SYNOPSIS
Sleep disordered breathing (SDB) is increasingly recognized as an important clinical problem in children. However, the clinical, anatomic and physiologic correlates of SDB have not been extensively studied in a general population sample using polysomnography to document the presence of SDB. The Tucson Children's Assessment of Sleep Apnea Study (TuCASA) is a longitudinal cohort study of 503 6-12 year old Caucasian and Hispanic children who underwent polysomnography and neurocognitive testing at the time of recruitment. Subsets of the cohort had additional MRI imaging and pulmonary physiologic testing. Initial cross-sectional analyses indicate that SDB is associated with behavioral abnormalities, hypertension, learning problems and clinical symptoms such as snoring and excessive daytime sleepiness. Future follow-up of the cohort will assess the impact of SDB on subsequent childhood development.
INTRODUCTION
That sleep disordered breathing (SDB) occurs in children has been long known. However, emerging recognition of the frequent occurrence and impact of SDB in the pediatric population has led to an increased clinical and academic interest in this condition. The presenting symptoms of childhood SDB include snoring, arousals, enuresis, restlessness during sleep, daytime sleepiness and hyperactivity 1, 2. Of particular importance is that unlike SDB in adults, hyperactivity and not daytime sleepiness may be the most evident manifestation. Moreover, the presence of SDB has been suggested to result in an adverse impact on neurobehavioral development 3-6.
The Tucson Children's Assessment of Sleep Apnea study (TuCASA) is a prospective cohort study aimed at assessing the objective prevalence of SDB in pre-adolescent children aged 6—12 years using polysomnography (PSG). While there have been some studies evaluating the presence of symptoms and correlates of SDB in Caucasian and African American children 7, TuCASA is the first one to study a large population of Hispanic children. Further, the study has investigated the symptoms, anatomic and/or physiologic correlates, and consequences of SDB, including neuropsychological performance, in children. This review examines the methodological procedures and the cross-sectional outcome data from this study.
STUDY DESIGN
Participants
The study design for TuCASA has been described in detail previously 8. Five hundred and three 6-12 year old Caucasian and Hispanic children were recruited from selected elementary schools in the Tucson Unified School District to undergo unattended home polysomnography. Incentives were offered to schools in order to encourage participation. The selected schools were pre-screened in order to ensure that between 25%-75% of their children were of Hispanic descent. The children were asked to take home a short screening questionnaire with sleep, anthropometric and demographic items. Parents were requested to complete the questionnaire, return it to the investigators and indicate whether they would allow study personnel to call them. The screening questionnaire consisted of a 1-page survey designed to assess the severity of OSAS-related symptoms in children. Questions such as “How often does your child snore loudly?”, “Is your child sleepy during the daytime?”, “Does your child stop breathing during sleep?”, and “Does your child have learning problems?” were evaluated by a parent on the scale of Never, Rarely, Occasionally, Frequently, Almost Always or Don't Know. A total of 7055 questionnaires were distributed with 33% return rate. Of the children for whom the surveys were returned, 45.4% were Hispanic and 38% were Caucasian. Of those returning questionnaires, 1219 (52.4%) supplied recruitment information, and from this group, 503 children were selected to undergo unattended home PSG. Children with chronic medical conditions and attention deficit hyperactivity disorder (ADHD) were excluded. A full unattended PSG was performed at the home within one month of recruitment and another visit was scheduled at the medical center for neurocognitive evaluation several weeks later.
Home Polysomnography
A two-person, mixed gender team arrived at the participant's home approximately one hour prior to the child's regular bedtime to prepare the child for an unattended PSG using the Compumedics PS-2 system (Abbotsford, Victoria, Australia), a portable monitor capable of recording a full polysomnographic montage 9. Use of this system in TuCASA has been described in detail elsewhere 8. Briefly, sensors included C3/A2 and C4/A1 electroencephalogram (EEG), right and left electrooculogram (EOG), a bipolar submental electromyogram (EMG), thoracic and abdominal displacement (inductive plethysmography bands), airflow (nasal/oral thermistor), nasal pressure cannula, oximetry, ECG (single bipolar lead), snoring (microphone attached to the vest), body position (Hg gauge sensor), and ambient light (sensor attached to the vest to record on/off). The thermistor and nasal pressure signals were collected simultaneously by taping a nasal/oral thermistor on the superior surface of a nasal cannula. The data from the PSG were stored in real time on a 40MB PCMCIA flashcard and reviewed in the morning. If the study had insufficient duration or quality of artifact free signal, less than 4 hours of oximetry, or equipment malfunction, it was categorized as a failed study. A study was classified as `good' if there were a minimum of 5 or more hours of scorable signal on at least 2 respiratory channels (airflow, thoracic or abdominal bands), oximetry, and 1 electroencephalogram signal. If 4-5 hours of scorable signal were present on at least 1 electroencephalogram, oximeter, and respiratory signal, the study was classified as `fair'. If an initial study was failed, the subject was asked to have another sleep recording. The final overall `pass rate' of the studies was 97%.
Sleep was manually staged according to Rechtschaffen and Kales criteria 10. Arousals were identified using criteria published by the American Academy of Sleep Medicine 11. . Obstructive apneas were identified if the magnitude of any ventilation signal decreased to below 25% of the baseline amplitude for at least 6 seconds or 2 or more consecutive breaths. Hypopneas were scored if the magnitude of any ventilation signal decreased to below approximately 70% of the baseline amplitude for at least 6 seconds or for 2 or more consecutive breaths. Central apneas were scored if both airflow and thoracoabdominal effort were absent. In order to describe a Respiratory Disturbance Index (RDI) defined by different magnitudes of oxygen desaturation and/or arousal, software linked various levels of minimum oxygen desaturation or the presence of arousal to each scored apneic or hypopneic event. In this way, a RDI could be generated characterized by no oxygen desaturation (RDI0) or by 2% (RDI2%), 3% (RDI3%) or an arousal (RDI-A).
