Abstract
Drug self-administration behavior has been one of the most direct and productive approaches for studying the reinforcing effects of psychoactive drugs, which are critical in determining their abuse potential. Cannabinoids, which are usually abused by humans in the form of marijuana, have become the most frequently abused illicit class of drugs in the United States. The early elucidation of the structure and stereochemistry of delta-9-tetrahydrocannabinol (THC) in 1964, which is now recognized as the principal psychoactive ingredient in marijuana, activated cannabinoid research worldwide. This review examines advances in research on cannabinoid self-administration behavior by humans and laboratory animals. There have been numerous laboratory demonstrations of the reinforcing effects of cannabinoids in human subjects, but reliable self-administration of cannabinoids by laboratory animals has only recently been demonstrated. It has now been shown that strong and persistent self-administration behavior can be maintained in experimentally and drug-naïve squirrel monkeys by doses of THC comparable to those in marijuana smoke inhaled by humans. Furthermore, reinforcing effects of some synthetic CB1 cannabinoid agonists have been recently reported using intravenous and intracerebroventricular self-administration procedures in rats and mice. These findings support previous conclusions that THC has a pronounced abuse liability comparable to other drugs of abuse under certain experimental conditions. Self-administration of THC by squirrel monkeys provides the most reliable animal model for human marijuana abuse available to date. This animal model now makes it possible to study the relative abuse liability of other natural and synthetic cannabinoids and to preclinically assess new therapeutic strategies for the treatment or prevention of marijuana abuse in humans.
Keywords: Cannabis, Cannabinoids, Delta-9-tetrahydrocannabinol, Humans, Marijuana, Squirrel Monkeys, Primates, Reinforcement, Reward, Rodents, Self-Administration, THC
Introduction
Cannabis derivatives are the most frequently abused illicit drugs in the United States and other countries (Johnston et al., 2003; Compton et al., 2004). In the United States, cannabinoids are most often abused by humans in the form of marijuana, made from the dry leaves and flowers of the plant Cannabis sativa, which has been used for centuries, primarily for its euphoric effects. Other names for the plant or its products include hemp, weed, hashish, charas, ganja, dagga and bhang. The potency of the marijuana product depends on the method of plant growing and processing, which can lead to different levels of psychoactive cannabinoid components.
At the end of 19th century the compound cannabinol was isolated from Cannabis sativa and was mistakenly considered the psychoactive principle of marijuana. It was later proven that it is only a minor constituent of cannabis, with weak psychotropic properties. More progress was made in the 1940’s when the major inactive component cannabidiol was isolated. This was of considerable importance, since the elucidation of the structure of cannabidiol (Mechoulam and Shvo, 1963) was a basis for the later elucidation of the structure and stereochemistry of delta-9-tetrahydrocannabinol (THC), which is now recognized as the principal psychoactive component of marijuana. THC was first isolated in the laboratory of Raphael Mechoulam (Gaoni and Mechoulam, 1964) and this discovery reactivated cannabinoid research worldwide.
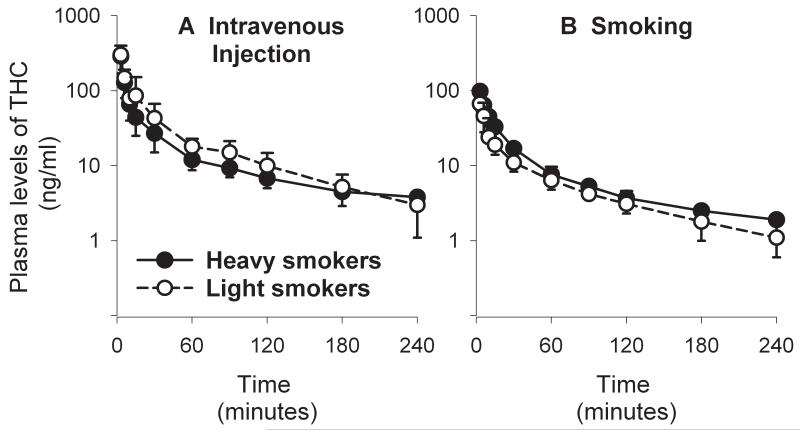
Although cannabinoids are usually smoked, they are also eaten in fat-containing foods or drunk as a tea and are very rarely injected intravenously. When marijuana is smoked, THC is rapidly absorbed into the bloodstream from the lungs and reaches the brain within 8-10 seconds. This rate of absorption accounts for the rapid onset of effects from smoked marijuana. Plasma concentration of THC peaks immediately at the end of smoking (Huestis et al., 1992b) and is usually in the range of 70 to 160 ng/ml (Lindgren et al., 1981; Heishman et al., 1990; Azorlosa et al., 1992; Huestis et al., 1992a) (Figure 1b). Because THC is a highly lipophilic molecule, it leaves the blood rapidly and is deposited in the fatty tissues of the body. As a result, after smoking, plasma THC concentration rapidly falls below 20 ng/ml within 30-45 minutes (Figure 1b). A very similar profile of THC plasma levels is produced by intravenous injection of THC (e.g., Lindgren et al., 1981; Figure 1a). Also, reports of THC-produced “highs” peak at around 20 minutes and are virtually gone 3 hours after both smoking of marijuana and intravenous administration of THC in human subjects (Lindgren et al., 1981; Ohlsson et al., 1980).
Figure 1.
Average plasma levels (± SD) of Δ9-THC in two groups of marijuana smokers (heavy and light users) after intravenous administration of Δ9-THC (5 mg; panel A) and after smoking a marijuana cigarette (heavy: 12.7 ± 1.3 mg of THC smoked; light: 13.4 ± 1.6 mg of THC smoked; panel B). See Lindgren et al., 1981 for more detailed information on methods and plasma levels. Modified from Lindgren et al., 1981.
Subjective and motivational effects of cannabinoids
Marijuana produces clear subjective reports of pleasurable effects, associated with motivational responses in humans, like drug-seeking and drug-taking behavior (Maldonado, 2002). Many different animal and human models are used to assess the consequences of acute and chronic exposure to THC and the abuse liability of related cannabinoids. Tolerance and physical dependence development can be assessed with specific behavioral and biochemical tests, but they provide only a partial correlate of the abuse liability of cannabinoids. The ability of abused cannabinoids to induce drug-seeking behavior due to positive reinforcing or reward related effects is likely the best correlate of motivational properties that contribute to their abuse liability and these effects can be evaluated using several models of animal behavior.
The administration of a psychoactive drug to humans produces a set of interoceptive, subjective feelings, that include reports of liking, and these subjective reports are often used as indirect measures of a drug’s reinforcing effects. The presence or absence of discriminable interoceptive CNS effects of a compound can be evaluated in animals with two-lever choice drug-discrimination procedures. Discriminative-stimulus effects of THC in animals show a high degree of pharmacological specificity and provide a reliable animal model of subjective effects of marijuana or THC in humans (Balster and Prescott, 1992; Barrett et al., 1995; Wiley et al., 1995b). Usually, only other cannabinoids that are active at central cannabinoid CB1 receptors reliably produce THC-like discriminative effects in animals (Barrett et al., 1995; Wiley et al., 1995b; Jarbe et al., 2001). It is interesting to note, however, that the endogenous cannabinoid CB1 receptor ligand anandamide either does not produce THC-like discriminative effects in monkeys or rats (Wiley et al., 1997; Burkey and Nation, 1997; Wiley et al., 1998; Jarbe et al., 2001) or does so to limited degree only at extremely high doses that markedly depress responding by the subjects (Wiley et al., 1995a; Alici and Appel, 2004). The rapid disappearance of anandamide after its administration due to its fast metabolism through the fatty acid amide hydrolase (FAAH) enzyme and the fact that studies of anandamide have employed the intraperitoneal (i.p.) route of administration, which does not favor rapid entry into the brain before metabolic breakdown, may explain the difficulties encountered in demonstrating THC-like discriminative effects of anandamide in animals. Among non-cannabinoid drugs, only pentobarbital and diazepam have been found to produce partial generalization to a cannabinoid training stimulus (Mokler et al., 1986; Balster and Prescott, 1992; Barrett et al., 1995; Wiley and Martin, 1999; Alici and Appel, 2004). Since this effect of diazepam is not blocked by the cannabinoid CB1 receptor antagonist rimonabant (SR 141716), it is probably mediated by an interaction through the GABAergic system (Wiley and Martin, 1999).
Another way to indirectly assess the reinforcing or rewarding effects of cannabinoids in experimental animals is to study their ability to modulate the reinforcing effects of other rewarding events. These models include intracranial electrical self-stimulation techniques. Consistent with a role of cannabinoids in the motivational effects of other events that can function as rewards or reinforcers, it has been shown that THC lowers the threshold for electrical brain-stimulation reward in Lewis and Sprague-Dawley rat strains, and that withdrawal from a single administration of THC can elevate brain-stimulation reward thresholds (Gardner et al., 1988; 1989; Lepore et al., 1996; Gardner and Vorel, 1998). However, these findings contrast with the lack of THC effects in the Fisher rat strain (Lepore et al., 1996), and the lack of effects of the synthetic CB1 receptor agonist CP 55,940 using the same procedure and a comparable range of doses (Arnold et al., 2001).
A more direct way to assess the reinforcing or rewarding effects of cannabinoids in experimental animals is to study the development of cannabinoid-induced conditioned place preferences. Surprisingly, THC, as well as synthetic cannabinoid agonists, like CP 55,940 (McGregor et al., 1996), WIN 55,212-2 (Chaperon et al., 1998) and HU 210 (Cheer et al., 2000), generally induce conditioned place avoidance or aversion rather than place preference in rats (Parker and Gillies, 1995; Sanudo-Pena et al., 1997; Hutcheson et al., 1998; Mallet and Beninger, 1998) and mice (Valjent and Maldonado, 2000), although THC-induced conditioned place preferences have been reported at limited dose-ranges and under restricted experimental conditions in Long Evans rats and in mice (Lepore et al., 1995; Valjent and Maldonado, 2000; Ghozland et al., 2002) and CP 55,940-induced conditioned place preferences have been reported in Wistar rats (Braida et al., 2001a).
There has been a great deal of work done during the last two decades using biochemical and electrophysiological methods to elucidate neurobiological mechanisms underlying the reinforcing or rewarding effects of THC and other cannabinoids. It has been confirmed that Δ9-THC shares many features with classical drugs of abuse, such as cocaine and heroin (for review see Gardner and Vorel, 1998; Tanda and Goldberg, 2003; Lupica et al., 2004). For example, THC lowers electrical brain-stimulation reward thresholds (Gardner et al., 1988), and increases the firing rate of ventral tegmental area (VTA) dopaminergic neurons projecting to the nucleus accumbens (French et al., 1997; Gifford et al., 1997), resulting in increased extracellular levels of dopamine in the nucleus accumbens (e.g., Chen et al., 1990; Tanda et al., 1997). It has also been shown that chronic cannabinoid administration leads to other significant neurobiological changes that resemble those already described for other drugs abused by humans (Rodriguez de Fonseca et al., 1997; Diana et al., 1998; Tanda et al., 1999; see Tanda and Goldberg, 2003 for review).
Evaluation of the reinforcing effects of THC and other synthetic cannabinoids with operant self-administration procedures
Drugs that are abused by humans are also typically self-administered by both human and non-human subjects under controlled laboratory conditions (Schuster and Thompson, 1969; Johanson and Balster, 1978; Griffiths, 1980; Goldberg et al., 1981; Fischman and Schuster, 1982; Goldberg and Henningfield, 1988; Foltin and Fischman, 1991; Preston and Jasinski, 1991). Reliable and persistent intravenous self-administration behavior has been demonstrated in laboratory animals for almost all drugs abused by humans, including psychostimulants, opiates, ethanol, and nicotine (Goldberg et al., 1981; Collins et al., 1984; Young and Herling, 1986; Yokel, 1987) and, more recently, THC (Tanda et al., 2000; Justinova et al., 2003). Reinforcing effects of a drug assessed by intravenous self-administration procedures in experimental animals are considered one of the most reliable predictors of abuse potential in humans (Deneau and Seevers, 1964; Schuster and Thompson, 1969; Johanson and Balster, 1978; Johanson, 1990; Henningfield et al., 1991; Brady, 1991). Providing access to drugs for self-administration during limited daily sessions not only provides a reliable way to study reinforcing effects but also a way to explore neuropharmacological mechanisms involved in these effects (Koob and Weiss, 1990).
