Abstract
BACKGROUND:
In a four-centre trial, the use of sputum cell counts (sputum strategy [SS]) to guide treatment had resulted in fewer and less severe exacerbations without the need for a higher corticosteroid dose, compared with the use of symptoms and spirometry (clinical strategy [CS]).
OBJECTIVE:
To compare the cost of the SS with the CS in the treatment of patients with moderate to severe asthma.
METHODS:
In 39 patients (19 in the SS, 20 in the CS) from one of the centres, the cost (third-party payer) of the two treatment strategies was compared. Resource use data were collected using a structured questionnaire. Corresponding unit costs in 2006 Canadian dollars were obtained.
RESULTS:
The clinical characteristics of the patients were similar to the study population at the four centres. In the SS, the number of visits to a family physician for health disorders indirectly related to asthma (P=0.003) and the amount of inhaled long-acting beta-agonists (P=0.007) were less than that of the CS. While the total estimated median cost per patient for spirometry ($393; range $299 to $487) was less than that for sputum induction ($1,008; range $907 to $1,411), the total cost of the SS ($2,265; range $1,466 to $4,347) was less than that of the CS ($3369; range $2208 to $3927) (P=0.216). This cost difference was due to lower costs of physician and hospital visits and services (P=0.078), of inhaled short-acting bronchodilators (P=0.067), of long-acting beta-agonists (P=0.002) and of inhaled corticosteroids (P=0.064) in the SS.
CONCLUSION:
In patients with moderate to severe asthma, the use of sputum cell counts to guide treatment is more effective and is likely to be less costly than management using symptoms and spirometry.
Keywords: Asthma, Cost, Spirometry, Sputum cell counts, Symptoms
Abstract
HISTORIQUE :
Dans une étude regroupant quatre centres, l’utilisation des numérations cellulaires des expectorations (stratégie « expectorations » [SE]) pour orienter le traitement a donné lieu à des poussées moins nombreuses et moins graves de la maladie, sans recours à des doses plus fortes de corticostéroïdes, comparativement à l’utilisation des symptômes et de la spirométrie (stratégie clinique [SC]).
OBJECTIF :
Comparer les coûts de la SE et de la SC dans le traitement des patients souffrant d’asthme de modéré à grave.
MÉTHODE :
Chez 39 patients (19 soumis à la SE et 20 à la SC) provenant de l’un des centres, les coûts (pour un tiers payeur) des deux stratégies thérapeutiques ont été comparés. Les auteurs ont recueilli les données d’utilisation des ressources à l’aide d’un questionnaire structuré. Ils ont ensuite obtenu les coûts unitaires correspondants en dollars canadiens de 2006.
RÉSULTATS :
Les caractéristiques cliniques des patients étaient similaires à celles de la population de l’étude dans les quatre centres. Dans le groupe soumis à la SE, le nombre de consultations en médecine familiale pour un problème de santé indirectement lié à l’asthme (p = 0,003) et la quantité de bêta-agonistes à longue action par inhalation utilisée (p = 0,007) ont été moindres que dans le groupe soumis à la SC. Tandis que le coût médian estimé total par patient pour la spirométrie (393 $, entre 299 $ et 487 $) a été inférieur au coût de l’induction des expectorations (1 008 $, entre 907 $ et 1 411 $) et le coût total de la SE (2 265 $, entre 1 466 $ et 4 347 $) a été inférieur à celui de la SC (3 369 $, entre 2 208 $ et 3 927 $) (p = 0,216). Cette différence de coût a été attribuable aux frais moindres associés aux consultations et services en cabinet médical et à l’hôpital (p = 0,078), aux bronchodilatateurs à action brève par inhalation (p = 0,067), aux bêta-agonistes à longue action (p = 0,002) et aux corticostéroïdes par inhalation (p = 0,064) dans le groupe soumis à la SE.
