Abstract
The purpose of this 12-week open trial was to evaluate the potential utility of atomoxetine for the treatment of attention deficit hyperactivity disorder (ADHD) in cocaine-dependent treatment seekers. The sample consisted of 20 participants with all participants meeting DSM-IV-TR criteria for ADHD and cocaine dependence (CD). Using several measures to assess ADHD, there was a significant reduction in ADHD symptoms. There was no significant decrease in cocaine use throughout the trial. Taken together, although cocaine-dependent individuals showed some reduction in ADHD symptoms while receiving atomoxetine, the high drop-out rate and lack of impact on cocaine use may limit its utility in ADHD adults who are currently abusing cocaine.
1. Introduction
ADHD, a disorder characterized by inattention, hyperactivity and impulsivity, is estimated to occur in approximately 2-5% of the United States population (Adler and Cohen, 2004,Barkley, et al., 2002, Kessler, et al., 2006). Adults with ADHD are at increased risk for having co-occurring psychiatric disorders, and in particular, substance use disorders (SUDS). Specifically, the National Comorbidity Survey Replication (NCS-R), a community-based epidemiologic study, found that approximately 15.2% of individuals with ADHD met the Diagnostic and Statistical Manual of Mental Disorders-Forth Edition (DSM-IV-TR) criteria for a substance use disorder (SUD) (Kessler, et al., 2006). When compared with the SUD prevalence of 5.6% among individuals without ADHD, this estimate shows an odds ratio of 3.0, suggesting a three times higher likelihood of SUD among adults with ADHD. Additionally, the NCS-R study found that 10.8% of individuals with SUDs met criteria for adult ADHD, demonstrating a substantially higher prevalence compared to a 3.8% prevalence in individuals without SUDs.
Among adults who seek treatment for SUD, ADHD is especially prevalent. Using DSM-IV criteria, several prevalence studies in clinical setting have found that the prevalence of adult ADHD ranges from 10-24% among treatment seekers (Clure, et al., 1999,King, et al., 1999, Levin, et al., 1998a,Schubiner, et al., 2000). This overrepresentation of ADHD in substance-abusing populations is important because the presence of comorbid ADHD may worsen substance treatment outcome (Carroll and Rounsaville, 1993,Levin, et al., 2004,Wise, et al., 2001). It is therefore important that in treating substance use, the comorbid presence of ADHD be addressed. However, it is currently unclear whether medications proven effective for adults with ADHD without co-occurring substance use disorders are effective for patients with ADHD who have co-occurring substance use disorder.
Growing evidence has shown that stimulants, particularly long-acting formulations, can be given safely and are not routinely abused in substance-abusing populations (Mariani and Levin, 2006, 2007;Wilens et al, 2008). However, clinicians continue to have concerns about the potential diversion or abuse of these medications by substance-abusing patients with adult ADHD. Although long-acting stimulants are less likely to be abused than short-acting stimulants, there remains some risk of abuse (Wilens et al., 2008). To date, atomoxetine is the only nonstimulant FDA-approved medication for adult ADHD. Several large clinical trials in adult populations have found atomoxetine to be superior to placebo in reducing ADHD symptoms in nondrug abusing populations (Michelson, et al., 2003, Michelson, et al., 2002). However, atomoxetine's benefits in active substance abusers have yet to be established.
Given that no published trials have assessed the therapeutic efficacy of atomoxetine in cocaine-dependent individuals with ADHD, we implemented an open-label preliminary trial to assess the utility and tolerability of the medication in this patient population. We hypothesized that adult cocaine-dependent individuals with ADHD who received atomoxetine along with individual cognitive behavioral therapy would have improvements in their ADHD symptoms and allow them to utilize the cognitive and behavioral tasks associated with cognitive behavioral therapy. This, in turn, might lead to a reduction in cocaine use. Further, to control for a possible placebo effect in this trial, we compared our data on ADHD with a historical placebo control from a recently completed double-blind placebo-controlled trial from our clinic that explored the efficacy of sustained-release methylphenidate in cocaine-dependent adults with ADHD (Levin et al., 2007).
2. Methods
2.1 Participants
All participants were seeking outpatient treatment for problems related to cocaine use and were recruited by local advertising or by referrals in the New York City metropolitan area. Two types of advertisements were placed: (1) those that recruited individuals who were seeking treatment for cocaine dependence, and (2) those that recruited individuals who were seeking treatment for cocaine dependence and might have problems with inattention and/or hyperactivity. The study was initiated in March 2004 and the last participant was entered in October, 2006.
Potential participants underwent a detailed medical and psychiatric assessment. All participants gave written informed consent before both the screening and the study procedures were initiated, and were told that the study medication might improve their ADHD symptoms and possibily reduce their cocaine use. The Institutional Review Boards of the New York State Psychiatric Institute, Columbia University, and the Long Island Jewish Medical Center approved the study.