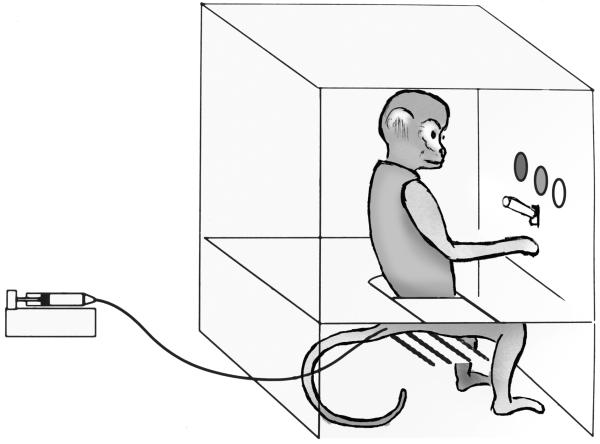
During intravenous drug self-administration studies in human subjects and experimental animals, subjects are allowed to self-administer a drug by making operant responses, such as pressing or pulling a lever or, with rodents, inserting their nose into a hole (a “nose-poke” response) and these operant responses then have the consequence of activating a pump, which intravenously delivers the drug (Figure 2). Behavioral measures usually employed include the rate of responding (i.e. lever presses/minute), the frequency of self-administered injections and the number of drug injections delivered within the session, together with the total drug-intake during the session. Although there are many variations to this basic experimental procedure, a commonly used method for assessing the reinforcing efficacy of a test drug in experimental animals is to compare self-administration of the drug to self-administration of a standard drug of known abuse potential and to a vehicle control in the same subject (e.g., Johanson and Balster, 1978; Young and Woods, 1981; Bergman and Johanson, 1985; Tanda et al., 2000). These studies are usually performed in rhesus monkeys (Macaca mulatta), squirrel monkeys (Saimiri sciureus) or rats that have learned to self-administer a prototypical drug of abuse, such as cocaine or codeine, under a schedule requiring a fixed number of responses to obtain each injection (e.g., a 10-response, fixed-ratio schedule of drug injection, FR10) (Goldberg et al., 1971; Goldberg, 1973; Marquis et al., 1989; Mansbach et al., 1994; Tanda et al., 2000). The drugs to be tested are then substituted for the training drug and evaluated for their ability to maintain levels of responding greater than those maintained during vehicle substitution. One must consider, however, that the functional state of brain reward circuits in drug naïve subjects versus experienced subjects that have chronically self-administered a training drug such as cocaine is likely different, as recently demonstrated by findings that dopamine elevations in the nucleus accumbens produced by cocaine are dramatically higher in experienced subjects compare to naïve subjects (Duvauchelle et al., 2000; Zapata et al., 2003). Neurobiological adaptations that occur over time in subjects that have repeatedly self-administered psychoactive drugs (e.g., Stefanski et al., 1999; Lu et al., 2003; Stefanski et al., 2004; Thompson et al., 2004) might predispose them to self-administer a test drug or limit the self-administration of a test drug (Young et al., 1981; Nader and Mach, 1996; Wojnicki and Glowa, 1996; Tella et al., 1996; Morgan et al., 2002). Thus, it can be important to study the acquisition of drug self-administration behavior in drug naïve subjects (e.g., Deneau and Seevers, 1964).
Figure 2.
Schematic drawing of a squirrel monkey sitting in a self-administration chair. During daily one-hour sessions, monkeys sat inside the experimental chamber, restrained in the seated position by a waist lock on the Plexiglas chair. At the start of each session, a white-house light was turned off and a green stimulus light was turned on; ten lever presses turned off the green light and produced a 2-s amber light paired with i.v. injection of THC (0.2 ml in 0.2 s) delivered from a syringe pump outside the chamber. There was a 60-s time-out period after each injection, during which the chamber was dark and lever presses had no programmed consequences (a 10-response, fixed-ratio schedule of i.v. THC injection with a 60-s time-out; FR 10, TO 60 s).
Substitution or acquisition studies, however, give only limited information on the reinforcing efficacy of tested drugs. One procedure that has been used to better assess reinforcing efficacy of self-administered drugs is the progressive-ratio schedule of intravenous drug-injection, in which the number of responses required for each injection increases progressively within a session. This allows an estimation of the maximal effort an individual will put forth under a specified set of conditions to obtain a particular dose of a test drug (e.g., Griffiths et al., 1979; Arnold and Roberts, 1997). The behavioral measure usually obtained is the maximal number of responses the subject will make in order to self-administer different doses of a drug, often called the “break-point.” This is taken as a measure of the motivational strength of the reinforcing event and is thought to predict reinforcing rewarding efficacy of test drugs (Hodos, 1961). So far, however, there have been no reports of successful self-administration of cannabinoid compounds under a progressive-ratio schedule.
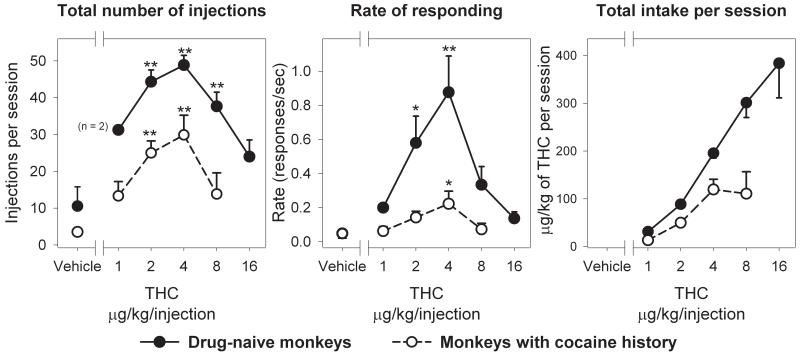
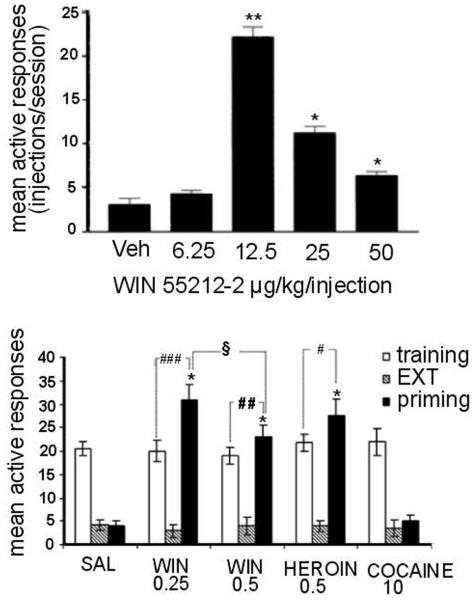
Human subjects and laboratory animals will self-administer addictive drugs by a variety of routes, including oral, intragastric, intraperitoneal, and intracranial routes. Cannabinoids have long been considered one of the exceptions to the close correspondence between drugs abused by humans and those self-administered by laboratory animals (e.g., Griffiths, 1980; Woods, 1983; Johanson, 1990; Henningfield et al., 1991). Although there have been numerous laboratory demonstrations of the reinforcing effects of cannabinoids in human subjects, reliable self-administration of cannabinoids by laboratory animals has only recently been successfully demonstrated. Self-administration of THC has now been repeatedly demonstrated in non-human primates (Tanda et al., 2000; Justinova et al., 2003; Justinova et al., 2004) (Figures 2 and 3) and reliable self-administration of synthetic cannabinoids has been demonstrated in rodents (Fattore et al., 2001; Braida et al., 2001b) (Figure 4).
Figure 3.
THC dose-response curves in squirrel monkeys with no history of exposure to other drugs (n = 3) and in squirrel monkeys with a history of cocaine self-administration (n = 4). Numbers of injections per session (left panel), overall rates of responding in the presence of a green light signalling THC availability (middle panel) and total THC intake per session (right panel) are presented as a function of injection dose of THC. Each symbol represents the mean (± S.E.M.) of the last three sessions under each THC injection dose condition and under a vehicle condition from three or four monkeys, with the exception of the values for the 1 μg/kg/injection dose of THC, which represent mean results from two monkeys. *P < 0.05, **P < 0.01 post-hoc comparisons with the vehicle conditions after significant one-way ANOVA for repeated measures main effect, Dunnett’s test. Modified from Tanda et al., 2000 and Justinova et al., 2003.
Figure 4.
A. Intravenous self-administration of WIN 55,212-2 by rats. Each bar represents the mean ± S.E.M number of injections per session during 6 consecutive sessions immediately after 3 days of stable responding (n = 12-14). Doses are expressed as μg/kg per injection. **P<0.01 and *P<0.05 significant difference from vehicle group. Modified from Fattore et al., 2001. B. Effects of acute priming injections of WIN 55,212-2, heroin or cocaine on reinstatement of cannabinoid-seeking behavior following prolonged abstinence. Each bar represents the mean ± S.E.M of active nose-pokes over the last 3 days of cannabinoid self-administration (training), over the last five consecutive sessions of extinction (EXT) and during reinstatement test sessions (priming). Doses are expressed as mg/kg (i.p.). *P<0.001 vs respective EXT; #P<0.05, ##P<0.01 and ###P<0.001 vs respective training; §P<0.05 between primings of the two doses of WIN 55,212-2. ANOVA followed by post hoc test (n=7-8). Modified from Spano et al., 2004.
Self-administration of cannabinoids in human subjects
Although THC is recognized as the main psychoactive ingredient of cannabis derivatives, there is still frequent debate about the therapeutic efficacy of whole plant extracts versus pure THC (See, for example, Wachtel et al., 2002; Russo and McPartland, 2003). Cannabis contains more than 400 different chemicals, in addition to THC (ElSohly, 2002). While it is likely that some of these compounds may themselves produce subjective and/or behavioral effects if consumed in high enough concentrations (Whalley et al., 2004), some of them may also have a so-called “entourage effect”, which has been described as a potentiation of the psychotropic effects of THC. However, previous research has clearly identified THC as the primary psychoactive agent associated with behavioral impairment following marijuana smoking (e.g., Heishman et al., 1989; Foltin et al., 1993; Kelly et al., 1993). Moreover, several studies have provided experimental data supporting THC’s integral role in the reinforcing effects of smoked marijuana, when subjects are given a choice between marijuana cigarettes with different THC contents (Mendelson and Mello, 1984; Chait and Zacny, 1992; Chait and Burke, 1994; Kelly et al., 1994b; Haney et al., 1997; Kelly et al., 1997). Marijuana cigarettes with a greater THC content have been shown to be consistently preferred to cigarettes with a lower THC content, even when the choice between the two was not mutually exclusive (Haney et al., 1997; Kelly et al., 1997; Ward et al., 1997). Also, the choice to self-administer a drug can often be shifted when an effective alternative reinforcer, such as money or tokens is available (e.g., Ward et al., 1997; Comer et al., 1998; Hart et al., 2000). However, there was little difference between the self-administration of low or high potency marijuana cigarettes when snacks were used as alternative reinforcers (Mendelson and Mello, 1984; Haney et al., 1997). In contrast, marijuana self-administration was substantially reduced when it preceded a task reinforced by money that required accurate and rapid performance (Haney et al., 1997; Ward et al., 1997).
