Abstract
Purpose
Cyclooxygenase-2 (COX-2, PTGS2) is considered to play an important role in colorectal carcinogenesis, and is often upregulated in colon cancers. However, previous data on the influence of COX-2 expression on patient outcome have been conflicting.
Experimental Design
Utilizing 662 colon cancers (stage I-IV) in two independent prospective cohorts (the Nurses' Health Study and the Health Professionals Follow-up Study), we detected COX-2 overexpression in 548 tumors (83%) by immunohistochemistry. Cox proportional hazard models were used to compute hazard ratios (HRs) of colon cancer-specific and overall mortalities, adjusted for patient characteristics and related molecular events, including the CpG island methylation phenotype (CIMP), microsatellite instability (MSI) and p53, KRAS and BRAF mutations.
Results
During follow-up of the 662 cases, there were 283 deaths, including 163 colon cancer-specific deaths. Patients with COX-2-positive tumors showed a trend towards an inferior colon cancer-specific mortality [HR 1.37; 95% confidence interval (CI), 0.87-2.14], which became significant after adjusting for tumor stage and other predictors of clinical outcome (multivariate HR 1.70; 95% CI, 1.06-2.74, p=0.029). Notably, the effect of COX-2 expression on survival might differ according to p53 status (Pinteraction=0.04). Compared to tumors with both COX-2 and p53 negative, COX-2-positive tumors were significantly associated with an increased cancer-specific mortality (multivariate HR 2.12; 95% CI, 1.23-3.65) regardless of p53 status. A similar trend was observed when overall mortality was used as an outcome.
Conclusion
COX-2 overexpression is associated with worse survival among colon cancer patients. The effect of COX-2 on clinical outcome may be modified by p53 status.
Keywords: colorectal cancer, PTGS2, outcome, survival, cyclooxygenase
INTRODUCTION
Cyclooxygenase-2 (COX-2, PTGS2) converts arachidonic acid to prostaglandins and related eicosanoids, and promotes inflammation and cell proliferation (1, 2). COX-2 is overexpressed in the majority of human colon cancers (2-4). Supporting the importance of COX-2 in colorectal carcinogenesis, randomized trials have demonstrated that aspirin and COX-2 selective inhibitors reduce risk of recurrent adenoma among high-risk patients (5-7).
Despite the well-accepted role of COX-2 in tumor development (2), studies are conflicting regarding prognostic significance of COX-2 in colorectal cancer with some (3, 8, 9) supporting and others (4, 10-16) refuting an independent adverse effect of COX-2 overexpression. COX-2 overexpression has been positively associated with p53 alteration (17, 18), and inversely associated with microsatellite instability (MSI) (18-20), which generally predicts longer survival of colon cancer patients (21). Moreover, COX-2 and p53 appear to regulate each other in a complex manner (17, 22, 23). Thus, effect of COX-2 on patient survival can possibly be confounded by p53 alteration, MSI and other related molecular events.
In this study using a large number (N=662) of colon cancer patients in two independent cohort studies, we have examined the effect of tumoral COX-2 expression on patient outcome, adjusted for tumor stage and other potential predictors of clinical outcome. Since we concurrently assessed tumoral molecular alterations including p53, KRAS and BRAF mutations, MSI, and the CpG island methylator phenotype (CIMP), we could evaluate the independent effect of COX-2 expression after controlling for these related molecular events.
MATERIALS AND METHODS
Study Population
We utilized the databases of two large prospective cohort studies; the Nurses' Health Study (N = 121,700 women followed since 1976) (24, 25), and the Health Professional Follow-up Study (N = 51,500 men followed since 1986) (25). On each biennial follow-up questionnaire, participants were asked whether they had a diagnosis of colon cancer during the previous 2 years. When a participant (or next of kin for decedents) reported colon cancer, we sought permission to obtain medical records. Study physicians, while blinded to exposure data, reviewed all records related to colon cancer, and recorded AJCC (American Joint Committee on Cancer) tumor stage and tumor location. For nonresponders, we searched the National Death Index to discover deaths and ascertain any diagnosis of colon cancer that contributed to death or was a secondary diagnosis. Approximately 96% of all incident colon cancer cases were identified through these methods. We collected paraffin-embedded tissue blocks from hospitals where colon cancer patients underwent resections of primary tumors (25). Tissue sections from all colon cancer cases were reviewed and confirmed by a pathologist (S.O.). Tumor grade was categorized as high (≤50% glandular area) or low (>50% glandular area). Based on availability of tissue samples, we included a total of 662 colon cancer cases (287 from the men's cohort and 375 from the women's cohort) diagnosed up to 2002. Written informed consent was obtained from all subjects. This study was approved by the Human Subjects Committees at Brigham and Women's Hospital and the Harvard School of Public Health.
Measurement of Mortality
Patients were observed until death or June 2006, whichever came first. Ascertainment of deaths included reporting by the family or postal authorities. In addition, the names of persistent nonresponders were searched in the National Death Index. The cause of death was assigned by physicians blinded to other clinical and lifestyle information. More than 98% of deaths in the cohorts were identified by these methods.
Immunohistochemistry for COX-2 and p53
Tissue microarrays (TMAs) construction and immunohistochemical examination for COX-2 and p53 were performed as previously described (18). p53 positivity was defined as 50% or more of tumor cells with unequivocal strong nuclear staining. These criteria were based on the observations that the 50% cutoff appeared to increase specificity of p53 immunohistochemistry to correlate with the presence of TP53 mutation (26-28). Our data also indicated that MSI-high or CIMP-high was uncommon (6.3-8.1%) in tumors with p53 positivity in ≥50% tumor cells, while CIMP-high or MSI-high was more frequent (22-27%) in tumors with p53 positivity in <50% tumor cells as well as tumors without p53 staining.
