Abstract
Moringa oleifera, an edible tree found worldwide in the dry tropics, is increasingly being used for nutritional supplementation. Its nutrient-dense leaves are high in protein quality, leading to its widespread use by doctors, healers, nutritionists and community leaders, to treat under-nutrition and a variety of illnesses. Despite the fact that no rigorous clinical trial has tested its efficacy for treating under-nutrition, the adoption of M. oleifera continues to increase. The “Diffusion of innovations theory” describes well, the evidence for growth and adoption of dietary M. oleifera leaves, and it highlights the need for a scientific consensus on the nutritional benefits.
Keywords: Diffusion of innovations theory, Moringa oleifera, nutrient supplement
BACKGROUND
Over 143 million children under the age of five in developing countries were undernourished in 2006 (UNICEF, 2007). Food insecurity, lack of access to health care (including international food aid), and social, cultural, and economic class, all play a major role in explaining the prevalence of under-nutrition (West et al., 2006). The regions most burdened by under-nutrition, (in Africa, Asia, Latin America, and the Caribbean) all share the ability to grow and utilize an edible plant, Moringa oleifera, commonly referred to as “The Miracle Tree” (Palada, 1996; Fuglie, 1999). For hundreds of years, traditional healers have prescribed different parts of M. oleifera for treatment of skin diseases, respiratory illnesses, ear and dental infections, hypertension, diabetes, cancer treatment, water purification, and have promoted its use as a nutrient dense food source (Anwar et al., 2007; Castellón and González, 1996; Fahey, 2005; Fuglie, 1999). The leaves of M. oleifera have been reported to be a valuable source of both macro- and micronutrients and is now found growing within tropical and subtropical regions worldwide, congruent with the geographies where its nutritional benefits are most needed.
Anecdotal evidence of benefits from M. oleifera has fueled a recent increase in adoption of and attention to its many healing benefits (Fahey, 2005), specifically the high nutrient composition of the plants leaves and seeds. Trees for Life, an NGO based in the United States has promoted the nutritional benefits of Moringa around the world, and their nutritional comparison has been widely copied and is now taken on faith by many: “Gram for gram fresh leaves of M. oleifera have 4 times the vitamin A of carrots, 7 times the vitamin C of oranges, 4 times the calcium of milk, 3 times the potassium of bananas, ¾ the iron of spinach, and 2 times the protein of yogurt” (Trees for Life, 2005). Other NGOs that have been active in promoting the use of M. oleifera include, but are not limited to: ECHO (Florida, USA), Church World Service (Indiana, USA), GIANT (Georgia, USA), Helen Keller International (Guinea), and Santé et Nature (Congo).
Feeding animals M. oleifera leaves results in both weight gain and improved nutritional status (Hunter & Stewart, 1993; Castellon & Gonzalez, 1996; Rocha & Mendieta, 1998; Nambiar & Seshadri, 2001; Sarwatt et al., 2004; Reyes-Sanchez et al., 2005; Kakengi et al., 2007). However, scientifically robust trials testing its efficacy for undernourished human beings have not yet been reported. If the wealth of anecdotal evidence (not cited herein) can be supported by robust clinical evidence, countries with a high prevalence of under-nutrition might have at their fingertips, a sustainable solution to some of their nutritional challenges.
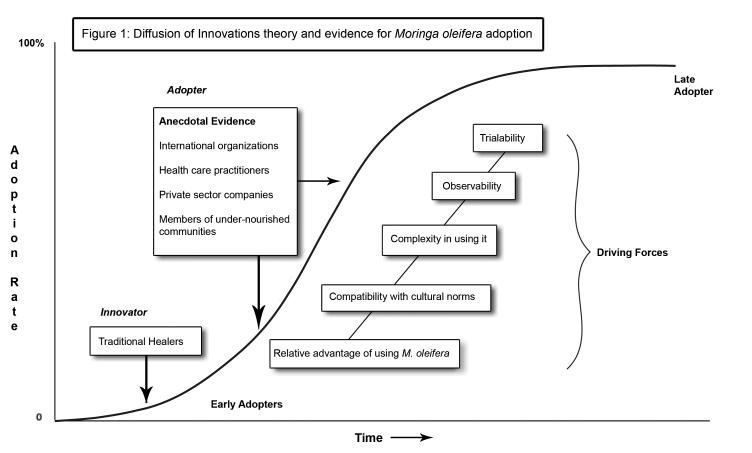
What started as traditional practice and knowledge is being disseminated by international aid agencies, health care workers, and the private sector, to educate people around the world as a sustainable innovation to combat under-nutrition including micronutrient deficiencies. The “Diffusion of Innovations” theory explains the recent increase in M. oleifera adoption by various international organizations and certain constituencies within undernourished populations, in the same manner as it has been so useful in explaining the adoption of many of the innovative agricultural practices in the 1940-1960s (Dearing, 2008).