CONCLUSION :
Chez les patients souffrant d’asthme de modéré à grave, l’utilisation des numérations cellulaires des expectorations permet de mieux orienter le traitement et est susceptible de coûter moins cher qu’une prise en charge établie en fonction des symptômes et de la spirométrie.
An important component of asthma is airway inflammation. When the percentage of eosinophils in sputum is used to measure the inflammation and to guide corticosteroid treatment in patients with moderate to severe asthma, exacerbations (1), specifically eosinophilic exacerbations (2), are reduced without the need for a higher steroid dose in comparison with optimum treatment that is guided by symptoms and spirometry. As part of a secondary analysis based on data collected from the main study (2), we compared the cost of the sputum strategy (SS) with that of the clinical strategy (CS), because the added cost of sputum (induced or spontaneous) cell counts is usually perceived incorrectly as one of the major limitations to its widespread use in clinical practice.
METHODS
Clinical study
The clinical trial was a multicentre, randomized, parallel-group effectiveness study that was conducted to determine whether the use of sputum cell counts was more effective than the usual CS to guide treatment and reduce exacerbations in asthma. A detailed description of the study and the findings can be found elsewhere (2). In brief, 117 adults in Hamilton (Ontario), Quebec City (Quebec), Montreal (Quebec) and Florianópolis (Brazil), who had symptoms of asthma for a minimum of one year, were randomly assigned to either the CS or SS over a two-year period (1999 to 2001). In the CS, symptoms and spirometry were used to identify clinical control, exacerbations and other treatments, while in the SS, sputum cell counts were used to guide corticosteroid treatment to keep eosinophil counts within the normal range at 2% or less. Of the 117 patients randomly assigned to the clinical or sputum strategy, minimum treatment to maintain control was identified in 107 patients during the initial phase (phase 1). This treatment was continued (phase 2) for the remainder of the two-year period, at the end of which 102 patients were eligible for final analysis.
During phase 2, the induced sputum cell counts and spirometry were recorded every three months and at exacerbations. The primary outcomes were the RR reduction for the occurrence of the first exacerbation and the length of time without exacerbation. In the SS patients, the RR ratio was lower by 49% and the time to first exacerbation was longer by 213 days. In one of the centres (Hamilton), data from both strategies on clinical and sociodemographic characteristics of patients, health care resource use and amount of drugs used to maintain control of the disease were collected because this was the phase during which the effectiveness of using sputum cell counts was examined. The economic analysis was approved by the Research Ethics Board of St Joseph’s Healthcare in Hamilton.
Use of health resources
The induced sputum cell counts were recorded at 163 routine visits and at 28 visits for exacerbations of asthma in phase 2 of the SS. Spirometry was recorded at 178 routine visits and at 35 visits for exacerbations of asthma in the CS. Information on health care resource use, such as consultation and follow-up visits to the family physician and specialist, as well as services provided by the physician and hospital, was collected using a resource use questionnaire. The services provided were categorized into those directly related to asthma and those indirectly related to asthma (Table 1). The visits to the physician and hospital that were directly related to asthma were for exacerbations of asthma. The visits to the physician and hospital that were indirectly related to asthma were for evaluation and control of the causes of exacerbations and the side effects of therapy for asthma. The information on the use of drugs, such as short-acting beta-agonists, long-acting beta-agonists (LABAs), inhaled corticosteroids, prednisone and antibiotics, was collected from the case report forms.
TABLE 1.