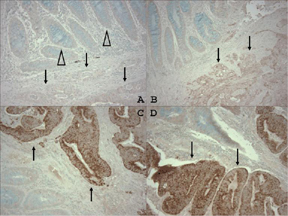
For COX-2 immunohistochemistry, antigen retrieval was performed by incubating deparaffinized tissue sections in citrate buffer (BioGenex) by a microwave for 15 min, and let the sections cool for at least 40 min. Tissue sections were incubated with 3% H2O2 (20 min) to block endogenous peroxidase, and then incubated with Avidin Block (Vector Laboratories) (15 min), than with Biotin Block (Vector Laboratories) (15 min). Primary anti-COX-2 antibody (Cayman Chemical, Ann Arbor, MI) (dilution 1:300) was applied for overnight at 4°C. Then, secondary anti-mouse antibody (Vector Laboratories) was applied (20 min), avidin biotin complex conjugate (Vector Laboratories) was added and sections were visualized by diaminobenzidine (DAB) (5 min) and methyl-green counterstain. For each assay run, we included a positive control (cancer with COX-2 overexpression) and a negative control (normal colonic tissue). We also treated a positive control specimen with phosphate-buffered saline without anti-COX-2 antibody. A pathologist (S.O.), unaware of other data, interpreted cytoplasmic COX-2 expression in tumor as either absent, weak, moderate, or strong staining compared to adjacent normal colonic epithelium. Inflammatory cells served as internal built-in positive controls (18, 25). Consistent with other investigators (8, 29), if immunostaining intensity was moderate or strong, tumors were classified as cancers with COX-2 overexpression. If immunostaining intensity was weak or absent, tumors were classified as cancers with negative COX-2 overexpression (Figure 1). This classification has been previously shown to associate well with p53 expression and inversely with MSI and CIMP in colorectal cancer (18).
Figure 1. COX-2 Expression in Colon Cancer.
A. No COX-2 overexpression in colon cancer (arrow) or normal colonic mucosa (empty arrowheads). B. Weak COX-2 overexpression (COX-2 negative) in colon cancer (arrows). C. D. Strong COX-2 overexpression in colon cancer (arrows).
Appropriate positive and negative controls were included in each run of immunohistochemistry. All immunohistochemically-stained slides were interpreted by a pathologist (S.O.) unaware of other data. A random sample of 108 cases was re-examined for COX-2 expression by a second observer (R.D.) unaware of other data, and the concordance between the two observers was 0.92 (κ=0.62, p<0.0001), indicating substantial agreement. Another random sample of 118 tumors were re-examined for p53 by another observer (K.N.) unaware of other data, and the concordance between the two observers was 0.87 (κ=0.75, p<0.0001).
Genomic DNA Extraction and Sequencing of KRAS and BRAF
Genomic DNA from paraffin-embedded tissue and whole genome amplification of genomic DNA was performed as previously described (30). PCR and sequencing targeted for KRAS codons 12 and 13, and BRAF codon 600 were performed as previously described (30, 31).
Microsatellite Instability (MSI) Analysis
MSI status was determined using a microsatellite marker panel consisting of D2S123, D5S346, D17S250, BAT25, BAT26, BAT40, D18S55, D18S56, D18S67 and D18S487 (i.e., 10-marker panel) (32). A high degree of MSI (MSI-high) was defined as the presence of instability in ≥30% of the markers, MSI-low as the presence of instability in <30% of markers, and microsatellite stability (MSS) as no unstable marker.
Real-Time PCR (MethyLight) for Quantitative DNA Methylation Analysis
Sodium bisulfite treatment on DNA and MethyLight assays were validated and performed as previously described (33). We used ABI 7300 (Applied Biosystems, Foster City, CA) for quantitative real-time PCR [MethyLight (34)] on 8 CIMP-specific markers (CACNA1G, CDKN2A (p16), CRABP1, IGF2, MLH1, NEUROG1, RUNX3 and SOCS1) (32, 35). CIMP-high was defined as ≥6/8 methylated markers using the 8-marker CIMP panel, CIMP-low/0 as ≤5/8 methylated markers, and CIMP-0 as 0/8 methylated markers, according to the previously established criteria (32).
Statistical Analysis
We used Cox proportional hazard models to calculate hazard ratios (HRs) of death according to tumoral COX-2 status, unadjusted as well as adjusted for age, sex, year of diagnosis, tumor location, stage, grade, MSI, CIMP, KRAS, BRAF and p53. For analyses of colon cancer-specific mortality, death as a result of colon cancer was the primary end point and deaths as a result of other causes were censored. To adjust for potential confounding, age and year of diagnosis were used as continuous variables, and all of the other covariates were used as categorical variables. We dichotomized tumor location (proximal vs. distal), tumor grade (high vs. low), CIMP (high vs. low/0), MSI (high vs. low/MSS), p53 (positive vs. negative), KRAS (mutated vs. wild-type) and BRAF (mutated vs. wild-type). We assigned a separate indicator variable to each tumor stage (as in Table 1) in order to minimize residual confounding. When there was missing information on tumor location (1.2% missing), stage (7.4%), tumor grade (0.5% missing), MSI (3.0% missing), p53 (0.8% missing), KRAS (2.6% missing) or BRAF (4.8% missing), we assigned a separate (“missing”) indicator variable and included those cases in the multivariate analysis models. We confirmed that excluding cases with a missing variable did not significantly alter results (data not shown). An interaction was assessed by including the cross product of the COX-2 variable and another variable of interest in a multivariate Cox model, and the likelihood ratio test was performed. To assess an interaction of COX-2 and stage, we dichotomized tumor stage (I-II vs. III-IV). The Kaplan-Meier method was used to describe the distribution of colon cancer-specific and overall survival time, and the log-rank test was performed. The chi square test was used to examine an association between categorical variables. The t-test assuming unequal variances was performed to compare mean age. All analyses used SAS version 9.1 (SAS Institute, Cary, NC) and all p values were two-sided.