“Diffusion is the process by which an innovation is communicated through certain channels over time among the members of a social system…it is a kind of social change” (Rogers, 1983). A sigmoidal curve (Figure 1), illustrates the adoption process starting with innovators (traditional healers in the case of M. oleifera), who communicate and influence early adopters, (international organizations), who then broadcast over time new information on M. oleifera adoption, in the wake of which adoption rate steadily increases. To date, over 1100 people are studying, growing, using, or implementing M. oleifera programs (MoringaNews, 2008). According to Rogers (1983), the rate of adoption and possibilities of overadoption can be predicted using five characteristics of a new innovation. In order for M. oleifera to be adopted and for its widespread use to be promoted, evidence must be provided for the following five attributes: relative advantage, compatibility, complexity, observability, and trialability. We describe these attributes in context, and provide the evidence pertinent to Moringa consumption, in the following sections.
Figure 1.
Diffusion of Innovations theory and evidence for Moringa oleifera adoption
Relative Advantage
Relative advantage is the extent to which an innovation is perceived as being better or more useful than the idea it supersedes (Rogers, 1983). International food supplement programs (e.g. feeding centers and delivery of industrially produced macro- and micronutrients) are highly effective in addressing under-nutrition in developing countries. However, a locally produced food-based approach such as adding M. oleifera leaves or powder to a local diet is arguably even more advantageous. Not only are the leaves nutrient-dense (Dhar & Gupta, 1982; D’Souza & Kulkarni, 1993; Seshadri & Nambiar, 2003; Trees for Life, 2005; Broin, 2006) the plant also provides individual households and agricultural societies a sustainable food source and possible microfinance opportunities (Nautiyal & Venkataraman, 1987). Though the benefits of M. oleifera are manifold, the primary advantage discussed herein is for undernourished populations to adopt M. oleifera for its nutritional benefits since it is rich in a variety of macro- and micronutrients and essential amino acids (see Table 1).
Table 1.
Table of Moringa oleifera leaf nutrient composition and nutrient RDAs* (Trees for Life 2005, The National Academies Press 2002, 2004, 2005)
| Fresh leaves a (value/100g edible portion) |
Dried leaves b (value/24g [≈3tbsp] edible portion) |
RDA for healthy children age 1-8 years old c |
|
|---|---|---|---|
| Calories | 92 cal | 49 cal | |
| Macronutrients | |||
| Protein | 6.70 g | 6.5 g | 13-19g/day |
| Fat | 1.70 g | 0.55 g | 30-40 g/day |
| Carbohydrates | 12.5 g | 9.2 g | 130 g/day |
| Micronutrients | |||
| Carotene (Vitamin A) | 6.78 mg | 4.54 mg | 300-400 μg/day |
| Thiamin (B1) | 0.06 mg | 0.63 mg | .5-.6 mg/day |
| Riboflavin (B2) | 0.05 mg | 4.92 mg | .5-.6 mg/day |
| Niacin (B3) | 0.8 mg | 1.97 mg | 6-8 mg/day |
| Vitamin C | 220 mg | 4.15 mg | 15-25 mg/day |
| Calcium | 440 mg | 480.72 mg | 500-800 mg/day** |
| Copper | 0.07 mg | 0.14 mg | 340-440 mg/day |
| Fiber | 0.90 g | 4.61 g | 19-25 g/day** |
| Iron | 0.85 mg | 6.77 mg | 7-10 mg/day |
| Magnesium | 42 mg | 88.32 mg | 80-130 mg/day |
| Phosphorus | 70 mg | 48.96 mg | 460-500 mg/day |
| Potassium | .26 g | 0.32 g | 3.0-3.8 g/day** |
| Zinc | 0.16 mg | .79 mg | 3-5 mg/day |
|
Essential Amino Acids 7 |
|||
| Histidine | 149.8 mg | 147.12 mg | 8 mg/g protein |
| Isoleucine | 299.6 mg | 198 mg | 25 mg/g protein |
| Leucine | 492.2 mg | 468 mg | 55 mg/g protein |
| Lysine | 342.4 mg | 318 mg | 51 mg/g protein |
| Methionine + Cysteine | 117.7 mg | 84 mg | 25 mg/g protein |
| Phenylalanine Tyrosine | 310.3 mg | 333.12 mg | 47 mg/g protein |
| Threonine | 117.7 mg | 285.12 mg | 27 mg/g protein |
| Tryptophan | 107 mg | 102 mg | 7 mg/g protein |
| Valine | 374.5 mg | 255.12 mg | 32 mg/g protein |
Trees for Life (2005) reported M. oleifera fresh leaf content from Gopalan et al. (1971).
Trees for Life (2005) reported M. oleifera dried leaf nutrient content from Fuglie (2001).