To assess the reliability of scoring, 5% of studies were rescored by the same scorer on a blinded basis. No significant difference was found between initial and repeat scoring. There was a good agreement between the two sets of studies regarding classification as sleep apnea (RDI0 < 1 or ≥ 1) (κ=0.78). A night-to-night variability study in 10 children showed no statistically significant differences in key sleep parameters between 2 different nights of recording.
During the home visit, anthropometric measurements, visual oropharynx inspection, seated blood pressure, a digital photograph of the oropharynx and tonsils, and a more extensive sleep habits questionnaire were also collected in addition to PSG. A post study survey was completed by the caregiver the morning after the PSG.
Behavioral evaluation
Behavior problems were measured using the Conners' Parent Rating Scale-Revised-long version (CPRS-R (L)) and the Child Behavior Checklist (CBCL). Families were paid $25 for completing the behavioral evaluation. The long version of CPRS-R contains 80 items 12. It is typically used with parents or caregivers when comprehensive information and DSM-IV consideration are required. Scales include: Oppositional, Cognitive Problems, Inattention, Hyperactivity, Anxious-Shy, Perfectionism, Social Problems, Psychosomatic, Conners' Global Index (Restless Impulsive, Emotional Lability), DSM-IV symptom subscales (Inattentive, Hyperactive Impulsive) and ADHD Index. Parents rate their children's behavior in the past month on a 4 point Likert scale (Not True At All; Just A Little True; Pretty Much True; Very Much True). Subscale T-scores can be calculated based on a large age and gender specific normative sample. A T-score (M = 50, SD =10) over 65 is considered to indicate moderate to severe clinical impairment. The Child Behavior Checklist (CBCL) was designed to assess social competence and behavior problems in children aged 4-18 13. It includes 118 items related to behavior problems which are scored on a 3-point scale (Not True, Somewhat True, or Very/Often True).
Neurocognitive Evaluation
A neurocognitive assessment was performed a mean of 38 days after the successful completion of the PSG. The 3 hour assessment battery consisted of a series of standardized neurocognitive measures completed in a fixed order and ending with a single standard 10-min visual psychomotor vigilence task (PVT) trial 14, 15. The cognitive measures administered to the children were completed as follows: the Wechsler Abbreviated Scale of Intelligence (WASI) 16; Letter-Word Identification, Applied Problems, and Dictation from the Woodcock-Johnson Psycho-Educational Battery-Revised Tests of Achievement (WJ-R; Woodcock & Johnson) 17; the Children's Auditory Verbal Learning Test-2 18. Dependent measures from each of these tests are age based standard scores that have a mean of 100 and standard deviation of 15, and higher scores indicate better performance. The WASI is a brief and reliable measure of intelligence and was used to facilitate characterization of the study participants. Measures of Full Scale IQ, Verbal IQ, and Performance IQ were obtained. The WJ-R academic achievement measures were used to assess learning of and memory for information learned prior to and outside of the evaluation setting. Letter-Word Identification assesses letter and single word reading. Applied Problems assesses math skills. Dictation is a measure of spelling, punctuation, grammar, and word usage. The Children's Auditory Verbal Learning Test-218 was administered to assess learning of and memory for novel information learned within the evaluation setting. This multi-trial word list learning task provides age based standard scores for each of the following: five list learning trials; overall learning across trials (Level of Learning), recall of a second word list (Interference Trial) presented after the learning trials, and immediate and delayed recall of the original list. Attention was evaluated using CPRS-R (L) questionnaire. The PVT-192 was used for the psychomotor vigilance testing. It is a simple task administered on a handheld device. Participants were asked to press a button as quickly as possible each time they saw a visual stimulus appear (a small red LED-digital counter). The stimulus was presented approximately 100 times during the 10-min task, with the interstimulus interval varying randomly from 2 to 10 s.
BASELINE CHARACTERISTICS OF THE TuCASA COHORT
Five hundred and three children underwent PSG in the TuCASA study. The baseline characteristics of this sample are shown in Table 1. Forty nine percent of the children were Hispanic and half the subjects were girls. The mean body mass index was 18.0 kg/m2 (SD=4.4) and 10.4% had body mass index greater than the 95th percentile for their age, sex, and ethnicity and were classified as `obese'. Of the 503 PSGs, 480 were found to be of good quality and were used for further analyses. The average total time in bed was 542.7±85.7 minutes, sleep latency was 18.5 ± 21.0 minutes and total sleep time was 487.0±79.7 minutes with the average sleep efficiency being 89.8% 8. These values were not significantly different in boys and girls.
Table 1.
Baseline Characteristics of the TuCASA cohort
| Age (Years) | ||||
|---|---|---|---|---|
| All | ≤ 7 | 8-9 | ≥ 10 | |
| Total Cohort | 503 | 165 | 184 | 154 |
| % Boys/Girls | 50/50 | 53/47 | 49/51 | 49/51 |
| % Caucasians/Hispanic | 59/41 | 59/41 | 59/41 | 56/44 |
| BMI (mean ± s.d.) | 18 ± 9 | 17 ± 4 | 18 ± 3 | 19 ± 5 |
| % Obese | 10.4 | 11.4 | 8.3 | 9.2 |
| Neck Circumference (cm) (mean ± s.d.) | 27 ± 3 | 26 ± 3 | 27 ± 4 | 29 ± 3 |
SLEEP DIORDERED BREATHING
Prevalence and Predictors of SDB
The primary goal of the TuCASA study was to determine the prevalence and correlates of sleep disordered breathing (SDB). The children were diagnosed as having SDB if the RDI3% ≥ 1 per hour of total sleep time. Composite variables were created based on a combination of selected survey items from the basic screening questionnaire and the more extensive sleep-habits questionnaire to elucidate the clinical correlates of sleep disordered breathing 19.