In human studies, there have been attempts to better define THC’s role as the primary psychoactive ingredient with reinforcing effects in marijuana by systematically manipulating THC content during smoked marijuana self-administration in controlled laboratory situations. For example, in one early study, Cappell and colleagues (1973), investigated whether experienced marijuana users would titrate their marijuana intake according to THC concentration in order to achieve a defined subjective state. Male volunteers were smoking cigarettes of defined concentration of delta-9-tetrahydrocannabinol (0.2, 0.4 or 0.8%) until they reached a subjectively determined “optimal” high. The puff duration, number of puffs taken, duration of inhalation holding, intervals between puffs and weight of material consumed were measured. The experimenters observed a tendency for titration of THC intake. The failure to see a higher degree of titration might have been partially due to the narrow range of THC content studied and to psychological variables. The influence of nonpharmacological variables, such as environmental setting, subject expectancy, and previous experience with drugs, on the subjective effects of marijuana had been noticed very early in the human research with marijuana (e.g., Hochman and Brill, 1971; Jones, 1971; Meyer et al., 1971).
During the following quarter century, scientists attempted, mostly with little success, to further demonstrate a THC dose-regulation phenomenon with smoked marijuana (Ashton et al., 1981; Perez-Reyes et al., 1982; Wu et al., 1988; Chait, 1989; Zacny and de Wit, 1991; Kelly et al., 1994a, but see also: Azorlosa et al., 1992; Harder and Rietbrock, 1997; Block et al., 1998). However, Herning and colleagues (1986) observed that experienced marijuana smokers took significantly more puffs with longer intervals between puffs while smoking high potency (3.9% THC) compared to low potency (1.2% THC) marijuana cigarettes and they also inhaled substantially larger (46%) volumes of air. The marijuana users appeared to be diluting the more potent marijuana smoke, perhaps as a mechanism to down-regulate THC dose. In contrast with these findings, Heishman and colleagues (1989) observed that moderate marijuana users were taking smaller and shorter puffs and inhaling less air with each puff of a 2.7% THC cigarette compared to a 1.3% THC cigarette. Another study by Nemeth-Coslett and colleagues (1986) showed some compensatory changes in marijuana smoking in response to THC dose manipulations in subjects who were given the opportunity to sample the different potency cigarettes before self-administration behavior was measured. They demonstrated that expired air carbon monoxide (CO) levels following marijuana smoking were inversely related to THC content of the marijuana (1.29, 2.84 or 4.0% THC), suggesting that subjects reduced their smoke intake as cigarette THC content increased. Despite the opportunity to learn the difference in THC content of different marijuana cigarettes from previous experience, subjects in a subsequent study by Chait (1989) failed to show a similar tendency to titrate their smoke intake, possibly due to the choice of THC concentrations chosen for study (0.9 to 2.7% THC).
Generally, the range of the THC content in marijuana appeared to be relevant to the outcomes of these studies in humans. Studies using marijuana cigarettes with maximal THC contents ranging from 1.24 to 2.54% did not observe a significant titration of THC intake (e.g., Cappell et al., 1973; Perez-Reyes et al., 1982; Wu et al., 1988; Chait, 1989; Kelly et al., 1994a), while studies which observed a significant titration of THC intake used cigarettes with maximal THC contents ranging from 3.9 to 4.0% (Herning et al., 1986; Nemeth-Coslett et al., 1986). It appears that the ability of subjects to discriminate between different dose levels of THC might affect their marijuana smoking behavior. It has been suggested that the differences in effects within the lower dose-range of 1.3 to 2.7% of THC may be difficult to discriminate (Heishman et al., 1989).
In one study investigating reports of subjective effects from smoking marijuana cigarettes with different THC concentrations (Chait et al., 1988), 1.4% THC marijuana cigarettes produced 100% drug-appropriate responding in subjects trained to discriminate between effects of a 2.7% THC marijuana cigarette and a placebo 0% THC cigarette, suggesting that subjects perceived the effects of 1.4 and 2.7% THC marijuana cigarettes as subjectively similar. Using a 100-point scale, the subjects in the study by Heishman et al. (1989), who adjusted their smoking of cigarettes that varied in THC content, also did not report differences in “drug high” or being “stoned” between 1.2 and 2.7% THC cigarettes, but they were not asked to evaluate potency. There have been dose-dependent increases in reports of subjective-effects ratings in several studies investigating regulation of smoking patterns with different THC-content marijuana cigarettes, regardless of the range of THC contents in the marijuana cigarettes used (low range of THC contents: Perez-Reyes et al., 1982; Chait, 1989; high range of THC contents: Herning et al., 1986; Zacny and de Wit, 1991). However, there have been studies using marijuana cigarettes with higher ranges of THC content that did not observe this tendency. For example, a study by Nemeth-Coslett and colleagues (1986) used a four-point scale to evaluate reported strength of the marijuana cigarettes and found no dose-related differences, but the four-point scale may not have been sufficiently sensitive to capture differences in subjective response between 1.29, 2.84 and 4.0% THC marijuana cigarettes. Despite using a potentially more sensitive 100-point scale to evaluate reported strength (THC content), “high” and drug effects, subjects in a study by Kelly et al. (1994) also failed to differentiate between 2.0 and 3.5% THC marijuana cigarettes. Drug ratings were sensitive to the presence or absence of THC in the cigarettes, but not to differences in the amount of THC. Taking into an account the marked differences in these different studies in the previous experience of subjects with marijuana smoking, in the methodologies and the experimental conditions employed and in the marijuana cigarettes preparations used, it is not possible to conclude that the THC dose-titration phenomenon was either satisfactorily demonstrated or ruled out. The large intersubject variability in puff and inhalation volume calls for precise methods of control over marijuana smoking patterns in order to improve the accuracy of dosage delivery in future studies (Heishman et al., 1989).
Although the majority of human studies have focused on the effects of smoked marijuana, a few studies have investigated the psychoactive and reinforcing effects of oral THC (given, for example, in the form of dronabinol - Marinol®). Dronabinol is a synthetic THC and is approved for use as an appetite stimulant in AIDS patients experiencing anorexia associated with weight loss and as an antiemetic in cancer patients experiencing nausea and vomiting associated with chemotherapy. Interviews with addiction medicine specialists, oncologists, and HIV treatment providers indicate no abuse or diversion of dronabinol for sale as a street drug (Calhoun et al., 1998). An important pharmacokinetic distinction with respect to abuse liability between dronabinol and smoked marijuana is the relatively slow onset of action of dronabinol. Peak plasma THC concentrations and psychoactive effects of oral THC occur 2-3 hours after ingestion and effects last up to 8 hours.
Several studies have demonstrated that oral THC in acute doses up to 20 mg produces subjective effects characterized as discriminable (“feel drug”), cannabis-specific (“stoned”), and related to abuse liability (“like drug” and “want more drug”) (Chesher et al., 1990; Kirk et al., 1998; Curran et al., 2002). Other studies have shown that the subjective effects produced by oral THC are of comparable intensity to those of smoked marijuana (Wachtel et al., 2002; Hart et al., 2002). For example, Hart and colleagues (2002) reported that oral THC (20 mg) and smoked marijuana (3.1% THC) produced comparable increases in ratings of “high,” “good effects,” and “liking.” Taken together, these subjective data suggest that oral THC may have abuse potential which has not materialized, due to the availability of marijuana for smoking, with its more rapid onset of pleasurable effects.
Only two studies have directly examined the reinforcing effects of oral THC. Chait and Zacny (1992) used a between-subject design to compare oral THC and smoked marijuana. The reinforcing effect of each drug was tested using a discrete-trial choice paradigm in which subjects chose to self-administer active THC versus placebo on two separate occasions. All subjects chose marijuana cigarettes containing THC over placebo cigarettes, and all but one subject chose active oral THC over placebo, leading the authors to conclude that oral THC can function as a positive reinforcer. However, these choices to self-administer THC either orally or by smoking were made in the absence of an alternative non-drug reinforcer, which can modify drug-reinforced behavior (Carroll et al., 1989; Petry and Bickel, 1999). Using a similar discrete-trial choice procedure, Hart et al. (in press) had subjects choose between different doses of oral THC (0, 10, or 20 mg) and a $2.00 voucher that was redeemable as cash at the end of the study. Subjects chose to self-administer active doses of THC significantly more often than placebo; THC was chosen on 5 of 11 choice opportunities and placebo on 2 of 11 opportunities. However, oral THC produced less than robust reinforcing effects since it was chosen on less than 50% of choice opportunities. The modest reinforcing effects of oral THC observed in the laboratory, together with the virtual absence of evidence of abuse potential in the treatment community (Calhoun et al., 1998), indicates that oral THC is not likely to be self-administered for recreational purposes.
Self-administration of cannabinoids by laboratory animals
Over the last three decades, many attempts to demonstrate intravenous self-administration of THC or of synthetic cannabinoid CB1 receptor agonists by experimental animals were relatively unsuccessful (Pickens et al., 1973; Kaymakcalan, 1973; Harris et al., 1974; Carney et al., 1977; van Ree et al., 1978; Mansbach et al., 1994) (Table 1). None of these studies clearly demonstrated persistent, dose-related, self-administration behavior maintained by THC or synthetic cannabinoids, which would be susceptible to vehicle extinction and subsequent reinstatement in the absence of unusual “foreign” conditions. A study by Kaymakcalan (1973) did demonstrate acquisition of THC self-administration behavior in two monkeys out of six studied, but only after withdrawal from forced automatic i.v. injections of THC, when signs of physical dependence on THC occurred. Takahashi and Singer (1979; 1980) reported THC self-administration behavior above placebo levels in diet-restricted rats maintained at 80% of normal body weight, under conditions where a food pellet was automatically delivered every minute. However, this self-administration behavior immediately decreased to placebo levels when food restriction was discontinued. It has been repeatedly shown that diet restriction can facilitate the initiation of drug self-administration behavior (Carroll et al., 1979; de la Garza and Johanson, 1987; Cabeza de Vaca and Carr, 1998) and can increase already established self-administration of drugs from each of the major classes of abused drugs (review by Carroll and Meisch, 1984).
Table 1.