Recommended Daily Allowance (RDA) values are given as an estimate of what an individual’s recommended intake should be. For the purpose of this paper, these values should be interpreted as a general comparison of leaf content to what an average healthy child should intake. Source: Food and Nutrition Board, Institute of Medicine, National Academy of Sciences Dietary Reference Intake database (2002)
Gopalan et al. (1971) originally expressed amino acid content per g N, and have been converted to mg per 100g leaves
Leaf nutrient composition will vary depending on the geographic region the leaves are sampled from and the type of analysis used. This table acts to give a general idea of the nutrient content of the leaves.
Represents Adequate Intake (AI) for an individual
While the reported nutrient content of M. oleifera leaves, both fresh, and in powdered form, appears promising in terms of a nutritional supplement, an understanding of the bioavailability of these nutrients is limited to vitamins A and B and calcium. M. oleifera is one of the richest natural sources of β-carotene or provitamin A (Kumar, 2004), thus prompting much research interest in India. β-carotene and lutein from M. oleifera leaves in India was found to be highly bioavailable in an in vitro model (Pullakhandam & Failla, 2007). Using a rat model, the comparative bioavailability of β-carotene in fresh and dried M. oleifera leaves was evaluated by Nambiar & Seshadri (2001). Rats receiving M. oleifera leaves increased their food intake and weight gain compared to rats given either synthetic vitamin A or vitamin A adequate diets. Though synthetic vitamin A fed rats had the highest serum and liver vitamin A levels, the M. oleifera fed rats had significantly higher serum and liver vitamin A levels than at baseline.
The bioavailability of thiamine, riboflavin and niacin from dried M. oleifera leaves was evaluated in six 17-20 year old subjects in India. Subjects were given a series of experimental curry-based diets with leaves of trees noted for their contents of thiamine, riboflavin, and niacin (Girija et al., 1982). Diets containing M. oleifera leaves resulted in urinary excretion of 11.72 % of thiamine, 10.78 % of riboflavin, and 9.44 % of niacin intake, leading the authors to conclude that this equated to bioavailability.
Dried M. oleifera leaves are high in calcium but also contain substantial quantities of oxalic acid, which interferes with the absorption of calcium. Rats were fed calcium-rich diets containing: (a) 15 g dried M. oleifera leaf powder, (b) 30 g milk powder, or (c) 4 g dried kilkeerai (Amaranthus tricolor) leaf powder per 100 g of basal diet. The calcium contents of the M. oleifera leaf diet and the milk diet were the same (ca. 635 mg per 100 g of diet), but the M. oleifera leaves had 160 mg oxalates per 100 g diet. Although milk did provide for the best absorption and retention of calcium, 73% of the calcium provided by M. oleifera was absorbed and 59% was retained, thus providing a good alternative or ancillary source of calcium when milk is not available (Pankaja & Prakash, 1994).
Conventional macro- and micronutrient supplements have well-proven efficacy, and M. oleifera is likely not a suitable replacement for these nutrient dense supplements. However, it is a sustainable and economically sound nutrient-rich food option for populations who suffer from chronic or seasonal micro- and macronutrient deficiencies. The M. oleifera tree costs little to plant -- in fact aid agencies who are working with M. oleifera often donate seeds, and individuals can easily grow, maintain and utilize the tree provided they are not in high-density urban centers. Comparisons have been made with other nutrient-dense leafy vegetables in Niger (Sena et al., 1998) and in Nigeria (Barminas et al., 1998)). No other plant, whose nutritional profile compares favorably with that of M. oleifera, appears able to match its combination of overall utility, micro- and macronutrient composition, rapid growth habit, high yield leaf production, and survival in harsh climates. This strongly suggests that M. oleifera is a unique pan-tropical dietary plant.
Compatibility
Compatibility, in the context of the diffusion of innovations theory, is a measure of how well an innovation is consistent with existing social and cultural practices, if it is likeable, and if it meets the needs of potential adopters (Rogers, 1983). Intake of wild plants as food and medicine lies at the foundation of many traditional healing systems. Many traditional agricultural societies rely heavily on edible wild plants to provide important energy and micronutrients throughout the year (Grivetti & Ogle, 2000). A study of dietary intake in the Usumbara Mountains of Tanzania found that wild vegetables accounted for over 80% of all leafy vegetables consumed. These wild vegetables were primary ingredients of side dishes to staple foods in 25-43% of meals (Fleuret, 1979). In more recent studies of local African diets, approximately half of vegetables consumed were from wild sources and they were used as major micronutrient sources especially in times of drought and famine (Uiso & Johns, 1996; Lockett et al., 2000; Grivetti & Ogle, 2000). Food trends show that the use of plants which once offered important flavor, texture satisfaction, and supplied essential nutrients has declined so much in recent years that now 80% of total dietary intake globally comes from: eight cereals (barley, maize, millet, rice, rye, sorghum, sugar cane and wheat) and four tubers (cassava, potato, sweet potato and yam) (Grivetti & Ogle, 2000). This poses potential barriers to nutritional security in developing country regions by: decreasing variety and biodiversity of dietary intake, increasing the possibility of a cereal virus or parasite attacking these grains and causing worldwide famine, and declining promotion of dietary and nutrition knowledge due to lack of food variation (Grivetti & Ogle, 2000). Adopting M. oleifera remains consistent with the use of green leafy plant sources and re-introduces diversity along with knowledge of local nutritious plant sources into the diet and the culture.