Study inclusion required participants between the ages of 18-60 to meet DSM-IV (APA, 1994) criteria for cocaine dependence, persistent adult attention deficit hyperactivity disorder (ADHD), and describe cocaine as their primary drug of abuse. Participants had to be capable of providing informed consent and complying with outlined study procedures. Women of child-bearing age were only included if they were not pregnant based on a blood pregnancy test results at screening and agreed to use a method of contraception with proven efficacy. Participants were excluded if they 1) met DSM-IV-TR criteria for current psychiatric disorders (other than ADHD or substance abuse), which required psychiatric intervention or had unstable physical disorders which might make participation hazardous.
2.2 Settings
Participants were treated at the Substance Treatment and Research Service (STARS) of the New York State Psychiatric Institute (NYSPI) or at Project Outreach of the Long Island Jewish Medical Center.
2.3 Study Procedures
This study was a 12-week open trial to assess the tolerability and clinical utility of atomoxetine in cocaine-dependent individuals with ADHD. Initially, atomoxetine was administered at 20 mg/day for three days. This dose was then increased to 40 mg/day for four days. During the second week of the trial, the dose was increased to 60 mg/day and later increased to 80 mg/day at the start of Week 3. Patients were then maintained at 80 mg per day for four weeks. At the beginning of Week 7, patients were maintained at 80 mg/day or increased to the maximal dose of 100 mg/day if there was less than a 50% reduction of ADHD symptoms (as determined by the Adult ADHD Rating Scale; Murphy, 1996) and if the patients were tolerating the medication well. They were then maintained at 80 mg/day or 100 mg/day for the remaining six weeks of the trial. All patients received two capsules to be taken once a day in the morning.
Twenty-five mg of riboflavin was added to each of the two prescribed capsules (approximately 50 mg/day) in an effort to track compliance, which was determined if patients' urine samples fluoresced under an ultraviolet (UV) lamp, signifying consumption of the study capsules. This approach has been shown to be sensitive and utilized in other pharmacologic trials (Brady, et al., 2005, Del Boca, et al., 1996, Pettinati, et al., 2001).
At each of the thrice-weekly visits, participants were asked to provide a urine specimen, complete self-report questionnaires, have their vital signs and side effects assessed. Medication was distributed once per week, and doses were adjusted based on compliance and tolerability. All clinical assessments of drug use and ADHD symptoms were conducted on a weekly basis. Monthly serum pregnancy tests for women were performed. All participants were expected to participate in weekly individual cognitive behavioral therapy (CBT). To ensure reliability of CBT across patients, a structured relapse prevention manual (Carroll, et al., 1994) was used. All participants were compensated $4.00 in cash for transportation costs per visit.
2.4 Assessments
2.4.1 Screening
During the screening appointments, participants were asked to fill out two self-report instruments: 1) the Wender Utah Rating Scale (WURS) (Ward, et al., 1993); and 2) the Adult ADHD Rating Scale (AARS) (Murphy, 1996). Those with elevated scores on either instrument were targeted as possibly having ADHD. To establish a current ADHD diagnosis, the Conners' Adult ADHD Diagnostic Interview for DSM-IV (CAADID) was administered and scored by clinical staff (Epstein, et al., 2001).
Diagnoses were determined by clinicians who had either a Ph.D., Psy.D., or M.A. in Clinical Psychology. The training of the clinicians was extensive and is well described in a previous paper (Levin, et al., 1998a). In addition, a calendar-based Drug Use Questionnaire (DUQ) was administered at screening to assess patterns of lifetime drug use and recent use during the 30 days prior to evaluation.
2.4.2 Treatment Phase
Weekly assessments of ADHD symptoms were carried out using the Adult ADHD Rating Scale (AARS); (DuPaul, et al., 1998), and the Conners' Adult ADHD Rating Scale-Self Report: Long Version and Observer: Screening Version (CAARS-S:L and CAARS-O:SV) (Conners, et al., 1999). The AARS was the primary measure used to assess ADHD outcome and is an 18-item self-report questionnaire that represents the most common problems experienced by adults with ADHD derived from the DSM-IV criteria. The CAARS-S:L is a 66-item self-report and the CAARS-O:SV is a 30-item clinician-rated instrument. T-scores of 50 represent normative means of ADHD symptoms based on age and gender. Every 10-point change from 50 represents 1 standard deviation from the mean.
Drug use assessments were completed at every visit and included a self-report questionnaire and a urine sample tested for benzoylecognine (the metabolite of cocaine), and scored as positive or negative based on standard NIDA guidelines for cut-off points (e.g. 300 ng/ml for cocaine). New cocaine use was determined and distinguished from carryover from previous use among cocaine positive urine samples based on a method developed by Preston et al. (1997), which utilizes pharmacokinetic criteria applied to quantitative benzoylecgonine concentration data. To be considered negative in a given week, patients had to have at least two negative urines or a greater than 50% reduction in quantitative levels within a 48 hour period. A negative urine week had to be corroborated by self-report or the week was considered positive (Levin, et al., 2007). If only one urine sample was available in the week, patients were considered positive for that week irrespective of the urine result. The rationale for using these rules is that one missed urine drug screen out of three urines can occur for reasons not related to drug use (e.g., work-related issues) and counting all missed urines screens as positive may overestimate drug use (McDowell, et al., 2005, Carroll, et al. 1994)
Sensitivity and specificity analyses using urine toxicology as the gold standard indicated that there was good concordance between urine analysis and self-report, suggesting that self-report was a considerably valid measure of cocaine use (Sensitivity=0.88 and Specificity=0.76). Furthermore, a positive predicted value of 0.94 indicated that for those patients who reported having used cocaine, the probability that they had indeed used was very high. Likewise, a negative predicted value of 0.60 suggested that the probability of actual no use among patients who reported not having used was fairly high. However, this value suggested that the validity of self-report was slightly weaker when used to assess negative use.