The children were classified as snorers if parents reported that their child snored loudly `frequently' or `almost always'. Witnessed apnea was defined by the parents' positive reply to the following items on the questionnaire: their child stopped or struggled to breathe, their child's lips turned blue, or they shook their child because they were worried about their child's breathing during sleep `frequently' or `almost always'. Subjects were diagnosed as having excessive daytime sleepiness if the parents had answered `frequently' or `almost always' on any of the following statements: their child was sleepy in the daytime, fell asleep while watching TV or in school, or had problems with falling asleep during the day. Insomnia was diagnosed if the parents reported that their child had trouble falling or staying asleep, did not have enough sleep, or was troubled by waking up too early and not being able to get back to sleep. Learning problems were considered present if the parent reported this happened frequently or almost always.
Of the children undergoing PSG, 15% had snoring, 5.2% had witnessed apnea, 16.3% had excessive daytime sleepiness, 29.4% had insomnia, and 5.8% had learning problems8. Polysomnographic evidence of SDB (RDI3% ≥ 1) was present in 24% of children (mean RDI3%=2.6±3.4). The mean RDI3% in children without SDB was 0.38±0.28. The likelihood of SDB was higher in boys than in girls [OR=1.9 (CI 1.2-3.0), P=0.006]. Snoring [OR=3.5 (CI 2.0-6.2), P= .001] and excessive daytime sleepiness [OR=2.1 (CI 1.2-3.8), P=.01] were also significantly associated with SDB. Furthermore, learning problems, as reported by parents, were more common in children in SDB than those without SDB (11.3% vs. 4.1%, P=0.004). However, reported witnessed apneas [OR=1.8 (CI 0.8-4.3), P=0.15] or insomnia [OR=0.9 (CI 0.6-1.5), P=0.85] were not associated with a higher likelihood of SDB. In addition, ethnicity (Hispanic or Caucasian), age, obesity or airway size were not significantly different between children with or without SDB.
Analyses were performed to assess the sensitivity, specificity and likelihood ratios of different predictors for SDB 8. The sensitivity of different variables for identifying SDB was usually low, with highest being that of male sex (60%). Frequent loud snoring, EDS and learning problems had low sensitivities of 29.5%, 24.4% and 11.3%, respectively. The sensitivities continued to be low when combinations of different symptoms and/ or demographic variables were assessed. However, the specificities of learning problems (95.9%), snoring (89.5%), and EDS (86.3%) for SDB were high. Similarly, the combinations of snoring and learning problems (98.9%), snoring and EDS (97.0%) and snoring and male gender (95.1%) had very high specificities for SDB.
These findings should be useful in the clinical evaluation of children with possible sleep disordered breathing. They suggest that while many children with SDB will not have habitual snoring and EDS, when the symptoms are present, a diagnosis of SDB should be sought. Positive likelihood ratios for snoring (2.8) learning problems (2.8) again suggest an increase in the chance of SDB being present when these are present. The results of the study also add to the current literature suggesting SDB as a possible contributing factor to poor academic achievement in children.
One limitation of the TuCASA study is the use of only a thermistor to monitor airflow. Because an end-tidal CO2 monitor was not used, it is possible that this may have led to some cases of obstructive hypoventilation being missed. Moreover, use of a nasal pressure transducer to identify hypopneas during PSG is now considered standard practice 20. Since most TuCASA analyses utilized thermistor-derived recordings, there may have been some underestimation of the presence and severity of SDB. Indeed, a comparison of nasal pressure transducer and thermistor in a sample from TuCASA cohort suggested that the former was able to detect a significantly higher number of events than the latter 21. However, there was a greater likelihood of signal loss with the nasal pressure transducer than with the thermistor which may make use of the former unfeasible in young children.
SDB and Learning
Kaemingk et al assessed the relationship between intelligence, learning and memory, and academic achievement and SDB in TuCASA subjects 14. A group of children with an RDI0 ≥ 5 (n = 77) was compared to a group with RDI0 < 5 (n = 72). There was a significant inverse relationship between RDI0 and full IQ, performance IQ, immediate recall and Applied Problems. Learning and memory were decreased in the group of otherwise healthy children with a RDI0 of 5 or more. These results suggest that SDB has an important adverse impact on the ability to learn during childhood. Whether such impairment has lasting consequences will not be known until long-term follow up of these children is completed.
SDB and Hypertension
There is strong literature implicating SDB as a risk factor for hypertension in adults 22, 23. However, the data assessing such an association in children have been meager. In TuCASA, blood pressure (BP) measurements were performed on children during the home visit for PSG recording. BP was measured in triplicate from the right arm of the child using a portable mercury sphygmomanometer and standardized techniques 24. The appropriate BP cuff was selected according to the measured arm circumference. In an analysis of the first 239 TuCASA study participants who completed unattended home PSGs, the association between hypertension and SDB was examined 25. Boys constituted 55% of this sample and 51% of the children were Hispanic. The mean age was 8.7 years, the BMI was 18.2 kg/m2 and mean neck size was 27 cm. Obesity was defined as a BMI of greater than the 95th percentile standardized for age, sex, and ethnicity 26 and 12% of the children were obese by this criterion.
The mean ± SD systolic and diastolic blood pressures were 98.4±10.6 mmHg and 62.0±8.9 mm Hg, respectively. Blood pressure elevation was systolic and diastolic BP above the 90th percentile when adjusted for age, height, and sex 27. Fifteen children (6%) had hypertension. Associations were noted between systolic and diastolic BP elevation and RDI defined by either 2%, 3% or 4% oxygen desaturations linked with apneic and hypopneic events. However, BP elevation was not noted in children with apneic/hypopneic events unassociated with oxygen desaturation. Obesity, poorer sleep efficiency, arousal index and RDI2% were independently associated with diastolic blood pressure elevation. Habitual loud snoring, witnessed apnea, RDI2% and poorer sleep efficiency were associated with systolic blood pressure elevation. Sex, ethnicity, total sleep time, and parental smoking were not associated with either systolic or diastolic hypertension.