Table 1.
Clinical and molecular features of colon cancer according to COX-2 expression.
| Clinical or molecular feature | All cases | COX-2 negative | COX-2 positive | P value |
|---|---|---|---|---|
| Total N | 662 | 114 | 548 | |
| Sex | 0.59 | |||
| Male (HPFS) | 287 (43%) | 52 (46%) | 235 (43%) | |
| Female (NHS) | 375 (57%) | 62 (54%) | 313 (57%) | |
| Mean age ± SD | 66.5 ± 8.3 | 66.8 ± 7.4 | 66.5 ± 8.4 | 0.69 |
| Year of diagnosis | 0.05 | |||
| Prior to 1990 | 101 (15%) | 17 (15%) | 84 (15%) | |
| 1990 to 1999 | 482 (73%) | 91 (80%) | 391 (71%) | |
| 2000 to 2002 | 79 (12%) | 6 (5.3%) | 73 (13%) | |
| Tumor location^ | 0.005 | |||
| Proximal | 434 (59%) | 80 (71%) | 305 (56%) | |
| Distal | 297 (41%) | 33 (29%) | 236 (44%) | |
| Tumor stage | 0.77 | |||
| I | 136 (21%) | 25 (22%) | 111 (20%) | |
| IIA | 207 (31%) | 40 (35%) | 167 (30%) | |
| IIB | 20 (3.0%) | 1 (0.9%) | 19 (3.5%) | |
| IIIA | 21 (3.2%) | 2 (1.8%) | 19 (3.5%) | |
| IIIB | 88 (13%) | 13 (11%) | 75 (14%) | |
| IIIC | 55 (8.3%) | 13 (11%) | 42 (7.7%) | |
| IV | 86 (13%) | 15 (13%) | 71 (13%) | |
| Unknown | 49 (7.4%) | 5 (4.4%) | 44 (8.0%) | |
| Tumor grade | 0.0003 | |||
| Low | 585 (89%) | 90 (79%) | 495 (91%) | |
| High | 74 (11%) | 24 (21%) | 50 (9.2%) | |
| p53* | <0.0001 | |||
| (-) | 404 (61%) | 91 (81%) | 313 (58%) | |
| (+) | 253 (39%) | 22 (19%) | 231 (42%) | |
| MSI | 0.0009 | |||
| MSI-low/MSS | 521 (81%) | 77 (69%) | 446 (84%) | |
| MSI-high | 121 (19%) | 33 (31%) | 88 (16%) | |
| CIMP | 0.004 | |||
| CIMP-0 | 277 (42%) | 32 (28%) | 245 (45%) | |
| CIMP-low | 257 (39%) | 53 (46%) | 204 (37%) | |
| CIMP-high | 128 (19%) | 29 (25%) | 99 (18%) | |
| KRAS mutation | 0.55 | |||
| (-) | 411 (64%) | 68 (61%) | 343 (64%) | |
| (+) | 234 (36%) | 43 (39%) | 191 (36%) | |
| BRAF mutation | 0.24 | |||
| (-) | 525 (83%) | 85 (79%) | 440 (84%) | |
| (+) | 105 (17%) | 22 (21%) | 83 (16%) |
(%) indicates the proportion of tumors with a specific clinical or molecular feature in a given COX-2 subtype.
Proximal colon includes cecum to transverse colon, and distal colon includes splenic flexure to sigmoid colon.
p53 status was determined by immunohistochemistry.
CIMP, CpG island methylator phenotype; COX-2, cyclooxygenase-2 (PTGS2); HPFS, Health Professionals Follow-up Study; MSI, microsatellite instability; MSS, microsatellite stable; NHS, Nurses' Health Study; SD, standard deviation.
RESULTS
Cyclooxygenase-2 (COX-2) expression in colon cancers
Among the 662 tumors, 548 (83%) were positive for overexpression of COX-2, while 114 (17%) were negative for COX-2. We also examined p53 status (by immunohistochemistry), microsatellite instability (MSI), the CpG island methylator phenotype (CIMP), KRAS and BRAF mutations, because expressions of COX-2 and p53 were inversely related with MSI and CIMP (18), and MSI, CIMP and BRAF mutation have been related with patient outcome (21, 36-39). Table 1 summarizes clinical and molecular features of colon cancer according to COX-2 status. Notably, compared to COX-2 negative tumors, COX-2 positive tumors are more likely distal, low-grade, and p53-positive, and less likely MSI-high and CIMP-high.
COX-2 expression and patient survival in colon cancer
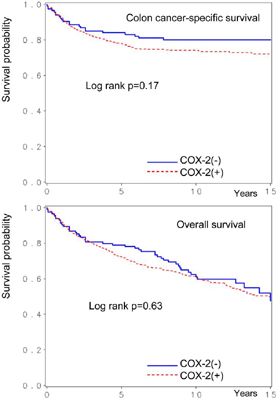
Among the 662 eligible patients with adequate follow-up, there were 283 deaths, including 163 colon cancer-specific deaths. We assessed the influence of COX-2 expression on patient survival. Five-year colon cancer-specific survival was 84% among patients with COX-2-negative tumors and 78% among patients with COX-2-positive tumors (log-rank p=0.17) (Figure 2). Five-year overall survival was 79% among patients with COX-2-negative tumors and 72% among those with COX-2-positive tumors (log-rank p=0.63).