Evaluation of the reinforcing effects of cannabinoids with operant self-administration procedures in laboratory animals
| Dose range Vehicle | Species | Procedure | Results | Reference |
|---|---|---|---|---|
| Delta-9-Tetrahydrocannabinol (Δ9-THC) | ||||
| 25-100 μg/kg/inj. PVP, Saline | Rhesus Monkeys | IVSA Substitution for phencyclidine Inhalation | Low rates of responding; No clear evidence that responding for THC could persist above vehicle control levels over repeated daily sessions | Pickens et al., 1973 |
| 100-400 μg/kg/inj. Tween-20, Saline | Rhesus Monkeys | IVSA Naïve subjects Substitution for cocaine | Negative results in naïve subjects 2 monkeys out of 6 acquired THC IVSA after physical dependence to THC had been established and 1 monkey following cocaine self-administration | 1Deneau and Kaymakcalan, 1971; Kaymakcalan et al., 1973 |
| 25-300 μg/kg/inj. PVP, Saline | Rhesus Monkeys | IVSA Naïve subjects; Substitution for cocaine, cocaine-THC mixture; IVSA measured after exposure to programmed infusions of THC | Negative results | Harris et al., 1974 |
| Cannabis resin suspension (contained 30-330 μg of THC/ml) | Rats | Oral SA | Negative results | 2Leite and Carlini, 1974 |
| Aqueous hashish suspension (contained 1.25-125 μg of THC/ml) | Rats | Oral SA | Negative results | 3Corcoran and Amit, 1974 |
| 3-300 μg/kg/inj. EL-620, Ethanol, Saline | Rhesus Monkeys | IVSA Substitution for cocaine | Negative results | Carney et al., 1977 |
| 7.5-300 μg/kg/inj. Tween-20, Saline | Rats | IVSA Naïve subjects | Low incidence of lever-pressing; Maximal incidence of rats self-administering THC was only 40 % on the highest dose | Van Ree et al., 1978 |
| 6.25-50 μg/kg/inj. Tween-80, Saline | Rats | IVSA Naïve Subjects | IVSA in food-deprived rats when a pellet of food was automatically delivered at 1-min intervals; Behavior immediately disappeared when a diet restriction or the automatic food presentation was discontinued | Takahashi and Singer, 1979; 1980 |
| 17-100 μg/kg/inj. EL-620, Ethanol, Saline | Rhesus Monkeys | IVSA Substituted for phencyclidine | Negative results | Mansbach et al., 1994 |
| 2-8 μg/kg/inj. Tween-80, Ethanol, Saline | Squirrel Monkeys | IVSA Substituted for cocaine after saline extinction period | Persistent and reliable IVSA; Peak rates of responding at THC dose of 4 μg/kg/inj. | Tanda et al., 2000 |
| 1-16 μg/kg/inj. Tween-80, Ethanol, Saline | Squirrel Monkeys | IVSA Naïve Subjects | Persistent and reliable IVSA; Peak rates of responding at THC dose of 4 μg/kg/inj. | Justinova et al., 2003; 2004 |
| WIN 55,512-2 | ||||
| 10-500 μg/kg/inj. Cremophor, Saline | Mice | IVSA Naïve subjects | Positive results; Single IVSA session in restrained mice | Martellotta et al., 1998 |
| 100 μg/kg/inj. Cremophor, Saline | Mice | IVSA Naïve subjects | Positive results in wild-type mice (CB1+/+) Negative results in CB1 receptor knockout mice (CB1-/-) | Ledent et al., 1999 |
| 10-100 μg/kg/inj. Cremophor, Saline | Mice | IVSA Naïve subjects | Positive results; Single IVSA session in restrained mice | Navarro et al., 2001 |
| 6.25-50 μg/kg/inj. Tween-80, Saline | Rats | IVSA Naïve subjects | Positive results; Peak rates of responding at WIN 55,212-2 dose of 12.5 μg/kg/inj.; Freely moving rats; Diet-restriction necessary to demonstrate self-administration | Fattore et al., 2001; Spano et al., 2004 |
| CP 55,940 | ||||
| 0.3-3 μg/kg/inj. EL-620, Ethanol, Saline | Rhesus Monkeys | IVSA Substituted for phencyclidine | Negative results | Mansbach et al., 1994 |
| 0.1-1.6 μg/infusion ACP, Cremophor, Saline | Rats | ICVSA Naïve subjects | Positive results, Water-restricted rats; Concurrent delivery of water with each infusion | Braida et al., 2001 |
| HU-210 | ||||
| 5 μg/kg/inj. Cremophor, Saline | Mice | IVSA Naïve subjects | Positive results; One-session self-administration in restrained mice | Navarro et al., 2001 |
| SR 141716 (Rimonabant) | ||||
| 1-100 μg/kg/inj. EL-620, Ethanol, Saline | Rhesus Monkeys | IVSA Substitution for cocaine | Negative results | Beardsley et al., 2002 |
IVSA - Intravenous Self-administration; ICVSA - Intracerebroventricular Self-administration; PVP - Polyvinylpyrollidone; EL-620 - Emulphor ; ACP - artificial cerebrospinal fluid
Deneau GA, Kaymakcalan S. Physiological and psychological dependence to synthetic Δ9-Tetrahydrocannabinol (THC) in rhesus monkeys. The Pharm acologist 1971;13:246;
Leite JR , Carlini E A . Failure to obtain “cannabis-directed behavior” and abstinence syndrome in rats chronically treated with cannabis sativa extracts. Psychopharmacologia. 1974;36(2):133-45;
Corcoran ME , Amit Z. Reluctance of rats to drink hashish suspensions: free-choice and forced consumption, and the effects of hypothalamic stimulation. Psychopharmacologia. 1974;35(2):129-47
Although THC has not been found to maintain persistent self-administration in mice or rats, there have been several recent reports of intravenous self-administration of synthetic cannabinoid CB1 receptor agonists in rodents. The synthetic CB1 agonist WIN 55,212 has been reported to maintain intravenous self-administration behavior in mice and rats (Martellotta et al., 1998; Fattore et al., 2001; Navarro et al., 2001) and the synthetic CB1 agonist HU-210 maintains intravenous self-administration behavior in mice (Navarro et al., 2001). However, these studies used experimental procedures that limited the generality of the findings. For example, in both studies with mice (Martellotta et al., 1998; Navarro et al., 2001), one-day experimental tests were used during which mice were severely restrained for acute intravenous administration through the tail vein. There is very limited information available about acquisition, extinction and relapse to drug self-administration behavior using this methodology. Fattore and colleagues (2001) utilized unrestrained, freely-moving rats, which were given the opportunity to intravenously self-administer WIN 55,212 over repeated sessions under a one-response fixed-ration (FR1) schedule of drug injection with a 10-second time-out after each injection. Rats acquired stable self-administration behavior in about 16 sessions with peak rates of responding of about 25 injections in a 3-hour session at a dose of 12.5 μg/kg per injection of WIN 55,212-2 (Figure 4a). However, when saline was substituted for WIN 55,212-2, responding did not immediately decrease, but instead increased dramatically to about 70 injections per session and remained high for six consecutive sessions, before decreasing to very low rates over the next five sessions. Also, chronic diet restriction (rats were maintained at 80% of their normal body weight) was a necessary condition in this study by Fattore and colleagues (2001), since rats on an unrestricted diet did not acquire cannabinoid self-administration behavior. The synthetic cannabinoid CB1 receptor agonist CP 55,940 has not yet been reported to be self-administered intravenously by experimental animals (e.g., Mansbach et al., 1994), but it has been reported to maintain self-administration behavior of rats when injected intracerebroventricularly (Braida et al., 2001b). Finally, self-administration behavior is not maintained by cannabinoid receptor antagonists in experimental animals (Beardsley et al., 2002) (Table 1).
Reliable and persistent intravenous self-administration of THC was first demonstrated in our laboratory using a primate species (the squirrel monkey), THC doses, a THC vehicle and a rapid injection speed not previously employed (Tanda et al., 2000; Justinova et al., 2003; Justinova et al., 2004). Monkeys utilized in our initial study (Tanda et al., 2000) were not diet restricted, but had a history of intravenous cocaine self-administration (Figure 3). In contrast to some earlier studies by others, THC was not substituted directly for cocaine in an attempt to facilitate acquisition of THC self-administration behavior. Instead, all monkeys had access to THC only after at least one week of saline extinction (wash-out from cocaine exposure and extinction of drug-seeking behavior) and one week of vehicle extinction was always kept between self-administration of different THC doses. THC was dissolved in a Tween-80 vehicle resulting in a clear solution that was rapidly delivered (0.2 ml injection delivered in 200 ms) through a chronic indwelling intravenous catheter. THC doses (1-8 μg/kg/injection) employed in this study were in a clinically relevant range, which means they were several times lower than doses generally used in previous attempts to demonstrate THC self-administration in monkeys and comparable to those delivered by an average marijuana cigarette (Agurell et al., 1986; Tanda et al., 2000). Monkeys were given the opportunity to intravenously self-administer THC under a 10-response, fixed-ratio (FR10) schedule of drug injection with a 60-second time-out after each injection (Figure 2). Under these conditions, monkeys rapidly acquired THC self-administration behavior and peak rates of responding were maintained by a 4 μg/kg injection dose of THC (mean values of 0.22 ± 0.07 response/sec, 29.92 ± 5.32 injections/session and 119.67 ± 21.27 μg/kg/session) (Figure 3). Once acquired, self-administration behavior was rapidly extinguished either by substituting vehicle injections for THC injections or by administering the cannabinoid CB1 receptor antagonist, rimonabant (SR 141716) before the session, demonstrating that the THC self-administration behavior was mediated by actions at cannabinoid CB1 receptors.
Since monkeys in our initial study (Tanda et al., 2000) had a history of cocaine self-administration, this raised the possibility that persistent neurobiological adaptations from prior cocaine exposure might subsequently predispose animals to self-administer THC (as suggested by Maldonado, 2002). This was unlikely, since earlier attempts to obtain THC self-administration behavior in monkeys with a previous cocaine self-administration experience had been unsuccessful, even when THC was directly substituted for cocaine with no intervening vehicle extinction (Harris et al., 1974). However, we resolved this issue in a recent study with squirrel monkeys that were experimentally and drug naive at the start of the experiments (Justinova et al., 2003). As in the previous study with squirrel monkeys (Tanda et al., 2000), low clinically relevant doses of THC (1-16 μg/kg/injection) were employed and THC was dissolved in a Tween-80 vehicle to produce a clear solution (Figure 3). Under the same FR10 schedule of intravenous drug injection and identical experimental conditions, THC self-administration behavior was rapidly initiated, subsequently maintained with very high rates of responding and easily extinguished, even though the monkeys had no history of exposure to other drugs. The peak rates of responding maintained by 4 μg/kg i.v. dose of THC in this study were similar to or greater than the peak rates of responding maintained by intravenous injections of cocaine, d-amphetamine, nicotine, methohexital or midazolam in previous studies using the same primate species and the same schedule of intravenous drug injection (Goldberg, 1973; Spear et al., 1991; Sannerud et al., 1994; Munzar et al., 2001). Interestingly, overall response rates, injections per session and total THC intake per session in this study with drug-naïve monkeys (Justinova et al., 2003) were greater than in our previous study (Tanda et al., 2000) in which monkeys had a previous history of cocaine self-administration. At a dose of 4 μg/kg per injection of THC, mean rate of responding was 0.88 ± 0.21 response/sec, with a mean of 48.89 ± 2.61 injections/session and a total session intake of THC of 195.6 ± 10.45 μg/kg, values almost three-fold higher than in our previous study with cocaine-experienced monkeys (Figure 3). There were no obvious differences in age or source of monkeys or laboratory conditions in these two studies. It appears, then, that rather than predisposing animals to self-administer THC, a history of cocaine self-administration might limit the intensity of subsequent THC self-administration behavior, although we cannot exclude the possibility of random interindividual variability.
The possibility of a limiting effect of contrasting drug history on subsequent drug self-administration has been previously reported with drugs from other pharmacological classes (e.g., Schlichting et al., 1970; Hoffmeister and Schlichting, 1972; Bergman and Johanson, 1985; Young and Herling, 1986; Hoffmeister et al., 1988). For example, the antitussive agent dextrorphan maintained self-administration behavior by monkeys above vehicle levels when substituted for ketamine but not when substituted for codeine (Young and Woods, 1981). In another study, diazepam maintained self-administration behavior by monkeys when substituted for pentobarbital but not when substituted for cocaine (Bergman and Johanson, 1985). A potential explanation for these observed effects is that ketamine and dextrorphan share some discriminative-stimulus effects (Holtzman, 1980; Herling et al., 1981), as do diazepam and pentobarbital (Colpaert et al., 1976; Shannon and Herling, 1983). However, attempts to directly substitute THC for drugs thought to have some discriminative-stimulus effects in common with THC (such as phencyclidine, phenobarbital or ethanol) have been unsuccessful in generating THC self-administration behavior (Harris et al., 1974; Mansbach et al., 1994).
The availability of an animal model of THC self-administration provides an opportunity to intervene behaviorally and pharmacologically in order to gain a better understanding of the neurobiological mechanisms underlying marijuana abuse and to test therapeutic strategies against marijuana abuse. For example, THC self-administration can be blocked by treatment with the cannabinoid CB1 receptor antagonist rimonabant (SR 141716) and these suppressant effects are not due to nonselective depressant effects on behavior, since rimonabant had no effect in monkeys responding for cocaine (Tanda et al., 2000) or food (Goldberg et al., unpublished observations) under identical conditions. Selective blockade of THC self administration behavior by rimonabant indicates that actions of THC at cannabinoid CB1 receptors are primarily responsible for its abuse-related reinforcing effects.