This study provides evidence for the association between poor sleep and SDB and hypertension in children. The results were in accordance with a prior study which reported higher BP in children with OSA compared to those with primary snoring 28. That BP was not elevated in the absence of oxygen desaturation suggests hypoxemia to be a major factor in the etiology of SDB-related hypertension. This finding is consistent with data in adults suggesting that hypoxemic stress may be the pivotal factor contributing to endothelial dysfunction, hypertension and SDB 29. Furthermore, obesity was associated with hypertension, suggesting that controlling obesity may help limit such adverse consequences.
SDB and Behavioral Problems
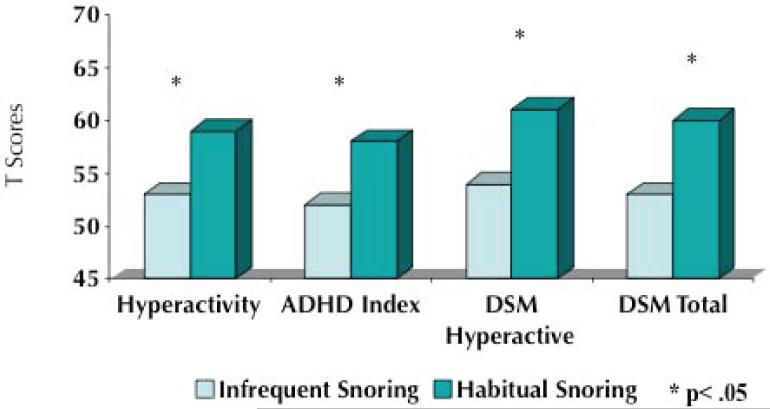
The correlation between SDB and behavioral problems was analyzed in one TuCASA study 30. The mean RDI0 for the sample evaluated in this study was 5.29 (SD = 4.80, range 0.10-72.4). Children in the upper 15% of the RDI distribution had higher mean CBCL scores on the Aggressive, Attention Problems, Social Problems, Thought Problems, Total and Externalizing scales. Hyperactivity, however, was not strongly associated with a higher RDI. Similarly, on the CPRS-R scale, high odds ratios for Oppositional, Cognitive Problems, Social Problems, Psychosomatic, ADHD Index, and DSM Total scales were seen in children in the upper 15% of the RDI distribution. Other data from TuCASA indicate that snoring children also have a higher prevalence of behavioral problems (Figure 1). These results indicate that SDB is associated with increased behavioral morbidity in children with SDB. Furthermore, they are consistent with clinical observations that behavioral problems are frequently the primary and/or initial manifestation of SDB in children.
Figure 1.
T Scores on the Conners' Parent Rating Scales. Values are higher in snoring children.
Parasomnias and SDB
Parasomnias in children have been associated with sleep disruption, SDB, psychiatric comorbidities and distress to the child and the family members 31-33. One TuCASA analysis characterized the relationship between parasomnias and SDB in these children. A Sleep Habits Questionnaire administered on the night of polysomnography in this study included questions such as: “Does this child sleepwalk?” and “Does this child talk in his or her sleep? (Talk without being fully awake?)” and “How often does this child awaken at night afraid or appearing tearful?” The choices of responses were “Never”, “Less than three times per month”, “Three to five times per month,” or “More than five times per month.” A child was classified as having parasomnias if sleepwalking was present more than 3 times per month, sleep talking was present more than 5 times per month or if the child had more than 5 fearful awakenings per month. Enuresis was defined as occurring more than 5 times per month 34.
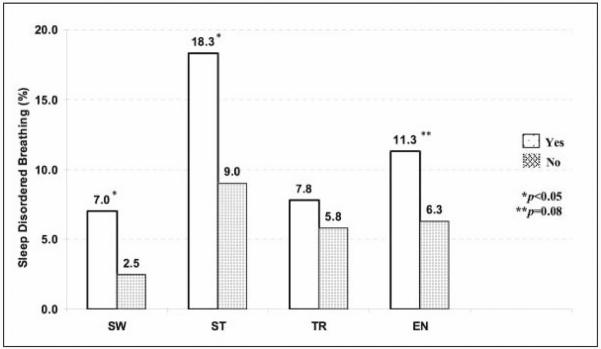
Of the children undergoing PSG, 3.5% had sleepwalking, 11.3% had sleep talking, 6.3% had fearful awakenings, and 7.5% had enuresis 8. Children with sleep talking were more likely to have concomitant sleepwalking and sleep terrors. However, there was no association between arousal parasomnias and enuresis. There was an increased prevalence of reported EDS, insomnia and learning problems and a trend towards more habitual snoring in children with sleepwalking 34. Children with sleep talking were more likely to have habitual snoring, insomnia, and learning problems, but not EDS. Children with fearful awakenings were more likely to have habitual snoring, EDS, insomnia, and learning problems. Enuresis was strongly associated with habitual snoring and witnessed apnea. As shown in Figure 2, sleepwalking [OR=2.9 (CI 1.1-7.8), P=.02] and sleeptalking [OR=2.2 (CI 1.2-4.1), P=.006] were associated with a higher likelihood of SDB. There was a non-significant trend for enuresis to be associated with SDB [OR=1.9 (CI 0.9-3.9), P=.08]. Fearful awakenings or child being fidgety were not associated with SDB 8.
Figure 2.