Figure 2. Kaplan-Meier survival curves in colon cancer according to tumoral COX-2 status.
In univariate Cox regression analysis, COX-2 positivity was associated with a non-significant increase in colon cancer-specific mortality [hazard ratio (HR) 1.37; 95% confidence interval (CI), 0.87-2.14] (Table 2). In a multivariate model that adjusted for other clinical, pathologic and molecular predictors of survival, COX-2 positivity was associated with a significant increase in colon cancer-specific mortality (multivariate HR 1.70; 95% CI, 1.06-2.74). The increase in the effect of COX-2 positivity on survival in the multivariate analysis was mainly the result of adjusting for tumor stage; when we simply adjusted for tumor stage, the HR for colon cancer-specific mortality in COX-2 positive tumors was 1.57 (95% CI, 1.00-2.46). When we excluded stage IV cases, multivariate HR for colon cancer-specific mortality in COX-2 positive cases (vs. COX-2 negative cases) was 1.60 (95% CI, 0.81-3.13). Thus, the results did not change substantially.
Table 2.
COX-2 expression and survival among colon cancer patients
| Total N | Colon cancer-specific mortality | Overall mortality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Deaths/person-years | Univariate HR (95% CI) | Stage-adjusted HR (95% CI) | Multivariate HR (95% CI) | Deaths/person-years | Univariate HR (95% CI) | Stage-adjusted HR (95% CI) | Multivariate HR (95% CI) | ||
| COX-2 negative | 114 (17%) | 22/1041 | 1 (referent) | 1 (referent) | 1 (referent) | 47/1041 | 1 (referent) | 1 (referent) | 1 (referent) |
| COX-2 positive | 548 (83%) | 141/4834 | 1.37 (0.87-2.14) | 1.57 (1.00-2.46) | 1.70 (1.06-2.74) | 236/4834 | 1.08 (0.79-1.48) | 1.21 (0.88-1.66) | 1.21 (0.87-1.69) |
| P value | 0.17 | 0.051 | 0.029 | 0.63 | 0.23 | 0.26 | |||
The multivariate Cox model includes age, year of diagnosis, sex, tumor location, stage, grade, and statuses of KRAS, BRAF, p53, microsatellite instability, and CpG island methylator phenotype.
CI, confidence interval; COX-2, cyclooxygenase-2 (PTGS2); HR, hazard ratio.
COX-2 expression did not significantly influence overall mortality in both univariate and multivariate analyses (Table 2). High tumor grade was associated with an increased colon cancer-specific mortality (multivariate HR 2.07; 95% CI, 1.17-3.66). p53 positivity was not a significant predictor of survival in both univariate and multivariate analyses (multivariate HR for colon cancer-specific mortality, 1.34, 95% CI, 0.92-1.94).
Association between COX-2 expression and patient survival in various strata
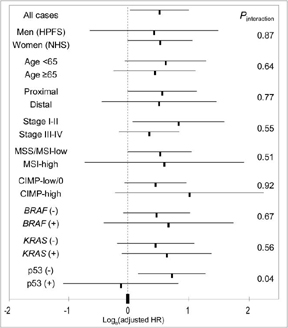
We examined whether the effect of COX-2 expression on survival was modified by any of the clinical and molecular variables (Figure 3). The effect of COX-2 overexpression on colon cancer-specific mortality was not significantly different across most strata of patient and disease characteristics. Notably, the effect of COX-2 overexpression was similar across the two independent cohort studies (p for interaction =0.87). We did observe an apparently significant effect of p53 expression on the association between COX-2 and mortality (p for interaction =0.04). A significant adverse effect of COX-2 overexpression was present in p53-negative tumors, but not among p53-positive tumors.
Figure 3. Stratified analysis of colon cancer-specific mortality in COX-2(+) tumors.
Loge(adjusted HR) with 95% CI for COX-2 positive tumors (vs. COX-2 negative tumors) in various strata is shown. Note that loge(HR) is great than 0 (i.e., HR>1) in most strata [except for p53(+)], indicating that COX-2 is associated with increased mortalities.
CI, confidence interval; CIMP, CpG island methylator phenotype; HPFS, Health Professionals Follow-up Study; HR, hazard ratio; MSI, microsatellite instability; NHS, Nurses' Health Study.
Combined COX-2 and p53 status and patient survival
Since there was evidence for effect modification by p53 status on the association between COX-2 and patient survival, we stratified tumors by combined status of COX-2 and p53 (Table 3). Compared to tumors that were negative for both COX-2 and p53, COX-2-positive tumors (regardless of p53 status) were associated with a significant increase in cancer-specific mortality (multivariate HR 2.12, 95% CI, 1.23-3.65). At the same time, p53 status had little effect on mortality among COX-2-positive tumors.
Table 3.