2.5 Outcomes
Weekly AARS scores (continuous, range 0-54) were examined with the baseline score compared to that at the last assessment obtained and change in these scores over time. Because 30% improvement is considered to be a clinically meaningful standard categorical response criterion applied in ADHD treatment trials (Levin, et al., 2006, Levin, et al., 2007, Spencer, et al., 1998,Wilens, et al., 1999,Wilens, et al., 1995), we also determined the percentage of patients who attained this response at the end of the trial. Furthermore, in an attempt to mitigate the placebo effect, we compared the change in AARS scores over time with that of a historical placebo control from a previous study (Levin et al., 2007). Two additional secondary approaches were used to assess ADHD treatment outcome: (1) a comparison of CAARS-S:L and CAARS-O:SV scores at baseline and end of study, and (2) the change in these scores over time during the course of the 12-week trial..
Three cocaine use outcome measures were examined: (1) the change in the probability of positive cocaine use week over time based on urine toxicology, (2) the change in the probability of positive cocaine use week over time based on self-report, and (3) the change in the probability of positive cocaine use week over time based on corroborated data from urine toxicology and self-report, before and after adjusting for baseline amount spent on cocaine. Further, the relationship between the change in AARS and cocaine use was explored in a time effect model.
2.6 Data Analysis
All analyses were conducted on patients who were enrolled in the trial for at least three weeks to allow for adequate exposure to a standard therapeutic dose. All tests were two-tailed with the alpha significance level set at 0.05. T-tests were used for continuous measures comparing baseline to end of study ratings and chi-squares were used for categorical measures. Longitudinal analyses of the ADHD outcome measures and probability of positive cocaine use week were performed using repeated measures models as implemented by SAS PROC MIXED, and PROC GENMOD (version 9.1; SAS Institute, Cary, NC).
3. Results
3.1 Participant Flow and Retention in Treatment
A total of 824 cocaine abusing treatment seekers contacted the clinic for an appointment but 70% either did not show for the initial screening appointment or lost interest in entering a treatment research study. The most common reason patients did not qualify for this study was the absence of ADHD, and that many of those patients entered other clinical trials. Twenty participants met inclusion/exclusion criteria and were enrolled into the study. Of those enrolled, thirteen (65%) reached the maintenance dose phase of treatment, and nine (45%) completed at least six weeks of treatment. Only five (25%) completed twelve weeks of treatment. The mean weeks completed for all entered participants was 5.4 (± 4.5) weeks. Ten participants voluntarily withdrew, and five were removed from the protocol. The primary reason for participants withdrawing from the study was lack of interest (n=8); one individual relocated and another decided to attend an inpatient treatment facility. Of the participants who were removed from the trial, three were removed because of non-compliance with study visits, and two were removed due to adverse side effects. One participant developed chest pain without any untoward effects and one patient complained of nervousness, anxiety, insomnia, and fatigue effecting his functioning.
3.2 Sample Description
The sample (n=20) consisted of 19 males, 75% Caucasian, 15% African-American and 15% Hispanic. The average age was 39.3 (± 6.6) years and 14.1 (± 2.4) years of education. Fifty percent were employed full time, 15% worked part-time and the rest were unemployed or not disclosed. Forty percent had a current alcohol use disorder and 30% had a current cannabis use disorder. Average days of cocaine used in the past 30 days was 13.8 (± 9.6) and average amount used in the past month prior to treatment entry was $1,177 ± ($1,243). Seventy-five percent used cocaine intranasally and the remaining patients smoked cocaine. There were no significant differences in baseline demographic and clinical characteristics between completers and non-completers except that completers had achieved significantly more education [16.4 (± 3.1) vs. 13.4 (± 1.6) years; t= −2.89; p=.01] and began regularly using cocaine at a significantly later age [30 (± 8.6) vs. 22.1 ± 4.3 years old; t= −2.68; p=.015]. There were no significant differences in baseline ADHD symptoms based on the AARS or CAARS ratings between completers and non-completers.
3.3 Medication Compliance
Medication compliance was monitored every visit through two methods: self-report and riboflavin fluorescence testing. The mean percent of self reported missed doses for all the participants who entered the trial was 4.84 (± 7.5)%. Using riboflavin florescence as an indicator of capsule ingestion, the mean percent of riboflavin positive urines for all participants was 82.8 (± 31.9) %. Both approaches suggest fairly good compliance.
3.4 ADHD Symptoms Outcome
Regarding the primary ADHD outcome measure, ten participants (50%) of the total sample met the standard response criterion of at least a 30% reduction from baseline in AARS scores compared to the end of the trial. Of the nine participants who completed at least six weeks, seven (78%) had at least a 30% reduction in AARS scores. As illustrated in Table 1, when comparing overall baseline scores to end of study scores for patients who had at least two AARS weekly measures completed, there was a significant decrease in AARS [ 31.4 (± 9.8) vs. 21.2 (± 12.4), t17 =4.13, p=0.0007].
