Abstract
Continuous axenic culture of Pneumocystis carinii has been achieved. A culture vessel is used that allows for frequent medium exchange without disturbance of organisms that grow attached to a collagen-coated porous membrane. The growth medium is based on Minimal Essential Medium with Earle’s salt supplemented with S-adenosyl-l-methionine, putrescine, ferric pyrophosphate, N-acetyl glucosamine, putrescine, p-aminobenzoic acid, l-cysteine and l-glutamine, and horse serum. Incubation is in room air at 31°C. The pH of the medium begins at 8.8 and rises to ≈9 as the cells grow. Doubling times calculated from growth curves obtained from cultures inoculated at moderate densities ranged from 35 to 65 hours. With a low-density inoculum, the doubling time is reduced to 19 hours. The morphology of cultured organisms in stained smears and in transmission electron micrographs is that of P. carinii, and P. carinii-specific mAbs label the cultured material. Cultured organisms are infective for immunosuppressed rats and can be stored frozen and used to reinitiate culture.
Pneumocystis carinii is an opportunistic fungal pathogen causing P. carinii pneumonia (PCP) in immunocompromised mammals. PCP is associated with AIDS, malnourished infants, and immunosuppressive drugs used for cancer, organ transplant, and rheumatic diseases (1). Although prophylaxis has reduced the incidence of PCP in AIDS patients, it remains common (2, 3) even after the introduction of improved antiretroviral therapy (4).
Research on this important pathogen has been greatly impeded by the lack of a continuous culture system despite the need for P. carinii culture having been obvious since the organism was first studied (5). This lack of progress has not been because of lack of interest and investment of resources, as there are many published reports of culture attempts (5). Incremental optimization of some aspects of short-term P. carinii culture has been achieved, but overall there has been little or no improvement in P. carinii multiplication in vitro beyond the initial description of a short-term system utilizing a cocultivated mammalian cell line in 1979 (6). Although a report of an axenic system (i. e., one not requiring cocultivation with another cell type) using a fungal culture medium was published in 1990 (7), there have been no further reports using this system by the original workers or others, so it was apparently unsuccessful. Another system using a cocultivated mammalian cell line did allow retention of infectivity for experimental animals for 42 days, although P. carinii numbers declined steadily after 10 days (8). Improved production efficiency was gained by using a suspension of beads coated with mammalian cells, but the improvement was one of scale, because total multiplication of P. carinii was not increased over other systems (9). Here we report a system that is both axenic and supports continuous multiplication in vitro.
METHODS
Culture Conditions and Medium.
The growth medium is based on Minimal Essential Medium with Earle’s salts (GIBCO) supplemented with the following: 20% horse serum (GIBCO), 500 μg/ml S-adenosyl-l-methionine sulfate (Research Biochemicals, Natick, MA), 80 μg/ml each of the following: p-aminobenzoic acid, putrescine, ferric pyrophosphate, l-cysteine, l-glutamine, and N-acetyl-d-glucosamine (Sigma) and antibiotics (500 units/ml penicillin and 500 μg/ml streptomycin; Sigma). Culture is at 31°C in normal atmosphere. Primary cultures are inoculated with P. carinii isolated from homogenized infected rat lungs (10) with penicillin (500 units/ml) and streptomycin (500 μg/ml) added to all buffers during isolation. After isolation, P. carinii are suspended in medium and pipetted into 0.4 μM pore size, collagen-coated Transwell inserts (Costar, catalogue nos. 3491, 3493, and 3495 for 24-, 12-, and 6-mm insert diameters, respectively) held in appropriate multiwell culture plates. The volume of medium containing P. carinii placed in the insert and the volume of media placed in the well below the insert are according to manufacturer’s recommendations.
Preparation of Frozen Stabilates.
Dimethyl sulfoxide and additional horse serum are added to final concentrations of 10% and 50%, respectively, and the organisms are placed in freezing tubes and put directly into liquid nitrogen.
Estimation of Growth by Measurement of Total DNA.
