Abstract
A series of ruthenium olefin metathesis catalysts bearing N-heterocyclic carbene (NHC) ligands with varying degrees of backbone and N-aryl substitution have been prepared. These complexes show greater resistance to decomposition through C–H activation of the N-aryl group, resulting in increased catalyst lifetimes. This work has utilized robotic technology to examine the activity and stability of each catalyst in metathesis, providing insights into the relationship between ligand architecture and enhanced efficiency. The development of this robotic methodology has also shown that, under optimized conditions, catalyst loadings as low as 25 ppm can lead to 100% conversion in the ring-closing metathesis of diethyl diallylmalonate.
Introduction
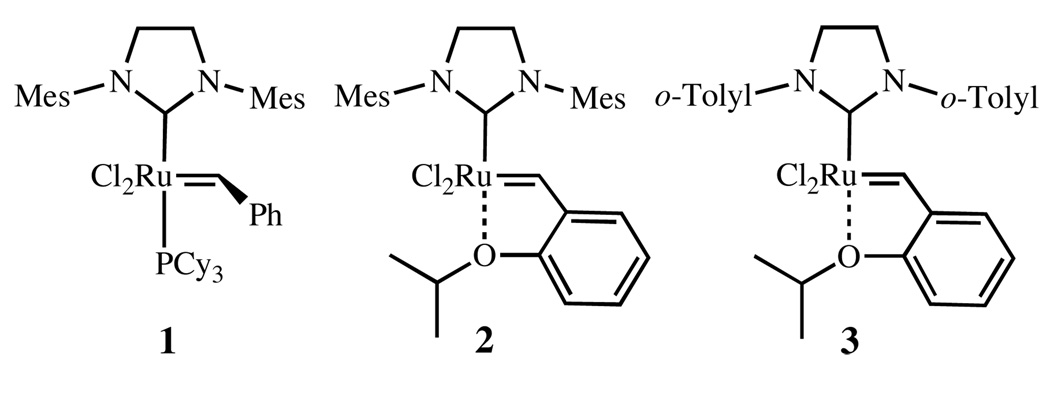
Olefin metathesis has emerged as a valuable tool in both organic and polymer chemistry.1 Ruthenium-based catalysts, in particular, have received considerable attention because of their tolerance to moisture, oxygen, and a large number of organic functional groups.2 Following the report of the increased activity of complex 1 (H2IMes)(PCy3)Cl2Ru=CHPh (H2IMes = 1,3-dimesitylimidazolidine-2-ylidene),3 and Hoveyda’s subsequent exchange of the phosphine ligand with a chelating ether moiety (2),4 many researchers have focused on increasing catalytic activity, selectivity and stability through modification of the N-heterocyclic carbene (NHC) ligand.5
As ligand modification has led to improved catalyst activity, a variety of applications have become possible, including ring-closing metathesis (RCM), cross metathesis (CM), ring-opening cross metathesis (ROCM), acyclic diene metathesis polymerization (ADMET), and ring-opening metathesis polymerization (ROMP). Among those metathesis reactions, ring-closing metathesis has become the most commonly employed metathesis reaction in organic synthesis.6 For this transformation, NHC catalysts, such as 1, 2, and more recently 3, have allowed both high activity and increased catalyst lifetime to be realized (Chart 1).3,4,5c
Chart 1.
Representative NHC-bearing olefin metathesis catalysts.
Despite these advances, still more efficient catalysts are sought to increase the applicability of RCM in industry. In many cases, olefin metathesis is still plagued by catalyst deactivation and the requirement of high catalyst loadings.6 Furthermore, decomposition products of olefin metathesis catalysts have been shown to be responsible for unwanted side reactions such as olefin isomerization.7 Increased catalyst loading could also potentially increase the level of residual ruthenium impurities in the final products, which becomes especially critical where reaction products are intended for pharmaceutical use.8 Collectively, these issues have a direct influence on the operational cost of metathesis transformations. With these factors in mind, the next challenge in RCM is to substantially decrease the catalyst loading, thereby reducing both reaction cost and the challenges in product purification. To this effect, our goal has been to increase catalyst efficiency by developing even more stable and robust catalysts that still retain a high catalytic activity.
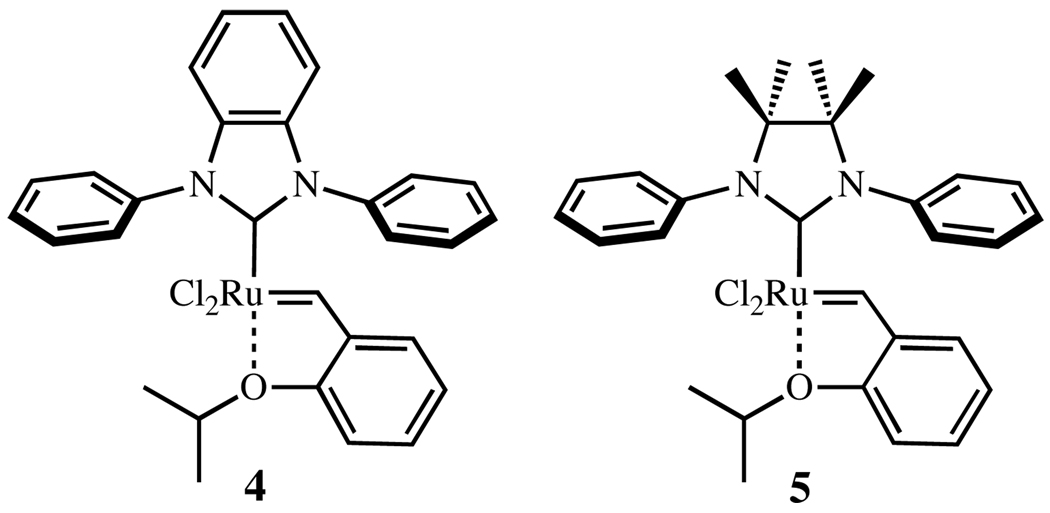
Recently, studies by our group and others have unveiled the decomposition pathways at play during metathesis reactions.9 Among other degradation products, complexes derived from C–H activation of N-aryl substituents were reported. Since the NHC ring and the aryl substituent must approach co-planarity for C–H activation, it was anticipated that decomposition via C–H activation processes might be slowed by restriction of N-aryl group of the NHC ligand, and this might be achieved by placing sterically hindered groups on the NHC backbone. This hypothesis was confirmed by successfully preparing N-phenyl complexes 4 and 5 that are more resistant to the decomposition initiated by C–H activation (Chart 2).5a,b Having unsubstituted N-phenyl groups, these complexes display good and exceptional reactivity, respectively, in the formation of highly substituted olefins. Despite these improvements, complexes 4 and 5 are more prone to decomposition than 1 and 2.10
Chart 2.
N-phenyl substituted complexes.
To address and further understand the balance between activity and stability of 5, we sought to investigate a homologous series of ruthenium catalysts bearing NHCs with varying degrees of backbone and aryl substitution. Molecular modeling and the calculations of Jensen et al. suggest that a catalyst bearing an NHC with mesityl groups at nitrogen and a fully methylated backbone would be an improvement over existing catalysts.11 We expected that the degree of substitution could be central to increased activity and catalyst lifetimes.
Herein, we report the preparation and characterization of a series of catalysts bearing NHCs with varying degrees of backbone and aryl substitution. Initial evaluation of their performance in olefin metathesis demonstrated that the common assays were not effective at measuring the relative efficiencies of these catalysts at standard catalyst loadings.12 While the standard conditions are excellent in evaluating the activity of new catalysts, they are not sensitive to small variations in the efficiency profile accompanying subtle modification in catalyst architecture.
In order to examine these small changes, we have developed a highly sensitive ppm level assay utilizing the precision and consistency of Symyx robotic technology. We utilized these techniques to examine the activity and stability of these catalysts in RCM at low catalyst loadings, providing increased insight into the relationship between ligand architecture and catalyst efficiency. The development of this methodology has also shown that, under optimized conditions, complete conversion in the RCM of diethyl diallylmalonate is observed with catalyst loadings as low as 25 ppm (0.0025 mol%).