Associations between sleep disordered breathing and parasomnias. Sleep disordered breathing (SDB) was defined as an RDI3% of more than one per hour of total sleep time.
From BMC Med. 2004; 2: 14 34.
http://www.biomedcentral.com/1741-7015/2/14, accessed June 23, 2008 Published online 2004 April 28. doi: 10.1186/1741-7015-2-14.
Copyright © 2004 Goodwin et al; licensee BioMed Central Ltd. This is an Open Access article: verbatim copying and redistribution of this article are permitted in all media for any purpose, provided this notice is preserved along with the article's original URL.
Parasomnias such as sleepwalking and sleeptalking have traditionally been considered usual and inconsequential childhood occurrences. However, the TuCASA study demonstrated a greater likelihood of SDB as well as other sleep symptoms and learning problems among children with parasomnias. Parasomnias may be a result of SDB and can improve with treatment for SDB 35. It remains to be elucidated whether the learning problems encountered in children with parasomnias are a function of concomitant SDB or whether parasomnias are an independent risk factor for the learning difficulties.
SDB and Ventilatory Drive
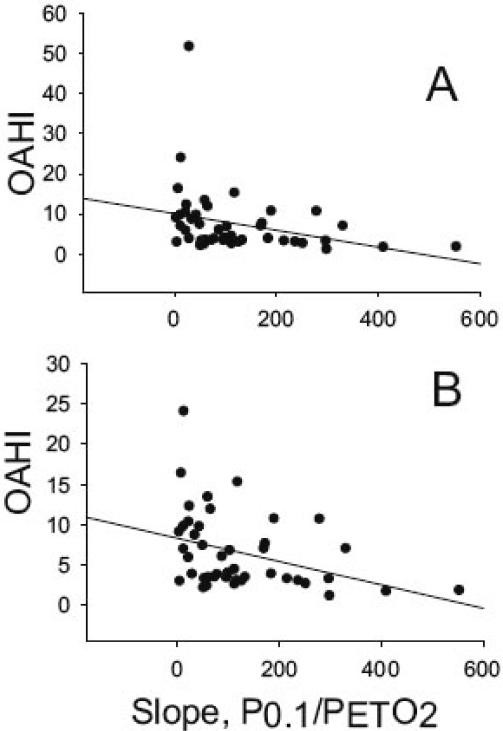
Hypoxic and hypercapnic ventilatory drive in relation to the severity of SDB was examined in 50 children recruited from the TuCASA cohort 36. The authors assessed ventilatory drive by measuring the mouth occlusion pressure response (P0.1) in normoxia, at two levels of isocapnic hypoxia, and at three levels of hyperoxic hypercapnia. They found a significant correlation between resting pressure of end-tidal CO2 (PETCO2) with the obstructive apnea hypopnea index (OAHI). The hypoxic, but not the hypercapnic occlusion pressure response, was significantly related to the OAHI (Figure 3). These results suggest that CO2 retention and reduced hypoxic ventilatory drive occur in children with a high OAHI. CO2 retention during wakefulness in children with severe SDB may be consequent to perturbed central ventilatory control. While mechanical obstruction from enlarged tonsils causing a narrowing of the pharyngeal airway may contribute to CO2 retention, a study demonstrating failure of adenotonsillectomy to abolish resting CO2 retention during wakefulness in children with SDB argues against this being a primary mechanism 37. The reduced hypoxic responsiveness seen in children in the study may be a paraphenomenon of SDB or may predispose children to SDB.
Figure 3.
Correlation between each subject's OAHI and the slope of the P0.1/PETO2 curve. The top panel represents data from all subjects, and the bottom panel shows that the relation is strengthened when the outlier with the very high OAHI is removed from the data set. The slope of the P0.1/PETO2 curves were obtained for each subject during the response tests, as described in Methods.
From BMC Pulm Med. 2004; 4: 4 36.
http://www.biomedcentral.com/1471-2466/4/4, accessed June 23, 2008 Published online 2004 April 29. doi: 10.1186/1471-2466-4-4.
Copyright © 2004 Fregosi et al; licensee BioMed Central Ltd. This is an Open Access article: verbatim copying and redistribution of this article are permitted in all media for any purpose, provided this notice is preserved along with the article's original URL.
Upper airway collapsibility in SDB
Children with severe obstructive sleep apnea have a more collapsible pharynx than that of normal children 38. Seventeen children from the TuCASA cohort underwent testing to assess whether children with mild SDB comprised primarily of hypopneas rather than apneas also have more collapsible airways than normal children 39. Airway collapsibility was estimated in 7 children with mild SDB (11.5 ± 0.1 hypopneas/hour) and 10 age-matched controls (1.9 ± 0.2 hypopneas/hour) during stable, non-rapid eye movement sleep. The groups were similar in regards to body mass index, neck circumference and the estimated airway size. The intermittent Pcrit method [brief (2-breath duration) and sudden reductions in pharyngeal pressure by connecting the breathing mask to a negative pressure source] revealed more collapsible airways in children in the SDB group than the age-matched controls. These observations point to an intrinsic abnormality in the pharyngeal airway of children with even mild SDB that may predispose to this disorder.