A method described for quantitation of malaria parasites in culture (11) was adapted for measurement of P. carinii growth in culture. This method is based on a dye (Hoechst dye 33258; Polysciences) that becomes fluorescent when conjugated to DNA. To measure DNA in P. carinii culture, organisms in the inserts are suspended by agitation, transferred to microcentrifuge tubes, and sedimented by centrifugation for 10 min at 13,000 × g. The pelleted organisms are disrupted and nucleic acids freed by vortexing after adding 25 μl of a buffer containing 6 M guanidinium HCl and 0.1 M sodium acetate, pH 5.5. To the extract is added 75 μl of a buffer composed of 3.3 μg/ml dye, 2 M NaCl, and 50 mM Tris⋅HCl, pH 7.8. The 100 μl samples are transferred to HPLC autosampler vials and loaded into an HPLC system with the column removed (Waters model 715 autosampler, MS 625 pump, 470 fluorescence detector, controlled by Waters millennium software). The mobile phase is 140 mM sodium acetate and 17 mM triethanolamine, pH 5.05. Fluorescence detector excitation is set at 265 λ and emission at 395 λ. The injection volume is 70 μl and a “chromatogram” is collected. The area under the “void volume peak” is integrated and becomes a raw measure of the DNA in the original culture well.
This method was calibrated and validated by making four sets of independent paired measurements P. carinii nuclei in Giemsa-stained slides counted by microscope (12) and the DNA quantitation method. P. carinii cells isolated from infected rat lungs were used for calibration rather than cultured cells because the lung homogenization and isolation process helps separate the cells allowing them to be counted more readily (10). When all four sets of measurements were plotted together, the correlation coefficient was >0.99.
Estimation of Growth by Measurement of Total ATP.
In principle, this ATP assay is similar to those used by others to measure the number of P. carinii in a suspension (13–15). After suspending cultured organisms as for DNA analysis, a cell extract is prepared by one of two methods. One method (used for data in Fig. 1a) involved centrifugation of the organisms, resuspension in ATP-free water (Sigma), and sonication for 10 min (Heat Systems/Ultrasonics model W380 at full output and 40% duty cycle). The other (Fig. 1b) involved direct sonication of the suspended culture, treatment in a boiling water bath for 3 min, microcentrifugation for 10 min and collection of the supernatant. For the assay, 10 μl of cell extract and 90 μl of ATP-free water are added to microcentrifuge tubes. The tubes are placed into the luminometer (model FB12, Xylux, Maryville, TN, controlled by a computer running manufacturer-supplied software) and 100 μl of luciferin/luciferase reagent (Firelight LB, Analytical Luminescence Laboratory, San Diego) are automatically injected by an attached pump. Beginning 20 sec after injection of the reagent, photons are automatically counted for 20 sec. A standard curve of “relative light units” (i.e., machine output) vs. known amounts of ATP is obtained before each day’s set of analyses and a correlation coefficient of ≥0.99 is required. We find that the yield of ATP is greatly altered by details of the extraction process.
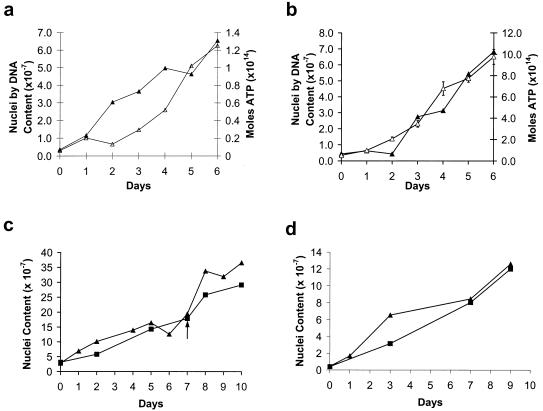
Figure 1.
Growth curves of P. carinii in axenic culture. Each data point in each curve represents an individual culture inoculated on day 0 and harvested at the indicated day. Three different means of growth assessment were used: total DNA (▴), total ATP (▵), and counts of P. carinii nuclei (■); when compared with other methods, the total DNA method always indicated the same overall rate of growth. The ATP scale differs in a and b because different methods of ATP extraction were used; because the extraction method used for b produced a more sensitive assay, it was possible to perform multiple analyses of the ATP for each culture, and error bars were calculated. After thawing and before being used to inoculate well inserts, the stock was grown for 21 days with 3 subcultures (a), 81 days with 9 subcultures (b), 88 days with 10 subcultures (c), and 96 days with 12 subcultures (d). The arrow in c indicates that an additional subculture was performed during the experiment; a dilution was made to reduce the cell concentration to the concentration on day 0, and subsequent time points were corrected by this dilution factor.