Combined COX-2 and p53 status and patient survival in colon cancer
| Combined COX-2/p53 status | Total N | Colon cancer-specific mortality | Overall mortality | ||||
|---|---|---|---|---|---|---|---|
| Deaths/person-years | Univeriate HR (95% CI) | Multivariate HR (95% CI) | Deaths/person-years | Univeriate HR (95% CI) | Multivariate HR (95% CI) | ||
| COX-2(-) p53(-) | 91 | 16/844 | 1 (referent) | 1 (referent) | 37/844 | 1 (referent) | 1 (referent) |
| COX-2(-) p53(+) | 22 | 5/197 | 1.30 (0.48-3.55) | 2.58 (0.89-7.48) | 9/197 | 1.04 (0.50-2.16) | 1.52 (0.71-3.24) |
| COX-2(+) p53(-) | 313 | 85/2689 | 1.61 (0.95-2.75) | 2.09 (1.19-3.65) | 134/2689 | 1.13 (0.79-1.63) | 1.35 (0.92-1.98) |
| COX-2(+) p53(+) | 231 | 56/2089 | 1.41 (0.81-2.46) | 2.20 (1.20-4.03) | 102/2089 | 1.12 (0.77-1.63) | 1.40 (0.93-2.11) |
| COX-2(+) total | 544 | 141/4778 | 1.53 (0.91-2.56) | 2.12 (1.23-3.65) | 236/4778 | 1.12 (0.79-1.59) | 1.37 (0.95-1.98) |
The multivariate analysis model includes age at diagnosis, year of diagnosis, sex, tumor location, stage, grade, and statuses of KRAS, BRAF, microsatellite instability, and CpG island methylator phenotype.
p53 status was determined by immunohistochemistry.
CI, confidence interval; COX-2, cyclooxygenase-2 (PTGS2); HR, hazard ratio.
DISCUSSION
We conducted this study to examine the influence of cyclooxygenase-2 (COX-2, PTGS2) expression on outcome of colon cancer patients. We have found that COX-2 overexpression appears to predict an inferior cancer-specific survival, independent of various clinical and molecular variables. The adverse effect of COX-2 overexpression was consistent across most strata of patient and tumoral characteristics, in particular across the two independent cohort studies. Our data support an adverse effect of COX-2 overexpression on survival of colon cancer patients.
Considerable experimental evidence supports a role of COX-2 in colorectal carcinogenesis (2). Randomized, placebo-controlled trials have uniformly shown that selective COX-2 inhibitors prevent adenoma recurrence among patients with a prior history of adenoma (5, 6). COX-2, possibly through production of inflammatory prostaglandins, may regulate angiogenesis, apoptosis, or tumor cell invasiveness (2, 40). We have previously shown that aspirin use decreases a risk for colon cancers that are positive for COX-2, but not a risk for COX-2-negative cancers, providing additional evidence for a role of COX-2 in colon carcinogenesis (25).
Studying molecular alterations and clinical outcome is important in cancer research (41-48). Our data support a role of COX-2 in determining biological behavior of colon cancer. COX-2 has been examined as a predictive biomarker in cancer (3, 8, 9). Previous studies are conflicting regarding prognostic significance of COX-2 in colorectal cancer with some (3, 8, 9) supporting and others (4, 10-16) refuting independent adverse effect of COX-2. These discrepant results are likely due to differences in patient cohorts, COX-2 detection methods, criteria for COX-2 overexpression, and multivariate survival analysis models. Our current study has comprehensively examined the effect of COX-2 on patient survival independent of clinical characteristics and other molecular events, including statuses of p53 alterations, mutations in KRAS and BRAF, microsatellite instability (MSI) and the CpG island methylator phenotype (CIMP). All of these molecular events are potential confounders for the association between COX-2 and patient survival.
The relationship between COX-2 overexpression and p53 alteration has been examined previously. In one in vivo study, inhibition of COX-2 by celecoxib led to p53 activation in colon cancer cells (22). In other studies, COX-2 expression was inhibited by wild-type p53 in murine embryo cell lines (17), whereas COX-2 overexpression was induced by p53 and nuclear factor-kappa B (NFKB1) in esophageal and colon cancer cells (23). It may be possible that COX-2 and p53 regulate each other to form a feedback loop. Thus, it may not be surprising to find a significant interactive effect of COX-2 and p53 alterations on patient survival. This possible interaction of COX-2 and p53 alterations needs to be further examined and confirmed by future studies.
Our study has several advantages including a large number of colon cancers in the two prospective cohort studies with adequate follow-up, as well as extensive data on disease characteristics and other important tumoral molecular events. Thus, we have been able to demonstrate an effect of COX-2 on patient survival, independent of clinical and other tumoral predictors of clinical outcome.
As a limitation of this study, data on cancer treatment are limited in our cohorts. Nonetheless, it is unlikely that chemotherapy use differed according to tumoral COX-2 status, especially since such data were not available to patients or treating physicians. In addition, beyond cause of mortality, data on cancer recurrences were not available in these cohorts. Nonetheless, given the median survival for metastatic colon cancer was approximately 10 to 12 months during much of the time period of this study, colon cancer-specific survival should be a reasonable surrogate for cancer-specific outcomes. Despite the apparent effects of COX-2 expression on colon cancer-specific mortality, the influence of COX-2 on all-cause mortality was considerably attenuated. This is likely due to deaths unrelated to colon cancer in our cohort studies.
There is variability in grading COX-2 expression and presently there is no widely accepted standardized classification scheme. False positive and false negative results are well-known problems in immunohistochemistry. Nonetheless, previous studies have demonstrated that Western and Northern blot analysis highly correlate with immunohistochemical expression of COX-2 (49), and our classification of COX-2 overexpression resulted in a similar proportion of COX-2 overexpressing tumors as other investigators (3, 4, 8-16). Moreover, we assessed COX-2 overexpression through central, blinded review of tumor specimens with rigorous comparison to internal controls with the substantial inter-observer agreement (92%, κ=0.62). Our COX-2 expression data in relation to MSI and CIMP are in agreement with studies by other investigators (18, 19, 50). Finally, any random misclassification of COX-2 status would have conservatively biased our results toward finding no significant difference in patient survival according to tumoral COX-2 expression.