SDB and Oropharyngeal Volume
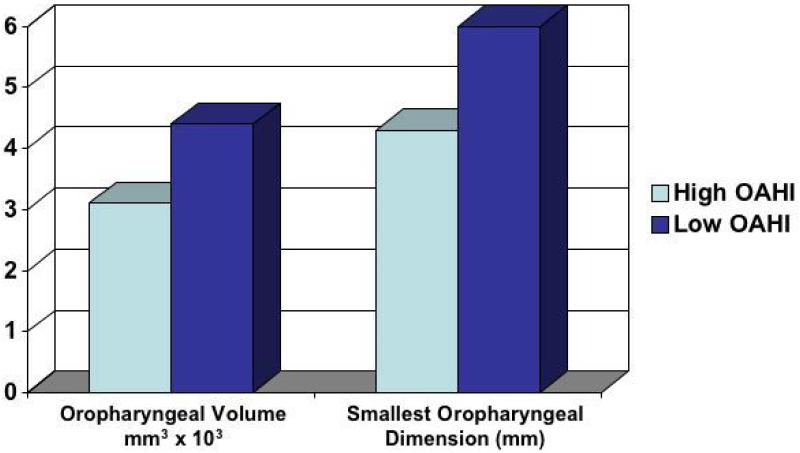
Whether childhood OSA is associated with a smaller airway was evaluated in another analysis in the TuCASA cohort. Magnetic resonance images of the pharynx were obtained in 18 awake children 7-12 years of age, with OAHI values ranging from 1.81 to 24.2 events/h, to assess the correlation between pharyngeal geometry and soft tissue anatomy and the severity of sleep-disordered breathing 40. The investigators found a positive correlation between the obstructive AHI and the size of tonsils (r2 = 0.42, P = 0.024) and soft palate (r2 = 0.33, P = 0.049) and an inverse correlation between the obstructive AHI and the oropharygeal volume (r2 = 0.42, P = 0.038) and the retropalatal air space (ratio of the retropalatal airway cross section area to the cross section area of the soft palate, r2 = 0.49, P = 0.001). As shown in Figure 4, both the oropharyngeal volume and the narrowest anterior-posterior oropharyngeal diameter were reduced in the high OAHI group. The high OAHI group had a narrower retropalatal airway where the adenoids, tonsils, and soft palate overlap than the low-OAHI group. The narrow upper airway likely contributes to worse sleep disordered breathing in children with a high OAHI..
Figure 4.
Oropharyngeal volume (p<.001) and the smallest oropharyngeal anterior posterior dimension (p=.024) in the high OAHI group (mean OAHI=13.5 /hour) were reduced in comparison to the low OAHI group (mean=2.8 /hour).
Data from Fregosi et al, Sleep-disordered breathing, pharyngeal size and soft tissue anatomy in children 40.
PSYCHOMOTOR VIGILANCE TASK PERFORMANCE
Although the PVT is a commonly employed as a marker of vigilance in adults, there are no normative data in children. In a TuCASA analysis by Venker et al15, normal PVT performance values were derived by analyzing data from a subsample comprised of children with RDI3% < 1 and no parent-reported sleep problems (n=162). Approximately 51% of this subsample was female. The reaction time (RT) decreased with increasing age, with children 11 years of age (n=15) having a mean RT of 396.3 ms compared to 721.15 ms in children 6 years of age (n=27). Boys and girls had statistically significantly different median RT (p<0.001). Boys had shorter median RT (659.4 vs. 787.6 ms) and fewer lapses (39.0 vs. 58.2) than girls at age 6, but performance on both measures was approximately equal by age 11. The interaction between gender and age was also significant. For boys, mean reciprocal RT (calculated because the distribution of mean RT was positively skewed,) is expected to be 2.06 s-1 at age 6, and expected to increase by 0.191 s-1 with each additional year of age. For girls, mean reciprocal RT is expected to be 1.61 s-1 at age 6, and expected to increase by 0.274 s-1 with each additional year of age. The results were not different in Hispanic or Caucasian participants. The median number of total errors was lower for girls (4 vs. 9) than for boys. This improvement in performance among school-aged children is consistent with the neurocognitive and physiological development taking place during these years.
BEHAVIORAL PROBLEMS ASSOCIATED WITH OVERWEIGHT
Of the 480 sleep studies completed in TuCASA, 402 (83.7%) had complete anthropometric and behavioral data available and constituted the sample for a study assessing the relation between obesity, SDB and behavioral problems 41. Fifty nine out of these 402 children (~15%) were classified as overweight (at or above the 95th percentile for their age and gender group). The children in the overweight group were slightly older (9.2 vs. 8.7 years, P=0.04) and more likely to be Hispanic (55.9% vs. 35.6%, P<0.01) compared to the non-overweight group. There were no significant differences in gender, RDI0, or parent education between the two groups. Overweight children were more likely to be classified in clinical range for psychosomatic complaints (OR 2.15, CI 1.02-4.54) on the CPRS-R scale. The Conners' psychosomatic complaints scale includes items related to headaches, stomach aches in general, stomach aches before school, vague complaints that are not supported by physical illness, and fatigue. However, this difference was not significant when adjusted for SDB.
Overweight children also had a higher probability of being classified within the clinical range for internalizing symptoms (OR 2.23, CI 1.05-4.72), withdrawal (OR 4.69, CI 2.05-10.73), and social problems (3.18, 1.53-6.60) on the CBCL. When adjusted for SDB, probability of clinically relevant withdrawal (OR 3.83 CI 1.59-9.22) and social problems (OR 2.49 CI 1.14-5.44) remained significantly higher for overweight subjects. The CBCL withdrawn scale includes items related to inhibition and withdrawal, and predicts anxiety and depression 42. Social scale items are related to social incompetence and being teased and disliked by peers. Overweight children may be bullied and teased by their peers because of their weight, while withdrawal may be a secondary to self-conscious behavior or judgment by peers.
CONCLUSION
TuCASA is a prospective cohort study of children aged 6-12 years that has helped elucidate anatomic and physiologic correlates of sleep disordered breathing as well as associated clinical outcomes in this age group. The analyses from this epidemiological study suggest that both obesity and SDB are associated with increased behavioral morbidity. Parasomnias, erstwhile considered benign occurrences in children, were shown to be associated with SDB. Furthermore, learning was impaired in children with PSG documented SDB. Anatomic and physiologic substudies revealed that children with SDB have smaller upper airways, increased tonsillar and palatal soft tissue volume and more collapsible upper airways. Furthermore, SDB may be associated with increased PETCO2 and depressed hypoxic responsiveness. In the future, results from a 4 year follow-up of the TuCASA cohort will be available which should result in further information concerning the potential impact of SDB on childhood learning, development and physiology.