Recently we have extended the study of the neurobiological basis of cannabinoid dependence to endogenous opioid systems. Most of the evidence for a role of endogenous opioid systems in the modulation of the reinforcing or rewarding effects of THC or cannabinoids is indirect and comes from behavioral studies of locomotion (Ghozland et al., 2002) or electrical brain stimulation reward (Gardner et al., 1989) or from in-vivo brain microdialysis studies in rodents (Chen et al., 1990; Tanda et al., 1997). More direct evidence for a role of opioid neurotransmitter systems in the modulation of the reinforcing effects of cannabinoids comes from recent rodent drug self-administration studies in which naloxone pretreatment reduced intravenous drug self-administration behavior maintained by the synthetic cannabinoid CB1 receptor agonists WIN 55,212-2 and HU-210 (Navarro et al., 2001) and CP 55,940 (Braida et al., 2001a; b). In a recent study by Spano and colleagues (2004), priming injections of either WIN 55,212-2 or heroin, but not cocaine, given before the session were shown to reinstate extinguished WIN 55,212-2 drug-seeking behavior (Figure 4b) and these reinstatement effects were blocked by naloxone administration. In a recent study with squirrel monkeys self-administering THC under a second-order schedule, we found that extinguished THC-seeking behavior was reinstated by priming injections of either THC or morphine, but not cocaine, before the session (Goldberg et al., 2002; Justinova et al., unpublished observations). However, previous studies in other assays in primates (e.g., withdrawal precipitation in THC-dependent monkeys, antinociception) did not detect robust interactions between opioid and cannabinoid systems (Beardsley et al., 1986; Vivian et al., 1998). The findings that cocaine does not reinstate cannabinoid-seeking behavior, though increasing, like cannabinoid CB1 agonists, dopamine transmission in the mesolimbic system, cannot be fully explained at this time because of the complexity of behavioral and pharmacological history preceding the reinstatement tests, and the complexity of the, still not well characterized, role of the mesolimbic dopamine transmission in cannabinoid-seeking behavior.
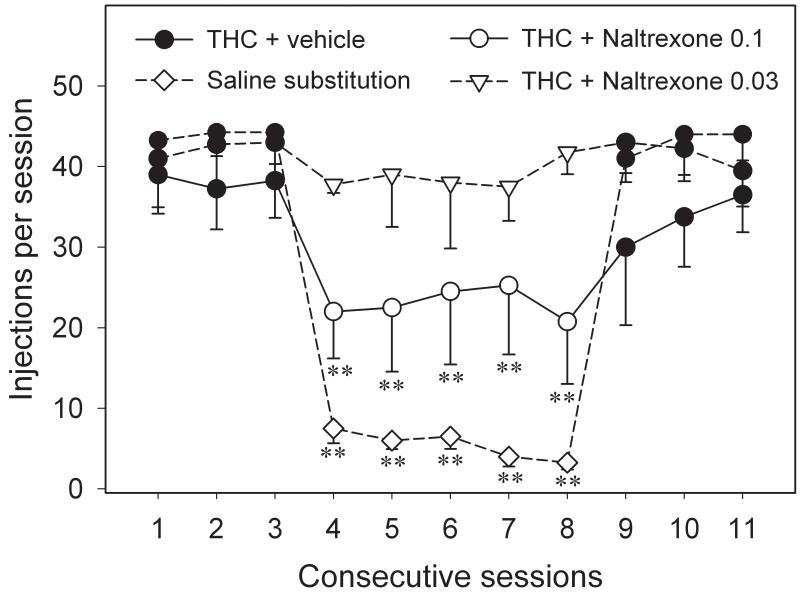
We tested the hypothesis that endogenous opioid systems may play an important role in modulating the reinforcing effects of THC by treating squirrel monkeys self-administering different doses of THC with the opioid antagonist naltrexone, a drug that shows some therapeutic value in the treatment of opiate (e.g., Mello et al., 1981; Kreek et al., 2002) and alcohol (e.g., Volpicelli et al., 1992; Sinclair, 2001) dependence. Pretreatment with naltrexone significantly reduced THC self-administration behavior by the monkeys (by about 50%; Figure 5; Justinova et al., 2004). These findings support the hypothesis that blockade of opioid receptors can modulate addictive effects of THC in non-human primates and are in agreement with a number of other preclinical studies showing that blockade of opioid receptors also modulates other behavioral and neurochemical effects of cannabinoids (Chen et al., 1990; Tanda et al., 1997; Braida et al., 2001a; b). Naltrexone has also been used in several recent studies with humans investigating opioid system involvement in the subjective responses to smoked marijuana or oral THC, but effects with naltrexone have either been limited and small (Greenwald and Stitzer, 2000), non-existent (Wachtel and de Wit, 2000) or in the opposite direction (Haney et al., 2003). Further studies are needed to reconcile the different results in human and animal studies.
Figure 5.
Effects of pretreatment with 0.03 and 0.1 mg/kg naltrexone on self-administration responding maintained by THC over consecutive sessions. Number of injections per session during THC (4 μg/kg/injection) self-administration sessions after pretreatment with vehicle (sessions 1-3 and 9-11) or naltrexone (sessions 4-8), and numbers of injections per session during self-administration sessions when saline was substituted for THC (sessions 4-8) are shown. Symbols represent the means (± S.E.M.) of injections per session from 4 monkeys. **P<0.01, post-hoc comparisons with the last THC session before naltrexone pretreatment or saline substitution (session 3) after significant one-way ANOVA for repeated measures main effect, Dunnett’s test. Modified from Justinova et al., 2004.
Summary
The preclinical findings with squirrel monkeys support the notion that cannabis derivatives have a pronounced abuse liability comparable to other drugs of abuse. Moreover, THC has the ability to support the acquisition and persistent maintenance of robust drug-taking behavior in subjects with no history of exposure to other drugs. Finally, endogenous opioid systems appear to play an important facilitative role in modulating the reinforcing effects of THC in squirrel monkeys. Although parallel effects have been found with synthetic cannabinoid CB1 receptor agonists such as WIN 55,212-2 in rodents, there is no evidence yet that THC itself can support intravenous self-administration behavior by rodents. Intravenous self-administration of THC by squirrel monkeys provides a reliable animal model of human marijuana abuse, suitable for comparative studies of the relative abuse liability of THC and other natural and synthetic cannabinoids and for preclinical assessment of new therapeutic strategies for the treatment or prevention of marijuana abuse in humans.
Acknowledgements
Work supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services.
References
- Agurell S, Halldin M, Lindgren JE, Ohlsson A, Widman M, Gillespie H, Hollister L. Pharmacokinetics and metabolism of delta 1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacol Rev. 1986;38:21–43. [PubMed] [Google Scholar]
- Alici T, Appel JB. Increasing the selectivity of the discriminative stimulus effects of D9-tetrahydrocannabinol: complete substitution with methanandamide. Pharmacol Biochem Behav. 2004 doi: 10.1016/j.pbb.2004.08.020. In press: manuscript PBB-69517. [DOI] [PubMed] [Google Scholar]
- Arnold JC, Hunt GE, McGregor IS. Effects of the cannabinoid receptor agonist CP 55,940 and the cannabinoid receptor antagonist SR 141716 on intracranial self-stimulation in Lewis rats. Life Sci. 2001;70:97–108. doi: 10.1016/s0024-3205(01)01366-2. [DOI] [PubMed] [Google Scholar]
- Arnold JM, Roberts DC. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Ashton H, Golding J, Marsh VR, Millman JE, Thompson JW. The seed and the soil: effect of dosage, personality and starting state on the response to delta 9 tetrahydrocannabinol in man. Br J Clin Pharmacol. 1981;12:705–720. doi: 10.1111/j.1365-2125.1981.tb01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azorlosa JL, Heishman SJ, Stitzer ML, Mahaffey JM. Marijuana smoking: effect of varying delta 9-tetrahydrocannabinol content and number of puffs. J Pharmacol Exp Ther. 1992;261:114–122. [PubMed] [Google Scholar]
- Balster RL, Prescott WR. Delta 9-tetrahydrocannabinol discrimination in rats as a model for cannabis intoxication. Neurosci Biobehav Rev. 1992;16:55–62. doi: 10.1016/s0149-7634(05)80051-x. [DOI] [PubMed] [Google Scholar]
- Barrett RL, Wiley JL, Balster RL, Martin BR. Pharmacological specificity of delta 9-tetrahydrocannabinol discrimination in rats. Psychopharmacology (Berl) 1995;118:419–424. doi: 10.1007/BF02245942. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Balster RL, Harris LS. Dependence on tetrahydrocannabinol in rhesus monkeys. J Pharmacol Exp Ther. 1986;239:311–319. [PubMed] [Google Scholar]
- Beardsley PM, Dance ME, Balster RL, Munzar P. Evaluation of the reinforcing effects of the cannabinoid CB1 receptor antagonist, SR41716, in rhesus monkeys. Eur J Pharmacol. 2002;435:209–216. doi: 10.1016/s0014-2999(01)01597-7. [DOI] [PubMed] [Google Scholar]
- Bergman J, Johanson CE. The reinforcing properties of diazepam under several conditions in the rhesus monkey. Psychopharmacology (Berl) 1985;86:108–113. doi: 10.1007/BF00431693. [DOI] [PubMed] [Google Scholar]
- Block RI, Erwin WJ, Farinpour R, Braverman K. Sedative, stimulant, and other subjective effects of marijuana: relationships to smoking techniques. Pharmacol Biochem Behav. 1998;59:405–412. doi: 10.1016/s0091-3057(97)00453-x. [DOI] [PubMed] [Google Scholar]
- Brady JV. Animal models for assessing drugs of abuse. Neurosci Biobehav Rev. 1991;15:35–43. doi: 10.1016/s0149-7634(05)80089-2. [DOI] [PubMed] [Google Scholar]
- Braida D, Pozzi M, Cavallini R, Sala M. Conditioned place preference induced by the cannabinoid agonist CP 55,940: interaction with the opioid system. Neuroscience. 2001a;104:923–926. doi: 10.1016/s0306-4522(01)00210-x. [DOI] [PubMed] [Google Scholar]
- Braida D, Pozzi M, Parolaro D, Sala M. Intracerebral self-administration of the cannabinoid receptor agonist CP 55,940 in the rat: interaction with the opioid system. Eur J Pharmacol. 2001b;413:227–234. doi: 10.1016/s0014-2999(01)00766-x. [DOI] [PubMed] [Google Scholar]
- Burkey RT, Nation JR. (R)-methanandamide, but not anandamide, substitutes for delta 9-THC in a drug-discrimination procedure. Exp Clin Psychopharmacol. 1997;5:195–202. doi: 10.1037//1064-1297.5.3.195. [DOI] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Carr KD. Food restriction enhances the central rewarding effect of abused drugs. J Neurosci. 1998;18:7502–7510. doi: 10.1523/JNEUROSCI.18-18-07502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun SR, Galloway GP, Smith DE. Abuse potential of dronabinol (Marinol) J Psychoactive Drugs. 1998;30:187–196. doi: 10.1080/02791072.1998.10399689. [DOI] [PubMed] [Google Scholar]
- Cappell H, Kuchar E, Webster CD. Some correlates of marihuana self-administration in man: a study of titration of intake as a function of drug potency. Psychopharmacologia. 1973;29:177–184. doi: 10.1007/BF00414031. [DOI] [PubMed] [Google Scholar]
- Carney JM, Uwaydah IM, Balster RL. Evaluation of a suspension system for intravenous self-administration studies of water-insoluble compounds in the rhesus monkey. Pharmacol Biochem Behav. 1977;7:357–364. doi: 10.1016/0091-3057(77)90232-5. [DOI] [PubMed] [Google Scholar]
- Carroll ME, France CP, Meisch RA. Food deprivation increases oral and intravenous drug intake in rats. Science. 1979;205:319–321. doi: 10.1126/science.36665. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST, Nygaard SL. A concurrently available nondrug reinforcer prevents the acquisition or decreases the maintenance of cocaine-reinforced behavior. Psychopharmacology (Berl) 1989;97:23–29. doi: 10.1007/BF00443407. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Meisch RA. Increased drug-reinforced behavior due to food deprivation. In: Thompson T, Dews PB, Barrett JE, editors. Advances in Behavioral Pharmacology. Vol. 4. Academic Press; New York: 1984. pp. 47–88. [Google Scholar]