Table 1.
Baseline and end of study scores for AARS and CAARS-S:L and CAARS-O:SV*
| Baseline | End of study | Change | T value | p-value | |
|---|---|---|---|---|---|
| Rating Scales | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Overall | |||||
| AARS | 31.4 (9.8) | 21.2 (12.4) | 10.3 (10.6) | 4.13 | 0.0007 |
| CAARS-S:L | 76.2 (13.5) | 62.2 (15.1) | 13.9 (16.3) | 3.50 | 0.003 |
| CAARS-O:SV | 67.9 (11.2) | 58.9 (13.2) | 8.9 (15.2) | 2.35 | 0.039 |
| Subscale | |||||
| Inattentiveness | |||||
| CAARS-S:L | 72.8 (13.7) | 61.7 (14.3) | 11.1 (16.1) | 2.84 | 0.0118 |
| CAARS-O:SV | 64.0 (12.3) | 57.0 (12.5) | 7.0 (15.1) | 1.86 | 0.083 |
| Hyperactivity | |||||
| CAARS:S:L | 70.8 (14.5) | 59.5 (15.7) | 11.4 (17.5) | 2.68 | 0.0165 |
| CAARS-O:SV | 67.5 (14.7) | 58.1 (13.5) | 9.4 (16.2) | 2.32 | 0.0349 |
AARS: Adult ADHD Rating Scale; CAARS-S:L: Conners' Adult ADHD Rating Scale-Self Report: Long Version; CAARS-O:SV: Conners' Adult ADHD Rating Scale-Observer: Short Version
Analysis of AARS over time using a mixed effect model revealed that there was a significant time effect on patients' AARS scores (F1,94=8.17, p=0.0053). For every week, the mean AARS score decreased by 1. The effect of baseline AARS was also significant (F1,16 =20.54, p=0.0003), suggesting that patients with high AARS scores at baseline tended to have consistently higher AARS scores during the treatment compared to patients with low AARS scores at baseline.
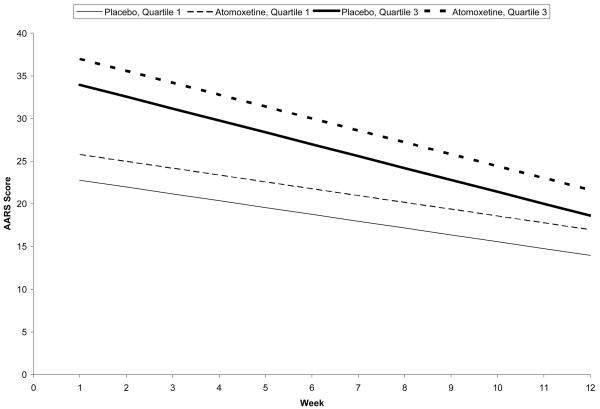
When compared to a historical placebo control from a previous study (Levin et al., 2007), no significant treatment effect or time effect was found. However, there existed a significant baseline effect (F1,67 =26.41, p<0.0001) and a time and baseline interaction (F1,540 =3.26, p=0.072). Patients with high AARS at baseline continued to have high AARS scores throughout the trial, irrespective of treatment. Figure 1 and Table 2 illustrate the effect of a time and baseline interaction comparing the first and third quartiles of baseline AARS scores for purposes of illustration. Patients with higher AARS at baseline experienced a more substantial decrease in AARS throughout the trial, when compared to patients with lower AARS at baseline.
Figure 1.
Mean AARS scores for atomoxetine and historical placebo control over the course of the 12-week trial, by treatment and baseline AARS score (1st quartile vs 3rd quartile).
Table 2.
Regression estimates for Adult ADHD Rating Scale (AARS) Scores
| Effect | β estimate (SE) | p-value |
|---|---|---|
| Intercept | 1.42 (5.04) | 0.7793 |
| Week* | 0.16 (0.72) | 0.8203 |
| Baseline AARS** | 0.74 (0.14) | <0.0001 |
| Treatment*** | 3.02 (2.24) | 0.1815 |
| Week x Baseline AARS | −0.04 (0.02) | 0.0716 |
Week corresponds to weeks in trial (1-12).
Baseline AARS represents AARS score at Week 0, prior to trial start.
Treatment is atomoxetine relative to historical placebo.
Using the secondary outcome measures, the CAARS-S:L and CAARS-O:SV significant changes in ADHD emerged. For the overall CAARS-S:L rating, there was a significant difference between the self-rated baseline CAARS-S:L, and the last self-rated CAARS-S:L [76.2 (±13.5)vs. 62.2 (±15.1), t16 =3.54, p=0.003]. The clinician based mean on the CAARS-O:SV was 67.9 (± 11.2) at baseline compared to last completed clinician-rated mean of 58.9 (± 13.2) (t15 =2.35, p=0.039) (Table 1).