ACKNOWLEDGMENTS
The authors thank Dr. James Goodwin, all of the other TuCASA investigators and the numerous research staff for their perseverance and dedication to this project, without whom, the success of TuCASA would not have occurred.
TuCASA is supported by NHLBI grant HL 62373
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gaultier C. Clinical and therapeutic aspects of obstructive sleep apnea syndrome in infants and children. Sleep. 1992 Dec;15(6 Suppl):S36–38. doi: 10.1093/sleep/15.suppl_6.s36. [DOI] [PubMed] [Google Scholar]
- 2.Guilleminault C. Obstructive sleep apnea. The clinical syndrome and historical perspective. Med Clin North Am. 1985 Nov;69(6):1187–1203. doi: 10.1016/s0025-7125(16)30982-8. [DOI] [PubMed] [Google Scholar]
- 3.Chervin RD, Archbold KH. Hyperactivity and polysomnographic findings in children evaluated for sleep-disordered breathing. Sleep. 2001 May 1;24(3):313–320. doi: 10.1093/sleep/24.3.313. [DOI] [PubMed] [Google Scholar]
- 4.Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics. 1998 Sep;102(3 Pt 1):616–620. doi: 10.1542/peds.102.3.616. [DOI] [PubMed] [Google Scholar]
- 5.Owens J, Opipari L, Nobile C, Spirito A. Sleep and daytime behavior in children with obstructive sleep apnea and behavioral sleep disorders. Pediatrics. 1998 Nov;102(5):1178–1184. doi: 10.1542/peds.102.5.1178. [DOI] [PubMed] [Google Scholar]
- 6.Hansen DE, Vandenberg B. Neuropsychological features and differential diagnosis of sleep apnea syndrome in children. J Clin Child Psychol. 1997 Sep;26(3):304–310. doi: 10.1207/s15374424jccp2603_9. [DOI] [PubMed] [Google Scholar]
- 7.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999 May;159(5 Pt 1):1527–1532. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 8.Goodwin JL, Kaemingk KL, Mulvaney SA, Morgan WJ, Quan SF. Clinical screening of school children for polysomnography to detect sleep-disordered breathing--the Tucson Children's Assessment of Sleep Apnea study (TuCASA) J Clin Sleep Med. 2005 Jul 15;1(3):247–254. [PMC free article] [PubMed] [Google Scholar]
- 9.Redline S, Sanders MH, Lind BK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998 Nov 1;21(7):759–767. [PubMed] [Google Scholar]
- 10.Rechtschaffen A, Kales A, editors. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. Brain Information Service/ Brain Research Institute, UCLA; Los Angeles: 1968. [Google Scholar]
- 11.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992 Apr;15(2):173–184. [PubMed] [Google Scholar]
- 12.Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised Conners' Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998 Aug;26(4):257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- 13.Achenbach T. Integrative guide for the 1991 CBCL/4-18, YSR, and TRF profiles. University of Vermont Department of Psychiatry; Burlington, VT: 1991. [Google Scholar]
- 14.Kaemingk KL, Pasvogel AE, Goodwin JL, et al. Learning in children and sleep disordered breathing: findings of the Tucson Children's Assessment of Sleep Apnea (tuCASA) prospective cohort study. J Int Neuropsychol Soc. 2003 Nov;9(7):1016–1026. doi: 10.1017/S1355617703970056. [DOI] [PubMed] [Google Scholar]
- 15.Venker CC, Goodwin JL, Roe DJ, Kaemingk KL, Mulvaney S, Quan SF. Normative psychomotor vigilance task performance in children ages 6 to 11--the Tucson Children's Assessment of Sleep Apnea (TuCASA) Sleep Breath. 2007 Dec;11(4):217–224. doi: 10.1007/s11325-007-0103-4. [DOI] [PubMed] [Google Scholar]
- 16.The Psychological Corporation . Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: 1999. [Google Scholar]
- 17.Woodcock RWJ. Woodcock-Johnson Psycho-Educational Battery-Revised. DLM Teaching Resources; Allen, TX: 1989. [Google Scholar]
- 18.Talley J. Children's Auditory Verbal Learning Test-2. Psychological Assessment Resources, Inc.; Odessa, FL: 1993. [Google Scholar]
- 19.Goodwin JL, Kaemingk KL, Fregosi RF, et al. Clinical outcomes associated with sleep-disordered breathing in Caucasian and Hispanic children--the Tucson Children's Assessment of Sleep Apnea study (TuCASA) Sleep. 2003 Aug 1;26(5):587–591. doi: 10.1093/sleep/26.5.587. [DOI] [PubMed] [Google Scholar]
- 20.Redline S, Budhiraja R, Kapur V, et al. The scoring of respiratory events in sleep: reliability and validity. J Clin Sleep Med. 2007 Mar 15;3(2):169–200. [PubMed] [Google Scholar]
- 21.Budhiraja R, Goodwin JL, Parthasarathy S, Quan SF. Comparison of nasal pressure transducer and thermistor for detection of respiratory events during polysomnography in children. Sleep. 2005 Sep 1;28(9):1117–1121. doi: 10.1093/sleep/28.9.1117. [DOI] [PubMed] [Google Scholar]
- 22.Budhiraja R, Sharief I, Quan SF. Sleep disordered breathing and hypertension. J Clin Sleep Med. 2005 Oct 15;1(4):401–404. [PubMed] [Google Scholar]