Electron Microscopic Examination.
Cultured organisms are fixed for electron microscope examination by suspension in a buffer composed of 0.15 M Na/K phosphate (pH 8.0), 4% paraformaldehyde, 3.1% glutaraldehyde, and 2.5% dimethyl sulfoxide. After an initial 15-min fixation at room temperature and sedimentation by microcentrifugation, three changes of cold fixative are made over several hours followed by rinsing with the same buffer without paraformaldehyde or glutaraldehyde. Postfixation is with 1% OsO4 and 1.5% K3Fe(CN)6 in water on ice for 2 hours. After rinsing, standard procedures are followed and sections are examined in a Zeiss EM 910 microscope.
RESULTS AND DISCUSSION
Because P. carinii in axenic culture grows in clumps, it is difficult to use the traditional method of enumerating cells by counting nuclei in Giemsa-stained smears. Therefore, the primary method used was to measure the total amount of DNA in culture lysates and a secondary method was an assay for the total amount of ATP in culture lysates. Any DNA or ATP remaining from the source used for the initial culture inoculation would have been diluted out by the early subcultures. Even if DNA or ATP had remained from the initial inoculum, such inert material could not increase, so it could not interfere with growth assessment. Both of these methods have the advantages of greater precision and more objectivity and are established, reliable means of monitoring microbial growth in culture—provided the culture is truly axenic, i.e., there are no other types of cells contaminating the culture. Several lines of evidence indicate that this condition was satisfied. Giemsa-stained smears (12) were prepared each time a subculture was made and each time cell numbers were measured by DNA or ATP content. Microscopic examinations never revealed another cell type after the first several subcultures. This allowed us to eliminate completely the possibility that the cultures were contaminated with host cells and most other microbes. Because Mycoplasma species can be introduced into a culture by a variety of routes and these small bacteria are not readily detected by light microscopy, cultures were tested repeatedly for Mycoplasma species infection by using two different types of commercial systems (Immu-Mark Myco test, ICN and Mycoplasma T. C., Gen-Probe, San Diego); in the culture lines reported here, results were always negative. Furthermore, no structures similar to Mycoplasma were observed in electron micrograph preparations. In addition to the negative data for contaminating microbes, the cultured material was positively identified by using a commercially obtained set of three fluorescein isothiocyanate-conjugated mAbs specific for P. carinii (Chemicon, catalog no. 910F). By using phase contrast microscopy in combination with fluorescence, we observed that all of the material in each of five samples from several stocks of P. carinii was stained with the antibodies (data not shown). Finally, for two growth curves (Fig. 1 c and d), essentially parallel curves were obtained with the classic method of counting nuclei in Giemsa-stained smears and the DNA-measurement method. Barring the unlikely presence of an undetected microbe growing at the same rate as P. carinii, these parallel curves indicate that the cultures were axenic.
Fig. 1 presents a series of growth curves from a single stock of P. carinii established with organisms isolated from an infected rat lung (10). After initiation of the primary culture, the stock was maintained for 9 days with one subculture, preserved as a frozen stabilate, and reestablished in culture for an additional 21 days with three subcultures before being used to generate the data presented in Fig. 1. Additional subcultures were made during the intervals between growth curves as noted in the legend. To obtain each of these growth curves in Fig. 1, a series of parallel cultures in 6-mm-diameter inserts was inoculated with aliquots of the same suspension of P. carinii; at the days indicated, the individual cultures were harvested and the amount of P. carinii measured. This approach was necessary to avoid repeated disruption of P. carinii attachment to the supporting membrane, which would have occurred if a single culture were sampled repeatedly. Growth assessment by measurement of the total DNA was used for each curve presented. For two curves (Fig. 1 a and b) total ATP in the culture was also used. As noted above, conventional counting of P. carinii nuclei by microscopic examination of Giemsa-stained smears (12) was used for two curves (Fig. 1 c and d).