Acceptance of M. oleifera as a nutritional supplement or a food additive in undernourished populations is compatible in those cultures that currently use green leafy plant sources in traditional dishes. Rural populations, and those populations who rely heavily on subsistence farming, may find using M. oleifera leaves more compatible than purchasing non-locally produced food. Because households can produce their own M. oleifera or find it in local markets, they are able to use it just as they would with other locally grown foods such as grains, legumes, root and/or tuber vegetables.
Traditional dishes around the world include green leafy plant sources which can be substituted or augmented with M. oleifera leaves. An Indian study evaluated food attitudes in children and infants, where they were given 30 g and 15 g respectively of M. oleifera leaves mixed with 10 g of legumes and the dish was found to be coarse and bitter (Gopaldas et al., 1973). In Malawi, 63% of households preferred M. oleifera leaves over commonly used pumpkin leaves (Babu, 2000). Nambiar et al. (2003) evaluated the feasibility and acceptability of M. oleifera leaf powder used in preschool meals prepared by staff at the Indian Integrated Child Development Scheme (ICDS). Forty children aged 1-5 years were given 5-7g dried leaf powder added to their daily salty snack. Acceptability (gauged by facial expression, demand for food, and measurement of food left at the end of the meal), was no different than the acceptability of diet to a control group (n=20) receiving the regular recipe (Nambiar et al., 2003). A similar acceptability trial utilized a panel of 12 women age 18-21 years old given traditional Indian recipes with 6-25g freshly blanched M. oleifera leaves (Nambiar & Parnami, 2008). Pulse recipes that included 20 g of leaves, calculated to give the women 82.5% to 83.3% of their RDA for vitamin A, were the most acceptable. Other studies have demonstrated that peoples’ taste perceptions of M. oleifera leaves varies from “tasteless” to “slightly bitter” depending on the geographic region from which the leaves come. Children given M. oleifera leaves in a variety of traditional Indian recipes revealed no specific like or dislike although mothers reported that children preferred having the leaves incorporated into the cereal-pulse dough which is used in several traditional dishes (Seshadri & Nambiar, 2003). In populations where traditional medicine is practiced and preferred, M. oleifera may likely be accepted as a way of treating under-nutrition. However, tradition does play a large role in adoption of certain food sources in which the use of M. oleifera leaves or other green leafy vegetables might be challenged.
Complexity
Complexity is the level to which the innovation is perceived to be difficult to understand or use (Rogers, 1983). M. oleifera is relatively easy to obtain, grow, and use on a regular basis. The tree grows well in climates ranging from warm tropical at sea level, to sub tropical climates up to an altitude of 3000 feet (Palada & Chang, 2003). The maximum temperature for growth varies from 38 to 48°C and minimum temperature from -1 to 3°C. M. oleifera prefers sandy soil, though it grows in most soils other than stiff clay of shallow hilly soils (Nautiyal & Venkataraman, 1987; Palada & Chang, 2003).
M. oleifera can be grown either seeds or from cuttings. Sprouting usually occurs within two weeks but can occur in rich soils in as little as three to four days. In one growing season, the tree can grow between nine and fifteen vertical feet. A full grown tree can be pruned to ground level and new shoots with leaves will emerge. Higher leaf production can be achieved if the trees are regularly pruned close to ground level (coppicing) or if individual shoots are regularly harvested. There is an extensive literature, not elaborated upon herein, on proper upkeep and care of M. oleifera (Palada & Chang, 2003; Price, 2007).
Once the leaves are harvested and cleaned, they can either be used fresh in meals or dried in the shade to be used at another time. In countries that suffer from annual drought or famine before harvest season, dried M. oleifera leaves can be made into a powder and used throughout the year. M. oleifera powder is made by crushing and sifting dried leaves. There have been studies on the retention of heat sensitive vitamins, such as vitamin A, during the drying and storage of M. oleifera leaves (Seshadri et al., 1997; Seshadri & Nambiar., 2003). For many rural agricultural societies storing grains is common practice (Lockett & Grivetti, 2000), and for many grains shade drying or blanching, is used prior to storage of the food source. Retention of total carotene, β-carotene, and ascorbic acid (vitamin C) was measured following storage for 0, 1, 2, and 3 months (Seshadri et al., 1997). Leaves that were blanched and sulfited compared to blanched-only leaves initially retained more total carotenes, β-carotene, and ascorbic acid but within about 3 months β-carotene levels were about half of original levels, with either method of drying. On the other hand, there were significant benefits of sulfiting on ascorbic acid retention.