Reasons for visits to a physician and hospital
| Directly related to asthma | Indirectly related to asthma | |
|---|---|---|
| Visits to family physician | Exacerbation of asthma* | Respiratory infection |
| Flu shot | ||
| Allergy shot | ||
| Referral for bone density scan (osteoporosis or osteopenia) | ||
| Electrocardiogram (palpitations related to beta-agonist therapy) | ||
| Blood work (easy bruising related to corticosteroid therapy) | ||
| Visits to specialist | Exacerbation of asthma* | Respirologist (respiratory infection and cardiopulmonary exercise test) |
| Ear, nose and throat disorders (sinus disease) | ||
| Ophthalmologist (to check for glaucoma caused by inhaled corticosteroid therapy) | ||
| Allergist | ||
| Geriatrist (osteoporosis or osteopenia) | ||
| Rheumatologist (osteoporosis or osteopenia) | ||
| Internist (palpitations related to beta-agonist therapy) | ||
| Visits to hospital | Exacerbation of asthma* | Chest radiograph |
| X-ray of neck (calcification in tonsil) | ||
| X-ray of nose | ||
| Computed tomography of the lungs | ||
| Computed tomography of the sinuses | ||
| Ultrasonography of the sinuses | ||
| Nasal polypectomy | ||
| Holter monitoring | ||
| Stress test | ||
| Cardiopulmonary exercise test | ||
| Bone density scan |
The number of patients that visited the emergency room for exacerbations of asthma was similar in the sputum strategy group and the clinical strategy group (3), and the maximum time spent in the emergency room was up to one day
Measurement of costs
The cost analysis was undertaken from the perspective of a third-party payer. Although the use of health care resources and the use of drugs were recorded during the trial, the associated unit costs were collected after the trial for the fiscal year 2006 to 2007. The unit costs of physician and hospital services were obtained from the Ontario fee schedule (3). The total cost of visits to the family physician or specialist was obtained by multiplying the unit cost of physician fees for the visit by the number of visits during phase 2 of the trial. The total cost of physician or hospital service was obtained by multiplying the unit cost of each service by the number of times the patient availed that service during phase 2 of the study. The total cost of health care resources in each strategy was the sum of the costs of visits to the family physician, visits to the specialist, and physician and hospital services. The unit costs of drugs were obtained from drug plans regulated by Health Canada. The total cost of each drug was obtained by multiplying the cumulative dose of the drug used during phase 2 of the trial by the respective unit cost. The total cost of drugs used in each strategy was the sum of the costs of the prescribed short-acting beta-agonists, LABAs, inhaled corti-costeroids, prednisone and antibiotics. The total cost of each strategy was the sum of the cost of health care resources and the cost of drugs. The cost of sputum induction (which included spirometry) ($48), processing and examination of sputum ($20.80), and the professional fee for supervision and interpretation of the report ($32) was added to the total cost for patients in the SS only. The costs of the technical and professional components of spirometry were $21.93 and $15.50, respectively, and were added to the total cost for patients in the CS only. The costs of these tests are according to the current government insurance plan billing codes (3).
Statistical analysis
Because of a secondary analysis of data from one of the four centres that participated in the main study (2), the sample size was limited by the available data. Descriptive statistics were used to summarize the clinical and sociodemographic characteristics of the patients, physician and hospital-related visits, use of drugs and cost data. Values are reported as median (minimum to maximum) or mean ± SD for continuous or discrete variables, and count (per cent) for categorical variables. The t test was used to examine the cost differences between the SS and CS, and as a sensitivity analysis. The quantile-quantile plot was used to assess the normality assumption, and the Mann-Whitney U test was used to compare the groups. Statistical significance was set at alpha = 0.05. All statistical analyses were performed using SPSS version 13 (SPSS Inc, USA) for Windows statistical software.
RESULTS
The clinical characteristics of the 39 Hamilton patients at the end of phase 1 were similar to the population in the effectiveness study (Table 2).
TABLE 2.