P. carinii doubling times were 35, 36, 65, and 44 hours (Fig. 1 a–d, respectively). However, cultures can be maintained by weekly or even less frequent splitting which allows for only a much slower growth with a doubling time equal to the weekly 1:1 split interval; i. e., 168 hours. Conversely, cultures started with very small inocula produced more rapid growth. Using 24-mm-diameter inserts, cultures were inoculated with 3.7, 18.4, and 36.9 (103) P. carinii. After 7 days of growth, the cultures contained 3.4, 4.3, and 4.9 (106) cells, respectively. For comparison, the inocula for the cultures used for the growth curves in Fig. 1 ranged from ≈2 to 30 (106) organisms in 6-mm inserts producing initial densities >1,000-fold higher. The low-inoculum cultures increased in density by factors of 920, 230, and 130, respectively, over the 7-day period as determined by measurement of total DNA. The 920-fold increase over 7 days calculates to an average doubling time of about 19 hours, the 230-fold increase to 21 hours, and the 130-fold increase to 24 hours. This response to inoculum size correlates with the fact that (among the growth curves presented) the culture using the highest inoculum (Fig. 1c) produced the slowest growth. It is not surprising that a cell that grows attached to a substrate or to other cells divides more slowly as the culture becomes crowded and membrane space becomes limited. Undoubtedly, if conditions allowing unrestricted (exponential) growth were devised, a P. carinii doubling time even shorter than 19 hours would be observed. However, there are limitations in this culture system at the current state of development such that no growth was observed after dilutions to the point that only 5–10 clusters of P. carinii could be observed on the membrane of an insert on examination with an inverted microscope.
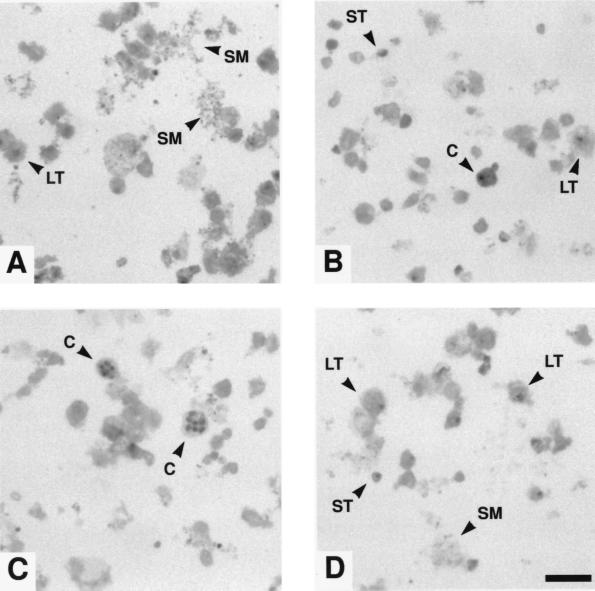
Fig. 2 presents photomicrographs of Giemsa-stained smears, and Fig. 3 presents electron micrographs of cultured P. carinii. These are similar in appearance to micrographs of P. carinii isolated from infected rat lungs.
Figure 2.
Giemsa-stained smears of cultured P. carinii. These organisms had been maintained for 50 days with 6 subcultures. Typical large trophozoites (LT) are shown in A, B, and D, and small trophozoites (ST) are shown in B and D. Intermediate size forms are visible in all panels. Cysts (C) can be seen in B and C. The labeled cyst in B has what appears to be a bud, residual trophozoite cytoplasm, or attached membranous material; although the nature of this is uncertain, such structures appear frequently. What seems to be shed material (SM), most likely membranous in nature, can always be seen in stained smears and is labeled in A and D. (Bar = 10 μm.)
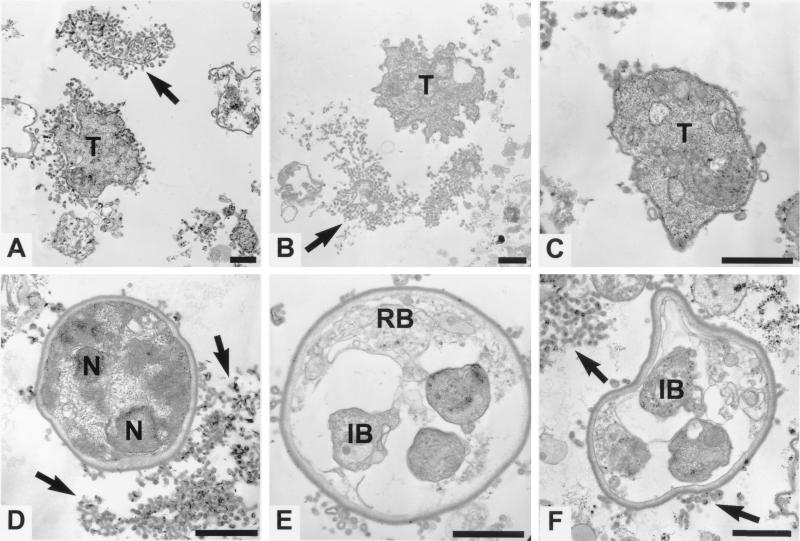
Figure 3.