Cooking with green leafy plant sources is relatively common, thus the overall complexity of using M. oleifera leaves should not deter adoption. However, proper cooking methods resulting in good nutrient retention can become complex. Three recipes of traditional Indian dishes using M. oleifera dried leaves were evaluated for retention of β-carotene. “Dhebra”, a shallow fried cereal and M. oleifera leaf recipe, a steamed cereal and leaf recipe called “muthia”, and “dal soup” a boiled pulse and leaf recipe were compared resulting in β-carotene retention per serving being: 69%, 73%, and 35% respectively (Seshadri et al., 1997). Recipes around the world that call for M. oleifera leaves often instruct to boil the leaves once to three times before the food is actually served (Fuglie, 1999). Boiling leaves can often degrade, inactivate, and eliminate heat-sensitive vitamins such (e.g. vitamin A). As more people adopt M. oleifera, cooking methods need to be evaluated to ensure that correct information is being disseminated and adopters are benefiting from the leaves as expected.
Observability
Observability is used to describe how well the results of the innovation can be seen and communicated to others (Rogers, 1983). The observability factor of M. oleifera is perhaps the greatest driving force for people to adopt its use. Not only is the plant appearance unique and its rapid growth almost palpable, but now extensive anecdotal evidence strongly supports the dissemination of information and adoption of its use. Currently there are 1,182 members of the privately run organization that represents itself as “the global Moringa network” (MoringaNews, 2008). MoringaNews was started in 2002 to: “enhance communication and coordination between actors, to provide reliable and checked information to members of the network, and to undertake research when the information is lacking” (MoringaNews, 2008). Fully half of these members claim to be working with Moringa in a health/nutrition capacity. Many of these members represent reputable international organizations and their local counterparts (the balance of governmental, non-governmental, and commercial entities), who are working in rural areas around the world to promote and disseminate knowledge of the benefits of M. oleifera. These campaigns are almost exclusively based upon personal testimonials and observation, fueled by traditional medicine knowledge.
In addition to international organizations, commercial retailers and private companies have used anecdotal evidence to promote and sell various products of M. oleifera. Among these products are oils extracted from seeds and leaves for skin and cosmetic purposes, in addition to capsules and beverages containing Moringa extracts that are promoted by some as vitamin- and nutrient-rich tonics, and [irresponsibly] as panaceas or miracle cures for a large variety of ailments.
Trialability
Trialability, in the context of the diffusion of innovations theory, refers to the ability of an innovation to be experimented with (Rogers, 1983). People around the world experiment with M. oleifera leaves on a daily basis, consuming it personally as a vitamin source, or providing it to friends and family members in the form of a beverage, a capsule, powder, and/or fresh leaves. Though trialable, M. oleifera has not been rigorously “proven” in the Western medical tradition. Because very few such studies have been completed, the use of M. oleifera for treatment of under-nutrition lacks a scientific base in clinical studies demonstrating either efficacy, or lack of toxicity. Furthermore, the studies that have been completed on nutrient content in various global regions are not fully congruent. This is partially due to natural variation among the source of leaves, such as genetic background, environment, and cultivation methods. This also includes the variation due to sample preparation and analysis including time between collection and analysis, mode of preservation between collection and analysis, and the analytical methods (Broin, 2006). Nutrient content data has been published in various forms (e.g. fresh leaf content, dried leaf content or both), and those reporting dried leaf data must differentiate sun drying from shade drying. Though most studies elaborate upon methods of collection and preparation of leaves, the apparent wide variation in the values published in journals reduces credibility in the eyes of some critics.
The lack of robust clinical trials data increases the uncertainty regarding M. oleifera nutritional benefits. Uncertainty about adoption of an innovation can lead both to overadoption by individuals, and to complete lack of adoption due to the inability of thought leaders and lead policy organizations to endorse the innovation. In the case of M. oleifera, the innovators or early adopters, (e.g. organizations that disseminate M. oleifera information), should provide sound scientific evidence of Moringa’s benefits and provide guidance for correct methods and application for use of the leaves. This will provide the comfort and confidence level required by policymakers and decision makers in large international and governmental organizations to support M. oleifera as a nutrition intervention, and will also decrease the threat of overadoption (e.g. use when and where it may not be appropriate for either public health or medical reasons).
DISCUSSION
Clinical trials in human beings needs to be implemented in order to provide researchers, international aid agencies, and health care practitioners with the sound scientific evidence needed to support or discourage the adoption of M. oleifera. Such studies should embrace (but not be limited to) the following:
Adherence to widely accepted ethical guidelines, approval by a local institutional review board, and oversight by a medical professional.
A randomized double blind placebo-controlleda study using an appropriately powered number of infants and/or children within the age range of 6 months to five years.b Blinding or masking may be problematic in this instance due to the intense green color of Moringa leaves.
