Abstract
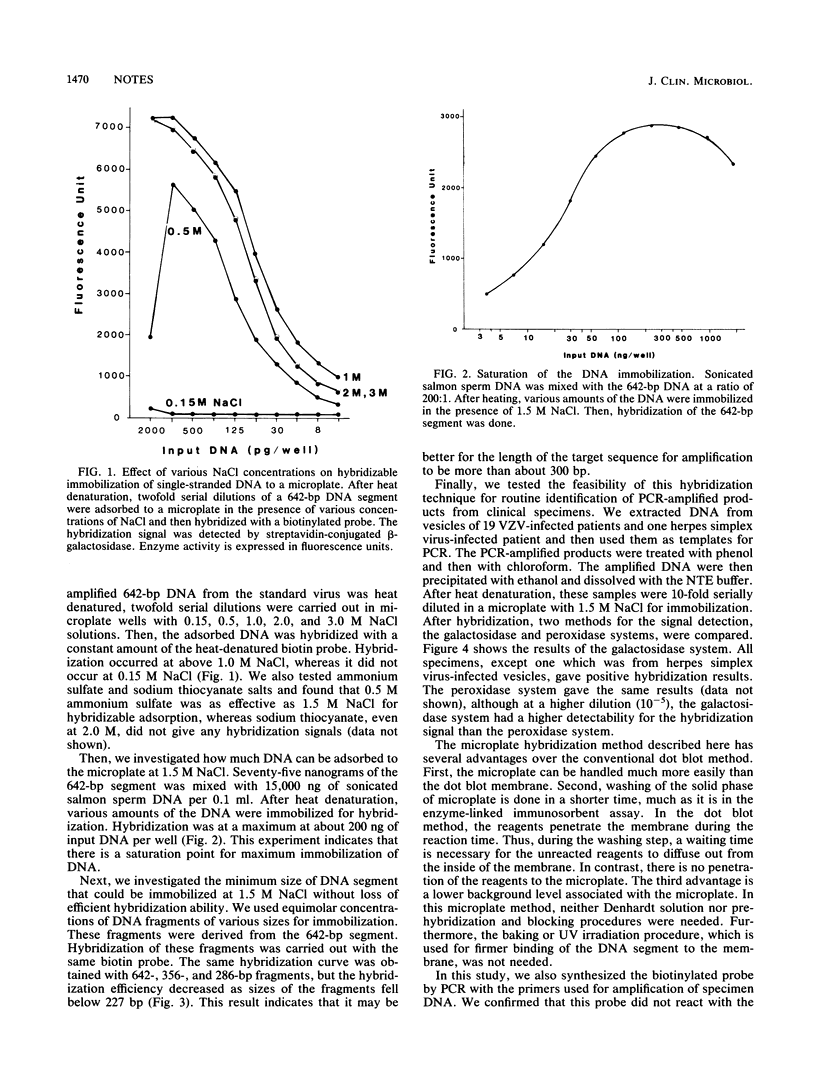
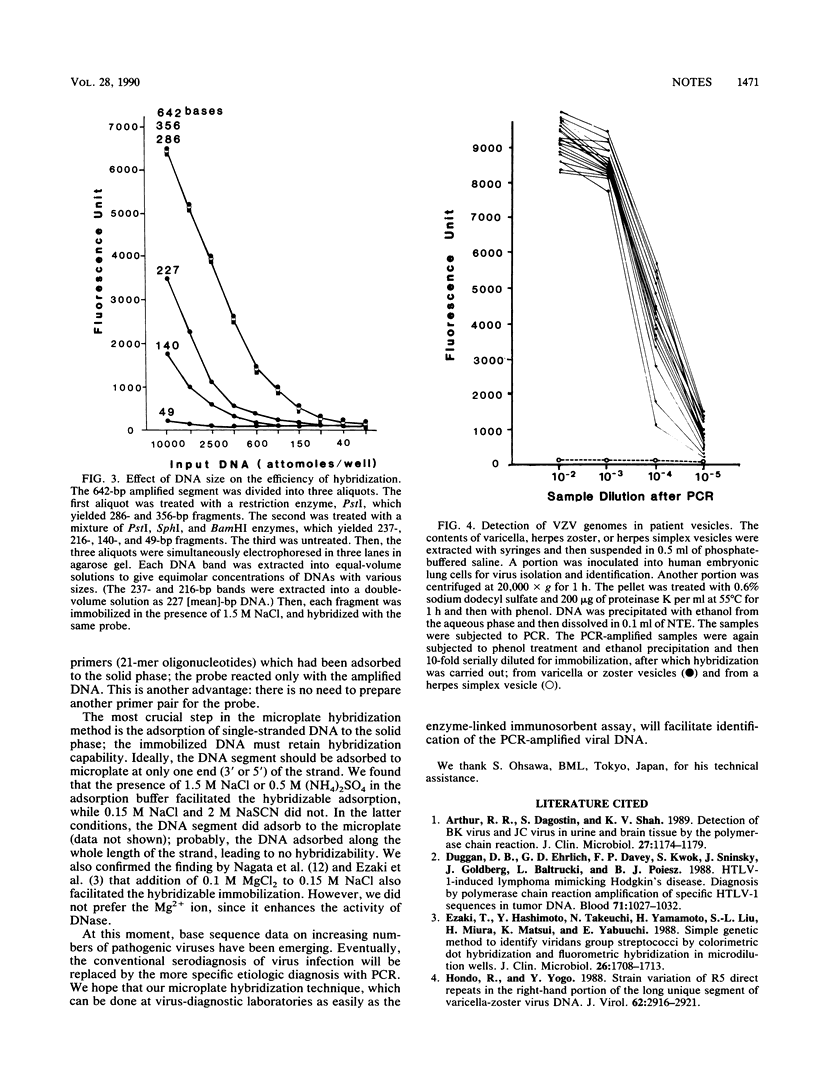
We have developed a simple hybridization method for a DNA segment which is amplified by the polymerase chain reaction: after heat denaturation, the amplified DNA segment with a length of more than 300 bases is adsorbed to microplate wells in the presence of 1.5 M NaCl or 0.5 M ammonium sulfate; the immobilized DNA is hybridized with a biotin-labeled DNA probe; then, the hybridization signal is detected by streptavidin-conjugated beta-galactosidase or peroxidase. This method has several advantages over the conventional dot blot hybridization method: (i) radioisotopes are not used, (ii) synthetic oligonucleotide for the probe is not needed, (iii) the time required for washing of the solid phase is greatly reduced, and (iv) the baking and prehybridization procedures are eliminated. By this method, we were able to detect viral genomes in vesicle specimens from patients infected with varicella-zoster virus.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur R. R., Dagostin S., Shah K. V. Detection of BK virus and JC virus in urine and brain tissue by the polymerase chain reaction. J Clin Microbiol. 1989 Jun;27(6):1174–1179. doi: 10.1128/jcm.27.6.1174-1179.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan D. B., Ehrlich G. D., Davey F. P., Kwok S., Sninsky J., Goldberg J., Baltrucki L., Poiesz B. J. HTLV-I-induced lymphoma mimicking Hodgkin's disease. Diagnosis by polymerase chain reaction amplification of specific HTLV-I sequences in tumor DNA. Blood. 1988 Apr;71(4):1027–1032. [PubMed] [Google Scholar]
- Ezaki T., Hashimoto Y., Takeuchi N., Yamamoto H., Liu S. L., Miura H., Matsui K., Yabuuchi E. Simple genetic method to identify viridans group streptococci by colorimetric dot hybridization and fluorometric hybridization in microdilution wells. J Clin Microbiol. 1988 Sep;26(9):1708–1713. doi: 10.1128/jcm.26.9.1708-1713.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondo R., Yogo Y. Strain variation of R5 direct repeats in the right-hand portion of the long unique segment of varicella-zoster virus DNA. J Virol. 1988 Aug;62(8):2916–2921. doi: 10.1128/jvi.62.8.2916-2921.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondo R., Yogo Y., Yoshida M., Fujima A., Itoh S. Distribution of varicella-zoster virus strains carrying a PstI-site-less mutation in Japan and DNA change responsible for the mutation. Jpn J Exp Med. 1989 Dec;59(6):233–237. [PubMed] [Google Scholar]