In conclusion, this large prospective study of colon cancer patients suggests that COX-2 upregulation is independently associated with a worse colon cancer-specific mortality. In addition, when compared to patients with tumors negative for both COX-2 and p53, patients with tumors positive for COX-2 exhibit longer survival regardless of p53 status. Our finding that COX-2 overexpression is associated with poor patient outcome may have significant clinical implications, considering an emerging role of COX-2 and its pathway as chemotherapeutic and chemopreventive targets.
ACKNOWLEDGEMENTS
We thank the Nurses' Health Study and Health Professionals Follow-up Study cohort participants who have generously agreed to provide us with biological specimens and information through responses to questionnaires. We thank hospitals and pathology departments throughout the United States for generously providing us with tissue materials from the participants who underwent resection of their colon cancer. We thank Frank Speizer, Walter Willett, Susan Hankinson, Graham Colditz, Meir Stampfer, and many other staff members who implemented and maintained the cohort studies.
This work was supported by the U.S. National Institute of Health (NIH) grants P01 CA87969, P01 CA55075, P50 CA127003 and K07 CA122826 (to S.O.), and in part by the Bennett Family Fund for Targeted Therapies Research, and by the Entertainment Industry Foundation (EIF) through the National Colorectal Cancer Research Alliance (NCCRA). K.N. was supported by a fellowship grant from the Japanese Society for Promotion of Science. Any of these funding agencies has not had any role in design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
This work was supported by the US National Institute of Health (NIH) grants P01 CA87969, P01 CA55075, P50 CA127003 and K07 CA122826 (to S.O.), and in part by the Bennett Family Fund for Targeted Therapies Research, and by the Entertainment Industry Foundation (EIF) through the EIF National Colorectal Cancer Research Alliance (NCCRA). K.N. was supported by a fellowship grant from the Japanese Society for Promotion of Science.
Abbreviations and the HUGO Gene Nomenclature Committee (HGNC)-approved official gene symbol
- AJCC
American Joint Committee on Cancer
- CI
confidence interval
- CIMP
CpG island methylator phenotype
- COX-2
cyclooxygenase-2 (PTGS2)
- HR
hazard ratio
- MSI
microsatellite instability
- MSS
microsatellite stable
- PTGS2 (the official symbol for COX-2)
prostaglandin-endoperoxide synthase 2
Footnotes
Statement of Translational Relevance COX-2 (cyclooxygenase-2) has been shown to play an important role in carcinogenesis in various organ systems including colon. COX-2 inhibitors (aspirin, NSAIDs and celecoxib) have been shown to be effective in preventing colorectal adenoma and cancer. However, the relation between COX-2 expression in colon cancer and patient survival has been controversial. We have utilized the database of more than 600 colon cancer in two independent, prospective cohort studies, with available clinical information, adequate follow-up, and other important molecular events in colon cancers. To our knowledge, this is the first study to demonstrate adverse effect of COX-2 overexpression on clinical outcome independent of related molecular events including BRAF mutation, MSI (microsatellite instability) and CIMP (the CpG island methylator phenotype), all of which are associated with both COX-2 expression and clinical outcome in colon cancer. Thus, our findings are relevant to practice in oncology.
REFERENCES
- 1.Buchanan FG, DuBois RN. Connecting COX-2 and Wnt in cancer. Cancer Cell. 2006;9:6–8. doi: 10.1016/j.ccr.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 2.Brown JR, DuBois RN. COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol. 2005;23:2840–55. doi: 10.1200/JCO.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 3.Soumaoro LT, Uetake H, Higuchi T, Takagi Y, Enomoto M, Sugihara K. Cyclooxygenase-2 expression: a significant prognostic indicator for patients with colorectal cancer. Clin Cancer Res. 2004;10:8465–71. doi: 10.1158/1078-0432.CCR-04-0653. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Sun XF. Overexpression of cyclooxygenase-2 correlates with advanced stages of colorectal cancer. Am J Gastroenterol. 2002;97:1037–41. doi: 10.1111/j.1572-0241.2002.05625.x. [DOI] [PubMed] [Google Scholar]
- 5.Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–84. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 6.Arber N, Eagle CJ, Spicak J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–95. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 7.Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369:1603–13. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 8.Tomozawa S, Tsuno NH, Sunami E, et al. Cyclooxygenase-2 overexpression correlates with tumour recurrence, especially haematogenous metastasis, of colorectal cancer. Br J Cancer. 2000;83:324–8. doi: 10.1054/bjoc.2000.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gustafsson A, Hansson E, Kressner U, et al. EP1-4 subtype, COX and PPAR gamma receptor expression in colorectal cancer in prediction of disease-specific mortality. Int J Cancer. 2007;121:232–40. doi: 10.1002/ijc.22582. [DOI] [PubMed] [Google Scholar]