Food sources given to both the intervention and the control group should be likeable and culturally appropriate.
The amount of M. oleifera used in the intervention group should be compared to the dietary reference intakes for the study population to assess adequate nutrient supplementation.
Anthropometry (e.g. height, weight, skin fold, and/or upper arm circumference measurements), should be conducted pre-intervention, during, and post-intervention.
Blood chemistries would be highly instructive to assess bioavailability of nutrients.
Physical examinations are critical in order to expedite study termination in the event that there are signs of toxicity at any intake level.
Health care professionals and researchers should be adequately trained to take measurements.
Findings must be reported in widely accessible journals. Such obligation to report either positive or negative results should be a pre-requisite for the granting of any research funding.
The state of world hunger and under-nutrition is slowly improving and could in some developing regions be close to its achieving the UN Millennium Development goal -- halving the proportion of people who suffer from hunger by 2015 (United Nations, 2000). To achieve this goal, nations have relied on food aid programs and manufactured nutrient supplementation. These programs that use manufactured nutrient-rich powders, high-energy food sources, vitamin A drops, and nutrition centers for severely undernourished children have adequately addressed the once bleak world nutritional status. However, as more emphasis is put on local food based approaches, assessment of local resources for improving nutritional status should be further investigated. M. oleifera is one example of a nutrient source that can be grown and used at the individual or societal level. By partnering with appropriate educational modalities to describe its uses and nutritional benefits, communities around the world will be able to participate directly in halving the world’s hunger and improving nutritional deficiencies.
The evidence provided herein, pursuant to each of the five attributes of the diffusion of innovations theory, support the adoption of M. oleifera as a nutrient supplement. However, there are many gaps in the data that will keep large policy advocates and international aid groups from recommending adoption. In accordance with the diffusion of innovations theory, the anecdotal evidence and the currently extant data suggests that even without an appropriately controlled [by Western medical standards] clinical study, a variety of organizations and individuals will continue to use and promote M. oleifera. The uncertainty, regardless of how slight, that M. oleifera might not be effective in addressing under-nutrition in disadvantaged populations, requires that additional rigorous trials with human volunteers be carried out rapidly, and that the results, whether positive or negative, be disseminated in peer reviewed, widely accessible journals, so that they can receive the imprimatur of the world nutritional science community.
ACKNOWLEDGEMENTS
Special thanks go to Balbir Mathur, Jeffrey Faus, Lowell Fuglie, Lydia da Ragos, Beth Doerr, Amaglo Newton, Doris Strong, Monica Marcu, and Deepak Acharya, all of whom have generously shared their knowledge and expertise with the authors. We are grateful to the Lewis B. and Dorothy Cullman Foundation and NIH Grant #1R01 CA 93780-05A2 for partial financial support.
Footnotes
Though M. oleifera does not have a strong, noticeable taste, the food the leaves are added to will be notably green. Therefore the placebo should be similar in taste and color, such as lettuce.
WHO recommends infants under the age of 6 months should be exclusively breastfed and therefore should not be included in such a study where complementary feeding is involved.
None of the authors has any conflicts of interest.
REFERENCES
- Anwar F, Latif S, Ashraf M, Gilani AH. Moringa oleifera: A Food Plant with Multiple Medicinal Uses. Phytotherapy Research. 2007;21:17–25. doi: 10.1002/ptr.2023. [DOI] [PubMed] [Google Scholar]
- Babu S. Rural nutrition interventions with indigenous plant foods-a case study of vitamin A deficiency in Malawi. Biotechnology, Agronomy, Society, and Environment. 2000;4(3):169–179. [Google Scholar]
- Barminas JT, Charles M, Emmanuel D. Mineral composition of non-conventional leafy vegetables. Plant Foods for Human Nutrition. 1998;53:29–36. doi: 10.1023/a:1008084007189. [DOI] [PubMed] [Google Scholar]
- Broin M. The nutritional value of Moringa oleifera Lam. Leaves: what can we learn from figures? [Accessed April 14, 2008];2006 Moringanews Workshop. 2006 [Poster] [Online] Available at: http://www.moringanews.org/doc/GB/Posters/Broin_poster.pdf.
- Castellón CV, González CJR. Thesis Lic. Zootecnia Managua; Nicaragua, UCA: 1996. Utilización del marango (Moringa oleifera) en la alimentación de novillos en crecimiento bajo régimen de estabulación; p. 44. [Google Scholar]
- Dearing JW. Evolution of Diffusion and Dissemination Theory. Journal of Public Health Management Practice. 2008;14(2):99–108. doi: 10.1097/01.PHH.0000311886.98627.b7. [DOI] [PubMed] [Google Scholar]
- Dhar B, Gupta OP. Nutritional value of Shigru (Moringa oleifera Lam.) Bulletin of Medico-Ethno-Botanical Research. 1982;3(24):280–288. [Google Scholar]
- D’Souza J, Kulkarni AR. Comparitive studies on nutritive values of tender foliage of seedlings and mature plants of Moringa oleifera Lam. Journal of Economic Taxonomy. 1993;17(2):479–485. [Google Scholar]
- Fahey J. Moringa oleifera: A Review of the medical Evidence for Its Nutritional Therapeutic, and Prophylactic Properties. Part 1. [Accessed on July 23, 2008];Trees for Life Journal. 2005 1(5) [Online] Available at: http://www.tfljournal.org/article.php/20051201124931586.