Results and Discussion
Catalyst Syntheses
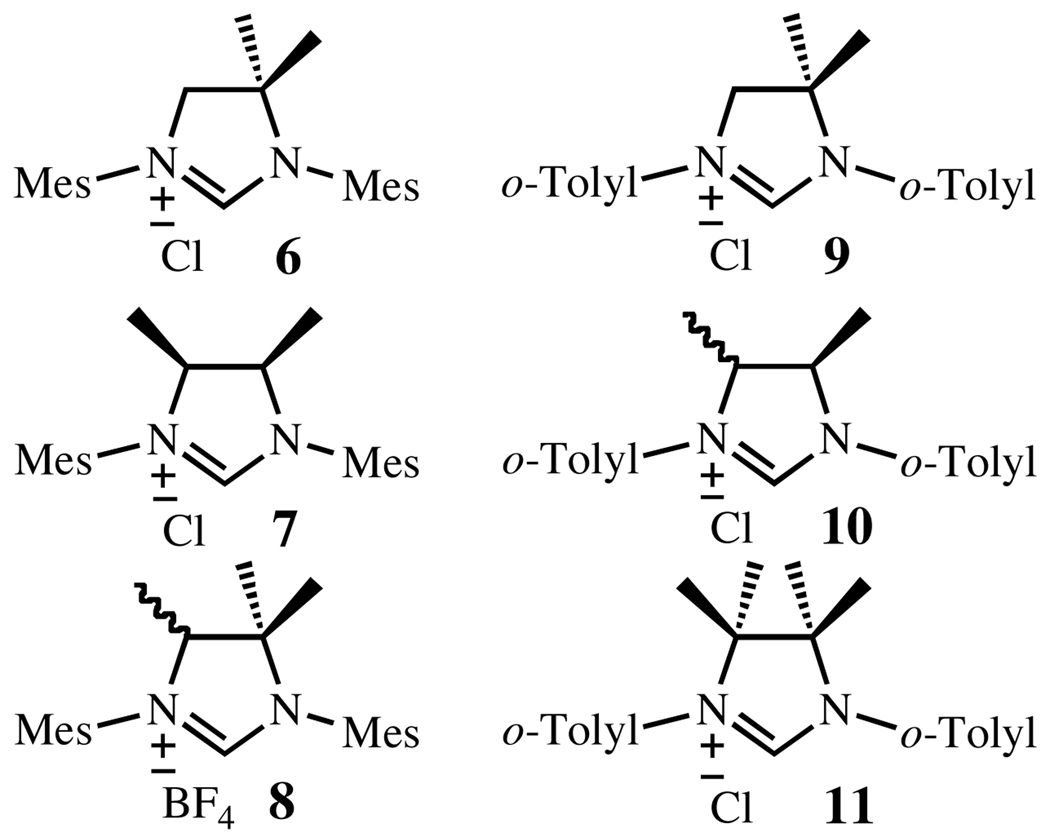
The preparation of the 1,1’-dimethyl- and 1,2-dimethyl-substituted imidazolinium chlorides 6 and 7 (Chart 3) have been previously reported by Bertrand and Çetinkaya, respectively.13 Under analogous experimental conditions, imidazolinium chlorides 9 and 10, featuring 2-methylphenyl (o-tolyl) groups were obtained in good yields. Unfortunately, separation of the syn- and anti- isomers of 10 proved to be extremely difficult, requiring the mixture to be carried forward.
Chart 3.
Imidazolinium salts.
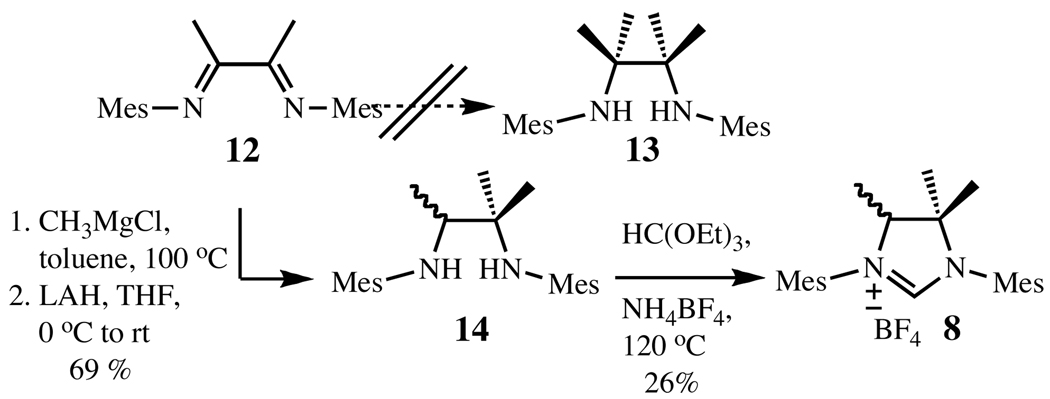
Following the procedures previously reported by our group to access the NHC of complex 5, we then attempted the preparation of the highly substituted imidazolinium salts bearing four methyl substituents.5a While imidazolinium chloride 11 was prepared without incident, we were unable to synthesize the intermediate tetramethylated diamine 13 of the corresponding N-mesityl analogue under various conditions (Scheme 1). Considering the trimethylated NHC to be sufficiently encumbered to prevent N-aryl rotation, we prepared 8 instead by Grignard addition followed by reduction and imidazolinium salt formation.
Scheme 1.
Synthesis of imidazolinium chloride 8.
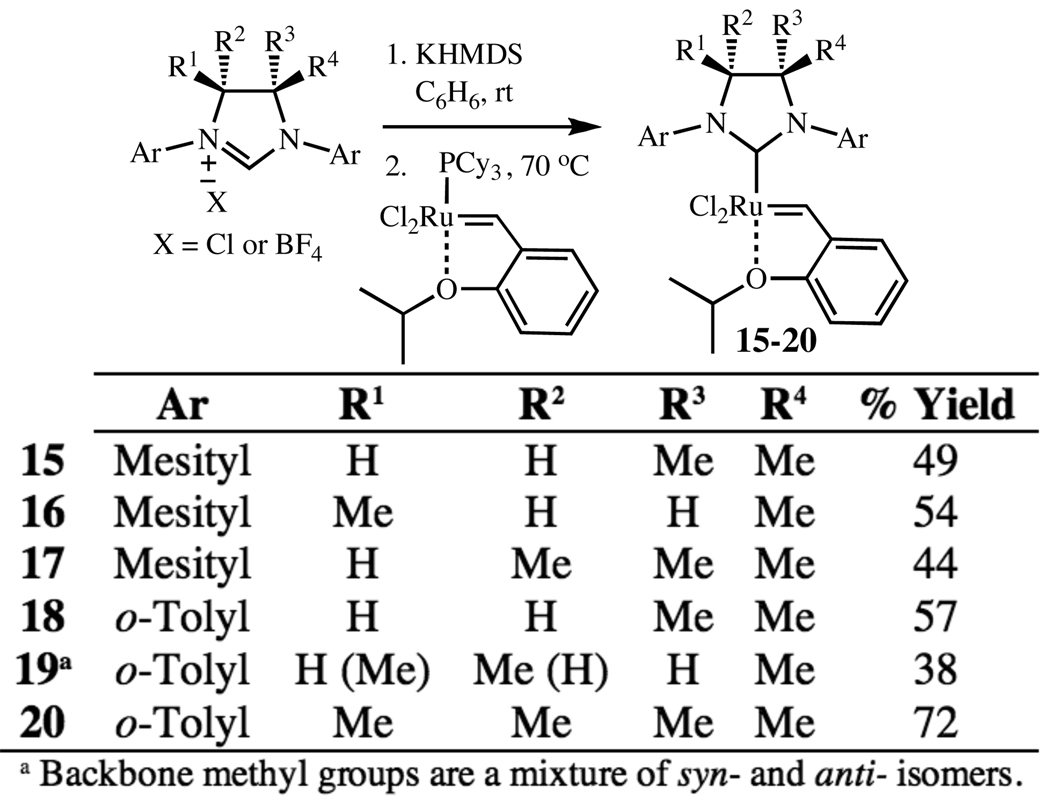
With precursors 6–11 in hand, the corresponding free carbenes were generated by treatment of the imidazolinium salts with potassium hexamethyldisilazide (KHMDS) at room temperature (Figure 1). These carbenes (prepared in situ) were reacted with commercially available (PCy3)RuCl2=CH(o-OiPrC6H4) at 70 °C, affording the phosphine-free chelating ether complexes 15–20. These complexes were isolated as crystalline green solids after flash column chromatography, and as solids are both air-and moisture-stable under standard conditions.
Figure 1.
Synthesis of ruthenium complexes 15–20.
Structural Analyses
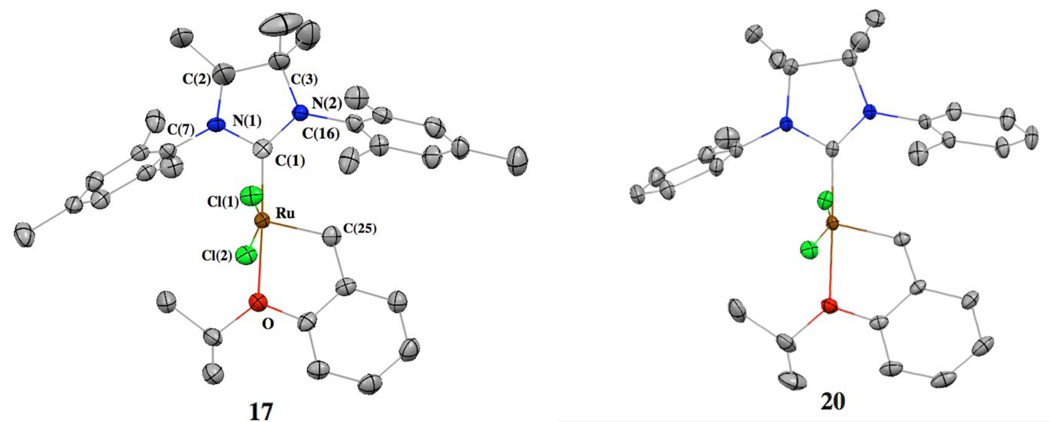
To probe the electronic and steric effects of backbone substitution, crystals of 17 and 20 were grown and their molecular structures were confirmed by single-crystal X-ray crystallographic analysis (Figure 2). The complexes exhibit a distorted square pyramidal geometry with the benzylidene moiety occupying the apical position. When compared with its unsubstituted analogue 2, the backbone substitution of 17 results in significant differences in three key structural parameters summarized in Table 1: (1) Ru-C(1) bond length, (2) C(1)-Ru-C(25) bond angle, and (3) the C(3)-N2-C(16) bond angle. Surprisingly, there are no major differences between the solid-state structures of complexes 3 and 20.