Clinical characteristics of asthma patients in the Hamilton centre and the study population at the end of phase 1 of the clinical trial
| Clinical characteristic |
Hamilton cohort |
Total cohort |
||
|---|---|---|---|---|
| CS (n=20) | SS (n=19) | CS (n=52) | SS (n=50) | |
| Age, years* | 46.3±11.0 | 46.6±12.0 | 44±14 | 46±14 |
| Male sex, % | 40 | 26.3 | 29 | 30 |
| Duration of disease, years* | 20.4 ±14.2 | 14.9±10.6 | 19.3±12.2 | 20±17 |
| Atopy, % | 90 | 94.7 | 90.2 | 90.2 |
| FEV1, % predicted* | 75.5±21.2 | 81.8±17.3 | 78.7±18.9 | 78.4±18.3 |
| Dose of inhaled corticosteroid, (μg)† | 800 (0 to 3200) | 800 (0 to 3200) | 500 (0 to 2000) | 500 (0 to 4000) |
Values are expressed as mean ± SD;
Values are expressed as median (minimum to maximum). CS Clinical strategy; FEV1 Forced expiratory volume in 1 s; SS Sputum strategy
The total number of patients in Hamilton was 39 (19 in the SS and 20 in the CS). Their clinical (Table 2) and sociodemographic (Table 3) characteristics; visits to the family physician, specialist and hospital for exacerbations of asthma; visits to the specialist and hospital for health disorders indirectly related to asthma (Table 4); and drugs used to maintain control of the disease were similar in both treatment strategies (Table 5). However, in the SS, the number of visits to the family physician for health disorders indirectly related to asthma (Table 4) and the dose of LABA (Table 5) were significantly less than those in the CS.
TABLE 3.
Sociodemographic characteristics of patients in the sputum and clinical strategies
| Sociodemographic characteristic, % | Sputum strategy (n=19) | Clinical strategy (n=20) |
|---|---|---|
| Working full time | 42.1 | 63.2 |
| Working part time | 26.3 | 21.1 |
| Retired | 21.1 | 5.3 |
| Government disability | 5.3 | 5.3 |
| Student and working part time | 5.3 | 5.3 |
| Annual income ≤$20,000 | 31.6 | 36.8 |
| Lost income from days missed from work | 71.4 | 70.6 |
| Has a drug plan | 78.9 | 85.0 |
| Pays a certain percentage of the drug plan | 83.3 | 92.9 |
TABLE 4.
Visits to family physician, specialist and hospital
| Sputum strategy (n=19) | Clinical strategy (n=20) | P | |
|---|---|---|---|
| Asthma directly related to | |||
| Family physician visits | 0 (0 to 2) | 0 (0 to 3) | 0.629 |
| Specialist visits | 0 (0) | 0 (0 to 1) | 0.330 |
| Hospital visits | 0 (0 6) | 0 (0 to 1) | 0.262 |
| Asthma indirectly related to | |||
| Family physician visits | 0 (0 to 3) | 1 (0 to 9) | 0.003 |
| Specialist visits | 0 (0 to 3) | 0 (0 to 6) | 0.911 |
| Hospital visits | 0 (0 to 1) | 0 (0 to 2) | 0.716 |
All values are expressed as median (minimum to maximum)
TABLE 5.
Use of drugs in the two treatment strategies
| Sputum strategy (n=19) | Clinical strategy (n=20) | P | |
|---|---|---|---|
| SABA puffs | 0 (0 to 1100) | 165.3 (0 to 1430) | 0.067 |
| LABA dose (mg) | 0 (0 to 52) | 15.6 (0 to 61) | 0.007 |
| ICS dose* (mg) | 294 (0 to 1965) | 565.6 (159 to 1294) | 0.109 |
| Proportion of patients requiring prednisone, % | 21 | 25 | 0.535 |
| Prednisone tablets† | 0 (0 to 360) | 0 (0 to 135) | 0.79 |
| Antibiotic courses | 1 (0 to 4) | 1 (0 to 6) | 0.905 |
All values are expressed as median (minimum to maximum).