For the inattentive subscales, there was a significant difference in the inattentive ratings based on the self-report ratings of the CAARS-S:L from baseline to end of study-[72.8 (±13.7) versus 61.7 (±14.3); t16=2.84, p=0.012]. Similarly, a trend was observed for the change in the inattentive subscale for the clinician rated CAARS-O:SV from baseline to end of study [64.0 (±12.3) vs.57.0 (±12.5); t15=1.85, p=0.083]. In terms of hyperactive symptomatology, a significant difference was also observed in the self-rated CAARS-S:L at baseline compared to end of study [70.8 (± 14.5) vs. 59.5 (± 15.7); t16=2.68, p= 0.017]. The clinician rated CAARS-O:SV hyperactivity sub-scale also showed a statistically significant change as well, with a baseline mean of 67.5 (±14.7) vs. a last recorded mean of 58.1 (±13.5) (t15=2.32, p=0.035) (Table 1).
Using a mixed-effects model (SAS-Proc Mixed- need ref) there was a significant time effect on patients' CAARS-S:L value (F1,92=9.07, p=0.0034). For every week, the mean value of CAARS decreased by 1.5. The effect of baseline CAARS was also significant (F1,15=7.61, p=0.0146) with high baseline CAARS values resulting in high scores throughout the trial.
3.5 Substance Use Outcome
According to a GEE analysis of cocaine use, defined using Preston's rule (1997), there was an overall decreasing trend in cocaine use over time. However, this time effect was not statistically significant (X2(1)=0.18, p=0.67). Similar results were obtained when cocaine use was determined based on urine toxicology or self-repot alone. To assess whether the change in AARS scores from baseline influenced cocaine use behavior, an additional GEE analysis was conducted to model this relationship over time. The results indicated no significant effects of time and change in AARS scores.
3.8 Safety
Side effects were rated on a scale of 0-3 (0=none, 1= mild, 2=moderate, 3= severe). The most commonly reported side effects rated as moderate or severe were nausea, mild headache, and mild dizziness. Several patients needed re-titration after missing doses during the maintenance phase. Although most patients tolerated the medication well and did not require a dose reduction, two participants was ultimately discontinued from the medication component of treatment due to adverse side effects. One participant developed chest pain during the first week of the trial and the other participant developed symptoms during the titration phase in the second week of the trial that were interfering with daily functioning such as nervousness, anxiety, insomnia, and fatigue.
4. Discussion
The results of this open-label trial suggest that atomoxetine in conjunction with cognitive-behavioral therapy reduced ADHD symptoms in actively using cocaine-dependent individuals but does not impact on cocaine use. This is consistent with other studies demonstrating that ADHD medication may be useful for ADHD symptoms but is less likely to produce reductions in cocaine use (Riggs, et al., 2004, Schubiner, et al., 2002). However, it is noteworthy that for this pilot study there was no evidence of a reduction in cocaine use, unlike earlier pilot studies evaluating bupropion and stimulant medications for the treatment of ADHD and cocaine dependence (Somoza et al., 2004; Levin, et al., 2002, Levin, et al., 1998b).
Our hypothesis that improvement in ADHD symptoms might lead to a reduction in cocaine use was not supported. Given that cocaine use was virtually unchanged, several factors need to be considered to explain this poor treatment response. Although ADHD symptoms improved, the mean end of study ratings on the CAARS scores on the self-report and clinician-rated scales were still one standard deviation above normative population data, suggesting that substantial ADHD symptoms persisted. Thus, CBT may have been difficult for these patients to effectively utilize. A recent review found that CBT is modestly effective in substance abusers without ADHD (Knapp, et al., 2007) and that it may take several months for this type of treatment to “take hold” (Carroll, et al., 1994). The persistence of some ADHD symptoms, the short nature of this trial and high drop-out may have precluded our ability to detect a reduction in cocaine use.
In this trial we were focused on assessing the feasibility and utility of atomoxetine in treating active cocaine abusers with ADHD. However, we were struck by the high drop-out rates. In earlier trials with methylphenidate (Levin, et al., 1998b) and bupropion (Levin, et al., 2002), the retention rate was substantially greater (83% and 67%, respectively). Further, although the majority showed a 30% of improvement in AARS scores in this trial, compared to the placebo group in a previous trial, the improvement in AARS scores was not superior. The reasons for the high drop-out in this trial are somewhat unclear. It may be that cocaine-dependent individuals with ADHD who are interested in treatment in the past several years, may be more impulsive or have more chaotic lives than those who used cocaine 5-10 years ago. Another possibility is that methylphenidate or bupropion was better tolerated than atomoxetine resulting in better retention. This is mitigated by the fact that only two patients were discontinued from medication due to intolerable side effects. However, it is possible that some individuals dropped out without providing a reason, might have done so because they did not find the medication helpful or found it aversive.