Figure 2.
X-ray crystal structures of complexes 17 and 20 are shown. Displacement ellipsoids are drawn at 50% probability. For clarity, hydrogen atoms have been omitted.
Table 1.
Selected X-ray data for 2, 17, 3, and 20.a
| 2b | 17 | 3c | 20 | |
|---|---|---|---|---|
| Bond Length (Å) | ||||
| Ru-C(1) | 1.980 | 1.968 | 1.962 | 1.964 |
| RuC(25) | 1.824 | 1.840 | 1.823 | 1.835 |
| Ru-O | 2.262 | 2.255 | 2.244 | 2.261 |
| Bond Angles (deg) | ||||
| C(3)-N(2)-C(16) | 118.22 | 122.60 | 119.91 | 119.82 |
| C(2)-N(1)-C(7) | 118.32 | 123.82 | 120.69 | 120.26 |
| C(1)-Ru-C(25) | 101.42 | 103.08 | 102.48 | 103.14 |
| C(1)-Ru-Cl(2) | 156.42 | 161.26 | 159.49 | 160.80 |
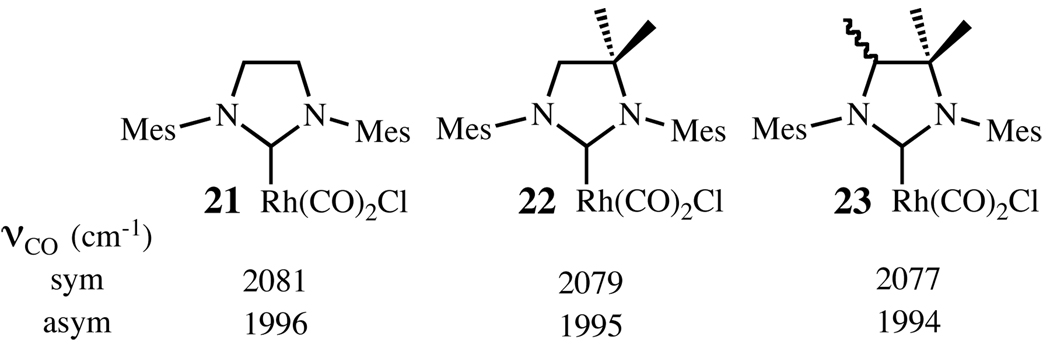
The crystal structure of complex 17 suggests that the backbone methyl substituents push the N-mesityl groups toward the ruthenium center and as a result the NHC-Ru-benzylidene bond angle is also increased. However, the bond distance between the NHC carbene carbon and the Ru center is shorter in 17 (1.968 Å) than in 2 (1.980 Å). This effect can be explained by noting that the backbone methyl substituents increase the electron-donating ability of the NHC ligand. This effect is also seen in the IR carbonyl stretching frequencies of the cis-[RhCl(CO)2(NHC)] complexes 21–23 (Chart 4), where increased substitution resulted in lower frequencies.14 These structural differences should have a significant impact on the efficiency of the different catalysts.
Chart 15.
IR carbonyl stretching frequencies of cis-[RhCl(CO)2(NHC)] complexes 21–23.
Ring-Closing Metathesis (RCM) Activity
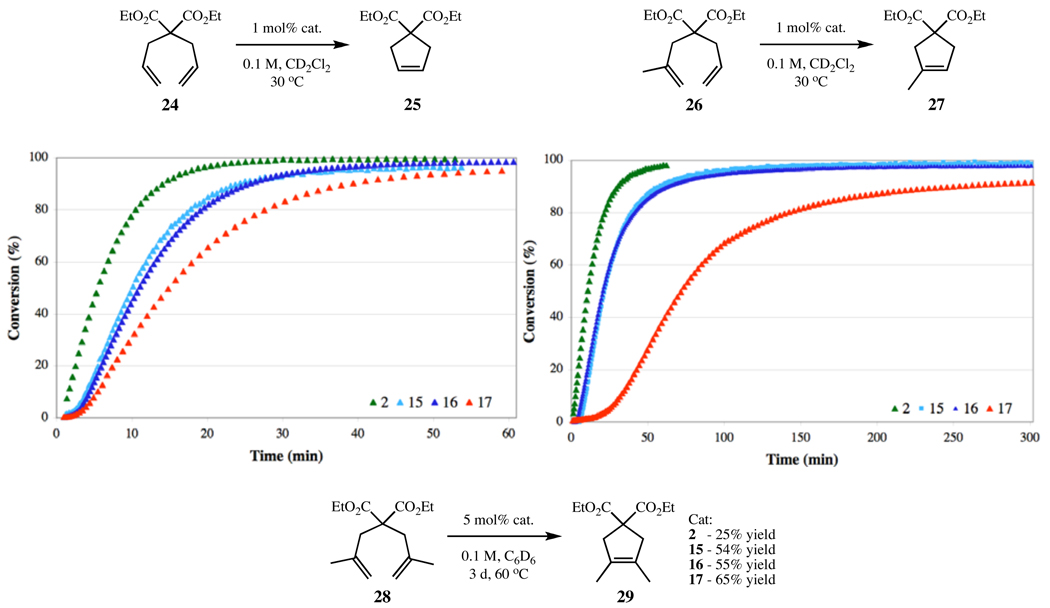
RCM is widely used in organic synthesis and serves as a standard assay to evaluate the relative efficiency of most ruthenium-based catalysts.6,12 With this in mind, we began our metathesis activity studies by focusing on the catalytic activity of the N-mesityl series (2, 15–17) in the RCM of diethyl diallylmalonate 24 to cycloalkene 25. The reactions, utilizing 1 mol% catalyst in CD2Cl2 at 30 °C, were monitored by 1H NMR spectroscopy (Figure 3). Interestingly, the plots of cycloalkene 25 concentration vs. time (Figure 3) revealed that the complexes effect the cyclization of 24, but with slower reaction rates as backbone substitution is increased. The same trend was observed for the cyclization of diethyl allylmethallylmalonate 26 to form trisubstituted cyclic olefin 27. However, in the very challenging RCM of diethyl dimethallylmalonate 28, using 5 mol% catalyst in C6D6 at 60 °C, increased substitution resulted in increased catalyst lifetimes and higher conversions to tetrasubstituted cyclic olefin 29.
Figure 3.
RCM of dienes 24, 26, and 28 to di-, tri-, and tetrasubstituted cycloalkenes 25, 27, and 29, respectively, using catalysts 2 and 15–17.
Several explanations could exist to explain these contradictory results. Along with decreased initiation rate, increased backbone substitution could also alter propagation rate, stability, or a combination of both. In any case, the results indicate that the assays reported by Ritter, et al., while useful for evaluating the activity of new catalysts,12 do not distinguish between catalysts that are both highly active15 and stable.16 Future improvements in, and understanding of, olefin metathesis catalysts will require a more sensitive assay to evaluate small variations in the efficiency profile accompanying subtle modification in catalyst architecture.
Development of a ppm Level Assay
In order to study subtle differences in activity and stability, the standard RCM reactions should be observed at the lower limit of productive catalyst loading and under optimized conditions. With this in mind, new techniques were developed using a Symyx robotic system to maintain a high degree of precision and consistency when working with ultra-low catalyst loadings. Our group has recently used these robotic systems to optimize reaction conditions and investigate new applications in olefin metathesis.17 Similarly, utilizing an automated Vantage system, Grela et al. recently reported the successful RCM of 24 at just 0.02 mol% 2.18
A robotic assay was developed utilizing the RCM of diene 24 by complex 2. Stock solutions of catalyst and substrate were prepared in a nitrogen-filled glovebox. While substrate stock solutions could be stored in septum-topped vials, catalyst solutions were prepared immediately prior to use. The Symyx core module was utilized to add all solutions to reaction vessels as well as to sample the reaction mixtures at programmed time intervals. Aliquots were added into ethyl vinyl ether solution at −20 °C,19 and then analyzed by gas chromatography with dodecane as an internal standard, measuring the change in the amounts of substrate and product with time. With minimal deviation in reaction results, 1 M (1 mL vials) and 0.1 M (20 mL vials) concentrations were employed depending on reaction scale and glassware to minimize substrate usage. The large vials were used in experiments where aliquots were withdrawn over the course of the reaction.