Fluticasone is equivalent to 432 mg and 1004 mg of beclomethasone dipropionate in SS and CS, respectively, and budesonide is equivalent to 543.4 mg and 570.4 mg of beclomethasone dipropionate in the SS and CS, respectively;
A course included 30 mg prednisone for five days, followed by a reduction of 5 mg daily. CS Clinical strategy; ICS Inhaled corticosteroid; LABA Long-acting beta-agonist; SABA Short-acting beta-agonist; SS Sputum strategy
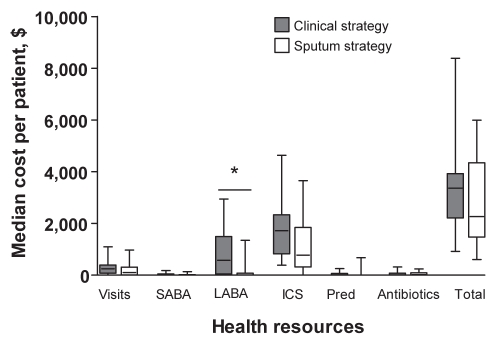
While the total estimated median cost per patient for spirometry ($393; range $299 to $487) was less than that for sputum induction ($1,008; range $907 to $1,411), the total cost of the SS ($2,265; range $1,466 to $4,347) was less than that of the CS ($3,369; range $2,208 to $3,927) (P=0.216), which amounts to a 19% reduction (95% CI 14% to 51%). This cost difference was the result of lower costs of physician and hospital-related visits and services ($106 [range $0 to $313] versus $247 [range $74 to $396]) (P=0.078), inhaled short-acting bronchodilators ($0 [range $0 to $23] versus $21 [range $0 to $44]) (P=0.067), inhaled LABAs ($0 [range $0 to $80] versus $573 [range $40 to $1,494]) (P=0.002) and inhaled corticosteroids ($775 [range $312 to $1845] versus $1722 [range $818 to $2337]) (P=0.064) in the SS versus CS. There was no significant difference in the cost of prednisone ($0 [range $0 to $0] versus $0 [range $0 to $63]) and antibiotics ($21 [range $0 to $92] versus $12 [range $0 to $78]) between the two treatment strategies (Figure 1).
Figure 1).
The costs of the different health resources in the two treatment strategies in the management of moderate to severe asthma. *P<0.05. ICS Inhaled corticosteroid; LABA Long-acting beta-agonist; Pred Prednisone; SABA Short-acting beta-agonist
When the cost of induction ($48) was not included in the cost of sputum examination at routine and exacerbation visits in the SS, the cost of the latter could be $28 up to $2197 less than that of the CS (P=0.045) (Table 6).
TABLE 6.
Sensitivity analysis – an independent t test to compare the costs of the two treatment strategies*
| Treatment strategy | Mean cost, $ | Mean difference, $ | P | 95% CI of the difference, $ |
|---|---|---|---|---|
| Sputum strategy (n=19) | 2,240±1,620 | 1,112 | 0.045 | 28 to 2,197 |
| Clinical strategy (n=20) | 3,352±1,717 |
When the cost of sputum induction was not included in the cost of sputum examination in the SS, the cost of the latter could be $28 up to $2,197 less than the CS
DISCUSSION
In addition to being more effective, the use of sputum cell counts to guide treatment was, on average, 19% less costly than the optimal clinical monitoring in patients with moderate to severe asthma. This cost difference was the result of lower costs of physician and hospital visits and services, and lower cost of drugs used to maintain control of the disease in the SS. Because the study was not adequately powered to examine cost differences between the two strategies, we cannot be certain that this is not due to chance.
The greatest strength of the present cost analysis is that our findings are based on effectiveness data of sputum cell counts from a randomized, controlled trial (2). The main weakness is that the economic analysis was evaluated in only one of the four centres, because we could not compare the costs across different provinces and countries, and funding to do the analysis was available in one centre only. The economic analysis was secondary and therefore was underpowered, which could explain the lack of overall statistically significant findings. We did not account for the number of days lost to short-term and long-term asthma-related disability, compensation payments made to people with disability, productivity loss for caretakers, travelling and waiting time. During the clinical trial, the cost of induction was considered for every routine visit and exacerbation. However, in a real-life setting, sputum induction might not be needed at every exacerbation, when it can be expectorated spontaneously, which would further reduce the cost of the SS. Indeed, when the sensitivity analysis was performed to assess the cost of spontaneous sputum in the SS, the cost of the latter was significantly less than that of the CS (Table 6). Also, once the minimum maintenance dose of corticosteroid has been established, it is not necessary to examine sputum cell counts every three months, as was done in the present study. In clinical practice, we would only examine them at exacerbations or when considering the need to readjust steroid treatment (4). Hence, in contrast to the research study, sputum will be examined less frequently in the real-life scenario, which may further reduce the cost of the SS.