- Chait LD. Delta-9-tetrahydrocannabinol content and human marijuana self-administration. Psychopharmacology (Berl) 1989;98:51–55. doi: 10.1007/BF00442005. [DOI] [PubMed] [Google Scholar]
- Chait LD, Burke KA. Preference for high-versus low-potency marijuana. Pharmacol Biochem Behav. 1994;49:643–647. doi: 10.1016/0091-3057(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Chait LD, Evans SM, Grant KA, Kamien JB, Johanson CE, Schuster CR. Discriminative stimulus and subjective effects of smoked marijuana in humans. Psychopharmacology (Berl) 1988;94:206–212. doi: 10.1007/BF00176846. [DOI] [PubMed] [Google Scholar]
- Chait LD, Zacny JP. Reinforcing and subjective effects of oral delta 9-THC and smoked marijuana in humans. Psychopharmacology (Berl) 1992;107:255–262. doi: 10.1007/BF02245145. [DOI] [PubMed] [Google Scholar]
- Chaperon F, Soubrie P, Puech AJ, Thiebot MH. Involvement of central cannabinoid (CB1) receptors in the establishment of place conditioning in rats. Psychopharmacology (Berl) 1998;135:324–332. doi: 10.1007/s002130050518. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Kendall DA, Marsden CA. Cannabinoid receptors and reward in the rat: a conditioned place preference study. Psychopharmacology (Berl) 2000;151:25–30. doi: 10.1007/s002130000481. [DOI] [PubMed] [Google Scholar]
- Chen JP, Paredes W, Li J, Smith D, Lowinson J, Gardner EL. Delta 9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology (Berl) 1990;102:156–162. doi: 10.1007/BF02245916. [DOI] [PubMed] [Google Scholar]
- Chesher GB, Bird KD, Jackson DM, Perrignon A, Starmer GA. The effects of orally administered delta 9-tetrahydrocannabinol in man on mood and performance measures: a dose-response study. Pharmacol Biochem Behav. 1990;35:861–864. doi: 10.1016/0091-3057(90)90371-n. [DOI] [PubMed] [Google Scholar]
- Collins RJ, Weeks JR, Cooper MM, Good PI, Russell RR. Prediction of abuse liability of drugs using IV self-administration by rats. Psychopharmacology (Berl) 1984;82:6–13. doi: 10.1007/BF00426372. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Desmedt LK, Janssen PA. Discriminative stimulus properties of benzodiazepines, barbiturates and pharmacologically related drugs; relation to some intrinsic and anticonvulsant effects. Eur J Pharmacol. 1976;37:113–123. doi: 10.1016/0014-2999(76)90014-5. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Wilson ST, Donovan MR, Foltin RW, Fischman MW. Effects of an alternative reinforcer on intravenous heroin self-administration by humans. Eur J Pharmacol. 1998;345:13–26. doi: 10.1016/s0014-2999(97)01572-0. [DOI] [PubMed] [Google Scholar]
- Compton WM, Grant BF, Colliver JD, Glantz MD, Stinson FS. Prevalence of marijuana use disorders in the United States: 1991-1992 and 2001-2002. JAMA. 2004;291:2114–2121. doi: 10.1001/jama.291.17.2114. [DOI] [PubMed] [Google Scholar]
- Curran HV, Brignell C, Fletcher S, Middleton P, Henry J. Cognitive and subjective dose-response effects of acute oral Delta 9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology (Berl) 2002;164:61–70. doi: 10.1007/s00213-002-1169-0. [DOI] [PubMed] [Google Scholar]
- de la Garza R, Johanson CE. The effects of food deprivation on the self-administration of psychoactive drugs. Drug Alcohol Depend. 1987;19:17–27. doi: 10.1016/0376-8716(87)90083-4. [DOI] [PubMed] [Google Scholar]
- Deneau GA, Seevers MH. Pharmacological aspects of drug dependence. Adv Pharmacol. 1964;55:267–283. doi: 10.1016/s1054-3589(08)61114-x. [DOI] [PubMed] [Google Scholar]
- Diana M, Melis M, Muntoni AL, Gessa GL. Mesolimbic dopaminergic decline after cannabinoid withdrawal. Proc Natl Acad Sci U S A. 1998;95:10269–10273. doi: 10.1073/pnas.95.17.10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvauchelle CL, Ikegami A, Asami S, Robens J, Kressin K, Castaneda E. Effects of cocaine context on NAcc dopamine and behavioral activity after repeated intravenous cocaine administration. Brain Res. 2000;862:49–58. doi: 10.1016/s0006-8993(00)02091-6. [DOI] [PubMed] [Google Scholar]
- ElSohly MA. Chemical constituents of cannabis. In: Grotenhermen F, Russo EB, editors. Cannabis and cannabinoids : pharmacology, toxicology, and therapeutic potential. Haworth Integrative Healing Press; New York: 2002. pp. 27–36. [Google Scholar]
- Fattore L, Cossu G, Martellotta CM, Fratta W. Intravenous self-administration of the cannabinoid CB1 receptor agonist WIN 55,212-2 in rats. Psychopharmacology (Berl) 2001;156:410–416. doi: 10.1007/s002130100734. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Schuster CR. Cocaine self-administration in humans. Fed Proc. 1982;41:241–246. [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Assessment of abuse liability of stimulant drugs in humans: a methodological survey. Drug Alcohol Depend. 1991;28:3–48. doi: 10.1016/0376-8716(91)90052-z. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Pippen PA, Kelly TH. Behavioral effects of cocaine alone and in combination with ethanol or marijuana in humans. Drug Alcohol Depend. 1993;32:93–106. doi: 10.1016/0376-8716(93)80001-u. [DOI] [PubMed] [Google Scholar]
- French ED, Dillon K, Wu X. Cannabinoids excite dopamine neurons in the ventral tegmentum and substantia nigra. Neuroreport. 1997;8:649–652. doi: 10.1097/00001756-199702100-00014. [DOI] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. Journal of American Chemical Society. 1964;86:1646–1647. [Google Scholar]
- Gardner EL, Paredes W, Smith D, Donner A, Milling C, Cohen D, Morrison D. Facilitation of brain stimulation reward by delta 9-tetrahydrocannabinol. Psychopharmacology (Berl) 1988;96:142–144. doi: 10.1007/BF02431546. [DOI] [PubMed] [Google Scholar]
- Gardner EL, Paredes W, Smith D, Zukin RS. Facilitation of brain stimulation reward by delta-9-tetrahydrocannabinol is mediated by an endogenous opioid mechanism. In: Cros J, Meunier J-C, Hamon M, editors. Progress In Opioid Research. Vol. 75. Pergamon Press; New York: 1989. pp. 671–674. [Google Scholar]
- Gardner EL, Vorel SR. Cannabinoid transmission and reward-related events. Neurobiol Dis. 1998;5:502–533. doi: 10.1006/nbdi.1998.0219. [DOI] [PubMed] [Google Scholar]
- Ghozland S, Matthes HW, Simonin F, Filliol D, Kieffer BL, Maldonado R. Motivational effects of cannabinoids are mediated by mu-opioid and kappa-opioid receptors. J Neurosci. 2002;22:1146–1154. doi: 10.1523/JNEUROSCI.22-03-01146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford AN, Gardner EL, Ashby CR., Jr. The effect of intravenous administration of delta-9-tetrahydrocannabinol on the activity of A10 dopamine neurons recorded in vivo in anesthetized rats. Neuropsychobiology. 1997;36:96–99. doi: 10.1159/000119369. [DOI] [PubMed] [Google Scholar]
- Goldberg SR. Comparable behavior maintained under fixed-ratio and second-order schedules of food presentation, cocaine injection or d-amphetamine injection in the squirrel monkey. J Pharmacol Exp Ther. 1973;186:18–30. [PubMed] [Google Scholar]
- Goldberg SR, Henningfield JE. Reinforcing effects of nicotine in humans and experimental animals responding under intermittent schedules of i.v. drug injection. Pharmacol Biochem Behav. 1988;30:227–234. doi: 10.1016/0091-3057(88)90450-9. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Hoffmeister F, Schlichting UU, Wuttke W. A comparison of pentobarbital and cocaine self-administration in rhesus monkeys: effects of dose and fixed-ratio parameter. J Pharmacol Exp Ther. 1971;179:277–283. [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD, Goldberg DM. Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science. 1981;214:573–575. doi: 10.1126/science.7291998. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Munzar P, Justinova Z, Tanda G. Drug-seeking behavior under a second-order schedule of THC self-administration in monkeys. Behav Pharmacol. 2002;13:509–510. [Google Scholar]
- Greenwald MK, Stitzer ML. Antinociceptive, subjective and behavioral effects of smoked marijuana in humans. Drug Alcohol Depend. 2000;59:261–275. doi: 10.1016/s0376-8716(99)00128-3. [DOI] [PubMed] [Google Scholar]
- Griffiths RR. Common factors in human and infrahuman drug self-administration. Psychopharmacol Bull. 1980;16:45–47. [PubMed] [Google Scholar]
- Griffiths RR, Bradford LD, Brady JV. Progressive ratio and fixed ratio schedules of cocaine-maintained responding in baboons. Psychopharmacology (Berl) 1979;65:125–136. doi: 10.1007/BF00433038. [DOI] [PubMed] [Google Scholar]
- Haney M, Bisaga A, Foltin RW. Interaction between naltrexone and oral THC in heavy marijuana smokers. Psychopharmacology (Berl) 2003;166:77–85. doi: 10.1007/s00213-002-1279-8. [DOI] [PubMed] [Google Scholar]
- Haney M, Comer SD, Ward AS, Foltin RW, Fischman MW. Factors influencing marijuana self-administration by humans. Behav Pharmacol. 1997;8:101–112. [PubMed] [Google Scholar]
- Harder S, Rietbrock S. Concentration-effect relationship of delta-9-tetrahydrocannabiol and prediction of psychotropic effects after smoking marijuana. Int J Clin Pharmacol Ther. 1997;35:155–159. [PubMed] [Google Scholar]
- Harris RT, Waters W, McLendon D. Evaluation of reinforcing capability of delta-9-tetrahydrocannabinol in rhesus monkeys. Psychopharmacologia. 1974;37:23–29. doi: 10.1007/BF00426679. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Comer SD, Foltin RW. Oral delta-9-THC produces modest reinforcing effects in marijuana smokers. Psychopharmacology. doi: 10.1007/s00213-005-2234-2. In press. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Foltin RW, Fischman MW. Alternative reinforcers differentially modify cocaine self-administration by humans. Behav Pharmacol. 2000;11:87–91. doi: 10.1097/00008877-200002000-00010. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Ward AS, Fischman MW, Foltin RW. Effects of oral THC maintenance on smoked marijuana self-administration. Drug Alcohol Depend. 2002;67:301–309. doi: 10.1016/s0376-8716(02)00084-4. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Huestis MA, Henningfield JE, Cone EJ. Acute and residual effects of marijuana: profiles of plasma THC levels, physiological, subjective, and performance measures. Pharmacol Biochem Behav. 1990;37:561–565. doi: 10.1016/0091-3057(90)90028-g. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Stitzer ML, Yingling JE. Effects of tetrahydrocannabinol content on marijuana smoking behavior, subjective reports, and performance. Pharmacol Biochem Behav. 1989;34:173–179. doi: 10.1016/0091-3057(89)90369-9. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Cohen C, Heishman SJ. Drug self-administration methods in abuse liability evaluation. Br J Addict. 1991;86:1571–1577. doi: 10.1111/j.1360-0443.1991.tb01750.x. [DOI] [PubMed] [Google Scholar]