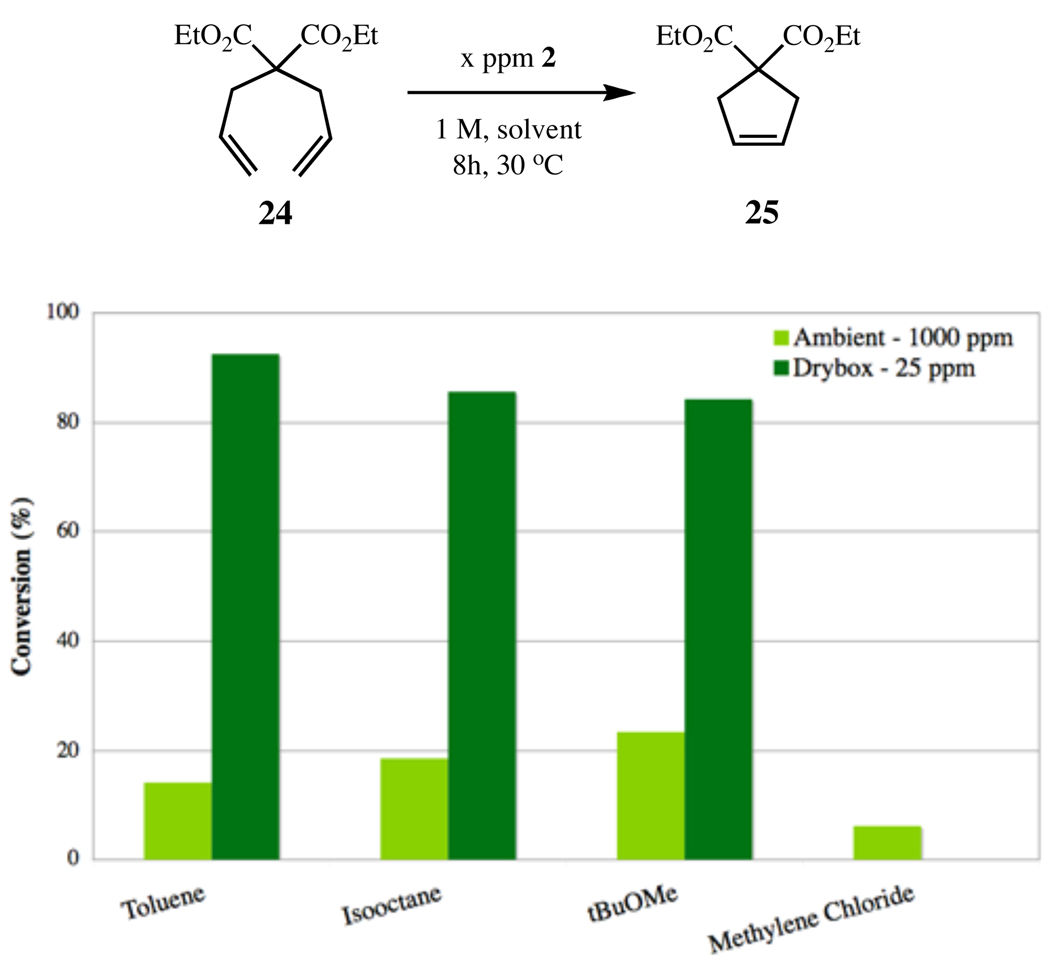
For practical reasons, most standard metathesis assays are performed in a closed system under inert atmosphere.12,18 However, we have observed variations in reaction rate and total conversion depending on the headspace of the reaction vessel. To circumvent this problem, reactions were carried out in open vials. Additionally, in order to minimize the potential for decomposition pathways related to oxygen, all reactions were conducted in a nitrogen-filled glovebox. While ruthenium-based catalysts are relatively stable under ambient conditions, at low catalyst loadings oxygen related decomposition becomes relevant. Control reactions were completed on a Symyx core module open to atmosphere, confirming the importance for oxygen-free reaction conditions (Figure 4).
Figure 4.
RCM of diene 24 to disubstituted cycloalkene 25, using catalyst 2 in a variety of solvents.
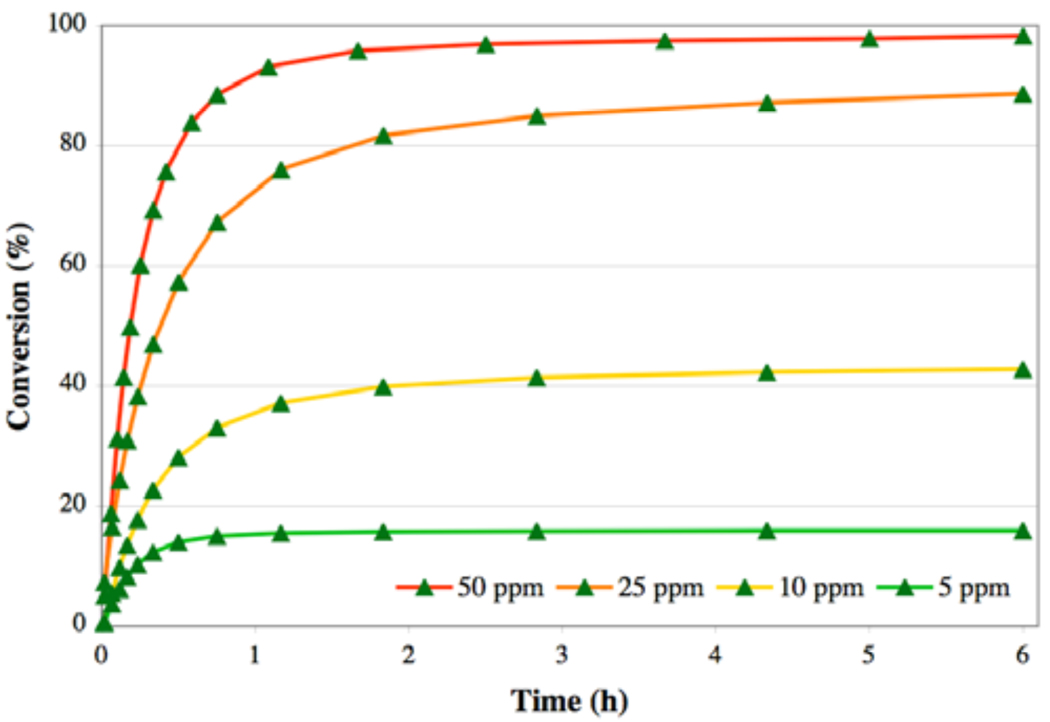
Other reaction considerations, including temperature and solvent, were optimized based on our recent complementary studies on the RCM of diallylamines with low catalyst loadings.17a A solvent screen identified toluene as the optimal solvent for RCM of these diallyl substrates (Figure 4). Toluene as solvent also allowed for an increased temperature of 50 °C. While increased temperatures have previously been shown to increase metathesis reaction rates,18,20 temperatures above 50 °C decreased assay consistency and resulted in significant solvent losses throughout the course of the reaction. The use of methylene chloride, the solvent most commonly used for RCM, resulted in considerable solvent loss even at 30 °C. Furthermore, its use resulted in decreased conversions, relative to other solvents. The RCM of 24 was then monitored over a variety of catalyst loadings to calibrate the new assay (Figure 5). Under optimized conditions (0.1 M, toluene, 50 °C), complex 2 afforded almost quantitative yields of 25 after 1 hour at just 50 ppm.
Figure 5.
RCM of diene 24 to disubstituted cycloalkene 25, using catalyst 2.
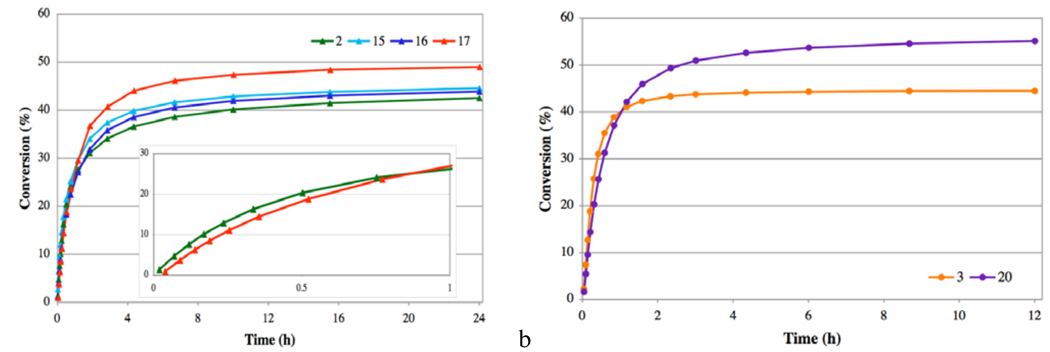
Under the optimized conditions, trimethylated complex 17 required only 25 ppm to reach full conversion to disubstituted cycloalkene 25; a catalyst loading near pharmaceutical impurity limits.8 In order to directly compare the N-mesityl series (2 and 15–17), catalyst loadings were further decreased to 15 ppm to ensure that no reactions would reach completion before the catalyst had completely decomposed. Again, at very low catalyst loadings, increased backbone substitution resulted in higher conversions to cyclic olefin 25. When conversions were monitored over the course of the reaction, the effects of backbone substitution became evident (Figure 6a). The data suggest that the higher conversions are a direct result of longer catalyst lifetimes. However, as observed during the NMR studies, increased backbone substitution decreases catalyst reaction rate. These results were supported through observation of the same trends when complexes 3 and 20 were studied using the same assay (Figure 6b).21
Figure 6.
Plot of the RCM of diene 24 to disubstituted cycloalkene 25, with conversion monitored over 24 h: (a) Using catalysts 2 and 15–17. The inset depicts a plot expansion over 1 h of the reaction. (b) Using catalysts 3 and 20.