A wide range of economic studies in asthma have examined end points such as total cost of disease (5), quality-adjusted life-years (6), improvement in airway function (7,8), symptom-free days (7), patient satisfaction (9), activity limitation (10), exacerbations (11) and cost benefit (12) in terms of a reduction in the use of health care resources. Sputum cell counts are a cost-effective alternative to peak expiratory flow for diagnosis of occupational asthma (13) and was stated to be less costly by Green et al (1); however, no cost analysis of SS versus CS has been published from a Canadian perspective. Our study differed in a number of ways from the latter. The resource use data are based on a different clinical trial, in a different clinical setting, based on recent estimates. We included a detailed analysis of the health care resources and drugs used by the patients during the trial. Despite these differences, the results of the current study confirm that using induced sputum cell counts to manage patients with moderate to severe asthma may be less costly than the traditional approach, which uses symptoms and spirometry.
The sociodemographic characteristics of patients were similar between the two treatment strategies, which further substantiates the need for a more effective and less costly strategy. Although there were no significant differences in the frequency of visits to the family physician, specialist and hospital for uncontrolled asthma, as well as the frequency of visits to the specialist and hospital for health disorders indirectly related to asthma, between the two treatment strategies, the cost of physician and hospital services were lower in the SS. This cost difference in the SS versus CS was because 47% versus 80% of patients, respectively, less frequently visited (zero to three visits versus zero to nine visits, respectively) the family physician for health disorders indirectly related to asthma, and 63% versus 95% of patients used physician or hospital services.
Because asthma was better controlled in the sputum arm, it follows that patients used short-acting bronchodilators less frequently in this group. The lack of sputum cell counts in the clinical arm meant that the physician had to guess whether continuing symptoms required an increase in the inhaled corticosteroid dose or the addition of an inhaled LABA, which could possibly account for the misuse of the latter two drugs in this group. The percentage of patients who required two or more courses of prednisone was less in the SS (15.8% versus 20%) and is consistent with better control of the disease in the SS. A greater proportion of patients in the CS than in the SS were treated with at least one course of antibiotics (30% versus 21.1%). Because the type of airway inflammation was identified in the sputum group, unnecessary treatment with antibiotics was avoided, in keeping with the paradigm of not treating eosinophilic exacerbations with antibiotics (14). However, there was no significant difference in the cost of prednisone and antibiotics between the two treatment strategies, which can be explained by one patient in the SS who required a greater amount of prednisone and antibiotics for uncontrolled asthma and recurrent respiratory tract infections, respectively.
In contrast to other noninvasive measurements of airway inflammation (such as exhaled nitric oxide, which was not effective in reducing asthma exacerbations [15]), the clinical study (2) and the present economic analysis together support a win-win situation, ie, the use of quantitative sputum cell counts is more effective and is likely to be less costly in the management of moderate to severe asthma. Although we need to conduct an adequately powered economic analysis, the current report emphasizes the need to consider implementing sputum induction into routine clinical practice, which will eventually reduce costs for asthma incurred by the health care system.
Footnotes
FUNDING: The Hamilton Community Health, Education and Research Fund supported this study. Dr Parameswaran Nair is supported by a Canada Research Chair in Airway Inflammometry.
DISCLOSURE OF POTENTIAL CONFLICT OF INTEREST: The authors declare no conflict of interests and have no personal or financial involvement in any organization with a financial interest in the subject matter.