- Fleuret A. The role of wild foliage plants in the diet. A case study from Lushoto, Tanzania. Ecology of Food and Nutrition. 1979;27:1–15. [Google Scholar]
- Fuglie LJ. The Miracle Tree: Moringa oleifera: Natural Nutrition for the Tropics. Church World Service; Dakar, Senegal: 1999. [Google Scholar]
- Girija V, Sharada D, Pushpamma P. Bioavailability of Thiamine, Riboflavin and Niacin from Commonly Consumed Green Leafy Vegetables in the Rural Areas of Andhra Pradesh in India. International Journal of Vitamin Nutrition Research. 1982;52:9–13. [PubMed] [Google Scholar]
- Gopaldas T, Ramakrishnan I, Grewal T, Rajalakshmi R, Devadas RP. Use of legumes and green leafy vegetables for infant and young child feeding: summary of results of studies in three different parts of India. PAG Bulletin. 1973;3(2):51–53. [Google Scholar]
- Grivetti LE, Ogle BM. Value of traditional foods in meeting macro- and micronutrient needs: the wild plant connection. Nutrition Research Reviews. 2000;13(1):31–46. doi: 10.1079/095442200108728990. [DOI] [PubMed] [Google Scholar]
- Hunter IR, Stewart JL. Foliar nutrient and nutritive content of Central American multipurpose tree species growing at Comayagua, Honduras. Commonwealth Forestry Review. 1993;72(3):193–197. [Google Scholar]
- Kakengi AMV, Kaijage JT, Sarwatt SV, Mutayoba SK, Shem MN, Fujihara T. Effect of Moringa oleifera leaf meal as a substitute for sunflower seed meal on performance of laying hens in Tanzania. Livestock Research for Rural Development. 2007;19(8) [Google Scholar]
- Kumar HD. Management of Nutritional and Health Needs of Malnourished and Vegetarian People in India. In: Cooper E, Yamaguchi N, editors. Complementary and Alternative Approaches to Biomedicine. Kluwer Academic/Plenum Publishers; 2004. pp. 311–321. [DOI] [PubMed] [Google Scholar]
- Lockett CT, Calvert CC, Grivetti LE. Energy and micronutrient composition of dietary and medicinal wild plants consumed during drought. Study of rural Fulani, Northeastern Nigeria. International Journal of Food Sciences and Nutrition. 2000;51:195–208. doi: 10.1080/09637480050029700. [DOI] [PubMed] [Google Scholar]
- Lockett CT, Grivetti LE. Food-related behaviors during drought: a study of rural Fulani, northeastern Nigeria. International Journal of Food Sciences and Nutrition. 2000;51:91–107. doi: 10.1080/096374800100796. [DOI] [PubMed] [Google Scholar]
- MoringaNews [Accessed on April 14, 2008];The MoringaNews network. 2008 [Online] Available at: http://www.moringanews.org/reseau_en.html.
- Nambiar VS, Bhadalkar K, Daxini M. Drumstick leaves as source of vitamin A in ICDS-SFP. Indian Journal of Pediatrics. 2003;70(5):383–387. doi: 10.1007/BF02723611. [DOI] [PubMed] [Google Scholar]
- Nambiar VS, Parnami S. Standardization and Organoleptic Evaluation of Drumstick (Moringa oleifera) Leaves Incorporated into Traditional Indian Recipes. [Accessed April 14, 2008];Trees for Life Journal. 2008 3(2) [Online] Available at: http://www.tfljournal.org/images/articles/20080407133437686_3.pdf.
- Nambiar VS, Seshadri S. Bioavailability trials of β-carotene from fresh and dehydrated drumstick leaves (Moringa oleifera) in a rat model. Plant Foods for Human Nutrition. 2001;56:83–95. doi: 10.1023/a:1008132503972. [DOI] [PubMed] [Google Scholar]
- National Academy of Science, Food and Nutrition Board, Institute of Medicine Dietary Reference Intakes for Energy, Carbohydrate. Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. [Accessed on April 14, 2008];The National Academies Press. 20022005 [Online] Available at: http://www.iom.edu/Object.File/Master/7/300/Webtablemacro.pdf.