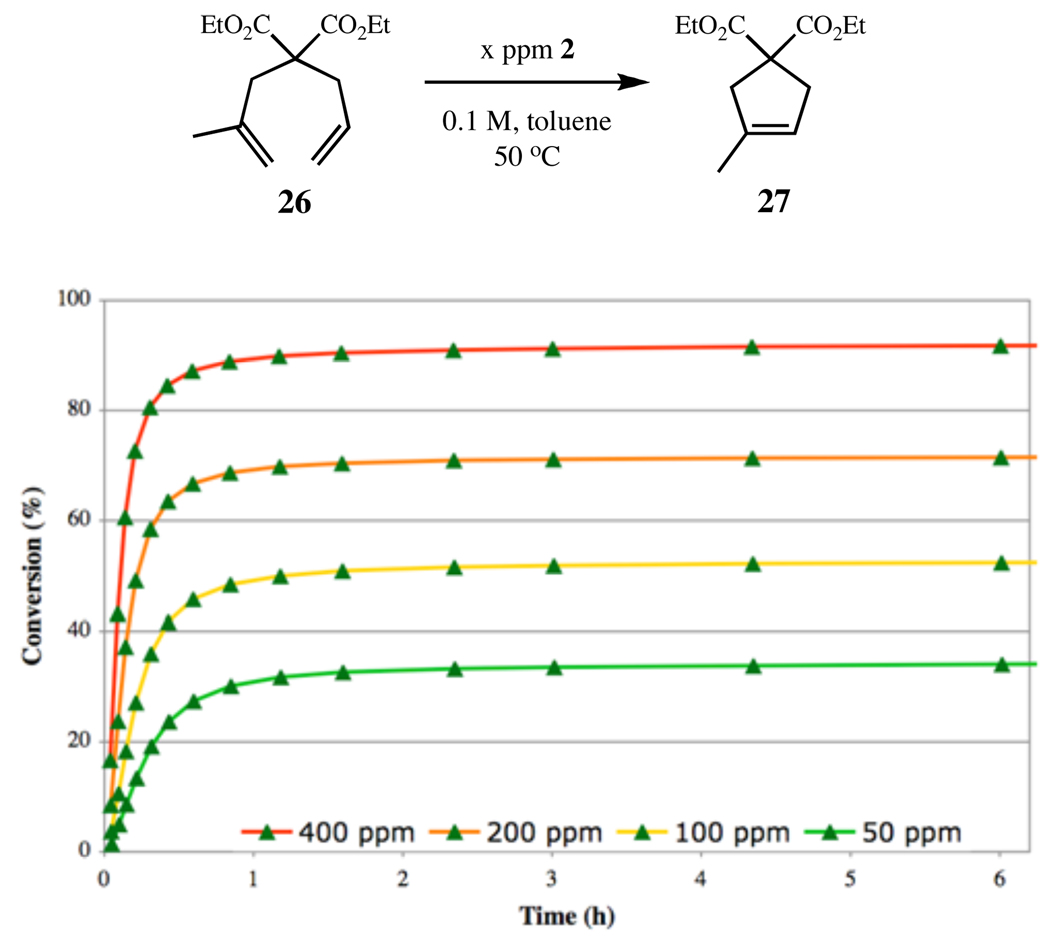
The catalyst efficiency assay was then expanded to study the RCM of 26 to give trisubstituted cycloalkene 27. Calibration using the more sterically challenging substrate revealed that significantly more catalyst is necessary to effect full conversion to 27, with complex 2 affording yields over 90% at 400 ppm catalyst loadings (Figure 7). The increase in required catalyst loading due to the addition of a single methyl group demonstrates the importance and effect of the olefin substrates steric environment.
Figure 7.
RCM of diene 26 to disubstituted cycloalkene 27, using catalyst 2.
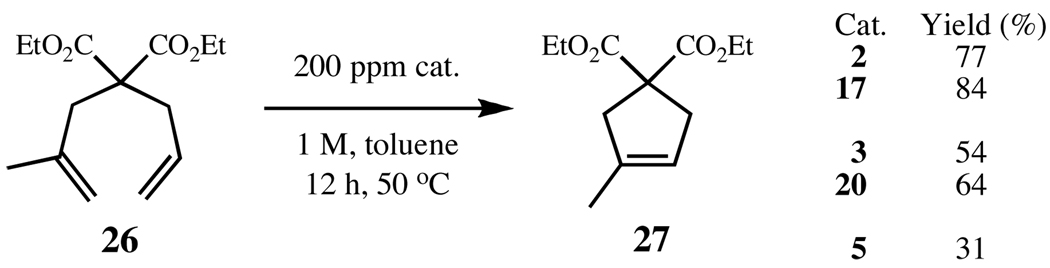
Catalyst comparison reactions, performed at 200 ppm, reveal that the addition of substituents to the NHC ligands has greater impact on the efficiency of the metathesis catalysts than with the previous substrate, with 17 and 20 both outperforming their unsubstituted analogues (Figure 8). Notably, the RCM of 26 also clearly highlights the difference in stability between the N-mesityl (2 and 17) and the N-o-tolyl catalysts (3 and 20). For this trisubstituted olefin substrate, catalyst stability is more significant than activity for success in RCM. Complex 5 is the most active ruthenium-based catalyst to date, but not particularly stable under prolonged reaction conditions. As expected, while 5 performs exceptionally well at standard loadings (1 mol%), it falters at low catalyst loadings.
Figure 8.
RCM of diene 26 to trisubstituted cycloalkene 27, using catalysts 2, 3, 5, 17, and 20.
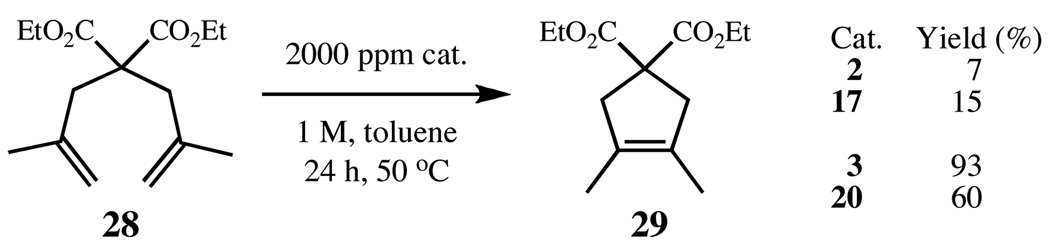
Finally, the ring-closing metathesis of 28 to tetrasubstituted cycloalkene 29 was examined using the same catalyst assay. Continuing the trend, at 0.2 mol% loading, complex 17 outperforms 2, yielding just 15% and 7% of the tetrasubstituted cycloalkene respectively (Figure 9). Despite the expected low yields, the result reaffirmed the conclusion that backbone substitution increases the stability of the resulting complex. In the case of the N-mesityl series, this increase in stability has not resulted in a detrimental decrease in activity.
Figure 9.
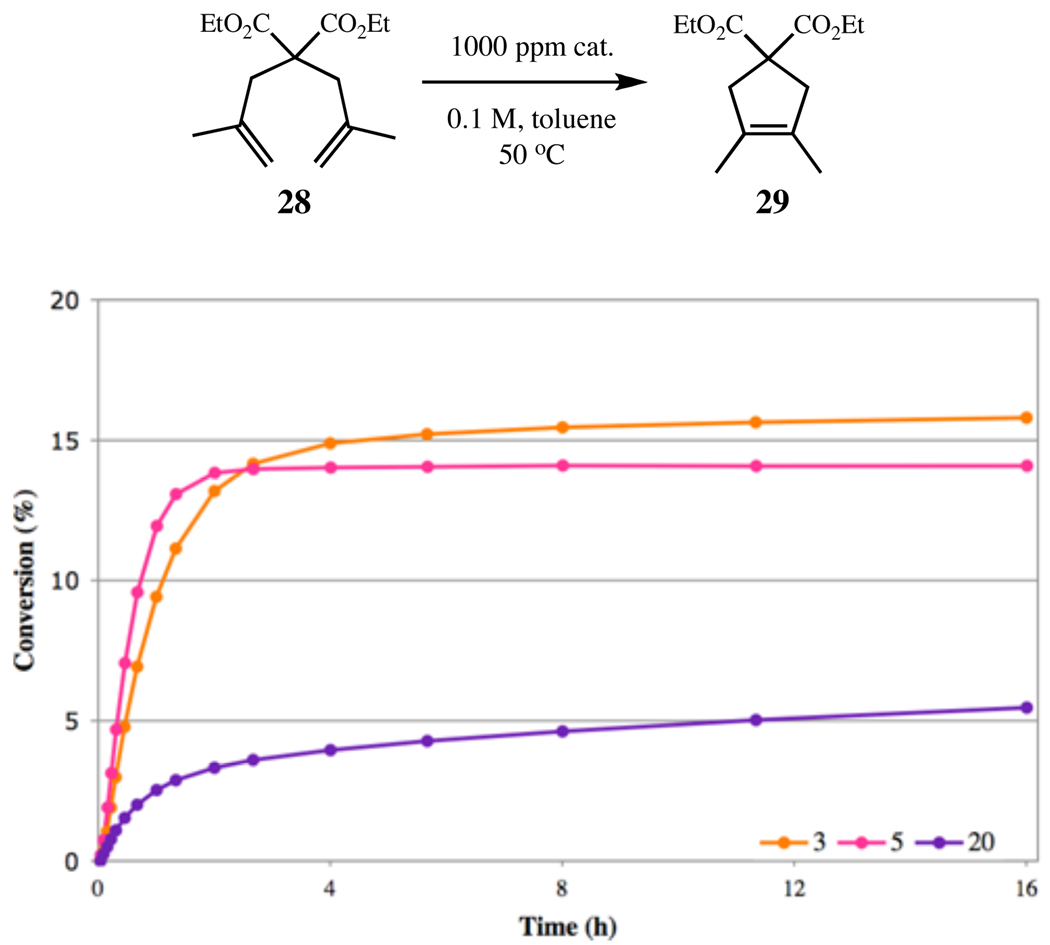
RCM of diene 28 to tetrasubstituted cycloalkene 29, using catalysts 2, 3, 17, and 20.