- 10.Sheehan KM, Sheahan K, O'Donoghue DP, et al. The relationship between cyclooxygenase-2 expression and colorectal cancer. Jama. 1999;282:1254–7. doi: 10.1001/jama.282.13.1254. [DOI] [PubMed] [Google Scholar]
- 11.Liang JT, Huang KC, Jeng YM, Lee PH, Lai HS, Hsu HC. Microvessel density, cyclooxygenase 2 expression, K-ras mutation and p53 overexpression in colonic cancer. Br J Surg. 2004;91:355–61. doi: 10.1002/bjs.4447. [DOI] [PubMed] [Google Scholar]
- 12.Fux R, Schwab M, Thon KP, Gleiter CH, Fritz P. Cyclooxygenase-2 expression in human colorectal cancer is unrelated to overall patient survival. Clin Cancer Res. 2005;11:4754–60. doi: 10.1158/1078-0432.CCR-04-2586. [DOI] [PubMed] [Google Scholar]
- 13.Yamac D, Celenkoglu G, Coskun U, et al. Prognostic importance of COX-2 expression in patients with colorectal cancer. Pathol Res Pract. 2005;201:497–502. doi: 10.1016/j.prp.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Joo YE, Kim HS, Min SW, et al. Expression of cyclooxygenase-2 protein in colorectal carcinomas. Int J Gastrointest Cancer. 2002;31:147–54. doi: 10.1385/IJGC:31:1-3:147. [DOI] [PubMed] [Google Scholar]
- 15.Wu AW, Gu J, Ji JF, Li ZF, Xu GW. Role of COX-2 in carcinogenesis of colorectal cancer and its relationship with tumor biological characteristics and patients' prognosis. World J Gastroenterol. 2003;9:1990–4. doi: 10.3748/wjg.v9.i9.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim SC, Lee TB, Choi CH, Ryu SY, Min YD, Kim KJ. Prognostic significance of cyclooxygenase-2 expression and nuclear p53 accumulation in patients with colorectal cancer. J Surg Oncol. 2008;97:51–6. doi: 10.1002/jso.20907. [DOI] [PubMed] [Google Scholar]
- 17.Subbaramaiah K, Altorki N, Chung WJ, Mestre JR, Sampat A, Dannenberg AJ. Inhibition of cyclooxygenase-2 gene expression by p53. J Biol Chem. 1999;274:10911–5. doi: 10.1074/jbc.274.16.10911. [DOI] [PubMed] [Google Scholar]
- 18.Ogino S, Brahmandam M, kawasaki T, Kirkner GJ, Loda M, Fuchs CS. Combined analysis of COX-2 and p53 expressions reveals synergistic inverse correlations with microsatellite instability and CpG island methylator phenotype in colorectal cancer. Neoplasia. 2006;8:458–64. doi: 10.1593/neo.06247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karnes WE, Jr., Shattuck-Brandt R, Burgart LJ, et al. Reduced COX-2 protein in colorectal cancer with defective mismatch repair. Cancer Res. 1998;58:5473–7. [PubMed] [Google Scholar]
- 20.Sinicrope FA, Lemoine M, Xi L, et al. Reduced expression of cyclooxygenase 2 proteins in hereditary nonpolyposis colorectal cancers relative to sporadic cancers. Gastroenterology. 1999;117:350–8. doi: 10.1053/gast.1999.0029900350. [DOI] [PubMed] [Google Scholar]
- 21.Popat S, Hubner R, Houlston RS. Systematic Review of Microsatellite Instability and Colorectal Cancer Prognosis. J Clin Oncol. 2005;23:609–18. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 22.Swamy MV, Herzog CR, Rao CV. Inhibition of COX-2 in colon cancer cell lines by celecoxib increases the nuclear localization of active p53. Cancer Res. 2003;63:5239–42. [PubMed] [Google Scholar]
- 23.Benoit V, de Moraes E, Dar NA, et al. Transcriptional activation of cyclooxygenase-2 by tumor suppressor p53 requires nuclear factor-kappaB. Oncogene. 2006;25:5708–18. doi: 10.1038/sj.onc.1209579. [DOI] [PubMed] [Google Scholar]
- 24.Colditz GA, Hankinson SE. The Nurses' Health Study: lifestyle and health among women. Nat Rev Cancer. 2005;5:388–96. doi: 10.1038/nrc1608. [DOI] [PubMed] [Google Scholar]
- 25.Chan AT, Ogino S, Fuchs CS. Aspirin and the Risk of Colorectal Cancer in Relation to the Expression of COX-2. New Engl J Med. 2007;356:2131–42. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 26.Baas IO, Mulder JW, Offerhaus GJ, Vogelstein B, Hamilton SR. An evaluation of six antibodies for immunohistochemistry of mutant p53 gene product in archival colorectal neoplasms. J Pathol. 1994;172:5–12. doi: 10.1002/path.1711720104. [DOI] [PubMed] [Google Scholar]
- 27.Curtin K, Slattery ML, Holubkov R, Edwards S, Holden JA, Samowitz WS. p53 alterations in colon tumors: a comparison of SSCP/sequencing and immunohistochemistry. Appl Immunohistochem Mol Morphol. 2004;12:380–6. doi: 10.1097/00129039-200412000-00017. [DOI] [PubMed] [Google Scholar]
- 28.Hall PA, McCluggage WG. Assessing p53 in clinical contexts: unlearned lessons and new perspectives. J Pathol. 2006;208:1–6. doi: 10.1002/path.1913. [DOI] [PubMed] [Google Scholar]
- 29.Knosel T, Yu Y, Stein U, et al. Overexpression of cyclooxygenase-2 correlates with chromosomal gain at the cyclooxygenase-2 locus and decreased patient survival in advanced colorectal carcinomas. Dis Colon Rectum. 2004;47:70–7. doi: 10.1007/s10350-003-0008-7. [DOI] [PubMed] [Google Scholar]