- 23.Budhiraja R, Quan SF. Sleep-disordered breathing and cardiovascular health. Curr Opin Pulm Med. 2005 Nov;11(6):501–506. doi: 10.1097/01.mcp.0000183058.52924.70. [DOI] [PubMed] [Google Scholar]
- 24.National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents: a working group report from the National High Blood Pressure Education Program. Pediatrics. 1996 Oct;98(4 Pt 1):649–658. [PubMed] [Google Scholar]
- 25.Enright PL, Goodwin JL, Sherrill DL, Quan JR, Quan SF. Blood pressure elevation associated with sleep-related breathing disorder in a community sample of white and Hispanic children: the Tucson Children's Assessment of Sleep Apnea study. Arch Pediatr Adolesc Med. 2003 Sep;157(9):901–904. doi: 10.1001/archpedi.157.9.901. [DOI] [PubMed] [Google Scholar]
- 26.Rosner B, Prineas R, Loggie J, Daniels SR. Percentiles for body mass index in U.S. children 5 to 17 years of age. J Pediatr. 1998 Feb;132(2):211–222. doi: 10.1016/s0022-3476(98)70434-2. [DOI] [PubMed] [Google Scholar]
- 27.Rosner B, Prineas RJ, Loggie JM, Daniels SR. Blood pressure nomograms for children and adolescents, by height, sex, and age, in the United States. J Pediatr. 1993 Dec;123(6):871–886. doi: 10.1016/s0022-3476(05)80382-8. [DOI] [PubMed] [Google Scholar]
- 28.Marcus CL, Greene MG, Carroll JL. Blood pressure in children with obstructive sleep apnea. Am J Respir Crit Care Med. 1998 Apr;157(4 Pt 1):1098–1103. doi: 10.1164/ajrccm.157.4.9704080. [DOI] [PubMed] [Google Scholar]
- 29.Budhiraja R, Parthasarathy S, Quan SF. Endothelial dysfunction in obstructive sleep apnea. J Clin Sleep Med. 2007 Jun 15;3(4):409–415. [PMC free article] [PubMed] [Google Scholar]
- 30.Mulvaney SA, Goodwin JL, Morgan WJ, Rosen GR, Quan SF, Kaemingk KL. Behavior problems associated with sleep disordered breathing in school-aged children--the Tucson children's assessment of sleep apnea study. J Pediatr Psychol. 2006 Apr;31(3):322–330. doi: 10.1093/jpepsy/jsj035. [DOI] [PubMed] [Google Scholar]
- 31.Gau SF, Soong WT. Psychiatric comorbidity of adolescents with sleep terrors or sleepwalking: a case-control study. Aust N Z J Psychiatry. 1999 Oct;33(5):734–739. doi: 10.1080/j.1440-1614.1999.00610.x. [DOI] [PubMed] [Google Scholar]
- 32.Owens J, Spirito A, Nobile C, Arrigan M. Incidence of parasomnias in children with obstructive sleep apnea. Sleep. 1997 Dec;20(12):1193–1196. [PubMed] [Google Scholar]
- 33.Mahowald MW, Rosen GM. Parasomnias in children. Pediatrician. 1990;17(1):21–31. [PubMed] [Google Scholar]
- 34.Goodwin JL, Kaemingk KL, Fregosi RF, et al. Parasomnias and sleep disordered breathing in Caucasian and Hispanic children - the Tucson children's assessment of sleep apnea study. BMC Med. 2004 Apr 28;2:14. doi: 10.1186/1741-7015-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guilleminault C, Palombini L, Pelayo R, Chervin RD. Sleepwalking and sleep terrors in prepubertal children: what triggers them? Pediatrics. 2003 Jan;111(1):e17–25. doi: 10.1542/peds.111.1.e17. [DOI] [PubMed] [Google Scholar]
- 36.Fregosi RF, Quan SF, Jackson AC, et al. Ventilatory drive and the apneahypopnea index in six-to-twelve year old children. BMC Pulm Med. 2004 Apr 29;4:4. doi: 10.1186/1471-2466-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kerbl R, Zotter H, Schenkeli R, et al. Persistent hypercapnia in children after treatment of obstructive sleep apnea syndrome by adenotonsillectomy. Wien Klin Wochenschr. 2001 Apr 17;113(78):229–234. [PubMed] [Google Scholar]
- 38.Marcus CL, Katz ES, Lutz J, Black CA, Galster P, Carson KA. Upper airway dynamic responses in children with the obstructive sleep apnea syndrome. Pediatr Res. 2005 Jan;57(1):99–107. doi: 10.1203/01.PDR.0000147565.74947.14. [DOI] [PubMed] [Google Scholar]
- 39.Fregosi RF, Quan SF, Morgan WL, et al. Pharyngeal critical pressure in children with mild sleep-disordered breathing. J Appl Physiol. 2006 Sep;101(3):734–739. doi: 10.1152/japplphysiol.01444.2005. [DOI] [PubMed] [Google Scholar]
- 40.Fregosi RF, Quan SF, Kaemingk KL, et al. Sleep-disordered breathing, pharyngeal size and soft tissue anatomy in children. J Appl Physiol. 2003 Nov;95(5):2030–2038. doi: 10.1152/japplphysiol.00293.2003. [DOI] [PubMed] [Google Scholar]
- 41.Mulvaney SA, Kaemingk KL, Goodwin JL, Quan SF. Parent-rated behavior problems associated with overweight before and after controlling for sleep disordered breathing. BMC Pediatr. 2006;6:34. doi: 10.1186/1471-2431-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kasius MC, Ferdinand RF, van den Berg H, Verhulst FC. Associations between different diagnostic approaches for child and adolescent psychopathology. J Child Psychol Psychiatry. 1997 Sep;38(6):625–632. doi: 10.1111/j.1469-7610.1997.tb01689.x. [DOI] [PubMed] [Google Scholar]