Surprisingly, the N-o-tolyl series does not continue in the expected trend. Complexes 3, and 20 were compared at 0.2 mol% catalyst loading (Figure 10), revealing complex 3 to be the most efficient catalyst for this tetrasubstituted olefin. To confirm this result, complexes 3, 5, and 20 were tested at a lower loading of 0.1 mol% and the reactions were monitored over time (Figure 9). At this loading, the effectiveness of the catalysts to complete the RCM dropped significantly, providing a reminder that more efficient catalysts still need to be developed.
Figure 10.
RCM of diene 28 to tetrasubstituted cycloalkene 29, using catalysts 3, 5, and 20.
Complex 3 outperformed both the more stable 20 and the more active 5. At low catalyst loadings, the decreased stability of 5 becomes a larger factor than its increased activity. Complex 20 faces the opposite challenge of substantially decreased activity. The differences between 3, 5, and 20 suggest that increased activity becomes more important than, but does not negate, increased stability for the RCM of very challenging substrates. While conversions were low, the experiment gives a clear result and is a reminder that the key to catalyst efficiency is the ratio of the rate of productive olefin metathesis relative to the rate of catalyst decomposition.
Conclusions
In summary, we describe the synthesis and characterization of a series of ruthenium-based olefin metathesis catalysts bearing NHCs with varying degrees of backbone and aryl substitution. In order to study their subtle differences in activity and stability, a highly sensitive assay was developed to operate at the lower limit of productive catalyst loading. These techniques were developed using a Symyx robotic system to maintain a high degree of precision and consistency when working with ultra-low catalyst loadings.
The development of this highly sensitive assay has provided increased insight into the relationship between ligand architecture and efficiency. In this study, both backbone and aryl substitution were found to significantly impact catalyst stability and activity. Whereas low N-aryl bulk on the NHC ligands led to increased activity, it also decreased stability. Increased backbone substitution, however, led to increased catalyst lifetimes and decreased reaction rates. Furthermore, it was found that the relative importance of stability and activity on efficiency is dependent on the steric encumbrance of the RCM reaction. For substrates with low steric demands, catalyst stability is quite important for success at low catalyst loadings. For sterically encumbered substrates, catalyst activity becomes much more important than increased stability. The ability to study the relationship between small changes in ligand architecture and efficiency will allow us to better explore new opportunities in catalyst design.
Experimental
General Information
NMR spectra were recorded using a Varian Mercury 300 or Varian Inova 500 MHz spectrometer. NMR chemical shifts are reported in parts per million (ppm) downfield from tetramethylsilane (TMS) with reference to internal solvent for 1H and 13C. Spectra are reported as follows: chemical shift (δ ppm), multiplicity, coupling constant (Hz), and integration. IR spectra were recorded on a Perkin-Elmer Paragon 1000 Spectrophotometer. Gas chromatography data was obtained using an Agilent 6850 FID gas chromatograph equipped with a DB-Wax Polyethylene Glycol capillary column (J&W Scientific). High-resolution mass spectroscopy (FAB) was completed at the California Institute of Technology Mass Spectrometry Facility. X-ray crystallographic structures were obtained by the Beckman Institute X-ray Crystallography Laboratory of the California Institute of Technology. Crystallographic data have been deposited at the CCDC, 12 Union Road, Cambridge CB2 1EZ, U.K., and copies can be obtained on request, free of charge, by quoting the publication citation and the deposition numbers 670930 (17) and 651007 (20).
All reactions involving metal complexes were conducted in oven-dried glassware under a nitrogen atmosphere with anhydrous and degassed solvents, using standard Schlenk and glovebox techniques. Anhydrous solvents were obtained via elution through a solvent column drying system.22 Silica gel used for the purification of organometallic complexes was obtained from TSI Scientific, Cambridge, MA (60 Å, pH 6.5–7.0). RuCl2(PCy3)(=CH–o-OiPrC6H4), 2, and 3 were obtained from Materia, Inc. Unless otherwise indicated, all compounds were purchased from Aldrich or Fisher and used as obtained. The compounds 6,13a 7,13b 12,13b 21,14 24–29,12 have been described previously and were prepared according to literature procedures or identified by comparison of their spectroscopic data. The initial screening of the catalysts, in RCM via 1H NMR spectroscopy was conducted according to literature procedures.12
Low ppm Level Assays
Experiments on the RCM of 24, 26, and 28 using the catalysts described were conducted using a SymyxTM Technologies Core Module (Santa Clara, CA) housed in a Braun nitrogen-filled glovebox and equipped with Julabo LH45 and LH85 temperature-control units for separate positions of the robot tabletop.
For experiments where aliquots were not taken during the course of the reaction, up to 576 reactions (6×96 well plates) could be performed simultaneously in 1 mL vials by an Epoch software-based protocol as follows. To prepare catalyst stock solutions (0. 25 mM), 20 mL glass scintillation vials were charged with catalyst (5 µmole) and diluted to 20.0 mL total volume in THF. Catalyst solutions, 6 to 800 µL depending on desired final catalyst loading, were transferred to reaction vials and solvent was removed via centrifugal evaporation. The catalysts were preheated to the desired temperature using the LH45 unit, and stirring was started. Substrates (0.1 mmol), containing dodecane (0.025 mmol) as an internal standard, were dispensed simultaneously to 4 reactions at a time using one arm of the robot equipped with a 4-needle assembly. Immediately following substrate addition, solvent was added to reach the desired reaction molarity, generally 1 M. All reactions were quenched by injection of 0.1 mL 5% v/v ethyl vinyl ether in toluene at a preprogrammed time. Samples were then analyzed by gas chromatography
Alternatively, where aliquots were taken during the course of the reaction, the entire operation was performed on 12 reactions simultaneously (4 catalyst loadings in triplicate or 2 catalysts at 3 catalyst loadings in duplicate) by an Epoch software-based protocol as follows. To prepare catalyst stock solutions (1.0 mM), 20 mL glass scintillation vials were charged with catalyst (5 µmole) and diluted to 5.0 mL total volume in toluene. Catalyst solutions, 10 to 400 µL depending on desired final catalyst loading, were transferred to glass 20 mL scintillation vials each capped with a septum having a 3 mm hole for the purpose of needle access, and were diluted to 10 mL total volume in toluene. The catalysts were preheated to 50.0 °C using the LH45 unit and stirred. Substrates (1 mmol), containing dodecane (0.25 mmol) as an internal standard, were dispensed simultaneously to 4 reactions at a time using one arm of the robot equipped with a 4-needle assembly. After the 2 minutes required for completion of the transfer, 50 µL aliquots of each reaction were withdrawn using the other robot arm and dispensed to 1.2 mL septa-covered vials containing 5% v/v ethyl vinyl ether in toluene cooled to −20 °C in two 96 well plates. The needle was flushed and washed between dispenses to prevent transfer of the quenching solution into the reaction vials. 16 timepoints were sampled at preprogrammed intervals and the exact times were recorded by the Epoch protocol. Samples were then analyzed by gas chromatography
General procedure for the preparation of catalysts 15–20
To a solution of imidazolinium salt in toluene (or benzene) was added KHMDS, and the resulting solution was stirred at room temperature for a few minutes. RuCl2(PCy3)(=CH–o-OiPrC6H4) was then added, and the mixture was stirred for the designated time and temperature (vide infra). After cooling to room temperature, the mixture was purified by column chromatography on TSI silica (eluent: hexane/diethyl ether = 2/1 → 1/1) to give the titled compounds as a green solid.
RuCl2(4,4-dimethyl-1,3-dimesityl-imidazolin-2-ylidene)(=CH–o-OiPrC6H4) (15)
6 (200 mg, 0.54 mmol), potassium hexamethyldisilazide (140 mg, 0.70 mmol), and RuCl2(PCy3)(=CH–o-OiPrC6H4) (250 mg, 0.42 mmol) was reacted according to the general procedure (stirred for 2 h at 70 °C) to give the desired ruthenium complex 15 as a green powder (135 mg, 0.21 mmol, 49%).
1H NMR (500 MHz, CD2Cl2, 25 °C): δ 16.46 (br s, 1H), 7.55 (ddd, J = 8.3 Hz, 2.0 Hz, 1H), 7.10 (br s, 2H), 7.05 (br s, 2H), 6.95 (dd, J = 7.5 Hz, 2.0 Hz, 1H), 6.91 (t, J = 7.5 Hz, 1H), 6.82 (d, J = 8.0 Hz, 1H), 4.86 (sept, J = 6.1 Hz, 1H), 3.93 (s, 2H), 2.50-2.25 (m, 18H), 1.47 (s, 6H), 1.21 (d, J = 6.1 Hz, 6H).
13C NMR (125 MHz, C6D6): δ 293.3 (m), 213.3, 153.0, 146.4, 141.3, 139.0, 138.6, 130.7, 130.0, 129.3, 122.7, 122.5, 113.6, 75.4, 68.2 (br), 65.6 (br), 28.1, 21.8, 21.5, 21.4.