- 30.Ogino S, Kawasaki T, Brahmandam M, et al. Sensitive sequencing method for KRAS mutation detection by Pyrosequencing. J Mol Diagn. 2005;7:413–21. doi: 10.1016/S1525-1578(10)60571-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogino S, Kawasaki T, Kirkner GJ, Loda M, Fuchs CS. CpG island methylator phenotype-low (CIMP-low) in colorectal cancer: possible associations with male sex and KRAS mutations. J Mol Diagn. 2006;8:582–8. doi: 10.2353/jmoldx.2006.060082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogino S, Kawasaki T, Kirkner GJ, Kraft P, Loda M, Fuchs CS. Evaluation of markers for CpG island methylator phenotype (CIMP) in colorectal cancer by a large population-based sample. J Mol Diagn. 2007;9:305–14. doi: 10.2353/jmoldx.2007.060170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogino S, kawasaki T, Brahmandam M, et al. Precision and performance characteristics of bisulfite conversion and real-time PCR (MethyLight) for quantitative DNA methylation analysis. J Mol Diagn. 2006;8:209–17. doi: 10.2353/jmoldx.2006.050135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eads CA, Danenberg KD, Kawakami K, et al. MethyLight: a high-throughput assay to measure DNA methylation. Nucleic Acids Res. 2000;28:E32. doi: 10.1093/nar/28.8.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weisenberger DJ, Siegmund KD, Campan M, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787–93. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- 36.Samowitz WS, Sweeney C, Herrick J, et al. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005;65:6063–9. doi: 10.1158/0008-5472.CAN-05-0404. [DOI] [PubMed] [Google Scholar]
- 37.Shen L, Catalano PJ, Benson AB, 3rd, O'Dwyer P, Hamilton SR, Issa JP. Association between DNA Methylation and Shortened Survival in Patients with Advanced Colorectal Cancer Treated with 5-Fluorouracil Based Chemotherapy. Clin Cancer Res. 2007;13:6093–8. doi: 10.1158/1078-0432.CCR-07-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward RL, Cheong K, Ku SL, Meagher A, O'Connor T, Hawkins NJ. Adverse prognostic effect of methylation in colorectal cancer is reversed by microsatellite instability. J Clin Oncol. 2003;21:3729–36. doi: 10.1200/JCO.2003.03.123. [DOI] [PubMed] [Google Scholar]
- 39.Ogino S, Nosho K, Kirkner GJ, et al. Gut 2009. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. in press (published online on 2 Oct 2008; doi.10.1136/gut.2008.155473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504–10. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- 41.Ogino S, Meyerhardt JA, Cantor M, et al. Molecular alterations in tumors and response to combination chemotherapy with gefitinib for advanced colorectal cancer. Clin Cancer Res. 2005;11:6650–6. doi: 10.1158/1078-0432.CCR-05-0738. [DOI] [PubMed] [Google Scholar]
- 42.de Heer P, Gosens MJ, de Bruin EC, et al. Cyclooxygenase 2 expression in rectal cancer is of prognostic significance in patients receiving preoperative radiotherapy. Clin Cancer Res. 2007;13:2955–60. doi: 10.1158/1078-0432.CCR-06-2042. [DOI] [PubMed] [Google Scholar]
- 43.Zlobec I, Terracciano LM, Lugli A. Local Recurrence in Mismatch Repair-Proficient Colon Cancer Predicted by an Infiltrative Tumor Border and Lack of CD8+ Tumor-Infiltrating Lymphocytes. Clin Cancer Res. 2008;14:3792–7. doi: 10.1158/1078-0432.CCR-08-0048. [DOI] [PubMed] [Google Scholar]
- 44.Henry LR, Lee HO, Lee JS, et al. Clinical implications of fibroblast activation protein in patients with colon cancer. Clin Cancer Res. 2007;13:1736–41. doi: 10.1158/1078-0432.CCR-06-1746. [DOI] [PubMed] [Google Scholar]
- 45.Ginty F, Adak S, Can A, et al. The Relative Distribution of Membranous and Cytoplasmic Met Is a Prognostic Indicator in Stage I and II Colon Cancer. Clin Cancer Res. 2008;14:3814–22. doi: 10.1158/1078-0432.CCR-08-0180. [DOI] [PubMed] [Google Scholar]
- 46.Rosen LS, Bilchik AJ, Beart RW, Jr., et al. New approaches to assessing and treating early-stage colon and rectal cancer: summary statement from 2007 Santa Monica Conference. Clin Cancer Res. 2007;13:6853s–6s. doi: 10.1158/1078-0432.CCR-07-1629. [DOI] [PubMed] [Google Scholar]
- 47.Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res. 2007;13:1472–9. doi: 10.1158/1078-0432.CCR-06-2073. [DOI] [PubMed] [Google Scholar]
- 48.Morris M, Platell C, Iacopetta B. Tumor-infiltrating lymphocytes and perforation in colon cancer predict positive response to 5-fluorouracil chemotherapy. Clin Cancer Res. 2008;14:1413–7. doi: 10.1158/1078-0432.CCR-07-1994. [DOI] [PubMed] [Google Scholar]
- 49.Cianchi F, Cortesini C, Bechi P, et al. Up-regulation of cyclooxygenase 2 gene expression correlates with tumor angiogenesis in human colorectal cancer. Gastroenterology. 2001;121:1339–47. doi: 10.1053/gast.2001.29691. [DOI] [PubMed] [Google Scholar]
- 50.Toyota M, Shen L, Ohe-Toyota M, Hamilton SR, Sinicrope FA, Issa JP. Aberrant methylation of the Cyclooxygenase 2 CpG island in colorectal tumors. Cancer Res. 2000;60:4044–8. [PubMed] [Google Scholar]