- Herling S, Coale EH, Jr., Hein DW, Winger G, Woods JH. Similarity of the discriminative stimulus effects of ketamine, cyclazocine, and dextrorphan in the pigeon. Psychopharmacology (Berl) 1981;73:286–291. doi: 10.1007/BF00422419. [DOI] [PubMed] [Google Scholar]
- Herning RI, Hooker WD, Jones RT. Tetrahydrocannabinol content and differences in marijuana smoking behavior. Psychopharmacology (Berl) 1986;90:160–162. doi: 10.1007/BF00181232. [DOI] [PubMed] [Google Scholar]
- Hochman JS, Brill NQ. Marijuana intoxication: pharmacological and psychological factors. Dis Nerv Syst. 1971;32:676–679. [PubMed] [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Hoffmeister F, Schlichting UU. Reinforcing properties of some opiates and opioids in rhesus monkeys with histories of cocaine and codeine self-administration. Psychopharmacologia. 1972;23:55–74. doi: 10.1007/BF00414414. [DOI] [PubMed] [Google Scholar]
- Hoffmeister F. A comparison of the stimulus effects of codeine in rhesus monkeys under the contingencies of a two lever discrimination task and a cross self-administration paradigm: tests of generalization to pentazocine, buprenorphine, tilidine, and different doses of codeine. Psychopharmacology (Berl) 1988;94:315–320. doi: 10.1007/BF00174682. [DOI] [PubMed] [Google Scholar]
- Holtzman SG. Phencyclidine-like discriminative effects of opioids in the rat. J Pharmacol Exp Ther. 1980;214:614–619. [PubMed] [Google Scholar]
- Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. II. Models for the prediction of time of marijuana exposure from plasma concentrations of delta 9-tetrahydrocannabinol (THC) and 11-nor-9-carboxy-delta 9-tetrahydrocannabinol (THCCOOH) J Anal Toxicol. 1992a;16:283–290. doi: 10.1093/jat/16.5.283. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Sampson AH, Holicky BJ, Henningfield JE, Cone EJ. Characterization of the absorption phase of marijuana smoking. Clin Pharmacol Ther. 1992b;52:31–41. doi: 10.1038/clpt.1992.100. [DOI] [PubMed] [Google Scholar]
- Hutcheson DM, Tzavara ET, Smadja C, Valjent E, Roques BP, Hanoune J, Maldonado R. Behavioural and biochemical evidence for signs of abstinence in mice chronically treated with delta-9-tetrahydrocannabinol. Br J Pharmacol. 1998;125:1567–1577. doi: 10.1038/sj.bjp.0702228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarbe TU, Lamb RJ, Lin S, Makriyannis A. (R)-methanandamide and Delta 9-THC as discriminative stimuli in rats: tests with the cannabinoid antagonist SR-141716 and the endogenous ligand anandamide. Psychopharmacology (Berl) 2001;156:369–380. doi: 10.1007/s002130100730. [DOI] [PubMed] [Google Scholar]
- Johanson CE. The evaluation of the abuse liability of drugs. Drug Saf. 1990;5(Suppl 1):46–57. doi: 10.2165/00002018-199000051-00008. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Balster RL. A summary of the results of a drug self-administration study using substitution procedures in rhesus monkeys. Bull Narc. 1978;30:43–54. [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG. Monitoring the Future National Survey Results on Drug Use, 1975-2002. National Institute on Drug Abuse; Bethesda, MD: 2003. [Google Scholar]
- Jones RT. Marihuana-induced “high”: influence of expectation, setting and previous drug experience. Pharmacol Rev. 1971;23:359–369. [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Munzar P, Goldberg SR. The opioid antagonist naltrexone reduces the reinforcing effects of Delta 9 tetrahydrocannabinol (THC) in squirrel monkeys. Psychopharmacology (Berl) 2004;173:186–194. doi: 10.1007/s00213-003-1693-6. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Redhi GH, Goldberg SR. Self-administration of Delta(9)-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology (Berl) 2003;169:135–140. doi: 10.1007/s00213-003-1484-0. [DOI] [PubMed] [Google Scholar]
- Kaymakcalan S. Tolerance to and dependence on cannabis. Bull Narc. 1973;25:39–47. [Google Scholar]
- Kelly TH, Foltin RW, Emurian CS, Fischman MW. Effects of delta 9-THC on marijuana smoking, dose choice, and verbal report of drug liking. J Exp Anal Behav. 1994a;61:203–211. doi: 10.1901/jeab.1994.61-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TH, Foltin RW, Emurian CS, Fischman MW. Are choice and self-administration of marijuana related to delta 9-THC content? Exp Clin Psychopharmacol. 1997;5:74–82. doi: 10.1037//1064-1297.5.1.74. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Foltin RW, Fischman MW. Effects of smoked marijuana on heart rate, drug ratings and task performance by humans. Behav Pharmacol. 1993;4:167–178. [PubMed] [Google Scholar]
- Kelly TH, Foltin RW, Mayr MT, Fischman MW. Effects of delta 9-tetrahydrocannabinol and social context on marijuana self-administration by humans. Pharmacol Biochem Behav. 1994b;49:763–768. doi: 10.1016/0091-3057(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Kirk JM, Doty P, de Wit H. Effects of expectancies on subjective responses to oral delta9-tetrahydrocannabinol. Pharmacol Biochem Behav. 1998;59:287–293. doi: 10.1016/s0091-3057(97)00414-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Weiss F. Pharmacology of drug self-administration. Alcohol. 1990;7:193–197. doi: 10.1016/0741-8329(90)90004-v. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, LaForge KS, Butelman E. Pharmacotherapy of addictions. Nat Rev Drug Discov. 2002;1:710–726. doi: 10.1038/nrd897. [DOI] [PubMed] [Google Scholar]
- Lepore M, Liu X, Savage V, Matalon D, Gardner EL. Genetic differences in delta 9-tetrahydrocannabinol-induced facilitation of brain stimulation reward as measured by a rate-frequency curve-shift electrical brain stimulation paradigm in three different rat strains. Life Sci. 1996;58:L365–L372. doi: 10.1016/0024-3205(96)00237-8. [DOI] [PubMed] [Google Scholar]
- Lepore M, Vorel SR, Lowinson J, Gardner EL. Conditioned place preference induced by delta 9-tetrahydrocannabinol: comparison with cocaine, morphine, and food reward. Life Sci. 1995;56:2073–2080. doi: 10.1016/0024-3205(95)00191-8. [DOI] [PubMed] [Google Scholar]
- Lindgren JE, Ohlsson A, Agurell S, Hollister L, Gillespie H. Clinical effects and plasma levels of delta 9-tetrahydrocannabinol (delta 9-THC) in heavy and light users of cannabis. Psychopharmacology (Berl) 1981;74:208–212. doi: 10.1007/BF00427095. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Shaham Y, Hope BT. Molecular neuroadaptations in the accumbens and ventral tegmental area during the first 90 days of forced abstinence from cocaine self-administration in rats. J Neurochem. 2003;85:1604–1613. doi: 10.1046/j.1471-4159.2003.01824.x. [DOI] [PubMed] [Google Scholar]
- Lupica CR, Riegel AC, Hoffman AF. Marijuana and cannabinoid regulation of brain reward circuits. Br J Pharmacol. 2004;143:227–234. doi: 10.1038/sj.bjp.0705931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R. Study of cannabinoid dependence in animals. Pharmacol Ther. 2002;95:153–164. doi: 10.1016/s0163-7258(02)00254-1. [DOI] [PubMed] [Google Scholar]
- Mallet PE, Beninger RJ. Delta9-tetrahydrocannabinol, but not the endogenous cannabinoid receptor ligand anandamide, produces conditioned place avoidance. Life Sci. 1998;62:2431–2439. doi: 10.1016/s0024-3205(98)00226-4. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Nicholson KL, Martin BR, Balster RL. Failure of Delta(9)-tetrahydrocannabinol and CP 55,940 to maintain intravenous self-administration under a fixed-interval schedule in rhesus monkeys. Behav Pharmacol. 1994;5:219–225. doi: 10.1097/00008877-199404000-00014. [DOI] [PubMed] [Google Scholar]
- Marquis KL, Webb MG, Moreton JE. Effects of fixed ratio size and dose on phencyclidine self-administration by rats. Psychopharmacology (Berl) 1989;97:179–182. doi: 10.1007/BF00442246. [DOI] [PubMed] [Google Scholar]
- Martellotta MC, Cossu G, Fattore L, Gessa GL, Fratta W. Self-administration of the cannabinoid receptor agonist WIN 55,212-2 in drug-naive mice. Neuroscience. 1998;85:327–330. doi: 10.1016/s0306-4522(98)00052-9. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Issakidis CN, Prior G. Aversive effects of the synthetic cannabinoid CP 55,940 in rats. Pharmacol Biochem Behav. 1996;53:657–664. doi: 10.1016/0091-3057(95)02066-7. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Shvo Y, Hashish I. The structure of cannabidiol. Tetrahedron. 1963:2073–2078. doi: 10.1016/0040-4020(63)85022-x. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Kuehnle JC, Sellers MS. Operant analysis of human heroin self-administration and the effects of naltrexone. J Pharmacol Exp Ther. 1981;216:45–54. [PubMed] [Google Scholar]
- Mendelson JH, Mello NK. Reinforcing properties of oral delta 9-tetrahydrocannabinol, smoked marijuana, and nabilone: influence of previous marijuana use. Psychopharmacology (Berl) 1984;83:351–356. doi: 10.1007/BF00428544. [DOI] [PubMed] [Google Scholar]
- Meyer RE, Pillard RC, Shapiro LM, Mirin SM. Administration of marijuana to heavy and casual marijuana users. Am J Psychiatry. 1971;128:198–204. doi: 10.1176/ajp.128.2.198. [DOI] [PubMed] [Google Scholar]
- Mokler DJ, Nelson BD, Harris LS, Rosecrans JA. The role of benzodiazepine receptors in the discriminative stimulus properties of delta-9-tetrahydrocannabinol. Life Sci. 1986;38:1581–1589. doi: 10.1016/0024-3205(86)90497-2. [DOI] [PubMed] [Google Scholar]
- Morgan D, Brebner K, Lynch WJ, Roberts DC. Increases in the reinforcing efficacy of cocaine after particular histories of reinforcement. Behav Pharmacol. 2002;13:389–396. doi: 10.1097/00008877-200209000-00012. [DOI] [PubMed] [Google Scholar]
- Munzar P, Yasar S, Redhi GH, Justinova Z, Goldberg SR. High rates of midazolam self-administration in squirrel monkeys. Behav Pharmacol. 2001;12:257–265. doi: 10.1097/00008877-200107000-00004. [DOI] [PubMed] [Google Scholar]
- Nader MA, Mach RH. Self-administration of the dopamine D3 agonist 7-OH-DPAT in rhesus monkeys is modified by prior cocaine exposure. Psychopharmacology (Berl) 1996;125:13–22. doi: 10.1007/BF02247388. [DOI] [PubMed] [Google Scholar]
- Navarro M, Carrera MR, Fratta W, Valverde O, Cossu G, Fattore L, Chowen JA, Gomez R, del A, Villanua MA, Maldonado R, Koob GF, Rodriguez de Fonseca F. Functional interaction between opioid and cannabinoid receptors in drug self-administration. J Neurosci. 2001;21:5344–5350. doi: 10.1523/JNEUROSCI.21-14-05344.2001. I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth-Coslett R, Henningfield JE, O’Keeffe MK, Griffiths RR. Effects of marijuana smoking on subjective ratings and tobacco smoking. Pharmacol Biochem Behav. 1986;25:659–665. doi: 10.1016/0091-3057(86)90156-5. [DOI] [PubMed] [Google Scholar]