HRMS Calc'd for C33H42Cl2N2ORu: 654.1718. Found: 654.1725.
RuCl2(1,3-dimesityl-4,5-dimethyl-imidazolin-2-ylidene)(=CH–o-OiPrC6H4) (16)
7 (100 mg, 0.27 mmol), potassium hexamethyldisilazide (70 mg, 0.35 mmol), and RuCl2(PCy3)(=CH–o-OiPrC6H4) (100 mg, 0.17 mmol) was reacted according to the general procedure (stirred for 2 h at 70 °C) to give the desired ruthenium complex 16 as a green powder (60 mg, 0.092 mmol, 54%).
1H NMR (500 MHz, C6D6, 25 °C): δ 16.74 (s, 1H), 7.14 (dd, J = 7.5, 1.5 Hz, 1H), 7.11 (ddd, J = 7.5, 1.5 Hz, 1H), 7.00 (br s, 4H), 6.65 (dt, J = 7.5, 1.0 Hz, 1H), 6.32 (d, J = 8.0 Hz, 1H), 4.49 (sept, J = 6.1 Hz, 1H), 4.12 (s, 2H), 3.00-2.30 (br s, 12H), 2.25 (s, 6H), 1.31 (br s, 6H), 0.81 (d, J = 6.5 Hz, 6H).
13C NMR (125 MHz, C6D6): δ 293.8, 213.4, 153.0, 146.4, 140.7, 138.7, 130.2, 129.9, 128.8, 122.8, 122.5, 113.6, 75.3, 62.4 (br), 21.8, 21.4, 13.9 (br).
HRMS Calc'd for C33H42Cl2N2ORu: 654.1718. Found: 654.1738.
RuCl2(1,3-dimesityl-4,4,5-trimethyl-imidazolin-2-ylidene)(=CH–o-OiPrC6H4) (17)
8 (200 mg, 0.46 mmol), potassium hexamethyldisilazide (120 mg, 0.60 mmol), and RuCl2(PCy3)(=CH–o-OiPrC6H4) (200 mg, 0.33 mmol) was reacted according to the general procedure (stirred for 2.5 h at room temperature and 4 h at 60 °C) to give the desired ruthenium complex 17 as a green powder (97 mg, 0.15 mmol, 44%). Crystals suitable for X-ray crystallography were grown at room temperature by slow diffusion of pentane into a solution of 17 in benzene.
1H NMR (500 MHz, C6D6, 25 °C): δ 16.65 (br s, 1H), 7.13-7.07 (m, 3H), 6.94 (br m, 3H), 6.63 (td, J = 7.6, 0.8 Hz, 1H), 6.31 (d, J = 8.0 Hz, 1H), 4.46 (sept, J = 6.1 Hz, 1H), 4.20 (br s, 1H), 2.85-2.47 (m, 12H), 2.24 (s, 3H), 2.21 (s, 3H), 1.28 (d, J = 6.1 Hz, 6H), 1.15 (br s, 3H), 0.88 (br s, 3H), 0.69 (br d, J = 6.9 Hz, 3H).
13C NMR (125 MHz, C6D6): δ 293.8 (m), 213.4 (br), 152.9, 146.5, 140.7, 138.7, 138.6, 130.9, 130.6, 130.3, 129.4, 122.7, 122.4, 113.6, 75.3, 71.0 (br), 68.4 (br), 25.1, 23.1 (br), 21.8, 21.5, 21.4, 12.1.
HRMS Calc'd for C34H44Cl2N2ORu: 668.1875. Found: 668.1898.
RuCl2(1,3-ditolyl-4,4-dimethyl-imidazolin-2-ylidene)(=CH–o-OiPrC6H4) (18)
9 (190 mg, 0.60 mmol), potassium hexamethyldisilazide (157 mg, 0.78 mmol), and RuCl2(PCy3)(=CH–o-OiPrC6H4) (200 mg, 0.33 mmol) was reacted according to the general procedure (stirred for 2 hours at 70 °C) to give the desired ruthenium complex 18 as a green powder (112 mg, 0.19 mmol, 57%).
1H NMR (500 MHz, CD2Cl2, 25 °C): δ 16. 41 (br s, 0.40H), 16.24 (br s, 0.60H), 8.59 (br s, 1.20H), 8.59 (br s, 0.80H), 7.60-7.20 (m, 7H), 6.88-6.81 (m, 3H), 4.91 (m, 1H), 4.40-3.60 (m, 2H), 2.62-2.40 (m, 6H), 1.64-1.07 (m, 12H).
13C NMR (125 MHz, CD2Cl2): δ 232.5, 152.2, 144.3, 141.9, 138.6, 134.3, 132.5, 131.4, 129.9, 129.5, 129.2, 128.9, 128.8, 127.6, 126.9, 122.3, 122.0, 121.8, 112.9, 74.8, 68.1, 66.6, 29.7, 27.3, 27.0, 26.9, 26.3, 24.6, 23.9, 21.5, 19.5.
HRMS Calc'd for C29H34Cl2N2ORu: 598.1092. Found: 598.1064.
RuCl2(1,3-ditolyl-4,5-dimethyl-imidazolin-2-ylidene)(=CH–o-OiPrC6H4) (19)
10 (100 mg, 0.32 mmol), potassium hexamethyldisilazide (70 mg, 0.35 mmol), and RuCl2(PCy3)(=CH–o-OiPrC6H4) (100 mg, 0.17 mmol) was reacted according to the general procedure (stirred for 2 h at 70 °C) to give the desired ruthenium complex 19 as a green (39 mg, 0.065 mmol, 38%).
1H NMR (500 MHz, C6D6, 25 °C): δ 16.64-16.41 (m, 1H), 9.00 (br s, 2H), 7.11-6.71 (m, 8H), 6.65 (m, 1H), 6.42 (t, J = 7.8 Hz, 1H), 4.57 (sept, J = 6.4 Hz, 1H), 4.29-3.55 (m, 2H), 2.65-2.25 (m, 6H),1.20-1.60 (m, 6H), 1.05-0.60 (m, 6H).
13C NMR (125 MHz, C6D6): δ 291.7, 290.9, 232.5, 210.74, 152.8, 144.2, 140.0, 139.6, 138.6, 137.4, 132.4, 132.2, 131.5, 131.3, 130.6, 130.3, 121.9, 121.8, 113.0, 112.8, 74.4, 61.1, 61.0, 60.4, 21.7, 21.6, 13.2, 12.9.
HRMS Calc'd for C29H34Cl2N2ORu: 598.1092. Found: 598.1097.
RuCl2(1,3-ditolyl-4,4,5,5-tetramethyl-imidazolin-2-ylidene)(=CH–o-OiPrC6H4) (20)
11 (41 mg, 0.12 mmol), potassium hexamethyldisilazide (24 mg, 0.12 mmol), and RuCl2(PCy3)(=CH–o-OiPrC6H4) (60 mg, 0.1 mmol) was reacted according to the general procedure described above to give the desired ruthenium complex 20 as a green powder as a ca. 3:1 mixture of isomers (45 mg, 0.072 mmol, 72%). Crystals suitable for X-ray crystallography were grown at room temperature by slow diffusion of pentane into a solution of 20 in benzene.
1H NMR (500 MHz, C6D6, 25 °C): δ 16.64 (s, 0.75H), 16.33 (s, 0.25H), 8.89 (d, J = 7.7 Hz, 0.75H), 8.84 (d, J = 7.9 Hz, 0.25H), 7.43-7.25 (m, 4H), 7.20-7.05 (m, 4H), 6.99-6.94 (m, 1H), 6.70-6.62 (m, 1H), 6.34 (d, J = 8.3 Hz, 1H), 4.45 (sept, J = 6.1 Hz, 1H), 2.74 (s, 0.75H), 2.68 (s, 2.25H), 2.47 (s, 0.75H), 2.44 (s, 2.25H), 1.38-1.20 (m, 10H), 1.04 (s, 2H), 0.76-0.70 (m, 6H).
13C NMR (125 MHz, C6D6): δ 214.0, 211.5, 153.1, 153.0, 145.8, 143.3, 143.2, 141.6, 140.8, 140.3, 139.8, 137.3, 136.5, 136.0, 134.7, 134.4, 132.3, 132.2, 131.9, 129.6, 129.5, 129.4, 129.1, 128.9, 127.6, 127.3, 126.9, 126.6, 122.7, 122.6, 122.6, 122.5, 113.5, 75.2, 75.1, 72.3, 71.8, 71.7, 71.4, 24.9, 24.3, 24.1, 23.9, 22.7, 22.5, 22.4, 22.2, 22.1, 22.0, 20.3, 20.1, 19.7, 19.4, 19.3.
HRMS Calc'd for C31H38Cl2N2ORu: 626.1405. Found: 626.1427.
Supplementary Material
Detailed experimental procedures and characterization data. Crystallographic details for 17 and 20 are provided in CIF format. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgement
We gratefully acknowledge financial support from the DOE (DE-FG02-08ER15933), the NIH (5RO1 GM31332), and the Gordon and Betty Moore Foundation. JBB would also like to thank Materia, Inc. for financial support. We thank Larry M. Henling and Dr. Michael W. Day (California Institute of Technology) for X-ray crystallographic analysis, and Materia, Inc. for a generous donation of ruthenium complexes, especially 2 and 3.