REFERENCES
- 1.Green RH, Brightling CE, McKenna S, et al. Asthma exacerbations and sputum eosinophil counts: A randomised controlled trial. Lancet. 2002;360:1715–21. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- 2.Jayaram L, Pizzichini MM, Cook RJ, et al. Determining asthma treatment by monitoring sputum cell counts: Effect on exacerbations. Eur Respir J. 2006;27:483–94. doi: 10.1183/09031936.06.00137704. [DOI] [PubMed] [Google Scholar]
- 3.Schedule of Benefits for Physician Services. Ministry of Health and Long-Term Care; Ontario: 2006. [Google Scholar]
- 4.Hargreave FE. Quantitative sputum cell counts as a marker of airway inflammation in clinical practice. Curr Opin Allergy Clin Immunol. 2007;7:102–6. doi: 10.1097/ACI.0b013e328013e3c2. [DOI] [PubMed] [Google Scholar]
- 5.Seung SJ, Mittmann N. Urgent care costs of uncontrolled asthma in Canada, 2004. Can Respir J. 2005;12:435–6. doi: 10.1155/2005/478764. [DOI] [PubMed] [Google Scholar]
- 6.Paltiel AD, Fuhlbrigge AL, Kitch BT, et al. Cost-effectiveness of inhaled corticosteroids in adults with mild-to-moderate asthma: Results from the asthma policy model. J Allergy Clin Immunol. 2001;108:39–46. doi: 10.1067/mai.2001.116289. [DOI] [PubMed] [Google Scholar]
- 7.Barnes NC, Thwaites RM, Price MJ. The cost-effectiveness of inhaled fluticasone propionate and budesonide in the treatment of asthma in adults and children. Respir Med. 1999;93:402–7. doi: 10.1053/rmed.1999.0577. [DOI] [PubMed] [Google Scholar]
- 8.Menendez R, Stanford RH, Edwards L, Kalberg C, Rickard K. Cost-efficacy analysis of fluticasone propionate versus zafirlukast in patients with persistent asthma. Pharmacoeconomics. 2001;19:865–74. doi: 10.2165/00019053-200119080-00008. [DOI] [PubMed] [Google Scholar]
- 9.Perera BJ. Efficacy and cost effectiveness of inhaled steroids in asthma in a developing country. Arch Dis Child. 1995;72:312–5. doi: 10.1136/adc.72.4.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolton MB, Tilley BC, Kuder J, Reeves T, Schultz LR. The cost and effectiveness of an education program for adults who have asthma. J Gen Intern Med. 1991;6:401–7. doi: 10.1007/BF02598160. [DOI] [PubMed] [Google Scholar]
- 11.Berggren F, Ekström T. A cost-effectiveness study comparing the as-needed use of formoterol (Oxis) and terbutaline (Bricanyl) in patients with moderate to severe asthma. Respir Med. 2001;95:753–8. doi: 10.1053/rmed.2001.1131. [DOI] [PubMed] [Google Scholar]
- 12.Evans MF, Frank J. Efficacy and cost benefit of inhaled corticosteroids in patients considered to have mild asthma in primary care practice. Can Fam Physician. 1997;43:632–3. [Google Scholar]
- 13.Kennedy WA, Girard F, Chaboillez S, et al. Cost-effectiveness of various diagnostic approaches for occupational asthma. Can Respir J. 2007;14:276–80. doi: 10.1155/2007/206519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pavord ID, Brightling CE, Woltmann G, Wardlaw AJ. Non-eosinophilic corticosteroid unresponsive asthma. Lancet. 1999;353:2213–4. doi: 10.1016/S0140-6736(99)01813-9. [DOI] [PubMed] [Google Scholar]
- 15.Smith AD, Cowan JO, Brassett KP, Herbison GP, Taylor DR. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med. 2005;352:2163–73. doi: 10.1056/NEJMoa043596. [DOI] [PubMed] [Google Scholar]