- Ohlsson A, Lindgren JE, Wahlen A, Agurell S, Hollister LE, Gillespie HK. Plasma delta-9 tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clin Pharmacol Ther. 1980;28:409–416. doi: 10.1038/clpt.1980.181. [DOI] [PubMed] [Google Scholar]
- Parker LA, Gillies T. THC-induced place and taste aversions in Lewis and Sprague-Dawley rats. Behav Neurosci. 1995;109:71–78. doi: 10.1037//0735-7044.109.1.71. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes M, Di Guiseppi S, Davis KH, Schindler VH, Cook CE. Comparison of effects of marihuana cigarettes to three different potencies. Clin Pharmacol Ther. 1982;31:617–624. doi: 10.1038/clpt.1982.86. [DOI] [PubMed] [Google Scholar]
- Petry NM, Bickel WK. Buprenorphine dose self-selection: effects of an alternative reinforcer, behavioral economic analysis of demand, and examination of subjective drug effects. Exp Clin Psychopharmacol. 1999;7:38–48. doi: 10.1037//1064-1297.7.1.38. [DOI] [PubMed] [Google Scholar]
- Pickens R, Thompson T, Muchow DC. Cannabis and phencyclidine self-administered by animals. In: Hoffmeister F, Goldberg L, editors. Psychic Dependence: Definition, Assessment In Animals And Man. Springer-Verlag; New York: 1973. pp. 78–86. [Google Scholar]
- Preston KL, Jasinski DR. Abuse liability studies of opioid agonist-antagonists in humans. Drug Alcohol Depend. 1991;28:49–82. doi: 10.1016/0376-8716(91)90053-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Carrera MR, Navarro M, Koob GF, Weiss F. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science. 1997;276:2050–2054. doi: 10.1126/science.276.5321.2050. [DOI] [PubMed] [Google Scholar]
- Russo EB, McPartland JM. Cannabis is more than simply delta(9)-tetrahydrocannabinol. Psychopharmacology (Berl) 2003;165:431–432. doi: 10.1007/s00213-002-1348-z. [DOI] [PubMed] [Google Scholar]
- Sannerud CA, Prada J, Goldberg DM, Goldberg SR. The effects of sertraline on nicotine self-administration and food-maintained responding in squirrel monkeys. Eur J Pharmacol. 1994;271:461–469. doi: 10.1016/0014-2999(94)90807-9. [DOI] [PubMed] [Google Scholar]
- Sanudo-Pena MC, Tsou K, Delay ER, Hohman AG, Force M, Walker JM. Endogenous cannabinoids as an aversive or counter-rewarding system in the rat. Neurosci Lett. 1997;223:125–128. doi: 10.1016/s0304-3940(97)13424-3. [DOI] [PubMed] [Google Scholar]
- Schlichting UU, Goldberg SR, Wuttke W, Hoffmeister F. D-Amphetamine self-administration by Rhesus-monkeys with different self-administration histories. Excerpta Medica International Congress Series No 220; proceedings of the Twelfth Meeting of the European Society for the Study of Drug Toxicity; Upsala. June 1970.1970. pp. 62–69. [Google Scholar]
- Schuster CR, Thompson T. Self administration of and behavioral dependence on drugs. Annu Rev Pharmacol. 1969;9:483–502. doi: 10.1146/annurev.pa.09.040169.002411. [DOI] [PubMed] [Google Scholar]
- Shannon HE, Herling S. Discriminative stimulus effects of diazepam in rats: evidence for a maximal effect. J Pharmacol Exp Ther. 1983;227:160–166. [PubMed] [Google Scholar]
- Sinclair JD. Evidence about the use of naltrexone and for different ways of using it in the treatment of alcoholism. Alcohol Alcohol. 2001;36:2–10. doi: 10.1093/alcalc/36.1.2. [DOI] [PubMed] [Google Scholar]
- Spano MS, Fattore L, Cossu G, Deiana S, Fadda P, Fratta W. CB1 receptor agonist and heroin, but not cocaine, reinstate cannabinoid-seeking behaviour in the rat. Br J Pharmacol. 2004;143:343–350. doi: 10.1038/sj.bjp.0705932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear DJ, Muntaner C, Goldberg SR, Katz JL. Methohexital and cocaine self-administration under fixed-ratio and second-order schedules. Pharmacol Biochem Behav. 1991;38:411–416. doi: 10.1016/0091-3057(91)90300-q. [DOI] [PubMed] [Google Scholar]
- Stefanski R, Ladenheim B, Lee SH, Cadet JL, Goldberg SR. Neuroadaptations in the dopaminergic system after active self-administration but not after passive administration of methamphetamine. Eur J Pharmacol. 1999;371:123–135. doi: 10.1016/s0014-2999(99)00094-1. [DOI] [PubMed] [Google Scholar]
- Stefanski R, Justinova Z, Hayashi T, Takebayashi M, Goldberg SR, Su TP. Sigma(1) receptor upregulation after chronic methamphetamine self-administration in rats: a study with yoked controls. Psychopharmacology (Berl) 2004;175:68–75. doi: 10.1007/s00213-004-1779-9. [DOI] [PubMed] [Google Scholar]
- Takahashi RN, Singer G. Self-administration of delta 9-tetrahydrocannabinol by rats. Pharmacol Biochem Behav. 1979;11:737–740. doi: 10.1016/0091-3057(79)90274-0. [DOI] [PubMed] [Google Scholar]
- Takahashi RN, Singer G. Effects of body weight levels on cannabis self-injection. Pharmacol Biochem Behav. 1980;13:877–881. doi: 10.1016/0091-3057(80)90222-1. [DOI] [PubMed] [Google Scholar]
- Tanda G, Loddo P, Di Chiara G. Dependence of mesolimbic dopamine transmission on delta9-tetrahydrocannabinol. Eur J Pharmacol. 1999;376:23–26. doi: 10.1016/s0014-2999(99)00384-2. [DOI] [PubMed] [Google Scholar]
- Tanda G, Goldberg SR. Cannabinoids: reward, dependence, and underlying neurochemical mechanisms-a review of recent preclinical data. Psychopharmacology (Berl) 2003;169:115–134. doi: 10.1007/s00213-003-1485-z. [DOI] [PubMed] [Google Scholar]
- Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci. 2000;3:1073–1074. doi: 10.1038/80577. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Tella SR, Ladenheim B, Andrews AM, Goldberg SR, Cadet JL. Differential reinforcing effects of cocaine and GBR-12909: biochemical evidence for divergent neuroadaptive changes in the mesolimbic dopaminergic system. J Neurosci. 1996;16:7416–7427. doi: 10.1523/JNEUROSCI.16-23-07416.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AM, Swant J, Gosnell BA, Wagner JJ. Modulation of long-term potentiation in the rat hippocampus following cocaine self-administration. Neuroscience. 2004;127:177–185. doi: 10.1016/j.neuroscience.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Valjent E, Maldonado R. A behavioural model to reveal place preference to delta 9-tetrahydrocannabinol in mice. Psychopharmacology (Berl) 2000;147:436–438. doi: 10.1007/s002130050013. [DOI] [PubMed] [Google Scholar]
- van Ree JM, Slangen JL, de Wied D. Intravenous self-administration of drugs in rats. J Pharmacol Exp Ther. 1978;204:547–557. [PubMed] [Google Scholar]
- Vivian JA, Kishioka S, Butelman ER, Broadbear J, Lee KO, Woods JH. Analgesic, respiratory and heart rate effects of cannabinoid and opioid agonists in rhesus monkeys: antagonist effects of SR 141716A. J Pharmacol Exp Ther. 1998;286:697–703. [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Wachtel SR, de Wit H. Naltrexone does not block the subjective effects of oral Delta(9)-tetrahydrocannabinol in humans. Drug Alcohol Depend. 2000;59:251–260. doi: 10.1016/s0376-8716(99)00127-1. [DOI] [PubMed] [Google Scholar]
- Wachtel SR, ElSohly MA, Ross SA, Ambre J, de Wit H. Comparison of the subjective effects of Delta(9)-tetrahydrocannabinol and marijuana in humans. Psychopharmacology (Berl) 2002;161:331–339. doi: 10.1007/s00213-002-1033-2. [DOI] [PubMed] [Google Scholar]
- Ward AS, Comer SD, Haney M, Foltin RW, Fischman MW. The effects of a monetary alternative on marijuana self-administration. Behav Pharmacol. 1997;8:275–286. doi: 10.1097/00008877-199708000-00001. [DOI] [PubMed] [Google Scholar]
- Whalley BJ, Wilkinson JD, Williamson EM, Constanti A. A novel component of cannabis extract potentiates excitatory synaptic transmission in rat olfactory cortex in vitro. Neurosci Lett. 2004;365:58–63. doi: 10.1016/j.neulet.2004.04.044. [DOI] [PubMed] [Google Scholar]
- Wiley J, Balster R, Martin B. Discriminative stimulus effects of anandamide in rats. Eur J Pharmacol. 1995a;276:49–54. doi: 10.1016/0014-2999(95)00010-i. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Golden KM, Ryan WJ, Balster RL, Razdan RK, Martin BR. Evaluation of cannabimimetic discriminative stimulus effects of anandamide and methylated fluoroanandamide in rhesus monkeys. Pharmacol Biochem Behav. 1997;58:1139–1143. doi: 10.1016/s0091-3057(97)00327-4. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Huffman JW, Balster RL, Martin BR. Pharmacological specificity of the discriminative stimulus effects of delta 9-tetrahydrocannabinol in rhesus monkeys. Drug Alcohol Depend. 1995b;40:81–86. doi: 10.1016/0376-8716(95)01193-5. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Martin BR. Effects of SR41716A on diazepam substitution for delta9-tetrahydrocannabinol in rat drug discrimination. Pharmacol Biochem Behav. 1999;64:519–522. doi: 10.1016/s0091-3057(99)00130-6. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Ryan WJ, Razdan RK, Martin BR. Evaluation of cannabimimetic effects of structural analogs of anandamide in rats. Eur J Pharmacol. 1998;355:113–118. doi: 10.1016/s0014-2999(98)00502-0. [DOI] [PubMed] [Google Scholar]
- Wojnicki FH, Glowa JR. Effects of drug history on the acquisition of responding maintained by GBR 12909 in rhesus monkeys. Psychopharmacology (Berl) 1996;123:34–41. doi: 10.1007/BF02246278. [DOI] [PubMed] [Google Scholar]
- Woods JH. Some thoughts on the relations between animal and human drug-taking. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7:577–584. doi: 10.1016/0278-5846(83)90028-3. [DOI] [PubMed] [Google Scholar]
- Wu TC, Tashkin DP, Rose JE, Djahed B. Influence of marijuana potency and amount of cigarette consumed on marijuana smoking pattern. J Psychoactive Drugs. 1988;20:43–46. doi: 10.1080/02791072.1988.10524370. [DOI] [PubMed] [Google Scholar]
- Yokel RA. Intravenous self-administration: response rates, the effects of pharmacological challenges, and drug preference. In: Bozarth MA, editor. Methods of assessing the reinforcing properties of abused drugs. Springer-Verlag; New York: 1987. pp. 1–33. [Google Scholar]
- Young AM, Herling S. Drugs as Reinforcers: Studies in Laboratory Animals. In: Goldberg SR, Stolerman IP, editors. Behavioral Analysis Of Drug Dependence. Academic Press; Orlando: 1986. pp. 9–67. [Google Scholar]
- Young AM, Herling S, Woods JH. History of drug exposure as a determinant of drug self-administration. NIDA Res Monogr. 1981;37:75–88. [PubMed] [Google Scholar]
- Young AM, Woods JH. Maintenance of behavior by ketamine and related compounds in rhesus monkeys with different self-administration histories. J Pharmacol Exp Ther. 1981;218:720–727. [PubMed] [Google Scholar]
- Zacny JP, de Wit H. Effects of food deprivation on subjective effects and self-administration of marijuana in humans. Psychol Rep. 1991;68:1263–1274. doi: 10.2466/pr0.1991.68.3c.1263. [DOI] [PubMed] [Google Scholar]
- Zapata A, Chefer VI, Ator R, Shippenberg TS, Rocha BA. Behavioural sensitization and enhanced dopamine response in the nucleus accumbens after intravenous cocaine self-administration in mice. Eur J Neurosci. 2003;17:590–596. doi: 10.1046/j.1460-9568.2003.02491.x. [DOI] [PubMed] [Google Scholar]