References
- 1.(a) Grubbs RH. Handbook of Metathesis. Weinheim, Germany: Wiley-VCH; 2003. and references cited therein. [Google Scholar]; (b) Hoveyda AH, Zhugralin AR. Nature. 2007;450:243–251. doi: 10.1038/nature06351. [DOI] [PubMed] [Google Scholar]; (c) Schrodi Y, Pederson RL. Aldrichimica Acta. 2007;40:45–52. [Google Scholar]; (d) Nicolaou KC, Bulger PG, Sarlah D. Angew. Chem., Int. Ed. 2005;44:4490–4527. doi: 10.1002/anie.200500369. [DOI] [PubMed] [Google Scholar]; (e) Grubbs RH. Tetrahedron. 2004;60:7117–7140. [Google Scholar]; (f) Furstner A. Angew. Chem., Int. Ed. 2000;39:3013–3043. [Google Scholar]
- 2.(a) Grubbs RH. J. Macromol. Sci. – Pure Applied Chem. 1994;A31:1829–1833. [Google Scholar]; (b) Trnka TM, Grubbs RH. Acc. Chem. Res. 2001;34:18–29. doi: 10.1021/ar000114f. [DOI] [PubMed] [Google Scholar]
- 3.Scholl M, Ding S, Lee CW, Grubbs RH. Org. Lett. 1999;1:953–956. doi: 10.1021/ol990909q. [DOI] [PubMed] [Google Scholar]
- 4.Garber SB, Kingsbury JS, Gray BL, Hoveyda AH. J. Am. Chem. Soc. 2000;122:8168–8179. [Google Scholar]
- 5.(a) Chung CK, Grubbs RH. Org. Lett. 2008;10:2693–2696. doi: 10.1021/ol800824h. [DOI] [PubMed] [Google Scholar]; (b) Berlin JM, Campbell K, Ritter T, Funk TW, Chlenov A, Grubbs RH. Org. Lett. 2007;9:1339–1342. doi: 10.1021/ol070194o. [DOI] [PubMed] [Google Scholar]; (c) Stewart IC, Ung T, Pletnev AA, Berlin JM, Grubbs RH, Schrodi Y. Org. Lett. 2007;9:1589–1592. doi: 10.1021/ol0705144. [DOI] [PubMed] [Google Scholar]; (d) Vougioukalakis GC, Grubbs RH. J. Am. Chem. Soc. 2008;130:2234–2245. doi: 10.1021/ja075849v. [DOI] [PubMed] [Google Scholar]; (e) Vehlow K, Maechling S, Blechert S. Organometallics. 2006;25:25–28. [Google Scholar]; (f) Despagnet-Ayoub E, Grubbs RH. Organometallics. 2005;24:338–340. [Google Scholar]; (g) Van Veldhuizen JJ, Garber SB, Kinsgbury JS, Hoveyda AH. J. Am. Chem. Soc. 2002;124:4954–4955. doi: 10.1021/ja020259c. [DOI] [PubMed] [Google Scholar]; (h) Funk TW, Berlin JM, Grubbs RH. J. Am. Chem. Soc. 2006;128:1840–1846. doi: 10.1021/ja055994d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Anderson DR, Lavallo V, O’Leary DJ, Bertrand G, Grubbs RH. Angew. Chem., Int. Ed. 2007;46:7262–7265. doi: 10.1002/anie.200702085. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Kuhn KM, Grubbs RH. Org. Lett. 2008;10:2075–2077. doi: 10.1021/ol800628a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Weigl K, Köhler K, Dechert S, Meyer F. Organometallics. 2005;24:4049–4405. [Google Scholar]
- 6.For recent examples, see: Enquist JE, Stoltz BM. Nature. 2008;453:1228–1231. doi: 10.1038/nature07046. White DE, Stewart IC, Grubbs RH, Stoltz BM. J. Am. Chem. Soc. 2008;130:810–811. doi: 10.1021/ja710294k. Pfeiffer MWB, Phillips AJ. J. Am. Chem. Soc. 2005;127:5334–5335. doi: 10.1021/ja0509836. Humphrey JM, Liao A, Rein T, Wong Y-L, Chen H-J, Courtney AK, Martin SF. J. Am. Chem. Soc. 2002;124:8584–8592. doi: 10.1021/ja0202964. Martin SF, Humphrey JM, Ali A, Hillier MC. J. Am. Chem. Soc. 1999;121:866–867. Yang Z, He Y, Vourloumis D, Vallberg H, Nicolaou KC. Angew. Chem., Int. Ed. 1997;36:166–168.
- 7.(a) Maynard HD, Grubbs RH. Tetrahedron Lett. 1999;40:4137–4140. [Google Scholar]; (b) Hong SH, Sanders DP, Lee CW, Grubbs RH. J. Am. Chem. Soc. 2005;127:17160–17161. doi: 10.1021/ja052939w. [DOI] [PubMed] [Google Scholar]
- 8.Governmental recommendations for residual ruthenium are now routinely less than 10 ppm. For recent guidelines, see: Zaidi K. Pharmacopeial Forum. 2008;34:1345–1348. (b) Criteria given in the EMEA Guideline on the Specification Limits for Residues of Metal Catalysts, available at: http://www.emea.europa.eu/pdfs/human/swp/444600.pdf
- 9.(a) Ulman M, Grubbs RH. J. Org. Chem. 1999;64:7202–7207. [Google Scholar]; (b) Hong SH, Day MW, Grubbs RH. J. Am. Chem. Soc. 2004;126:7414–7415. doi: 10.1021/ja0488380. [DOI] [PubMed] [Google Scholar]; (c) Hong SH, Wenzel AG, Salguero TT, Day MW, Grubbs RH. J. Am. Chem. Soc. 2007;129:7961–7968. doi: 10.1021/ja0713577. [DOI] [PubMed] [Google Scholar]; (d) Hong SH, Chlenov A, Day MW, Grubbs RH. Angew. Chem., Int. Ed. 2007;46:5148–5151. doi: 10.1002/anie.200701234. [DOI] [PubMed] [Google Scholar]; (e) Vehlow K, Gessler S, Blechert S. Angew. Chem., Int. Ed. 2007;46:8082–8085. doi: 10.1002/anie.200702560. [DOI] [PubMed] [Google Scholar]; (f) Leitao EM, Dubberley SR, Piers WE, Wu Q, McDonald R. Chem. Eur. J. 2008;14:11565–11572. doi: 10.1002/chem.200801584. [DOI] [PubMed] [Google Scholar]
- 10.Under inert atmosphere, heating a C6D6 solution of catalyst 5 for 3 days at 70 ° leads to its total decomposition, while catalyst 2 doesn’t readily decompose under those conditions.
- 11.Occhipinti G, Bjorsvik H-R, Jensen VR. J. Am. Chem. Soc. 2006;128:6952–6964. doi: 10.1021/ja060832i. [DOI] [PubMed] [Google Scholar]
- 12.Ritter T, Hejl A, Wenzel AG, Funk TW, Grubbs RH. Organometallics. 2006;25:5740–5745. and literature cited therein. [Google Scholar]
- 13.(a) Jazzar R, Bourg J-B, Dewhurst RD, Donnadieu B, Bertrand G. J. Org. Chem. 2007;72:3492–3499. doi: 10.1021/jo0703909. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Türkmen H, Çetinkaya B. J. Organomet. Chem. 2006;691:3749–3759. [Google Scholar]
- 14.Denk K, Sirsch P, Herrmann WA. J. Organomet. Chem. 2002;649:219–224. [Google Scholar]
- 15.In this paper, catalyst activity encompasses initiation and propagation rates. For more insight into initiation kinetic studies, see Sanford MS, Love JA, Grubbs RH. J. Am. Chem. Soc. 2001;123:6543–6554. doi: 10.1021/ja010624k.
- 16.Catalyst stability refers to the ability of a catalyst to do productive metathesis after extended period of time.
- 17.(a) Champagne TM, Hong SH, Lee CW, Ung TA, Stoianova DS, Pederson RL, Kuhn KM, Virgil SC, Grubbs RH. Abstracts of Papers; 236th ACS National Meeting; Philadelphia, PA. Washington, DC: American Chemical Society; 2008. ORGN-077. [Google Scholar]; (b) Matson JM, Virgil SC, Grubbs RH. J. Am. Chem. Soc. doi: 10.1021/ja809081h. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bieniek M, Michrowska A, Usanov DL, Grela K. Chem Eur. J. 2008;14:806–818. doi: 10.1002/chem.200701340. [DOI] [PubMed] [Google Scholar]
- 19.Ethyl vinyl ether functions as an effective catalyst quench, as the corresponding Fischer carbene complex is metathesis inactive. See: Louie J, Grubbs RH. Organometallics. 2002;21:2153–2164.
- 20.Wang H, Goodman SN, Dai Q, Stockdale GW, Clark WM., Jr Org. Process Res. Dev. 2008;12:226–234. [Google Scholar]
- 21.Complexes 15, 16, 18 and 19 underwent no further testing as experimentation continually demonstrated that complexes bearing disubstituted backbone ligands consistently gave results between the two extremes.
- 22.Pangborn AB, Giardello MA, Grubbs RH, Rosen RK, Timmers FJ. Organometallics. 1996;15:1518–1520. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed experimental procedures and characterization data. Crystallographic details for 17 and 20 are provided in CIF format. This material is available free of charge via the Internet at http://pubs.acs.org.