Transmission electron micrographs of cultured P. carinii. Before fixation, these organisms had been grown for 65 days with 7 subcultures. Trophozoites (T) with varying degrees of tubular extensions and crenations can be seen in A–C; D–F show cysts. Arrows point to membranous material that in some cases seem to be typical P. carinii cell membrane tubular extensions but in other cases may be similar material that has been shed from the organisms (B and D). D contains an immature cyst that has a typical thick cyst wall and multiple nuclei (N) but no developed intracystic bodies. The thickening of the cell membrane of the trophozoite in C may indicate an early stage in cyst formation. E and F show mature cysts with intracystic bodies (IB). A residual body (RB) can be seen in the cyst in E. (Bars = 1 μm.)
The data in Table 1 are presented to demonstrate that organisms grown in culture are infectious for immunosuppressed rats and produce PCP. Lung weights are included because an increase in lung weight compared with the controls is an independent indication of pneumonia. The mean lung weights in the table relate directly to the mean number of P. carinii in the lungs, confirming the development of PCP in the rats. From the ratio of the total inoculum to the total P. carinii burden in the lungs after 3 weeks, it is clear that the extent of multiplication within the animals was not the same for all inocula. The ratio of the total P. carinii cells in the control inocula prepared from frozen infected lungs to the P. carinii in the lungs at sacrifice 3 weeks later is 2.1. The equivalent ratio resulting from inoculation with cells in culture for 15 days is 10, and for cells in culture for 62 days the ratio is 16. Generally, the P. carinii we isolate from frozen lungs are 80–95% viable as indicated by intact cell membranes and enzymatically active cytoplasm (unpublished observation), so the small number of nonviable P. carinii in frozen-lung homogenate cannot account for the reduced multiplication in vivo. There is, however, an inverse relationship of inoculum size and multiplication in vivo, an observation parallel to what we observed in vitro. The data clearly show that cultured P. carinii cells are fully infective for immunosuppressed rats.
Table 1.
Infectivity of cultured organisms
| Inoculation source | n | Organisms for first inoculation,* ×104 | Organisms for second inoculation, ×104 | Mean lung weights, g† | P. carinii × 105 per lung‡ |
|---|---|---|---|---|---|
| Homogenate of infected frozen lung | 4 | 7.04 | 9.08 | 1.53 ± 0.15 | 3.38 ± 2.24 |
| P. carinii from 15-day culture | 5 | 2.30 | 1.89 | 1.56 ± 0.18 | 4.23 ± 2.07 |
| P. carinii from 62-day culture | 5 | 0.26 | 0.36 | 1.38 ± 0.13 | 1.01 ± 0.63 |
| Saline control | 5 | 0.0 | 0.0 | 1.14 ± 0.14 | 0.0 |
P. carinii cells were counted by microscopic examination (11). Homogenization of frozen lungs, rat immunosuppression to allow development of PCP, and P. carinii inoculation were as described (12). To avoid latent P. carinii infection commonly present in rats before immunosuppression, the test animals were treated for 3 weeks with the combination of trimethroprim and sulfamethoxazole (50 and 250 mg/kg, respectively). Two inoculations 3 days apart were used to reduce variations in the intensity of subsequent PCP.
PCP causes an increase in lung weight due to the presence of the organisms, foamy exudate filling the alveoli, and inflammation, so that the degree of increased lung weight compared to the control reflects the degree of the pneumonia in the animal (12). Lung weights were taken at the time of sacrifice (27 days after the first inoculation). Values are given as mean ± SEM.
Lung homogenates and P. carinii cell counts at the time of sacrifice were prepared as described (11).