Whereas, our early open trials with methyphenidate and bupropion suggested positive effects, double-blind trials with adult cocaine-dependent individuals have produced less robust findings (Levin, et al., 2007, Schubiner, et al., 2002). It is not uncommon to have greater treatment responses in open trials compared to double-blind studies, perhaps due to expectancy effects (i..e. the patient knows they are getting active medication and expect that the medication will work). However, in this open trial, while there was improvement in ADHD symptoms, there was no reduction in cocaine use. Further, using a historical control placebo group (Levin et al., 2007), the reduction in ADHD symptoms was not superior for those receiving atomoxetine. It may be medications that targeting ADHD symptoms may be less effective in actively-using cocaine-dependent individuals, both due to the ongoing intoxicating effects of the abused drug and/or the depletion of dopaminergic and noradrenergic transmission (Kalivas and Volkow, 2005) resulting in a blunted ADHD treatment response. (Levin, et al., 2007). Abstinence prior to initiation of treatment may give pre-synaptic stores time to replenish. As suggested in an earlier study, this blunted response may make it more difficult for the patient to engage in treatment and reduce his/her drug use (Levin, et al., 2007). It may be that if patients were cocaine-abstinent when medication was initiated, the therapeutic effect of atomoxetine on ADHD symptoms would have been greater resulting in less cocaine use and greater abstinence. Supporting this, a recently completed trial found that in alcohol-dependent adults with ADHD who were newly abstinent, atomoxetine was superior to placebo in reducing ADHD symptoms and on some outcome measures of alcohol use (Brady, et al., 2006). Further, unlike stimulants, which may have a direct agonist effect leading to a reduction in cocaine use (Grabowski, et al., 2001,Grabowski, et al., 2004), this has not been shown with atomoxetine.
Clearly, there are limitations of this study including the high drop-out rate, small sample size, and lack of a control group. This study was designed to assess the feasibility, tolerability and possible therapeutic utility of atomoxetine for actively abusing cocaine-dependent individuals with ADHD. Although the high drop-out can be viewed as a limitation, it may also be viewed as a finding. Without a control group, we cannot determine whether this was due to a change in the patient population or lack of “holding” power of the medication or overall treatment. Certainly, the drop-out rate is higher than studies carried out by our group in cocaine-dependent individuals with and without ADHD (Bisaga, et al., 2006, Levin, et al., 2002, Levin, et al., 1998b). Such a high drop-out rate led to only a small percentage of individuals receiving the maximum maintenance dose of atomoxetine and thus making it more difficult to obtain a therapeutic effect. Another limitation of the study was that the population was generally healthy, predominantly male with no additional severe psychopathology, thus limiting the generalizability of our findings.
In sum, administration of atomoxetine, along with cognitive behavioral therapy, was associated with a reduction in both inattentive and hyperactive-impulsive symptoms in cocaine-dependent individuals with ADHD. However, there was no notable reduction in cocaine use. The high drop-out rate may have limited our ability to observe more robust clinical effects. Future studies that compare atomoxetine to stimulant medication, perhaps in cocaine-dependent individuals who have achieved a period of abstinence, may allow a better assessment of the clinical utility in this dually-disordered population. However, the findings from this trial do not suggest that a double-blind, placebo-controlled trial in a current cocaine-abusing population with ADHD is warranted.
Acknowledgements
This research was supported by NIDA grants P50DA09236 and KO2 00465. We want to thank the staff of the Substance Treatment and Research Service (STARS) of the New York State Psychiatric Institute and of Project Outreach of the Long Island Jewish Medical Center for their clinical support. Some of these data were presented at the College on Drug Dependence 68th Annual Meeting, 2006. Dr. Levin is a past consultant for Eli Lily and Company, Shire Pharmaceuticals Group, AstraZeneca, and OrthoMcNeil Pharmaceutical Inc. Also she has also received research support from Eli Lily and Company, UCB Pharma Inc, Shire Pharmaceuticals Group, AstraZeneca and OrthoMcNeil Pharmaceutical Inc. Eli Lily provided the medication for this study.
References
- Adler L, Cohen J. Diagnosis and evaluation of adults with attention-deficit/hyperactivity disorder. Psychiatr Clin North Am. 2004;27:187–201. doi: 10.1016/j.psc.2003.12.003. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (APA) Diagnostic and Statistical Manual of Mental Disorders. 4th Edition American Psychiatric Press; Washington, DC: 1994. [Google Scholar]