- Hsia K., Spector D. H., Lawrie J., Spector S. A. Enzymatic amplification of human cytomegalovirus sequences by polymerase chain reaction. J Clin Microbiol. 1989 Aug;27(8):1802–1809. doi: 10.1128/jcm.27.8.1802-1809.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok S., Mack D. H., Mullis K. B., Poiesz B., Ehrlich G., Blair D., Friedman-Kien A., Sninsky J. J. Identification of human immunodeficiency virus sequences by using in vitro enzymatic amplification and oligomer cleavage detection. J Virol. 1987 May;61(5):1690–1694. doi: 10.1128/jvi.61.5.1690-1694.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larzul D., Guigue F., Sninsky J. J., Mack D. H., Bréchot C., Guesdon J. L. Detection of hepatitis B virus sequences in serum by using in vitro enzymatic amplification. J Virol Methods. 1988 Jul;20(3):227–237. doi: 10.1016/0166-0934(88)90126-7. [DOI] [PubMed] [Google Scholar]
- Loche M., Mach B. Identification of HIV-infected seronegative individuals by a direct diagnostic test based on hybridisation to amplified viral DNA. Lancet. 1988 Aug 20;2(8608):418–421. doi: 10.1016/s0140-6736(88)90412-6. [DOI] [PubMed] [Google Scholar]
- Melchers W. J., Schift R., Stolz E., Lindeman J., Quint W. G. Human papillomavirus detection in urine samples from male patients by the polymerase chain reaction. J Clin Microbiol. 1989 Aug;27(8):1711–1714. doi: 10.1128/jcm.27.8.1711-1714.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis K., Faloona F., Scharf S., Saiki R., Horn G., Erlich H. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):263–273. doi: 10.1101/sqb.1986.051.01.032. [DOI] [PubMed] [Google Scholar]
- Nagata Y., Yokota H., Kosuda O., Yokoo K., Takemura K., Kikuchi T. Quantification of picogram levels of specific DNA immobilized in microtiter wells. FEBS Lett. 1985 Apr 22;183(2):379–382. doi: 10.1016/0014-5793(85)80814-0. [DOI] [PubMed] [Google Scholar]
- Olive D. M., Simsek M., Al-Mufti S. Polymerase chain reaction assay for detection of human cytomegalovirus. J Clin Microbiol. 1989 Jun;27(6):1238–1242. doi: 10.1128/jcm.27.6.1238-1242.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou C. Y., Kwok S., Mitchell S. W., Mack D. H., Sninsky J. J., Krebs J. W., Feorino P., Warfield D., Schochetman G. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988 Jan 15;239(4837):295–297. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Shibata D. K., Arnheim N., Martin W. J. Detection of human papilloma virus in paraffin-embedded tissue using the polymerase chain reaction. J Exp Med. 1988 Jan 1;167(1):225–230. doi: 10.1084/jem.167.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]