- National Academy of Science, Food and Nutrition Board, Institute of Medicine Dietary Reference Intakes (DRIs): Recommended Intakes for Individuals, Vitamins. [Accessed on April 14, 2008];The National Academies Press. 2004 [Online] Available at: http://www.iom.edu/Object.File/Master/21/372/0.pdf.
- Nautiyal BP, Venkataraman KG. Moringa (Drumstick)-An ideal tree for Social Forestry: Growing conditions and uses-Part 1. MYFOREST. 1987;23(1):53–58. [Google Scholar]
- Palada MC. Moringa (Moringa oleifera Lam.): a versatile tree crop with horticultural potential in the subtropical United States. HortScience. 1996;31:794–797. [Google Scholar]
- Palada MC, Chang LC. Suggested Cultural Practices for Moringa. [Accessed April 14, 2008];AVRDC International Cooperators’ Guide. 2003 [Online] Available at: http://www.avrdc.org/LC/indigenous/moringa.pdf.
- Price ML. The Moringa Tree. [Accessed July 23, 2008];ECHO Technical Note. 2007 [Online] Available at: http://www.echotech.org/mambo/index.php?option=com_docman&task=doc_view&gid=170.
- Pankaja N, Prakash J. Availability of calcium from kilkeerai (Amaranthus tricolor) and drumstick (Moringa oleifera) greens in weanling rats. Die Nahrung. 1994;2:199–203. doi: 10.1002/food.19940380212. [DOI] [PubMed] [Google Scholar]
- Pullakhandam R, Failla ML. Micellarization and Intestinal Cell Uptake of β-carotene and Lutein from Drumstick (Moringa oleifera) Leaves. Journal of Medicinal Food. 2007;10(2):252–257. doi: 10.1089/jmf.2006.250. [DOI] [PubMed] [Google Scholar]
- Sánchez N. Reyes, Spörndly E, Ledin I. Effect of feeding different levels of foliage of Moringa oleifera to creole dairy cows on intake, digestibility, milk production and composition. Livestock Science. 2005;101(13):24–31. [Google Scholar]
- Rocha MLR, Mendieta B. Tesis Ing. Agron. Facultad de Ciencia Animal. Universidad Nacional Agraria Nicaragua; Managua: 1998. Effecto de la suplementación con follaje de Moringa oleifera sobre la producción de leche de vacas en pastoreo; p. 36. [Google Scholar]
- Rogers EM. Diffusion of Innovations, Third Edition. The Free Press, A Division of Macmillan Publishing Co., Inc.; New York, NY: 1983. [Google Scholar]
- Sarwatt SV, Milang’ha MS, Lekule FP, Madalla N. Moringa oleifera and cottonseed cake as supplements for smallholder dairy cows fed Napier grass. Livestock Research for Rural Development. 2004:16. [Google Scholar]
- Sena LP, Vanderjagt DJ, Rivera C, Tsin ATC, Muhamadu I, Mahamadou O, Millson M, Pastuszyn A, Glew RH. Analysis of nutritional components of eight famine foods of the Republic of Niger. Plant Foods for Human Nutrition. 1998;52:17–30. doi: 10.1023/a:1008010009170. [DOI] [PubMed] [Google Scholar]
- Seshadri S, Nambiar VS. Kanjero (Digera arvensis) and drumstick leaves (Moringa oleifera): Nutrient Profile and Potential for Human Consumption. World review of nutrition and dietetics. 2003;91:41–59. doi: 10.1159/000069927. [DOI] [PubMed] [Google Scholar]
- Seshadri S, Jain M, Dhabhai D. Retention and storage stability of beta-carotene in dehydrated drumstick leaves (Moringa oleifera) International Journal of Food Sciences and Nutrition. 1997;48:373–379. [Google Scholar]
- Trees for Life . Moringa Book [Brochure] Balbir Mathur; Wichita, Kansas: 2005. [Google Scholar]
- Uiso FC, Johns T. Consumption patterns and nutritional contribution of Crotalaria brevidens (mitoo) in Tarime District, Tanzania. Ecology of Food and Nutrition. 1996;35:59–69. [Google Scholar]
- UNICEF . Progress for Children: A World Fit for Children Statistical Review Number 6 revised. United Nations Children’s Fund; New York: 2007. [Accessed July 22, 2008]. [Online] Available at: http://www.unicef.org/publications/files/Progress_for_Children_No_6_revised.pdf. [Google Scholar]
- United Nations . United Nations Millennium Declaration. United Nations; New York: 2000. [Accessed July 23, 2008]. 8th Plenary Meeting. New York, NY 6-8 September 2000. [Online] Available at: http://www.un.org/millennium/declaration/ares552e.htm. [Google Scholar]
- West KP, Jr., Caballero B, Black RE. Nutrition. In: Merson M, Black RE, Mills AJ, editors. International Public Health: Diseases, Programs, Systems, and Policies. Jones and Bartlett Publishers; Sudbury, Massachusetts: 2006. pp. 187–272. [Google Scholar]