- Barkley RA, Fischer M, Smallish L, Fletcher K. The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. J Abnorm Psychol. 2002;111:279–289. [PubMed] [Google Scholar]
- Bisaga A, Aharonovich E, Garawi F, Levin FR, Rubin E, Raby WN, Nunes EV. A randomized placebo-controlled trial of gabapentin for cocaine dependence. Drug Alcohol Depend. 2006;81:267–274. doi: 10.1016/j.drugalcdep.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Brady KT, Sonne S, Anton RF, Randall CL, Back SE, Simpson K. Sertraline in the treatment of co-occurring alcohol dependence and posttraumatic stress disorder. Alcohol Clin Exp Res. 2005;29:395–401. doi: 10.1097/01.alc.0000156129.98265.57. [DOI] [PubMed] [Google Scholar]
- Brady K, Wilens T, Adler L, Weiss MD, Ramsey JL, Michelson D, Ahrbecker LM, Moore RJ, Renard D, Levine LR. Atomoxetine Treatment of Adults with ADHD and Comorbid Alcohol Abuse Disorder; College of Neuropsychopharmacology 45th Annual Meeting; Hollywood, Florida. 2006. [Google Scholar]
- Carroll KM, Rounsaville BJ. History and significance of childhood attention deficit disorder in treatment-seeking cocaine abusers. Compr Psychiatry. 1993;34::75–82. doi: 10.1016/0010-440x(93)90050-e. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Gordon LT, Nich C, Jatlow P, Bisighini RM, Gawin FH. Psychotherapy and pharmacotherapy for ambulatory cocaine abusers. Arch Gen Psychiatry. 1994;5:177–187. doi: 10.1001/archpsyc.1994.03950030013002. [DOI] [PubMed] [Google Scholar]
- Clure C, Brady KT, Saladin ME, Johnson D, Waid R, Rittenbury M. Attention-deficit/hyperactivity disorder and substance use: symptom pattern and drug choice. Am J Drug Alcohol Abuse. 1999;25:441–448. doi: 10.1081/ada-100101871. [DOI] [PubMed] [Google Scholar]
- Conners C, Erhardt JN, Sparrow E. Conner's Adult ADHD Rating Scale (CAARS) Multi-Health Systems; North Tonawanta, NY: 1999. [Google Scholar]
- Del Boca FK, Kranzler HR, Brown J, Korner PF. Assessment of medication compliance in alcoholics through UV light detection of a riboflavin tracer. Alcoholism: Clinical & Experimental Research. 1996;20(8):1412–1417. doi: 10.1111/j.1530-0277.1996.tb01142.x. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Power T, Anastopoulos A, Reid R. Checklistsm Norms and Clinical Interpretation. Guilford Press; New York, NY: 1998. ADHD Rating Scale, IV. [Google Scholar]
- Epstein JN, Johnson DE, Conners CK. Conner's Adult ADHD Diagnostic Interview for DSM-IV (CAADID) Multi-Health Systems, Inc; North Tonawanda, NY: 2001. [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, Moeller FG. Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J Clin Psychopharmacol. 2001;21:522–526. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav . 2004;29:1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, Faraone SV, Greenhill LL, Howes MJ, Secnik K, Spencer T, Ustun TB, Walters EE, Zaslavsky AM. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King VL, Brooner RK, Kidorf MS, Stoller KB, Mirsky AF. Attention deficit hyperactivity disorder and treatment outcome in opioid abusers entering treatment. J Nerv Ment Dis. 1999;187:487–495. doi: 10.1097/00005053-199908000-00005. [DOI] [PubMed] [Google Scholar]
- Knapp WP, Soares BG, Farrel M, Lima MS. Psychosocial interventions for cocaine and psychostimulant amphetamines related disorders. Cochrane Database Syst Rev. 2007:CD003023. doi: 10.1002/14651858.CD003023.pub2. [DOI] [PubMed] [Google Scholar]
- Levin FR, Evans SM, Kleber HD. Prevalence of adult attention-deficit hyperactivity disorder among cocaine abusers seeking treatment. Drug Alcohol Depend. 1998a;52:15–25. doi: 10.1016/s0376-8716(98)00049-0. [DOI] [PubMed] [Google Scholar]
- Levin FR, Evans SM, McDowell DM, Kleber HD. Methylphenidate treatment for cocaine abusers with adult attention-deficit/hyperactivity disorder: a pilot study. J Clin Psychiatry. 1998b;59:300–305. doi: 10.4088/jcp.v59n0605. [DOI] [PubMed] [Google Scholar]
- Levin FR, Evans SM, McDowell DM, Brooks DJ, Nunes E. Bupropion treatment for cocaine abuse and adult attention-deficit/hyperactivity disorder. J Addict Dis. 2002; 21:1–16. doi: 10.1300/J069v21n02_01. [DOI] [PubMed] [Google Scholar]
- Levin FR, Evans SM, Vosburg SK, Horton T, Brooks D, Ng J. Impact of attention-deficit hyperactivity disorder and other psychopathology on treatment retention among cocaine abusers in a therapeutic community. Addict Behav. 2004;29:1875–1882. doi: 10.1016/j.addbeh.2004.03.041. [DOI] [PubMed] [Google Scholar]
- Levin FR, Evans SM, Brooks DJ, Kalbag AS, Garawi F, Nunes EV. Treatment of methadone-maintained patients with adult ADHD: double-blind comparison of methylphenidate, bupropion and placebo. Drug Alcohol Depend. 2006;81:137–148. doi: 10.1016/j.drugalcdep.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Levin FR, Evans SM, Brooks DJ, Garawi F. Treatment of cocaine dependent treatment seekers with adult ADHD: Double-blind comparison of methylphenidate and placebo. Drug Alcohol Depend. 2007;87:20–29. doi: 10.1016/j.drugalcdep.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Mariani JJ, Levin FR. Stimulant pharmacotheraphy in attention deficit/hyperactivity disorder in patients with co-occurring substance use disorder. Advance in ADHD. 2006;1:47–52. [Google Scholar]