Success of the current system in maintaining continuous axenic culture of P. carinii depends on several factors. Twice-daily medium exchange is important and is facilitated by use of a culture vessel composed of an insert with a collagen-coated porous-membrane bottom held in a multiwell plate. The medium within the insert, containing P. carinii, is in contact with medium in the well; therefore, providing fresh medium requires only replacement of fluid in the well, allowing P. carinii adhering to the collagen-coated membrane to remain undisturbed. Adherence is necessary, since no growth was obtained in uncoated inserts. With 26 mM NaHCO3 in the Minimal Essential Medium, omission of a CO2 atmosphere creates a starting pH of 8.8, which rises to >9.0, the extent of the rise depending on the density of the culture. Supplements in this medium that had not been previously tested for support of P. carinii growth include S-adenosyl-l-methionine, putrescine, and ferric pyrophosphate. Other medium supplements (N-acetyl glucosamine, p-aminobenzoic acid, l-cysteine, l-glutamine, and horse serum) have been used previously, although not in this combination. Incubation at 31°C proved useful during development.
The data presented in this report were collected with a single stock of P. carinii, but many additional stocks have been prepared. Indeed, to date, every attempt at primary culture has been successful unless there was initial microbial contamination in the P. carinii isolated from rat lungs. Preliminary experiments with P. carinii isolated from bronchial alveolar fluid (obtained from patients for diagnosis of P. carinii pneumonia) suggest that human P. carinii grows as well in this system as P. carinii derived from rats.
The work performed to date demonstrates continuous axenic culture, but we expect that the system is not fully optimized. Qualitative observations indicate that at least some of the conditions and medium additives we use are helpful or possibly essential. Twice-daily medium exchange improves growth, but we have not determined at what cell density this becomes necessary. S-adenosyl-l-methionine clearly enhances P. carinii growth as well as the production and maturation of cysts and intracystic bodies. Inclusion of this nutrient was suggested by previous work showing stimulation of nucleic acid and protein biosynthesis in Saccharomyces cerevisae culture as well as outgrowth of ascospores (17). Because we found that the strong iron chelator deferoxamine is an active anti-PCP agent in an animal model (16, 18–20), we added the soluble, relatively weak chelate ferric pyrophosphate to provide an available source of iron, as was done for Legionella pneumophila culture (21). The requirement for P. carinii to be attached to a surface has been noted many times but always with reference to a host cell or, in culture, a mammalian cell feeder layer (22–27). Our finding that collagen-coated inserts support P. carinii growth but uncoated inserts do not leads us to speculate that the function of mammalian cells in previously reported short-term culture systems was to provide a suitable substrate for attachment. We have no indication that 31°C is the most appropriate incubation temperature, although it was helpful in initial culture attempts and is used currently. The addition of N-acetyl glucosamine was suggested by earlier culture work (8). P. carinii can grow if ferric pyrophosphate, p-aminobenzoic acid, or putrescine supplements are individually omitted, but these additions do seem to enhance growth.
In summary, we present a system which allows P. carinii to be cultured axenically and continuously, thus providing a starting point for optimization of culture conditions and improvement of efficiency. Continuous, axenic culture of P. carinii will allow, among other things, establishment of banks of isolates, investigations of the basis for species specificity, studies of the metabolism of growing organisms, detailed examination of the binding of P. carinii to supportive substrates, exploration of cell biology and life cycle stages, detection of drug resistance in clinical isolates, and scrutiny of the environment for viable, infectious P. carinii. With some modifications, perhaps cloning of individual P. carinii organisms can be achieved, making genetic manipulation feasible.
Acknowledgments
This work was supported by U. S. Public Health Service/National Institutes of Health Grant RO1 AI38825.
