking history. Addictive Behaviors. 1986;11:149–162. doi: 10.1016/0306-4603(86)90040-7. [DOI] [PubMed] [Google Scholar]
- Stifter CA, Braungart JM. The regulation of negative reactivity in infancy: Function and development. Developmental Psychology. 1995;31:448–455. [Google Scholar]
- Stifter CA, Corey JM. Vagal regulation and observed social behavior in infancy. Social Development. 2001;10:189–201. [Google Scholar]
- Stifter CA, Fox NA. Infant reactivity: Physiological correlates of newborn 5-month temperament. Developmental Psychology. 1990;26:582–586. [Google Scholar]
- Stifter CA, Fox NA, Porges SW. Facial expressivity and vagal tone in 5- and 10-month-old infants. Infant Behavior and Development. 1989;12:127–137. [Google Scholar]
- Stifter CA, Jain A. Psychophysiological correlates of infant temperament: Stability of behavior and autonomic patterning from 5 to 18 months. Developmental Psychobiology. 1996;29:379–391. doi: 10.1002/(SICI)1098-2302(199605)29:4<379::AID-DEV5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Martin DC. Maternal alcohol use and neonatal habituation assessed with the Brazelton scale. Child Development. 1983;54:1109–1118. [PubMed] [Google Scholar]
- Suess PE, Alpan G, Dulkerian SJ, Doussard-Roosevelt J, Porges SW, Gewolb IH. Respiratory sinus arrhythmia during feeding: A measure of vagal regulation of metabolism, ingestion, and digestion in preterm infants. Developmental Medicine & Child Neurology. 2000;42:169–173. doi: 10.1017/s001216220000030x. [DOI] [PubMed] [Google Scholar]
- Suess PE, Bornstein M. Task-to-task vagal regulation: Relations with language and play in 20-month-old children. Infancy. 2000;1:303–322. doi: 10.1207/S15327078IN0103_2. [DOI] [PubMed] [Google Scholar]
- Suess PE, Porges SW, Plude DJ. Cardiac vagal tone and sustained attention in school-age children. Psychophysiology. 1994;31:17–22. doi: 10.1111/j.1469-8986.1994.tb01020.x. [DOI] [PubMed] [Google Scholar]
- Tronick EZ. Emotions and emotional communication in infants. American Psychologist. 1989;44:112–119. doi: 10.1037//0003-066x.44.2.112. [DOI] [PubMed] [Google Scholar]
- Tucker DM, Williamson PA. Asymmetric neural control systems in human self-regulation. Psychological Review. 1984;91:185–215. [PubMed] [Google Scholar]