- Mariani JJ, Levin FR. Treatment strategies for co-occurring ADHD and substance use disorders. Am J Addict. 2007;1(16 Suppl):45–54. doi: 10.1080/10550490601082783. quiz 55-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell D, Nunes EV, Seracini AM, Rothenberg J, Vosburg SK, Ma GJ, Petkova E. Desipramine treatment of cocaine-dependent patients with depression: a placebo-controlled trial. Drug Alcohol Depend. 2005;80:209–221. doi: 10.1016/j.drugalcdep.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Michelson D, Allen AJ, Busner J, Casat C, Dunn D, Kratochvil C, Newcorn J, Sallee FR, Sangal RB, Saylor K, West S, Kelsey D, Wernicke J, Trapp NJ, Harder D. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry. 2002;159:1896–1901. doi: 10.1176/appi.ajp.159.11.1896. [DOI] [PubMed] [Google Scholar]
- Michelson D, Adler L, Spencer T, Reimherr FW, West SA, Allen AJ, Kelsey D, Wernicke J, Dietrich A, Milton D. Atomoxetine in adults with ADHD: two randomized, placebo-controlled studies. Biol Psychiatry. 2003;53:112–120. doi: 10.1016/s0006-3223(02)01671-2. [DOI] [PubMed] [Google Scholar]
- Murphy KR, Barkley RA. Prevalence of DSM-IV symptoms of ADHD in adult licensed drivers: Implications of clinical diagnosis. J Atten Disord. 1996;1:147–161. [Google Scholar]
- Pettinati HM, Volpicelli JR, Luck G, Kranzler HR, Rukstalis MR, Cnaan A. Double-blind clinical trial of sertraline treatment for alcohol dependence. J Clin Psychopharmacol. 2001;21:143–153. doi: 10.1097/00004714-200104000-00005. [DOI] [PubMed] [Google Scholar]
- Preston KL, Silverman K, Schuster CR, Cone EJ. Use of quantitative urinalysis in monitoring cocaine use. NIDA Res Monogr. 1997;175:253–264. [PubMed] [Google Scholar]
- Riggs PD, Hall SK, Mikulich-Gilbertson SK, Lohman M, Kayser A. A randomized controlled trial of pemoline for attention-deficit/hyperactivity disorder in substance-abusing adolescents. J Am Acad Child Adolesc Psychiatry. 2004;43:420–429. doi: 10.1097/00004583-200404000-00008. [DOI] [PubMed] [Google Scholar]
- Schubiner H, Saules KK, Arfken CL, Johanson CE, Schuster CR, Lockhart N, Edwards A, Donlin J, Pihlgren E. Double-blind placebo-controlled trial of methylphenidate in the treatment of adult ADHD patients with comorbid cocaine dependence. Exp Clin Psychopharmacol. 2002;10:286–294. doi: 10.1037//1064-1297.10.3.286. [DOI] [PubMed] [Google Scholar]
- Schubiner H, Tzelepis A, Milberger S, Lockhart N, Kruger M, Kelley BJ, Schoener EP. Prevalence of attention-deficit/hyperactivity disorder and conduct disorder among substance abusers. J Clin Psychiatry. 2000;61:244–251. doi: 10.4088/jcp.v61n0402. [DOI] [PubMed] [Google Scholar]
- Somoza EC, Winhusen TM, Bridge TP, Rotrosen JP, Vanderburg DG, Harrer JM, Mezinskis JP, Montgomery MA, Ciraulo DA, Wulsin LR, Barrett JA. An open-label pilot study of methylphenidate in the treatment of cocaine dependent patients with adult attention deficit/hyperactivity disorder. J Addict Dis. 2004;23:77–92. doi: 10.1300/J069v23n01_07. [DOI] [PubMed] [Google Scholar]
- Spencer T, Biederman J, Wilens T, Prince J, Hatch M, Jones J, Harding M, Faraone SV, Seidman L. Effectiveness and tolerability of tomoxetine in adults with attention deficit hyperactivity disorder. Am J Psychiatry. 1998;155:693–695. doi: 10.1176/ajp.155.5.693. [DOI] [PubMed] [Google Scholar]
- Ward MF, Wender PH, Reimherr FW. The Wender Utah Rating Scale: an aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. Am J Psychiatry. 1993;150:885–890. doi: 10.1176/ajp.150.6.885. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Spencer TJ, Prince J. Pharmacotherapy of adult attention deficit/hyperactivity disorder: a review. J Clin Psychopharmacol. 1995;15:270–279. doi: 10.1097/00004714-199508000-00006. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Spencer TJ, Bostic J, Prince J, Monuteaux MC, Soriano J, Fine C, Abrams A, Rater M, Polisner D. A pilot controlled clinical trial of ABT-418, a cholinergic agonist, in the treatment of adults with attention deficit hyperactivity disorder. Am J Psychiatry. 1999;156:1931–1937. doi: 10.1176/ajp.156.12.1931. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Alder LA, Adams J, Sgambati S, Rotrosen J, Sawtell R, Utzinger L, Fusillo S. Misuse and diversion of stimulants prescribed for ADHD: A systematic reivew of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(1):21–31. doi: 10.1097/chi.0b013e31815a56f1. [DOI] [PubMed] [Google Scholar]
- Wise BK, Cuffe SP, Fischer T. Dual diagnosis and successful participation of adolescents in substance abuse treatment. J Subst Abuse Treat. 2001;21:161–165. doi: 10.1016/s0740-5472(01)00193-3. [DOI] [PubMed] [Google Scholar]