ABBREVIATION
- PCP
Pneumocystis carinii pneumonia
References
- 1.Fishman J A. Antimicrob Agents Chemother. 1998;42:995–1004. doi: 10.1128/aac.42.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bozzette S A, Finkelstein D M, Spector S A, Frame P, Powderly W G, Weili H, Craven D, Van der Horst C, Feinberg J. N Engl J Med. 1995;322:693–699. doi: 10.1056/NEJM199503163321101. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson, M. A. & French, M. (1998) AIDS12, Suppl. A, S157–S163. [PubMed]
- 4.Sepkowitz K A. Clin Infect Dis. 1996;26:806–810. doi: 10.1086/513920. [DOI] [PubMed] [Google Scholar]
- 5.Sloand E, Laughon B, Armstrong M, Bartlett M S, Blumenfeld W, Cushion M, Kalica A, Kovacs J A, Martin W, Pitt E, et al. J Eukaryotic Microbiol. 1993;40:188–195. doi: 10.1111/j.1550-7408.1993.tb04902.x. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett M S, Verbanac P A, Smith J W. J Clin Microbiol. 1976;10:796–799. doi: 10.1128/jcm.10.6.796-799.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cushion M T, Ebbets D. J Clin Microbiol. 1990;28:1385–1394. doi: 10.1128/jcm.28.6.1385-1394.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirovsky P, Fishman J A. J Infect Dis. 1993;167:1470–1473. doi: 10.1093/infdis/167.6.1470. [DOI] [PubMed] [Google Scholar]
- 9.Lee C-H, Bauer N L, Shaw M M, Durkin M M, Bartlett M S, Queener S F, Smith J W. J Clin Microbiol. 1993;31:1659–1662. doi: 10.1128/jcm.31.6.1659-1662.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merali S, Clarkson A B., Jr Antimicrob Agents Chemother. 1996;49:973–978. doi: 10.1128/aac.40.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smeijsters L J, Zijlstra N M, Franssen F F, Overdulve J P. Antimicrob Agents Chemother. 1996;40:835–838. doi: 10.1128/aac.40.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarić M, Clarkson A B., Jr Antimicrob Agents Chemother. 1994;38:2545–2552. doi: 10.1128/aac.38.11.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cushion M T, Chen F, Kloepfer N. Antimicrob Agents Chemother. 1997;41:379–384. doi: 10.1128/aac.41.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyahira Y, Kakeuchi T. Comp Biochem Physiol A Physiol. 1991;100:1031–1034. doi: 10.1016/0300-9629(91)90332-7. [DOI] [PubMed] [Google Scholar]
- 15.Chen F, Cushion M T. J Clin Microbiol. 1994;32:2791. doi: 10.1128/jcm.32.11.2791-2800.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merali S, Chin K, Grady R W, Weissberger L, Clarkson A B., Jr Antimicrob Agents Chemother. 1995;39:1442–1444. doi: 10.1128/aac.39.7.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brawley J W, Ferro A J. J Bacteriol. 1980;142:608–614. doi: 10.1128/jb.142.2.608-614.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarkson A B, Jr, Sarić M, Grady R W. Antimicrob Agents Chemother. 1990;34:1833–1835. doi: 10.1128/aac.34.9.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merali S, Chin K, Del Angel L, Grady R W, Armstrong M, Clarkson A B., Jr Antimicrob Agents Chemother. 1995;39:2023–2026. doi: 10.1128/aac.39.9.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merali S, Chin K, Grady R W, Clarkson A B., Jr Antimicrob Agents Chemother. 1996;40:1298–1300. doi: 10.1128/aac.40.5.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang W L, Blaser M J, Cravens J, Johnson M A. Ann Intern Med. 1979;90:614–618. doi: 10.7326/0003-4819-90-4-614. [DOI] [PubMed] [Google Scholar]
- 22.Atzori C, Bruno A, Agostoni C, Novati S, Batti S, Scaglia M. Parasite. 1996;3:183–185. doi: 10.1051/parasite/1996032183. [DOI] [PubMed] [Google Scholar]
- 23.Pottratz S T, Weir A L. Exp Cell Res. 1995;221:357–362. doi: 10.1006/excr.1995.1385. [DOI] [PubMed] [Google Scholar]
- 24.Bartlett M S, Goheen M P, Lee C-H, Shaw M M, Durkin M M, Smith J W. Parasitol Res. 1994;80:208–215. doi: 10.1007/BF00932676. [DOI] [PubMed] [Google Scholar]
- 25.Aliouat E M, Dei-Cas E, Ouaissi A, Palluault F, Soulez B, Camus D. Biol Cell. 1993;77:209–217. doi: 10.1016/s0248-4900(05)80190-x. [DOI] [PubMed] [Google Scholar]
- 26.Garner R E, Walker A N, Horst M N. J Clin Microbiol. 1992;30:2467–2470. doi: 10.1128/jcm.30.9.2467-2470.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dei-Cas E, Jackson H, Palluault F, Aliouat E M, Hancock V, Soulez B, Camus D. J Protozool. 1991;38:205S–207S. [PubMed] [Google Scholar]